Abstract
Docosahexaenoic acid (DHA) is an omega-3 fatty acid that is considered to have applications in cancer prevention and treatment. The beneficial effects of DHA against cancer metastasis are well established; however, the mechanisms underlying these effects in breast cancer are not clear. Cell invasion is critical for neoplastic metastasis, and involves the degradation of the extracellular matrix by matrix metalloproteinase (MMP)-9. The present study investigated the inhibitory effect of DHA on MMP-9 expression and cell invasion induced by 12-O-tetradecanoylphorbol-13-acetate (TPA) in the MCF-7 breast cancer cell line. DHA inhibited the TPA-induced activation of mitogen-activated protein kinase (MAPK) and the transcription of nuclear factor (NF)-κB, but did not inhibit the transcription of activator protein-1. DHA increased the activity of peroxisome proliferator-activated receptor (PPAR)-γ, an effect that was reversed by the application of the PPAR-γ antagonist GW9662. In addition, combined treatment with GW9662 and DHA increased NF-κB-related protein expression. These results indicate that DHA regulates MMP-9 expression and cell invasion via modulation of the MAPK signaling pathway and PPAR-γ/NF-κB activity. This suggests that DHA could be a potential therapeutic agent for the prevention of breast cancer metastasis.
Keywords: DHA, MMP-9, PPAR-γ, invasion, NF-κB, MCF-7
Introduction
Docosahexaenoic acid (DHA) is an omega (ω)-3 fatty acid (22:6Δ4,7,10,13,16,19) and a member of a family of compounds known to possess multiple benefits for human health, including anticancer properties (1). Epidemiological evidence strongly links fish oil, which is rich in DHA and eicosapentaenoic acid (EPA), with a reduced incidence of several types of cancer, including breast cancer (2,3). Epidemiological studies, dietary studies in mice and humans, and tissue culture studies have substantiated the beneficial role of DHA in breast cancer prevention (4–7).
Cancer treatment failure largely occurs due to cancer cell proliferation, invasion and metastasis, which ultimately lead to mortality. Invasion and metastasis are the major causes of morbidity and mortality in breast cancer patients (3). Metastasis involves the penetration of cancer cells into the extracellular basement membrane by proteolytic degradation of components of the extracellular matrix (ECM) (8), including type IV collagen, laminin, heparin sulfate proteoglycan, nidogen and fibronectin (9), which normally provide biochemical and mechanical barriers to cell movement (10).
ECM degradation requires extracellular proteinases such as matrix metalloproteinases (MMPs), a group of zinc and calcium-dependent endopeptidases that can be divided into different subclasses (including collagenases, gelatinases and stromelysins) based on their substrate (8). MMP-9 is directly associated with invasion, metastasis and poor prognosis in breast cancer (11,12). MMP-9 is stimulated by growth factors (including fibroblast growth factor 2, epidermal growth factor and hepatocyte growth factor), cytokines (such as tumor necrosis factor α), oncogenes (such as Ras) and 12-O-tetradecanoylphorbol-13-acetate (TPA) (13–17). TPA is a selective activator of protein kinase C (14). TPA can stimulate MMP-9 synthesis and secretion during breast cancer cell invasion (18,19). Cytokine and TPA treatment induce MMP-9 expression via activation of transcription factors such as nuclear factor (NF)-κB and activator protein (AP)-1 (20–22), since the MMP-9 gene promoter contains binding sites for both factors (23). Mitogen-activated protein kinase (MAPK) signaling is important for AP-1 and NF-κB activation, and requires nuclear factor of κ light polypeptide gene enhancer in B cells inhibitor (IκB) kinase, extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) or p38 MAPK, depending on the cell type (17,24,25). Thus, inhibiting MMP-9 expression and/or its upstream regulatory pathways may aid in the treatment of malignant tumors, including breast carcinoma.
Peroxisome proliferator-activated receptors (PPARs) belong to the nuclear receptor superfamily of ligand-activated transcription factors. Three types of PPARs [-α, -β and -δ (β)] have been identified thus far (26). PPAR-γ is expressed in various cell types and tissues, and is important in the regulation of inflammatory (27) and immune reactions (28), as well as in NF-κB activation (29). In a previous study, agents that inhibit MMP-9 expression in MCF-7 cells were described (30–32). The present study reports for the first time that increased PPAR-γ expression inhibits breast cancer metastasis via regulation of NF-κB activation. These results provide an insight into the anti-cancer actions of an ω-3 fatty acid, which could aid in the development of new cancer therapeutic strategies involving the use of fish oil as a dietary supplement.
Materials and methods
Cells and reagents
MCF-7 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics at 37°C in a 5% CO2 incubator. TPA, cis-4,7,10,13,16,19-DHA, MTT, GW9662 (a PPAR-γ antagonist) and anti-β-actin antibody (catalog no. A5441) were obtained from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). Antibodies against p38 (catalog no. 9212), phosphorylated (p)-p38 (catalog no. 9211), JNK (catalog no. 9252), p-JNK (catalog no. 9251), ERK (catalog no. 9102) and p-ERK (catalog no. 9101) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Antibodies against PPAR-γ (catalog no. 7169), MMP-9 (catalog no. 12759), p50 (catalog no. 7178), p65 (catalog no. 372) and proliferating cell nuclear antigen (catalog no. 7907), and horseradish peroxidase (HRP)-conjugated immunoglobulin (Ig)G (catalog no. 2004, 2005) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). [γ-32P] adenosine triphosphate was obtained from GE Healthcare Life Sciences (Chalfont, UK). High glucose DMEM, FBS and PBS were acquired from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Cell viability assay
The effect of DHA on MCF-7 cell viability was determined by MTT assay. A stock solution of 200 mM DHA was prepared in dimethylsulfoxide (DMSO) and diluted with DMEM prior to the experiments. Briefly, 3xl04 cells/well were seeded in 96-well plates and incubated at 37°C for 24 h to allow attachment. Cells were then left untreated, or were treated with 50, 100 and 200 µM DHA for 24 h at 37°C. Cells were next washed with PBS prior to the addition of MTT (0.5 mg/ml in PBS), and incubated at 37°C for 30 min. Formazan crystals were dissolved with DMSO (100 µl/well), and absorbance was measured at 570 nm using a Model 3550 microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Western blot analysis
MCF-7 cells (5×105) were pre-treated with 50 and 100 µM DHA for 1 h, and then incubated with TPA for 24 h at 37°C. Cells were lysed with ice-cold M-PER® Mammalian Protein Extraction Reagent (Pierce; Thermo Fisher Scientific, Inc.). The protein concentration in the lysate was determined by the Bradford method (33). Samples (20 µg) were separated by 10% SDS-PAGE and transferred to Hybond polyvinylidene difluoride membranes (GE Healthcare Life Sciences). Membranes were blocked for 2 h with 2% bovine serum albumin (Sigma-Aldrich; Merck Millipore) or 5% skimmed milk, and then incubated overnight at 4°C with primary antibodies at 1:2,000 dilution, followed by incubation with HRP-conjugated IgG at 1:2,000 dilution for 2 h at 4°C. Protein expression levels were measured by signal analysis using an image analyzer (Fujifilm, Tokyo, Japan) and specific immunoreactive signals were visualized with an enhanced chemiluminescence kit (GE Healthcare Life Sciences).
Gelatin zymography assay
Conditioned media were collected after 24 h of cell stimulation, mixed with non-reducing sample buffer and resolved by PAGE containing 0.1% (w/v) gelatin. The gel was washed at room temperature for 30 min with 2.5% Triton X-100 solution, and incubated at 37°C for 16 h in 5 mM CaCl2, 0.02% Brij (Sigma-Aldrich; Merck Millipore) and 50 mM Tris-HCl (pH 7.5). The gel was stained for 30 min with 0.25% (w/v) Coomassie Brilliant Blue in 40% (v/v) methanol/7% (v/v) acetic acid, and photographed on an image analyzer (Fujifilm). Proteolysis was imaged as a white zone in a dark blue field. Densitometric analysis was performed using MultiGauge image analysis software (version 3.0; Fujifilm).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using a FastPure RNA kit (Takara Bio, Inc., Otsu, Japan). The RNA concentration and purity were assessed by measuring the absorbance value at 260/280 nm. Complementary DNA was synthesized from 1 µg total RNA using a PrimeScript RT reagent kit (Takara Bio, Inc.) at 37°C for 15 min and 85°C for 5 sec. MMP-9 and GAPDH messenger (m)RNA expression were determined by qPCR using the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems; Thermo Fisher Scientific, Inc.) and SYBR Green PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) with the following sense and antisense primer sets: MMP-9 (NM_004994), 5′-CCTGGAGACCTGAGAACCAATCT-3′ (forward) and 5′-CCACCCGAGTGTAACCATAGC-3′ (reverse), and GAPDH (NM_002046), 5′-ATGGAAATCCCATCACCATCTT-3′ (forward) and 5′-CGCCCCACTTGATTTTGG-3′ (reverse). To control for variation in mRNA concentration, the results were normalized to the level of GAPDH. Relative quantitation was performed using the comparative 2−∆∆Cq method (34), according to the manufacturer's protocol.
Preparation of nuclear extract
MCF-7 cells (2×106) were treated with DHA in the presence or absence of TPA for 4 h. Cells were immediately washed twice, scraped into 1.5 ml ice-cold PBS (pH 7.5) and pelleted at 1,500 × g for 3 min. Cytoplasmic and nuclear extracts were prepared from cells using the NE-PER® Nuclear and Cytoplasmic Extraction kit (Pierce; Thermo Fisher Scientific, Inc.).
Electrophoretic mobility shift assay (EMSA)
NF-κB activation was evaluated with a gel mobility shift assay using nuclear extracts. Oligonucleotides containing a binding site for the κ chain (κB, 5′-CCGGTTAACAGAGGGGGCTTTCCGAG-3′) or AP-1 (5′-CGCTTGATGAGTCAGCCGGAA-3′) binding site was synthesized and used as a probe (Promega, Madison, WI, USA). The two complementary strands were annealed and labeled with [α-32P] deoxycytidine triphosphate. Labeled oligonucleotides (10,000 cpm) were combined with 10 µg nuclear extracts and binding buffer [10 mM Tris-HCl (pH 7.6), 500 mM KCl, 10 mM EDTA, 50% glycerol, 100 ng poly (dI:dC) (Roche, Basel, Switzerland) and 1 mM dithiothreitol], and incubated for 30 min at room temperature in a final volume of 20 µl. The reaction products were analyzed by 4% PAGE in 0.5X Tris-borate buffer. The gels were then dried and examined by autoradiography. A 50-fold excess of cold κB oligonucleotide was used as a control to confirm binding specificity.
Invasion assay
The invasion assay was conducted in 24-well chambers (8-µm pore size) coated with 20 µl Matrigel diluted in DMEM. The Matrigel coating was re-hydrated in 0.5 ml DMEM for 30 min immediately prior to the experiment. Cells (2×105) were added to the upper chamber, with the chemoattractant in the bottom well. Conditioned medium (0.5 ml) was added to the lower compartment of the invasion chamber, followed by incubation for 24 h. Subsequently, cells on the upper side of the chamber were removed using cotton swabs, while those that had migrated were fixed and stained with Toluidine Blue solution. Invading cells were counted in five random areas of the membrane under a light microscope. Data from three individual experiments performed in triplicate were analyzed and presented as the mean ± standard error of the mean.
Statistical analysis
Data were evaluated by analysis of variance and Duncan's test using the Microsoft 2010 Excel program (Microsoft Corporation, Redmond, WA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
MCF-7 cell viability is unaffected by DHA treatment
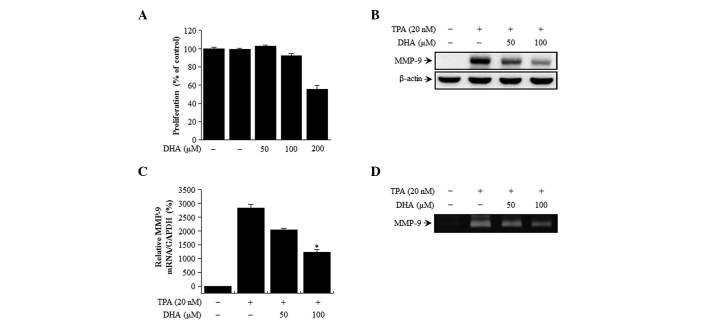
The cytotoxicity of DHA on MCF-7 cells was evaluated by MTT assay. There were no changes in cell viability or morphology upon treatment with the indicated concentrations of DHA for 24 h (Fig. 1A). Therefore, the subsequent experiments were performed at optimal, non-toxic DHA concentrations of 50 and 100 µM.
Figure 1.
Effect of DHA on cell viability and TPA-induced MMP-9 expression in MCF-7 cells. (A) The cytotoxicity of DHA was assessed using the MTT assay in cells exposed to the indicated concentrations of DHA for 24 h. The optical density value of control cells was considered as 100%. (B) MMP-9 expression was detected in cell lysates by western blotting, with β-actin used as a loading control. (C) MMP-9 mRNA level was analyzed by reverse transcription-quantitative polymerase chain reaction relative to the level of GAPDH. (D) MMP-9 activity was evaluated in conditioned medium by gelatin zymography. Values represent the mean + standard error of the mean of three independent experiments. *P<0.01 vs. TPA. TPA, 12-O-tetradecanoylphorbol-13-acetate; DHA, docosahexaenoic acid; MMP, matrix metalloproteinase; mRNA, messenger RNA.
DHA suppresses TPA-induced MMP-9 activation in MCF-7 cells
The effect of DHA on TPA-induced MMP-9 expression in MCF-7 cells was examined by western blot analysis, RT-qPCR and gelatin zymography. DHA treatment blocked the upregulation of MMP-9 protein expression induced by TPA, as determined by western blotting (Fig. 1B). Accordingly, RT-qPCR analysis revealed that the increase in MMP-9 expression induced by TPA treatment was abrogated by DHA in a dose-dependent manner (Fig. 1C). MMP-9 secretion was stimulated by TPA, but this effect was abrogated by treatment with DHA, as determined by zymography (Fig. 1D). These results indicate that DHA potently inhibits the TPA-induced increase in MMP-9 levels in MCF-7 cells.
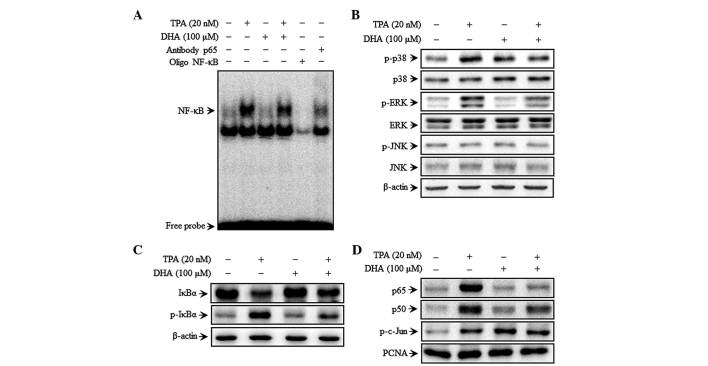
DHA inhibits TPA-induced NF-κB but not AP-1 DNA binding activity, as well as MAPK signaling
To investigate the mechanism of DHA-mediated inhibition of MMP-9 expression, the effect of DHA on TPA-induced NF-κB activation was evaluated by EMSA. TPA increased the NF-κB binding activity, whereas pre-treatment with DHA abolished this effect for NF-κB (Fig. 2A) but not for AP-1 (data not shown). These results suggest that DHA specifically blocks NF-κB activation in MCF-7 cells. DHA inhibited the phosphorylation of p38 and ERK, but not that of JNK, 30 min after TPA treatment (Fig. 2B). Additionally, TPA induced the phosphorylation of cytoplasmic IκBα and the consequent nuclear translocation of the NF-κB subunits p50 and p65, as determined by western blot analysis. In addition, c-Jun but not c-Fos expression was upregulated upon TPA treatment. Additionally, TPA also increased p-IκBα expression, as well as p65 and p50 translocation, which was suppressed by treatment with DHA (Fig. 2C and D). However, the TPA induced phosphorylation of c-Jun (a major subunit of AP-1) was unaffected by DHA (Fig. 2D). These results suggest that the effect of DHA on the regulation of TPA-induced MMP-9 expression is via modulation of MAPK signaling.
Figure 2.
DHA inhibits TPA-induced transcriptional activation of matrix metalloproteinase-9 and p38 phosphorylation in MCF-7 cells. Cells were treated with DHA in the presence of TPA for 4 h, followed by nuclear extraction. (A) NF-κB DNA binding was analyzed by electrophoretic mobility shift assay. (B) Cells were pretreated with TPA for 30 min in the presence or absence of DHA, and cell lysates were prepared for western blotting to detect p-p38, p38, p-JNK, JNK, p-ERK and ERK expression. (C) Cytoplasmic levels of NF-κB subunits, IκBα and p-IκBα, were determined by western blotting. (D) Nuclear levels of NFκB (p50 and p65) and activator protein 1 (p-c-Jun) subunits were determined by western blotting. TPA, 12-O-tetradecanoylphorbol-13-acetate; DHA, docosahexaenoic acid; NF, nuclear factor; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; IκB, nuclear factor of κ light polypeptide gene enhancer in B cells inhibitor; p, phosphorylated; PCNA, proliferating cell nuclear antigen.
DHA increases PPAR-γ expression
DHA treatment increased PPAR-γ expression in a dose-dependent manner in MCF-7 cells (Fig. 3A). To verify whether MMP-9 expression is regulated by PPAR-γ, TPA-treated cells were treated concurrently with DHA in the presence or absence of GW9662. MMP-9 expression was higher upon treatment in the presence of GW9662 than following treatment with DHA alone (Fig. 3B). GW9662 also rescued the expression of p65 and p50 that was abolished by DHA treatment (Fig. 3C). These results indicate that DHA inhibits MAPK signaling and MMP-9 expression by increasing PPAR-γ expression.
Figure 3.
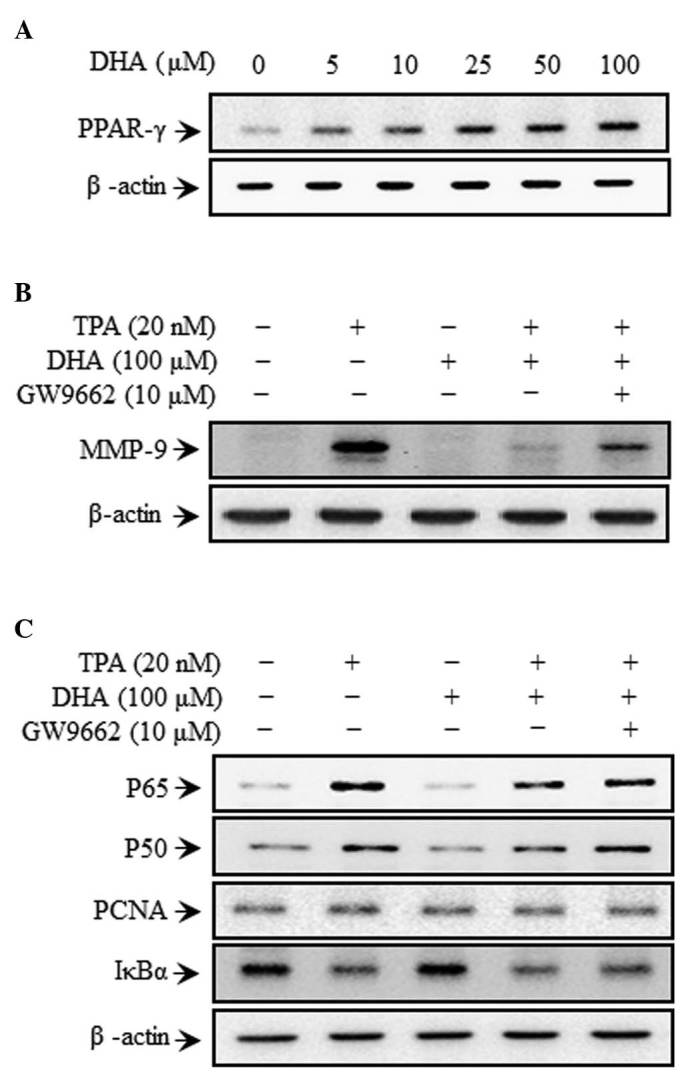
DHA induces upregulation of PPAR-γ in MCF-7 cells. Cells were treated with DHA and GW9662 in the presence of TPA for 24 h. Subsequently, cell lysates were prepared for western blotting to detect (A) PPAR-γ, (B) MMP-9 and (C) p50, p65 and IκBα expression. TPA, 12-O-tetradecanoylphorbol-13-acetate; DHA, docosahexaenoic acid; IκB, nuclear factor of κ light polypeptide gene enhancer in B cells inhibitor; PCNA, proliferating cell nuclear antigen; PPAR, peroxisome proliferator-activated receptor.
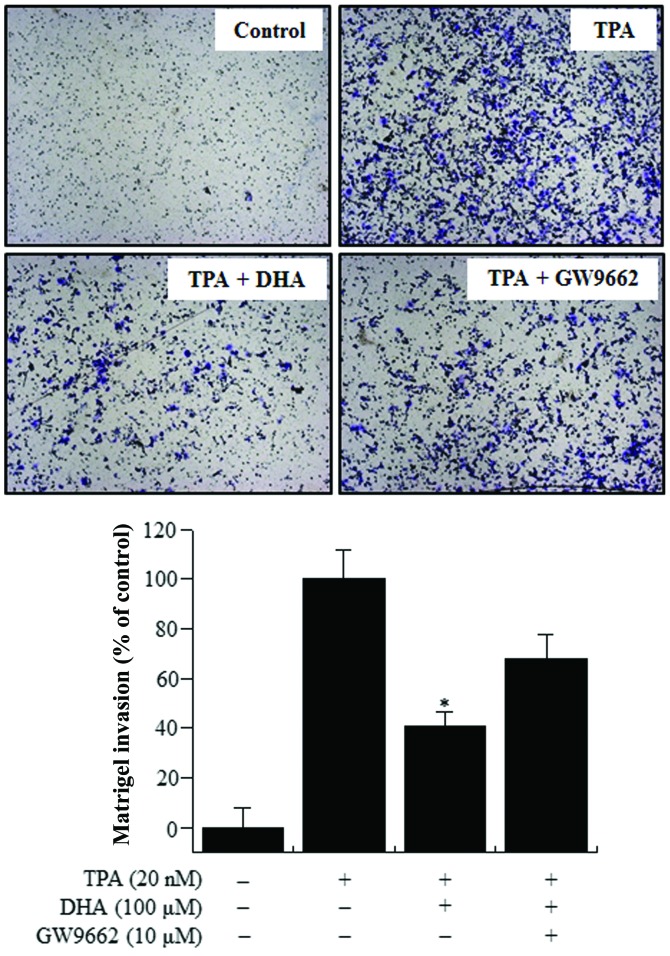
DHA treatment inhibits TPA-induced MCF-7 cell invasion
The upregulation of MMP-9 expression contributes to cancer cell invasion (10,27). Therefore, the effect of DHA on the invasive potential of MCF-7 cells was investigated by Matrigel assay. TPA treatment stimulated MCF-7 cell invasion 10-fold more, compared with untreated control cells. Treatment with DHA decreased TPA-induced cell invasion by 60%. However, a decrease of only 30% was observed in the presence of both GW9662 and DHA (Fig. 4), indicating that DHA suppresses the invasive potential of breast cancer cells via PPAR-γ activation.
Figure 4.
Effect of DHA on the TPA-induced invasion of MCF-7 cells. Cell invasion was determined by Matrigel assay. Cells were seeded in the upper chamber and drugs were placed in the well. After 24 h of incubation, invaded cells on the bottom of the filter were fixed, stained and counted (x100 magnification). Values represent the mean + standard error of the mean of three independent experiments. *P<0.01 vs. TPA. TPA, 12-O-tetradecanoylphorbol-13-acetate; DHA, docosahexaenoic acid.
Discussion
The protective effects of fish oil are attributable to ω-3 polyunsaturated fatty acids (PUFAs), including EPA (20:5 ω-3) and DHA (22:6 n-3), which are converted by fish from α-linolenic acid (LNA; 18:3 ω-3) present in ingested cold water vegetation (1). A study of metastatic mouse mammary carcinoma reported that a diet containing α-LNA-rich linseed oil effectively arrested tumor progression (12). Furthermore, tumor growth and metastasis were inhibited by diets containing fish oil, EPA or DHA (27,35). ω-3 PUFAs may affect carcinogenesis by altering transcription factor activity, gene expression and/or signal transduction (7,36). DHA is a particularly potent enhancer of tumor cell chemosensitivity (37). However, there is little information on the anti-metastatic effects of DHA in human breast cancer cells.
Metastasis is the primary cause of breast cancer mortality, and involves cell proliferation, ECM degradation, cell migration and tumor growth at secondary sites (19,20). Tumor cell invasion is an early step in this process, representing the transition from a benign state to malignancy (11). Tumor invasion is morphologically distinguished by a distortion of the primary tumor edge where individual or cohorts of tumor cells actively invade the surrounding ECM (38). MMP-9 is a critical molecule in tumor invasion and metastasis, and its activation is associated with the progression of mammary tumors (39). Inflammatory cytokines, growth factors or phorbol esters stimulate MMP-9 by activating different intracellular signaling pathways in breast cancer cells (40–42). Thus, inhibiting the expression and/or activity of MMP-9 may be an important strategy for slowing or preventing tumor metastasis.
AP-1 belongs to the basic region/leucine zipper motif group of DNA-binding proteins, and homo or heterodimerizes in response to signaling events to indirectly or directly activate c-Jun and c-Fos expression (43–46). NF-κB is a member of a family of inducible transcription factors that regulate host inflammatory and immune responses (47) as a result of MAPK signaling, which requires cell type-specific IκB kinase expression (17,24,25,48,49). Both AP-1 and NF-κB have been implicated in TPA-mediated MMP-9 gene induction (20,21). The present results demonstrate that DHA inhibits TPA-induced MMP-9 expression via activation of NF-κB, but not of AP-1, in MCF-7 breast cancer cells.
PPAR-γ is a member of the nuclear receptor and ligand-activated steroid hormone receptor-regulated transcription factor superfamily (26). ω-3 PUFAs are natural ligands of nuclear receptors such as PPAR-α and -γ, and can upregulate PPAR-γ expression (50). PPAR-γ has been reported to inhibit the NF-κB signaling pathway by reducing NF-κB binding activity and physically interacting with both p65 and p50 (51,26). The present study confirmed that PPAR-γ expression was increased by treatment with DHA, which consequently led to a decrease in p65 and p50 levels, and a decrease in MMP-9 expression. NF-κB inhibition by DHA was reversed by treatment with the PPAR-γ-specific antagonist GW9662, demonstrating that TPA-induced tumor cell invasion was suppressed by DHA through a PPAR-γ-dependent mechanism.
In conclusion, DHA is a potent inhibitor of TPA-induced MMP-9 expression, and blocks breast carcinoma cell invasion by modulating the NF-κB signaling pathway via PPAR-γ upregulation. These findings suggest that DHA may be an effective therapeutic agent for preventing breast tumor invasion and metastasis.
Acknowledgements
The present study was supported by the Korea Atomic Energy Research Institute (grant no. NRF-2015M2A2A6021673) and by a National Research Foundation of Korea grant funded by the Korean government (grant no. No-2011-0030130). The authors thank Editage (Cactus Communications Co. Ltd., Shanghai, China) for English language editing the present manuscript.
References
- 1.Stillwell W, Wassall SR. Docosahexaenoic acid: Membrane properties of a unique fatty acid. Chem Phys Lipids. 2003;126:1–27. doi: 10.1016/S0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- 2.Hirayama T. Epidemiology of breast cancer with special reference to the role of diet. Prev Med. 1978;7:173–195. doi: 10.1016/0091-7435(78)90244-X. [DOI] [PubMed] [Google Scholar]
- 3.Wynder EL, Rose DP, Cohen LA. Diet and breast cancer in causation and therapy. Cancer. 1986;58:S1804–S1813. doi: 10.1002/1097-0142(19861015)58:8+<1804::AID-CNCR2820581404>3.0.CO;2-N. (Suppl 8) [DOI] [PubMed] [Google Scholar]
- 4.Rose DP, Connolly JM, Coleman M. Effect of omega-3 fatty acids on the progression of metastases after the surgical excision of human breast cancer cell solid tumors growing in nude mice. Clin Cancer Res. 1996;2:1751–1756. [PubMed] [Google Scholar]
- 5.Wu M, Harvey KA, Ruzmetov N, Welch ZR, Sech L, Jackson K, Stillwell W, Zaloga GP, Siddiqui RA. Omega-3 polyunsaturated fatty acids attenuate breast cancer growth through activation of a neutral sphingomyelinase-mediated pathway. Int J Cancer. 2005;117:340–348. doi: 10.1002/ijc.21238. [DOI] [PubMed] [Google Scholar]
- 6.Bartsch H, Nair J, Owen RW. Dietary polyunsaturated fatty acids and cancers of the breast and colorectum: Emerging evidence for their role as risk modifiers. Carcinogenesis. 1999;20:2209–2218. doi: 10.1093/carcin/20.12.2209. [DOI] [PubMed] [Google Scholar]
- 7.Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am J Clin Nutr. 2004;79:935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 8.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima M, Welch DR, Belloni PN, Nicolson GL. Degradation of basement membrane type IV collagen and lung subendothelial matrix by rat mammary adenocarcinoma cell clones of differing metastatic potentials. Cancer Res. 1987;47:4869–4876. [PubMed] [Google Scholar]
- 10.Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–2154. [PubMed] [Google Scholar]
- 11.Velinov N, Poptodorov G, Gabrovski N, Gabrovski S. The role of matrixmetalloproteinases in the tumor growth and metastasis. Khirurgiia (Sofiia) 2010:44–49. (In Bulgarian) [PubMed] [Google Scholar]
- 12.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: A tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 13.Gum R, Wang H, Lengyel E, Juarez J, Boyd D. Regulation of 92 kDa type IV collagenase expression by the jun aminoterminal kinase- and the extracellular signal-regulated kinase-dependent signaling cascades. Oncogene. 1997;14:1481–1493. doi: 10.1038/sj.onc.1200973. [DOI] [PubMed] [Google Scholar]
- 14.Newton AC. Regulation of protein kinase C. Curr Opin Cell Biol. 1997;9:161–167. doi: 10.1016/S0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- 15.Zeigler ME, Chi Y, Schmidt T, Varani J. Role of ERK and JNK pathways in regulating cell motility and matrix metalloproteinase 9 production in growth factor-stimulated human epidermal keratinocytes. J Cell Physiol. 1999;180:271–284. doi: 10.1002/(SICI)1097-4652(199908)180:2<271::AID-JCP15>3.3.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Hozumi A, Nishimura Y, Nishiuma T, Kotani Y, Yokoyama M. Induction of MMP-9 in normal human bronchial epithelial cells by TNF-alpha via NF-kappa B-mediated pathway. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1444–L1452. doi: 10.1152/ajplung.2001.281.6.L1444. [DOI] [PubMed] [Google Scholar]
- 17.Weng CJ, Chau CF, Hsieh YS, Yang SF, Yen GC. Lucidenic acid inhibits PMA-induced invasion of human hepatoma cells through inactivating MAPK/ERK signal transduction pathway and reducing binding activities of NF-kappaB and AP-1. Carcinogenesis. 2008;29:147–156. doi: 10.1093/carcin/bgm261. [DOI] [PubMed] [Google Scholar]
- 18.Lin CW, Hou WC, Shen SC, Juan SH, Ko CH, Wang LM, Chen YC. Quercetin inhibition of tumor invasion via suppressing PKC delta/ERK/AP-1-dependent matrix metalloproteinase-9 activation in breast carcinoma cells. Carcinogenesis. 2008;29:1807–1815. doi: 10.1093/carcin/bgn162. [DOI] [PubMed] [Google Scholar]
- 19.Lee SO, Jeong YJ, Kim M, Kim CH, Lee IS. Suppression of PMA-induced tumor cell invasion by capillarisin via the inhibition of NF-kappaB-dependent MMP-9 expression. Biochem Biophys Res Commun. 2008;366:1019–1024. doi: 10.1016/j.bbrc.2007.12.068. [DOI] [PubMed] [Google Scholar]
- 20.Oh JH, Chung AS, Steinbrenner H, Sies H, Brenneisen P. Thioredoxin secreted upon ultraviolet A irradiation modulates activities of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 in human dermal fibroblasts. Arch Biochem Biophys. 2004;423:218–226. doi: 10.1016/j.abb.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 21.Hong S, Park KK, Magae J, Ando K, Lee TS, Kwon TK, Kwak JY, Kim CH, Chang YC. Ascochlorin inhibits matrix metalloproteinase-9 expression by suppressing activator protein-1-mediated gene expression through the ERK1/2 signaling pathway: Inhibitory effects of ascochlorin on the invasion of renal carcinoma cells. J Biol Chem. 2005;280:25202–25209. doi: 10.1074/jbc.M413985200. [DOI] [PubMed] [Google Scholar]
- 22.Woo MS, Jung SH, Kim SY, Hyun JW, Ko KH, Kim WK, Kim HS. Curcumin suppresses phorbol ester-induced matrix metalloproteinase-9 expression by inhibiting the PKC to MAPK signaling pathways in human astroglioma cells. Biochem Biophys Res Commun. 2005;335:1017–1025. doi: 10.1016/j.bbrc.2005.07.174. [DOI] [PubMed] [Google Scholar]
- 23.Eberhardt W, Huwiler A, Beck KF, Walpen S, Pfeilschifter J. Amplification of IL-1 beta-induced matrix metalloproteinase-9 expression by superoxide in rat glomerular mesangial cells is mediated by increased activities of NF-kappa B and activating protein-1 and involves activation of the mitogen-activated protein kinase pathways. J Immunol. 2000;165:5788–5797. doi: 10.4049/jimmunol.165.10.5788. [DOI] [PubMed] [Google Scholar]
- 24.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 25.Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 2001;276:18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 26.Delerive P, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol. 2001;169:453–459. doi: 10.1677/joe.0.1690453. [DOI] [PubMed] [Google Scholar]
- 27.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2:748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 28.Lee JH, Woo JH, Woo SU, Kim KS, Park SM, Joe EH, Jou I. The 15-deoxy-delta 12,14-prostaglandin J2 suppresses monocyte chemoattractant protein-1 expression in IFN-gamma-stimulated astrocytes through induction of MAPK phosphatase-1. J Immunol. 2008;181:8642–8649. doi: 10.4049/jimmunol.181.12.8642. [DOI] [PubMed] [Google Scholar]
- 29.Li CC, Yang HT, Hou YC, Chiu YS, Chiu WC. Dietary fish oil reduces systemic inflammation and ameliorates sepsis-induced liver injury by up-regulating the peroxisome proliferator-activated receptor gamma-mediated pathway in septic mice. J Nutr Biochem. 2014;25:19–25. doi: 10.1016/j.jnutbio.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Lee YR, Noh EM, Oh HJ, Hur H, Kim JM, Han JH, Hwang JK, Park BH, Park JW, Youn HJ, et al. Dihydroavenanthramide D inhibits human breast cancer cell invasion through suppression of MMP-9 expression. Biochem Biophys Res Commun. 2011;405:552–557. doi: 10.1016/j.bbrc.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 31.Kim JM, Noh EM, Kwon KB, Kim JS, You YO, Hwang JK, Hwang BM, Kim BS, Lee SH, Lee SJ, et al. Curcumin suppresses the TPA-induced invasion through inhibition of PKCalpha-dependent MMP-expression in MCF-7 human breast cancer cells. Phytomedicine. 2012;19:1085–1092. doi: 10.1016/j.phymed.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Kim JM, Noh EM, Kwon KB, Kim JS, You YO, Hwang JK, Hwang BM, Kim MS, Lee SJ, Jung SH, et al. Suppression of TPA-induced tumor cell invasion by sulfuretin via inhibition of NF-κB-dependent MMP-9 expression. Oncol Rep. 2013;29:1231–1237. doi: 10.3892/or.2012.2218. [DOI] [PubMed] [Google Scholar]
- 33.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Altenburg JD, Bieberich AA, Terry C, Harvey KA, Vanhorn JF, Xu Z, Jo Davisson V, Siddiqui RA. A synergistic antiproliferation effect of curcumin and docosahexaenoic acid in SK-BR-3 breast cancer cells: Unique signaling not explained by the effects of either compound alone. BMC Cancer. 2011;11:149. doi: 10.1186/1471-2407-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J, Lim SY, Shin A, Sung MK, Ro J, Kang HS, Lee KS, Kim SW, Lee ES. Fatty fish and fish omega-3 fatty acid intakes decrease the breast cancer risk: A case-control study. BMC Cancer. 2009;9:216. doi: 10.1186/1471-2407-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun H, Berquin IM, Owens RT, O'Flaherty JT, Edwards IJ. Peroxisome proliferator-activated receptor gamma-mediated up-regulation of syndecan-1 by n-3 fatty acids promotes apoptosis of human breast cancer cells. Cancer Res. 2008;68:2912–2919. doi: 10.1158/0008-5472.CAN-07-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 39.Scorilas A, Karameris A, Arnogiannaki N, Ardavanis A, Bassilopoulos P, Trangas T, Talieri M. Overexpression of matrix-metalloproteinase-9 in human breast cancer: A potential favourable indicator in node-negative patients. Br J Cancer. 2001;84:1488–1496. doi: 10.1054/bjoc.2001.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho HJ, Kang JH, Kwak JY, Lee TS, Lee IS, Park NG, Nakajima H, Magae J, Chang YC. Ascofuranone suppresses PMA-mediated matrix metalloproteinase-9 gene activation through the Ras/Raf/MEK/ERK- and Ap1-dependent mechanisms. Carcinogenesis. 2007;28:1104–1110. doi: 10.1093/carcin/bgl217. [DOI] [PubMed] [Google Scholar]
- 41.Kajanne R, Miettinen P, Mehlem A, Leivonen SK, Birrer M, Foschi M, Kahari VM, Leppa S. EGF-R regulates MMP function in fibroblasts through MAPK and AP-1 pathways. J Cell Physiol. 2007;212:489–497. doi: 10.1002/jcp.21041. [DOI] [PubMed] [Google Scholar]
- 42.Srivastava AK, Qin X, Wedhas N, Arnush M, Linkhart TA, Chadwick RB, Kumar A. Tumor necrosis factor-alpha augments matrix metalloproteinase-9 production in skeletal muscle cells through the activation of transforming growth factor-beta-activated kinase 1 (TAK1)-dependent signaling pathway. J Biol Chem. 2007;282:35113–35124. doi: 10.1074/jbc.M705329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frigo DE, Tang Y, Beckman BS, Scandurro AB, Alam J, Burow ME, McLachlan JA. Mechanism of AP-1-mediated gene expression by select organochlorines through the p38 MAPK pathway. Carcinogenesis. 2004;25:249–261. doi: 10.1093/carcin/bgh009. [DOI] [PubMed] [Google Scholar]
- 44.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 45.Yokoo T, Kitamura M. Dual regulation of IL-1 beta-mediated matrix metalloproteinase-9 expression in mesangial cells by NF-kappa B and AP-1. Am J Physiol. 1996;270:F123–F130. doi: 10.1152/ajprenal.1996.270.1.F123. [DOI] [PubMed] [Google Scholar]
- 46.Lungu G, Covaleda L, Mendes O, Martini-Stoica H, Stoica G. FGF-1-induced matrix metalloproteinase-9 expression in breast cancer cells is mediated by increased activities of NF-kappaB and activating protein-1. Mol Carcinog. 2008;47:424–435. doi: 10.1002/mc.20398. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao J, Xiong S, Klos K, Nguyen N, Grijalva R, Li P, Yu D. Multiple signaling pathways involved in activation of matrix metalloproteinase-9 (MMP-9) by heregulin-beta1 in human breast cancer cells. Oncogene. 2001;20:8066–8074. doi: 10.1038/sj.onc.1204944. [DOI] [PubMed] [Google Scholar]
- 49.Amin AR Ruhul, Senga T, Oo ML, Thant AA, Hamaguchi M. Secretion of matrix metalloproteinase-9 by the proinflammatory cytokine, IL-1beta: A role for the dual signalling pathways, Akt and Erk. Genes Cells. 2003;8:515–523. doi: 10.1046/j.1365-2443.2003.00652.x. [DOI] [PubMed] [Google Scholar]
- 50.Marion-Letellier R, Déchelotte P, Iacucci M, Ghosh S. Dietary modulation of peroxisome proliferator-activated receptor gamma. Gut. 2009;58:586–593. doi: 10.1136/gut.2008.162859. [DOI] [PubMed] [Google Scholar]
- 51.Delerive P, Martin-Nizard F, Chinetti G, Trottein F, Fruchart JC, Najib J, Duriez P, Staels B. Peroxisome proliferator-activated receptor activators inhibit thrombin-induced endothelin-1 production in human vascular endothelial cells by inhibiting the activator protein-1 signaling pathway. Circ Res. 1999;85:394–402. doi: 10.1161/01.RES.85.5.394. [DOI] [PubMed] [Google Scholar]