Introduction
The critical importance of temperature regulation to “neurological” well-being has some of its earliest roots in the writings of Aristotle in the 4th century B.C, who stated “...man's superior intelligence depends on the fact that his larger brain is capable of keeping the heart cool enough for optimal mental activity (1).” Given that Aristotle professed that the heart was the center of nervous function, he appears to have recognized the importance of temperature control and fever prevention in neurocritical care, except he had the organs confused! Almost 2,100 years later, another chapter in the origins of therapeutic hypothermia (TH) and its potential in neurocritical care and resuscitation is discovered in the writings of Baron Dominique-Jean Larrey, surgeon to Napoleon Bonaparte, who suggested in the early 1800s that hypothermia was beneficial to victims of traumatic exsanguination given that the soldiers located farthest from the campfire survived the longest (2). Shortly thereafter, the neurosurgeon Charles Phelps wrote in his 1897 treatise, “Traumatic Injuries of the Brain and its Membranes,” that the application of the “Ice Cap” was beneficial in traumatic brain injury (TBI)—second in efficacy only to trephination (3).
In the modern era, the use of TH in neurocritical care and resuscitation has evolved from a largely unregulated application regarding depth, duration, and type of acute brain injury to the recognition that fever is generally implicated as deleterious to the injured brain across the spectrum of insults. And that if TH is used, mild temperature reductions should be applied for 24-72h but only in certain conditions. The precise spectrum of use remains to be defined. In the modern era, early clues to the potential efficacy of TH for acute brain injury were suggested by remarkable recoveries of cold-water drowning victims (4) in the work of Rosomoff and colleagues (5) in neurosurgery, Lundberg et al (6) in TBI, and Conn et al (7) in pediatric drowning. In these studies from the 1960s to the early 1980s, TH was often used to treat acute brain injury in an unregulated manner and taken to surprising depths and durations. In the seminal report by Lundberg et al (6) on the use of intracranial pressure (ICP)-directed management in TBI, in discussing the treatment of intracranial hypertension, it states “These waves disappeared when, after the induction of hypothermia, the rectal temperature had dropped below 29°C.” In another case “Hypothermia was induced, and at a rectal temperature of 26°C, a partial resection of the contused right temporal lobe was carried out.”
Over the past decade, we have begun to formulate a clearer vision of where TH exhibits efficacy and where it has failed. We have also gained insight into the potential value of rigorous temperature control and the prevention of fever after acute brain injury. Ironically, as the use of TH in neurocritical care and resuscitation waned related to complications reported in the late 1980s, particularly those associated with its more aggressive use, discussed above, resurgence in the appreciation of mild TH and temperature regulation and its impact on acute brain injury emerged from the pre-clinical literature. The importance of “small differences” in temperature in impacting outcome in acute brain injury and the recognition that brain and core temperature were often somewhat different is often attributed to Busto et al (7). In that study, a 2°C temperature difference during global ischemia–from 36 to 34°C markedly reduced neuronal death in rat brain, while increasing temperature to 39°C increased neuronal death. That paper impacted the field of experimental brain injury (8) and to this day, pre-clinical studies in experimental models of acute brain injury require temperature monitoring/control and often include brain temperature monitoring or surrogates such as temporalis muscle or tympanic membrane temperature. That report also challenged the notion that the benefits of TH were mediated solely by a reduction in energy demands below a critical threshold during ischemia—suggesting other undefined mechanisms. It was followed by studies in models of cardiac arrest (CA) in adult animals and of asphyxia in developing animals that set the stage for clinical trials showing benefit in both settings (9-14). The greatest efficacy of mild TH is seen in the treatment of birth asphyxia in term newborns with benefit vs. standard of care on death, major disability, long term outcome, and structural preservation on neuroimaging (15). It is also ironic that the greatest benefit of TH in acute brain injury is seen in infants, given the longstanding concerns in neonatology about the highly deleterious consequences of cold stress (16). Benefit of TH after asphyxia on mechanisms of special importance to the developing brain, such as apoptosis, has been suggested (17, 18). Also, non-shivering thermogenesis is the primary mechanism of heat production in infants vs. adults, which may necessitate a distinct series of developmentally regulated biochemical responses or gene regulatory events induced by cold stress in newborn patients.
Contrasting the success seen with the use of mild TH in CA, despite early work showing the ability of TH to lower ICP, multi-center studies in TBI have failed to show benefit on long-term outcome. This included use in both adults and children in a variety of strategies (19-23). Relatively unique complications associated with the use of TH in TBI have included concerns with hemodynamic instability during re-warming which may be deleterious if ICP is elevated and concerns with the many drugs used to treat TBI and toxicities from them given the inhibition of cytochrome P-450-mediated drug metabolism by TH (24-26). Some have suggested the need to use isolated brain cooling to achieve benefit without side effects in TBI (27). Alternatively, the mechanisms underlying secondary injury in TBI may simply be less favorably influenced by TH than those in CA and counterbalanced by greater side effects.
Targeted temperature management (TTM) in neurocritical care in the modern era
In panel discussions held by a committee charged to craft a consensus statement based on presentations made at the Society of Critical Care Medicine conference “Therapeutic Hypothermia–To Cool or Not To Cool?” in 2009, the editor of this journal, Dr. Buchman, suggested the term TTM to describe the scope and approach to the potential use of TH and temperature regulation across the field of critical care—and that term has been accepted. That result predicted some of the findings of clinical investigations that followed. In 2013, Nielsen et al (28) carried out an important study in >900 patients of the impact of 28h of mild TH (33°C) vs. rigorously controlled “normothermia” (36°C) and reported an identical outcome in both groups, with ~50% of patients with severe disability, coma, or death. Interestingly these outcomes mirrored those in the hypothermia groups in the successful CA trials reported a decade earlier, and although the generalizability of this study has been questioned due to the high percentage of cases with bystander CPR (73%), it suggested that rigorous fever control might underlie much of the benefit seen in the prior studies of TH after CA in adults. Currently, both mild TH and TTM are used to treat adult CA victims (29).
Recently, a landmark study (30) was published testing the effect of mild TH in 260 infants and children with out-of-hospital CA randomized to 33°C or 36.8°C for 48h—the TH after CA (THAPCA) trial. Although the study failed to show benefit of TH on the primary outcome (survival with good neurobehavioral outcome at 12-mo), a trend with an 8% benefit on the primary outcome and an even stronger trend to reduced mortality was seen in the TH group. The study was powered to detect a 20% improvement—challenging for any therapy to achieve given the marked heterogeneity and severity of children with out-of-hospital CA (~50% had an underlying medical condition and only 40% had a witnessed). It has been estimated that to detect a 10% benefit, >600 patients would need to be randomized to achieve 80% power. This important trial might be viewed as a window on future trial design for TH in pediatric CA, although some have suggested that given the lack of side effects observed, it actually supports use of TH in pediatric CA (31).
A new concept, TTM versus “ultra-mild hypothermia (UMH)”
These studies have raised many questions. Why has TH been so efficacious to treat birth asphyxia but less so after CA in children and adults? Is the efficacy of TTM the result of the prevention of fever? Does TTM represent all that we can hope to achieve from “hypothermia”? A recent publication in Nature (32) introduced a novel concept for the possible benefits of deep TH that may shed some light on the entire field of TTM and TH. In that study, Prion-infected or XFAD Alzheimer mice were briefly maintained in a hibernation-like state by rapid cooling over 1h to 16-18°C, kept at target temperature for 45min, and given a 5-AMP injection to further augment body heat reduction. Remarkably this single brief intervention dramatically blunted the long-term consequences of these two neurodegenerative diseases including neuronal death, synaptic loss, and cognitive deficits assessed between 6-wks and 3-mo later. Importantly, the benefit was shown to be mediated to a large degree by the induction of the cold shock protein RNA binding motif-3 (RBM-3). It is unclear if 5-AMP (a vasodilator and hypothermia inducer) is an important component mediating RBM3 induction in this cooling protocol. However, RBM-3 overexposure by lentivirus mediated gene delivery produced a similar benefit in normothermic mice. Recently, work from our laboratory has suggested that TTM may be producing benefit beyond simply preventing fever. Jackson et al (33) reported that clamping isolated rat primary neurons (day in vitro-6 [DIV-6]) in culture at 36°C for 24 or 48h markedly upregulates RBM-3 vs. normothermia—although not quite to the level seen with exposure of these neurons to 33°C. DIV-6 neurons model many features of newborn neurons (34). In contrast, DIV-26 neurons, modeling adult neurons exhibit a much more blunted upregulation of RBM-3 at 36° or 33°C (33). Given that a 1°C reduction in temperature is not believed to produce neuroprotection via mechanisms such as reducing energy demands, our findings suggest that TTM may exert benefit by mechanisms beyond simply preventing fever. TTM in its current form (controlling patients at 36°C) may represent a novel concept—namely UMH. Equally exciting is the fact that we also discovered that two compounds, fibroblast growth factor-21 and melatonin (both of which are linked to endogenous control of temperature regulation in mammals) can substantially augment the upregulation of RBM-3 by exposure to 36°C in DIV-6 neurons (33). This suggests that we may be able to pharmacologically augment novel benefits of extremely mild temperature reductions (1°C) with one or more drugs—attenuating long-term deleterious consequences of brain injury. Melatonin augmented neuroprotection by 33.5°C in a porcine model of perinatal asphyxia (34). One could speculate that this approach could ultimately lead to a pharmacological substitute for TH–since UMH regulates global gene transcription via RBM-3. These are exciting new avenues for TH research applications for neurocritical care and beyond.
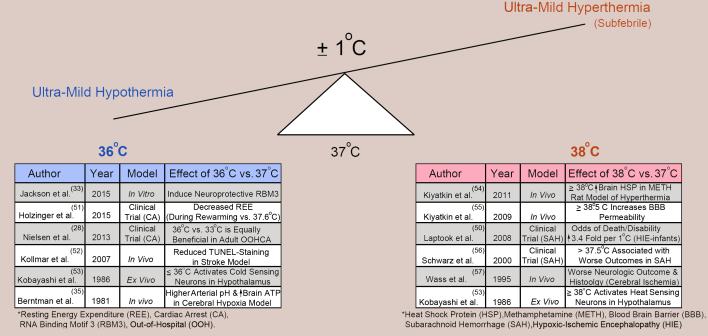
The concept that tiny shifts in temperature might impact CNS outcomes after injury, for better or worse, dates back to work in 1981, but was largely unnoticed. Berntman et al, (35) showed that cooling adult rats to 36°C (vs. 37°C) improved metabolic acidosis (blood pH and lactate) and increased ATP in the Levine model of hypoxic-ischemia. However, it is now better recognized, that shifts in core temperature by only ±1°C can engage a range of beneficial or deleterious biological, biochemical, and signaling responses, and our understandings of those nuanced effects on CNS recovery post-injury continues to evolve (Figure 1). Future studies using large-scale genomic, proteomic and metabolic approaches are warranted, and should provide greater insight into how small differences in temperature can modulate brain injury.
Figure 1.
Effect of ±1°C on brain function, biochemistry, and the injury response. (Left Table/Blue font) Selected studies supporting that 36°C activates neuroprotective pathways associated with targeted temperature management (TTM) or ultra-mild hypothermia (UMH) (28, 33, 35, 51-53). (Right Table/Red font) Selected studies supporting that 38°C activates detrimental pathways associated with mild (sub-febrile) hyperthermia. TTM/UMH holds promise as a potent and safe therapy to block detrimental pathways engaged on the right while simultaneously activating direct neuroprotective signaling pathways such as RBM3 (50, 53-57).
Mounting evidence suggests that organisms are unexpectedly sensitive to tiny temperature shifts. Might we be overdue to reevaluate factors of temperature control in optimal pre-clinical study design as well? As discussed, Busto et al (7) changed pre-clinical practice by bringing awareness to the need for rigorously monitoring/controlling temperature in brain injury research. Controversial studies suggest that the environmental temperature needed to achieve thermoneutrality for a mouse (a temperature perceived as “comfortable”) may be as high as ~30°C–whereas most animal housing facilities are kept at ambient temperatures 20-22°C (thermoneutral and comfortable for staff). This has led to the suggestion that animals used in research may be in a state of mild cold stress (36-39). TH has shown reproducible neuroprotection across brain injury models in rodents. It is possible that chronic exposure of rodents to perceived mild cold stress might pre-condition them to adopt an optimal TH response after injury and explain in part the failure in translation in some models to the clinical condition. What scenario best replicates the human condition?
Given that this review represents a discussion of novel approaches on the use of TH in neurocritical care and resuscitation, several others should be discussed (Figure 2). We mentioned that the failures of TH in RCTs studying TBI have greatly reduced enthusiasm for its use, despite the fact that it can reduce ICP (19-25). However, the role of spreading depression in exacerbating secondary injury after TBI has emerged as a therapeutic target and few drugs block it (40). TH attenuates spreading depression, suggesting that isolated brain cooling in TBI or injury phenotype-targeted use of TH might be worthy of investigation. In the treatment of CA in adults and children, additional investigations are needed to define the optimal approach to temperature management including all facets of its use and potential adjuncts. Many important unanswered questions remain including the possibility of differential use based on arrest severity or phenotype (41). In neonatology, where TH is standard of care for HIE in term newborns, identifying therapeutic adjuncts to TH and determining whether it is efficacious in premature infants with HIE are being investigated (42, 43). TH in various applications is currently under investigation in spinal cord injury, stroke, and status epilepticus (44-46). For example, in stroke, cooling might have potential via local delivery after clot retrieval to mitigate reperfusion injury (45). Finally, use of an emergency deep hypothermic preservation to treat otherwise lethal exsanguination CA in victims of penetrating trauma—an approach pioneered by the late Dr. Peter Safar and known as emergency preservation and resuscitation (EPR), will soon be tested in a clinical trial by a team led by Dr. Samuel Tisherman, at Maryland Shock Trauma (47).
Figure 2.
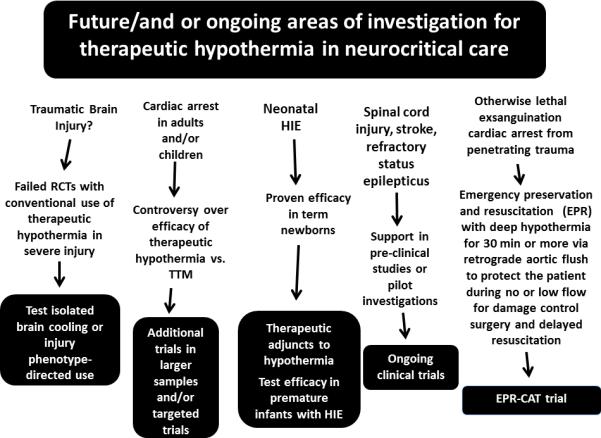
Future and or ongoing areas of investigation for therapeutic hypothermia in neurocritical care including traumatic brain injury, cardiac arrest in children and adults, perinatal asphyxia, spinal cord injury, stroke, refractory status epilepticus, and trauma induced exsanguination cardiac arrest. RCTs = randomized controlled trials, TTM = targeted temperature management, HIE = hypoxic ischemic encephalopathy, EPR = emergency preservation and resuscitation, CAT = cardiac arrest from trauma.
We would be remiss to not indicate that controversy and unknowns even remain about preventing fever after acute brain injury—such as 1) the window of vulnerability of the injured brain to fever, and 2) what is the optimal approach to fever in neurocritical care patients who are neither intubated nor sedated? Outside of pre-clinical (albeit compelling) studies (48), clinically detrimental effects of fever after acute brain injury are only known in association studies (49, 50).
Conclusions
We have come a long way in our understanding of the role of TH and TTM/UMH in neurocritical care. Recognition that in critical care we need careful titration of all therapies, limiting side effects, and maximizing benefit is paramount to success. We also have learned that in neurocritical care, each disease (and patient) may exhibit a different response to temperature manipulation. We also recognize the importance of continual re-examination of interventions that have potent impact on patients, as our field evolves. The use of TH in neurocritical care exemplifies that “the good stuff keeps coming back” and merits ongoing re-examination as other aspects of care evolve. We believe that hypothermia is a complex therapy—“not a pill.” And our understanding of how to optimize it is far from complete. Given the exciting new concepts that are emerging about the effects of TTM/UMH, we have come a long way from Aristotle's theory that the brain is merely a radiator to cool the heart!
Acknowledgement
This work was supported in part by NIH/NINDS grant to Travis C. Jackson (1R21NS088145).
Footnotes
Copyright form disclosure: Dr. Kochanek's institution received funding from United States Patent: US 8,628,512 B2 Title: Method of Inducing EPR Following Cardiopulmonary Arrest Filing Date: 6/22/05 Inventors: PM Kochanek, SA Tisherman, X Wu, SW Stezoski, LJ Yaffe; from United States Provisional Patent: Title: Compositions and Methods for Identifying Subjects at Risk for Traumatic Brain Injury Serial No: 62/113,292 Filing Date: February 6, 2015 Inventors: RP Berger, PM Kochanek, BJ Pak, PT Smith, MD Kolesnikova; from United States Invention Disclosure: Title: Small Molecule Inhibitors of RNA Binding MOTIF (RBM) Proteins for the Treatment of Acute Cellular Injury University of Pittsburgh Filing Date: November 13, 2014 Inventors: TC Jackson, J Verrier, PM Kochanek; and from United States Invention Disclosure: Title: Method to improve neurologic outcomes in temperature managed patients Application No.: 62/164,205 Country: United States Innovators: Travis C. Jackson (University of Pittsburgh); Patrick M. Kochanek. Dr. Jackson received funding from National Neurotrauma Society/Invited Speaker/$500 Travel Reimbursement. His institution received funding from American Heart Association National Scientist Development Grant, and from National Institutes of Health (NIH) (1R21NS088145). Dr. Jackson received support for article research from NIH. He disclosed other funding: Current, Pending, & Past Support: Current Support 1.PI: Travis C. Jackson Title: Pharmacological Inhibitors of PHLPP1: Novel Therapies to Treat Brain Injury after Cardiac Arrest. Time Commitment: 30% Effort Supporting Agency: American Heart Association Name and Address of Funding Agency's Procuring Contracting/Grants Officer: Research Administration 7272 Greenville Avenue Dallas, Texas 75231-4596 awards@heart.org Performance Period: 07/01/2014 – 06/30/2018 Goal: The proposed grant project will test if novel BBB permeable small-molecule (PHLPP1) inhibitors reduce cell death and cognitive impairment in a rat model of cardiac arrest. The project will also identify selective PHLPP1 versus PHLPP2 isoform inhibitors. Role: Principle Investigator 2. PI: Travis C. Jackson Title: The mRNA Splicing Factor RBM5: A New Therapeutic Target for TBI. Time Commitment: 20% Effort. Supporting Agency: National Institute of Health Name and Address of Funding Agency's Procuring Contracting/Grants Officer: NINDS - Neuroscience Center Division of Extramural Research 6001 Executive Boulevard Suite 3309 Bethesda, MD 20892- 9531* Performance Period: 07/01/2014 – 12/31/2016 Goal: The proposed grant project will test if pharmacological inhibition of RNA Binding Motif 5 (RBM5) with anthraquinone-2-sulfonic acid (AQ2S) is a novel therapeutic approach to improve histological and cognitive outcomes after traumatic brain injury. It will be the first study to test if targeting components of the spliceosome (a biological system responsible for cellular mRNA splicing) is a new therapeutic method to treat CNS injury. Role: Principle Investigator 3. PI: Patrick M. Kochanek, MD/Edwin Jackson, PhD Title: “2′,3′-cAMP in Traumatic Brain Injury” Time Commitment: 25 % Effort (3 calendar months) Supporting Agency: National Institutes of Health Name and Address of Funding Agency's Procuring Contracting/Grants Officer: Performance Period: July 1, 2014 - June 30, 2019 Goal: Our overall hypothesis is that the “2’,3’-cAMP-adenosine pathway” is an endogenous cytoprotective mechanism after traumatic brain injury (TBI). Using studies in cell culture and a mouse model of TBI, we will elucidate which CNS cell types produce 2’,3’-cAMP, what kinds of injury trigger 2’,3’-cAMP production, how 2’,3’-cAMP is transported out of cells, how downstream AMPs are converted to adenosine, and if manipulating the 2’,3’-cAMP-adenosine pathway alters secondary damage after TBI. Role: Co-Investigator 4. University of Pittsburgh, School of Medicine, Department of Critical Care Medicine. Salary Support. Pending Support 1. PI: Travis C. Jackson Title: FGF21 Activates RBM3 and is a Novel Drug to Revolutionize Temperature Management. Time Commitment: 20% Effort. Supporting Agency: National Institute of Health Name and Address of Funding Agency's Procuring Contracting/Grants Officer: NINDS - Neuroscience Center Division of Extramural Research 6001 Executive Boulevard Suite 3309 Bethesda, MD 20892- 9531* Performance Period: 04/01/2017 – 03/31/2019 Goal: The proposed project will test if targeted temperature management (TTM) to 36°C and/or therapeutic hypothermia (TH) to 33°C increases brain levels of the neuroprotective/cold-shock protein RNA Binding Motif 3 (RBM3) in rats. The study will also test if the hibernationassociated hormone fibroblast growth factor 21 (FGF21) augments the induction of RBM3 during TTM and/or TH, and reduces histological markers of cell death after a traumatic brain injury (TBI). Role: Principle Investigator 2. PI: Travis C. Jackson Title: New Reagents to Study Phosphorylated NSMF: A Major Target in Synaptic Activity. Time Commitment: 15% Effort. Supporting Agency: National Institute of Health Name and Address of Funding Agency's Procuring Contracting/Grants Officer: NINDS - Neuroscience Center Division of Extramural Research 6001 Executive Boulevard Suite 3309 Bethesda, MD 20892- 9531* Performance Period: 04/01/2017 – 03/31/2019 Goal: The proposed project will develop a new antibody to detect NSMF – a neuronal protein which is involved in synaptic activity. Role: Principle Investigator 3. PI: Patrick M. Kochanek, MD Title: Operation Brain Trauma Therapy: Resuscitation Funding/Supporting Agency, Name and Address of Funding Agency's Procuring Contracting/Grants Officer: US Army Medical Research and Materiel Command (USAMRMC) Brief Description of Projects Goals: Operation brain trauma therapy (OBTT) is a drug screening and biomarker development consortium that is proposing to test potential new therapies across multiple pre-clinical models of traumatic brain injury (TBI) plus hemorrhagic shock to identify the most promising therapies for ultimate testing in both blast TBI models and clinical trials. OBTT Resuscitation represents collaboration between the University of Pittsburgh (Safar Center), the University of Miami, WRAIR, Virginia Commonwealth University (VCU), Banyan Biomarkers, and the University of Florida. Role: Co-Investigator Previous Support 1. PI: Travis C. Jackson Title: PHLPP1, A Novel Drug Target to Limit Neuronal Death. Time Commitment: 91% Effort. Supporting Agency: American Heart Association Name and Address of Funding Agency's Procuring Contracting/Grants Officer: Research Administration 7272 Greenville Avenue Dallas, Texas 75231-4596 awards@heart.org Performance Period: 07/01/2011 – 07/01/2013 Goal: This project grant supported development of novel small-molecule drug inhibitors to block activity of pleckstrin homology domain and leucine rich repeat protein phosphatase 1 (PHLPP1), a potent pro-death protein, for the treatment of cardiac arrest brain injury. Role: Principle Investigator 2. PI: Patrick M. Kochanek, MD Title: Operation Brain Trauma Therapy Extended Studies Time Commitment: 30% Effort (3.60 calendar months) Funding/Supporting Agency, Name and Address of Funding Agency's Procuring Contracting/Grants Officer: US Army Medical Research and Materiel Command (USAMRMC) Performance Period: 04/01/14 through 03/31/16 Brief Description of Projects Goals: Operation brain trauma therapy (OBTT) is a drug screening and biomarker development consortium that is testing potential new therapies across multiple pre-clinical models of traumatic brain injury (TBI) to identify the most promising therapies for ultimate testing in both blast TBI models and clinical trials. OBTT represents collaboration between the University of Pittsburgh (Safar Center), the University of Miami, WRAIR, Virginia Commonwealth University (VCU), Banyan Biomarkers, and the University of Florida. Role: Co-Investigator Patents 1 Jackson, T.C., Verrier, J.D., Kochanek, P.M. Small Molecule Inhibitors of RNA Binding Motif (RBM) Proteins for the Treatment of Acute Cellular Injury (2014). Patent application filed with U.S. Patent and Trademark office, U.S. Department of Commerce by Klarquist Sparkman, LLP (assignee: University of Pittsburgh). Patent Application #14/401-088. 2 Jackson, T.C. and Kochanek, P.M. Method to Improve Neurologic Outcomes in Temperature Managed Patients.” (2015) Application filed with U.S. Patent and Trademark office, U.S. Department of Commerce by The WEBB Law Firm, (assignee: University of Pittsburgh). Provisional Application #62/164,205.
References
- 1.Gross CG. Aristotle on the brain. The Neuroscientist. 1995;1:245–250. [Google Scholar]
- 2.Remba SJ, Varon J, Rivera A, et al. The effects of therapeutic hypothermia and the first ambulance. Resuscitation. 2010;81:268–271. doi: 10.1016/j.resuscitation.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Phelps C. Traumatic Injuries of the Brain and Its Membranes. D. Appleton & Co; New York, NY: 1897. pp. 223–224. [Google Scholar]
- 4.Rosomoff HL. Protective effects of hypothermia against pathological processes of the nervous system. Ann NY Acad Sci. 1959;80:475–486. doi: 10.1111/j.1749-6632.1959.tb49225.x. [DOI] [PubMed] [Google Scholar]
- 5.Lundberg N, Troupp H, Lorin H. Continuous recording of the ventricular fluid pressure in patients with severe acute traumatic brain injury: a preliminary report. J Neurosurg. 1965;22:581–590. doi: 10.3171/jns.1965.22.6.0581. [DOI] [PubMed] [Google Scholar]
- 6.Conn AW. Near-drowning and hypothermia. Can Med Assoc J. 1979;120:397–400. [PMC free article] [PubMed] [Google Scholar]
- 7.Busto R, Dietrich WD, Globus MY, et al. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab. 1987;7:729–738. doi: 10.1038/jcbfm.1987.127. [DOI] [PubMed] [Google Scholar]
- 8.Busto R, Dietrich WD, Globus MY, et al. The importance of brain temperature in cerebral ischemic injury. Stroke. 1989;20:1113–1114. doi: 10.1161/01.str.20.8.1113. [DOI] [PubMed] [Google Scholar]
- 9.Leonov Y, Sterz F, Safar P, et al. Mild cerebral hypothermia during and after cardiac arrest improves neurologic outcome in dogs. J Cereb Blood Flow Metab. 1990;10:57–70. doi: 10.1038/jcbfm.1990.8. [DOI] [PubMed] [Google Scholar]
- 10.Gunn AJ, Gunn TR, de Haan HH, et al. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest. 1997;99:248–256. doi: 10.1172/JCI119153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nozari A, Safar P, Stezoski SW, et al. Critical time window for intra-arrest cooling with cold saline flush in a dog model of cardiopulmonary resuscitation. Circulation. 2006;113:2690–2696. doi: 10.1161/CIRCULATIONAHA.106.613349. [DOI] [PubMed] [Google Scholar]
- 12.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 13.The Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 14.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013 Jan 31;(1):CD003311. doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hey EN. The relation between environmental temperature and oxygen consumption in the new-born baby. J Physiol. 1969;200:589–603. doi: 10.1113/jphysiol.1969.sp008710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards AD, Yue X, Squier MV, et al. Specific inhibition of apoptosis after cerebral hypoxia-ischaemia by moderate post-insult hypothermia. Biochem Biophys Res Commun. 1995;217:1193–1199. doi: 10.1006/bbrc.1995.2895. [DOI] [PubMed] [Google Scholar]
- 18.Fukuda H, Tomimatsu T, Watanabe N, et al. Post-ischemic hypothermia blocks caspase-3 activation in the newborn rat brain after hypoxia-ischemia. Brain Res. 2001;910:187–191. doi: 10.1016/s0006-8993(01)02659-2. [DOI] [PubMed] [Google Scholar]
- 19.Marion DW, Penrod LE, Kelsey SF, et al. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med. 1997;336:540–546. doi: 10.1056/NEJM199702203360803. [DOI] [PubMed] [Google Scholar]
- 20.Clifton GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344:556–563. doi: 10.1056/NEJM200102223440803. [DOI] [PubMed] [Google Scholar]
- 21.Andrews PJ, Sinclair HL, Rodriguez A, et al. Hypothermia for intracranial hypertension after traumatic brain injury. N Engl J Med. 2015;373:2403–2412. doi: 10.1056/NEJMoa1507581. [DOI] [PubMed] [Google Scholar]
- 22.Clifton GL, Valadka A, Zygun D, et al. Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): A randomised trial. Lancet Neurol. 2011;10:131–139. doi: 10.1016/S1474-4422(10)70300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adelson PD, Wisniewski SR, Beca J, et al. Comparison of hypothermia and normothermia after severe traumatic brain injury in children (Cool Kids): A phase 3, randomised controlled trial. Lancet Neurol. 2013;12:546–553. doi: 10.1016/S1474-4422(13)70077-2. [DOI] [PubMed] [Google Scholar]
- 24.Hutchison JS, Ward RE, Lacroix J, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358:2447–2456. doi: 10.1056/NEJMoa0706930. [DOI] [PubMed] [Google Scholar]
- 25.Tortorici MA, Kochanek PM, Poloyac SM. Effects of hypothermia on drug disposition, metabolism, and response: A focus of hypothermia-mediated alterations on the cytochrome P450 enzyme system. Crit Care Med. 2007;35:2196–2204. doi: 10.1097/01.ccm.0000281517.97507.6e. [DOI] [PubMed] [Google Scholar]
- 26.Anderson KB, Poloyac SM, Kochanek PM, et al. Effect of hypothermia and targeted temperature management on drug disposition and response following cardiac arrest: A comprehensive review of preclinical and clinical investigations. Ther Hypothermia Temp Manag. 2016 Sep 13; doi: 10.1089/ther.2016.0003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu XC, Shear DA, Deng-Bryant Y, et al. Comprehensive evaluation of neuroprotection achieved by extended selective brain cooling therapy in a rat model of penetrating ballistic-like brain injury. Ther Hypothermia Temp Manag. 2016;6:30–39. doi: 10.1089/ther.2015.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 29.Donnino MW, Andersen LW, Berg KM, et al. Temperature management after cardiac arrest: An advisory statement by the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Resuscitation. 2016;98:97–104. doi: 10.1016/j.resuscitation.2015.09.396. [DOI] [PubMed] [Google Scholar]
- 30.Moler FW, Silverstein FS, Holubkov R, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med. 2015;372:1898–1908. doi: 10.1056/NEJMoa1411480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vincent JL, Taccone FS. Difficulty interpreting the results of some trials: the case of therapeutic hypothermia after pediatric cardiac arrest. Crit Care. 2015;19:391. doi: 10.1186/s13054-015-1121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peretti D, Bastide A, Radford H, et al. RBM3 mediates structural plasticity and protective effects of cooling in neurodegeneration. Nature. 2015;518:236–239. doi: 10.1038/nature14142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson TC, Manole MD, Kotermanski SE, et al. Cold stress protein RBM3 responds to temperature change in an ultra-sensitive manner in young neurons. Neuroscience. 2015;305:268–278. doi: 10.1016/j.neuroscience.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robertson NJ, Faulkner S, Fleiss B, et al. Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model. Brain. 2013;136:90–105. doi: 10.1093/brain/aws285. [DOI] [PubMed] [Google Scholar]
- 35.Berntman L, Welsh FA, Harp JR. Cerebral protective effect of low-grade hypothermia. Anesthesiology. 1981;55:495–498. doi: 10.1097/00000542-198111000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Speakman JR, Keijer J. Not so hot: Optimal housing temperatures for mice to mimic the thermal environment of humans. Mol Metab. 2013;2:5–9. doi: 10.1016/j.molmet.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaskill BN, Garner JP. Letter-to-the-editor on “Not so hot: Optimal housing temperatures for mice to mimic the thermal environment of humans.”. Mol Metab. 2014;3:335–336. doi: 10.1016/j.molmet.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Speakman JR, Keijer J. Not so nuanced: Reply to the comments of Gaskill and Garner on 'Not so hot: Optimal housing temperatures for mice to mimic the environment of humans.’. Mol Metab. 2014;3:337. doi: 10.1016/j.molmet.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maher RL, Barbash SM, Lynch DV, et al. Group housing and nest building only slightly ameliorate the cold stress of typical housing in female C57BL/6J mice. Am J Physiol Regul Integr Comp Physiol. 2015;308:R1070–1079. doi: 10.1152/ajpregu.00407.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartings JA, Wilson JA, Hinzman JM, et al. Spreading depression in continuous electroencephalography of brain trauma. Ann Neurol. 2014;76:681–694. doi: 10.1002/ana.24256. [DOI] [PubMed] [Google Scholar]
- 41.Takaoka S, Pearlstein RD, Warner DS. Hypothermia reduces the propensity of cortical tissue to propagate direct current depolarizations in the rat. Neurosci Lett. 1996;218:25. doi: 10.1016/0304-3940(96)13112-8. [DOI] [PubMed] [Google Scholar]
- 42.Reynolds JC, Callaway CW. All [post-cardiac arrest patients] are [not] created equal. Resuscitation. 2015;96:A1–2. doi: 10.1016/j.resuscitation.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 43.Davidson JO, Wassink G, van den Heuij LG, et al. Therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy - Where to from here? Front Neurol. 2015;6:198. doi: 10.3389/fneur.2015.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmad FU, Levi AD. Hypothermia for spinal cord injury. J Neurosurg Spine. 2014;21:843–844. doi: 10.3171/2014.4.SPINE14330. [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Liu L, Zhang H, et al. Endovascular hypothermia in acute ischemic stroke: Pilot study of selective intra-arterial cold saline infusion. Stroke. 2016;47:1933–1935. doi: 10.1161/STROKEAHA.116.012727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Legriel S, Pico F, Tran-Dinh YR, et al. Neuroprotective effect of therapeutic hypothermia versus standard care alone after convulsive status epilepticus: protocol of the multicentre randomised controlled trial HYBERNATUS. Ann Intensive Care. 2016;6:54. doi: 10.1186/s13613-016-0159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kutcher ME, Forsythe RM, Tisherman SA. Emergency preservation and resuscitation for cardiac arrest from trauma. Int J Surg. 2015 Oct 20;:S1743–9191(15)01279-0. doi: 10.1016/j.ijsu.2015.10.014. doi: 10.1016/j.ijsu.2015.10.014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Dietrich WD, Alonso O, Halley M, et al. Delayed posttraumatic brain hyperthermia worsens outcome after fluid percussion brain injury: a light and electron microscopic study in rats. Neurosurgery. 1996;38:533–541. doi: 10.1097/00006123-199603000-00023. [DOI] [PubMed] [Google Scholar]
- 49.Hickey RW, Kochanek PM, Ferimer H, et al. Hypothermia and hyperthermia in children after resuscitation from cardiac arrest. Pediatrics. 2000;106:118–122. doi: 10.1542/peds.106.1.118. [DOI] [PubMed] [Google Scholar]
- 50.Laptook A, Tyson J, Shankaran S, et al. Elevated temperature after hypoxic-ischemic encephalopathy: risk factor for adverse outcomes. Pediatrics. 2008;122:491–499. doi: 10.1542/peds.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holzinger U, Brunner R, Losert H, et al. Resting energy expenditure and substrate oxidation rates correlate to temperature and outcome after cardiac arrest - a prospective observational cohort study. Crit Care. 2015;19:128. doi: 10.1186/s13054-015-0856-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kollmar R, Blank T, Han JL, et al. Different degrees of hypothermia after experimental stroke: short- and long-term outcome. Stroke. 2007;38:1585–1589. doi: 10.1161/STROKEAHA.106.475897. [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi S. Warm- and cold-sensitive neurons inactive at normal core temperature in rat hypothalamic slices. Brain Res. 1986;362:132–139. doi: 10.1016/0006-8993(86)91406-x. [DOI] [PubMed] [Google Scholar]
- 54.Kiyatkin EA, Sharma HS. Expression of heat shock protein (HSP 72 kDa) during acute methamphetamine intoxication depends on brain hyperthermia: neurotoxicity or neuroprotection? J Neural Transm. 2011;118:47–60. doi: 10.1007/s00702-010-0477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kiyatkin EA, Sharma HS. Permeability of the blood-brain barrier depends on brain temperature. Neuroscience. 2009;161:926–939. doi: 10.1016/j.neuroscience.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwarz S, Häfner K, Aschoff A, et al. Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology. 2000;54:354–361. doi: 10.1212/wnl.54.2.354. [DOI] [PubMed] [Google Scholar]
- 57.Wass CT, Lanier WL, Hofer RE, et al. Temperature changes of > or = 1 degree C alter functional neurologic outcome and histopathology in a canine model of complete cerebral ischemia. Anesthesiology. 1995;83:325–335. doi: 10.1097/00000542-199508000-00013. [DOI] [PubMed] [Google Scholar]