Abstract
Secondary mitral valve regurgitation (MR) remains a challenging problem in the diagnostic work-up and treatment of heart failure patients. Although secondary MR is characteristically dynamic in nature and sensitive to changes in ventricular geometry and loading, current therapy is mainly focused on resting conditions. Exercise-induced increase in secondary MR, however, is associated with impaired exercise capacity and increased mortality. In an era where a multitude of percutaneous solutions are emerging for the treatment of HF patients it becomes important to address the dynamic component of secondary MR during exercise as well. A critical reappraisal of the underlying disease mechanisms, and in particular of the dynamic component during exercise is of timely importance. This review summarizes the pathophysiologic mechanisms involved in the dynamic deterioration of secondary MR during exercise, its functional and prognostic impact, and the way current treatment options affect the dynamic lesion and exercise hemodynamics in general.
Keywords: secondary mitral valve regurgitation, exercise testing, heart failure, mitral valve surgery, exercise capacity
Introduction
Secondary mitral valve regurgitation (MR) is a challenging problem in the diagnostic work-up and treatment of heart failure (HF). It occurs in 11 to 59% of patients after a myocardial infarction1, 2 and is present in more than half of patients with dilated cardiomyopathy3–5. Despite this high prevalence, the optimal approach to secondary MR remains a matter of debate, and the level of evidence for guideline recommendations is disappointingly low6, 7. Particularly controversial remains the question whether moderate and mild-moderate MR should be regarded as treatment targets: It is well known that outcomes and exercise capacity are impaired in secondary MR, even when only mild8, 9. This association may simply reflect the fact that the occurrence of MR, even if only mild, is a marker of more advanced disease. However, it could also be rooted in the characteristically dynamic behavior of secondary MR, often becoming more severe during exercise (Figure 1; Movie Clips 1–4) or with changing loading conditions10. To date, our knowledge of exercise dynamics in secondary MR is insufficient: Does relief of the dynamic lesion result in better outcome? Is the prognosis of severe exercise-induced MR (resting mild MR) comparable to resting severe MR? Should such patients be referred for surgical treatment? Do we even know the potential impact of the available therapies for secondary MR on the respective exercise hemodynamics? In an era where a multitude of percutaneous solutions are emerging for the treatment of HF patients it becomes important to understand who may benefit from a specific therapy and who not. A critical reappraisal of our knowledge of the underlying disease mechanisms, and in particular of the dynamic component of secondary MR during exercise, is therefore of timely importance.
Figure 1. Secondary mitral regurgitation (MR) at rest and during exercise.
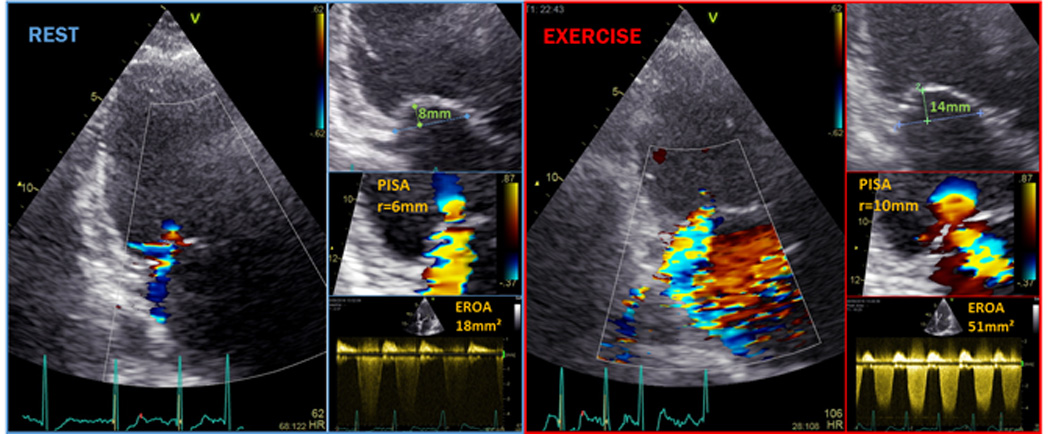
Apical long axis view with color Doppler in a typical ischemic cardiomyopathy patient with (eccentric) moderate MR at rest that significantly increases to severe MR already at modest exercise (Movie Clips 1 and 2). Proximal isovelocity surface area (PISA) and coaptation distance (i.e. a measure of tenting severity) was substantially higher during exercise (Movie Clips 3 and 4 respectively).
This review summarizes pathophysiological mechanisms involved in the dynamic deterioration of secondary MR, its functional and prognostic impact, and the manner in which current treatment options affect dynamic MR and exercise hemodynamics in general. Finally, potential targets for future research are highlighted.
Pathophysiology
The mitral valvular apparatus
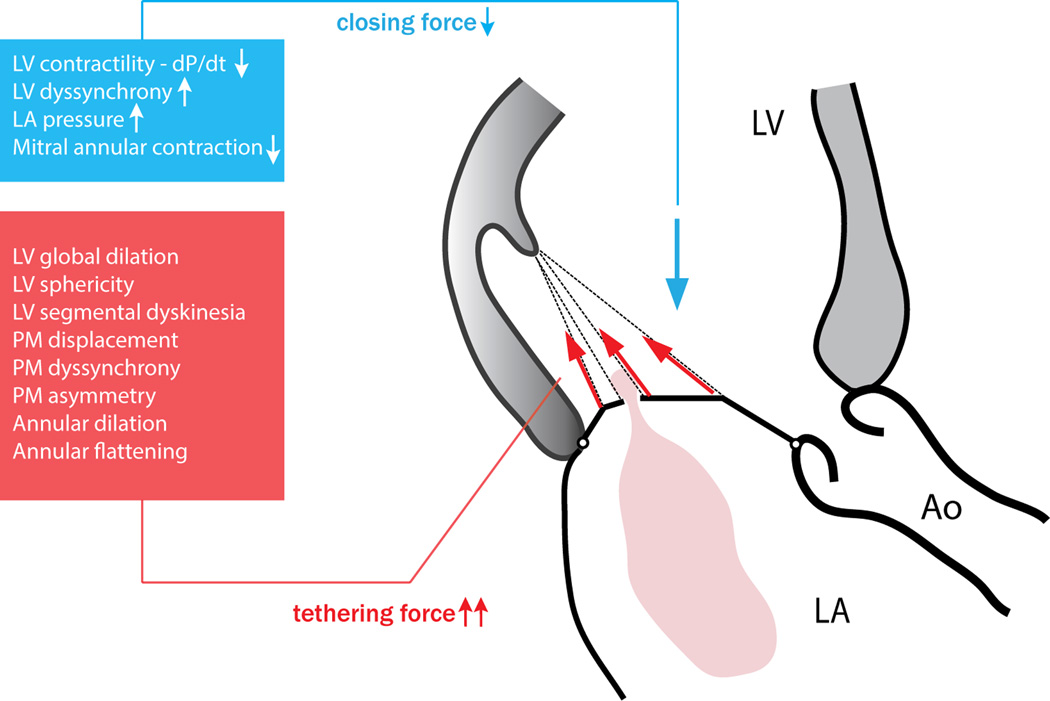
More than just a pair of leaflets, the mitral valve is an intricate apparatus comprised of multiple components (i.e., the mitral annulus, anterior and posterior leaflets, chordae tendineae, anterolateral and posteromedial papillary muscles and adjacent left ventricular wall) finely tuned to work together in ‘sealing’ the passage of blood from the left ventricle to the left atrium in systole, while at the same time enabling unrestricted inflow during diastole. Several mechanisms interplay during systole: (1) annular contraction and (2) left ventricular wall motion causing inward displacement of the papillary muscles, to facilitate leaflet coaptation while minimizing leaflet stress, and (3) measured contraction of the papillary muscles to avoid leaflet prolapse. A delicate balance is maintained between tethering forces (transmitted via the left ventricular wall, papillary muscles and chordae tendineae system influenced by annular/leaflet dimensions) and valve closing forces (left ventricular contractility and synchrony, mitral annular systolic contraction). Disruption in any of these mechanisms may unsettle the force balance and result in MR11.
A mechanistic insight into secondary MR
Secondary MR results from a functional imbalance between increased tethering forces (annular and/or left ventricular dilation, segmental or global left ventricular dysfunction with papillary muscle displacement/dysfunction) and decreased closing forces (reduced left ventricular contractility or synchrony) in the presence of a structurally normal valve. As a result, increased traction of the mitral valve leaflets – tethering – occurs during systole, resulting in incomplete coaptation and MR (Figure 2). This balance of forces creates a lesion that is evidently dynamic even within one cardiac cycle (Movie Clip 3): Schwammenthal et al12 demonstrated a typical decrease in MR during ventricular contraction with a minimum in mid-systole (at the time of peak orifice velocity and peak systolic pressure), and an increase again during ventricular relaxation, suggesting a decisive impact of the instantaneous left ventricular-to-atrial pressure difference on leaflet coaptation. While a mid-systolic decrease in annular area could also contribute to this phenomenon it was subsequently shown that variation in annular area in patients with left ventricular dysfunction is minor and insufficient to explain the degree of observed orifice area variation13. In addition, it was shown that an acute rise in closing force due to acute activation of cardiac resynchronization therapy (CRT) in patients with dilated hearts and secondary MR also reduces the degree of MR significantly (Figure 3)14. However, an impaired closing force is not the primary cause of secondary MR in HF. Numerous studies, both in vitro15 and in vivo16, 17, have demonstrated that increased tethering is a prerequisite for occurrence of secondary MR, and geometric disturbances of the left ventricle are key in the dynamic imbalance between closing and tethering forces. In an animal model of left ventricular systolic dysfunction without ventricular dilation, only trace MR was observed and tenting depth was the only predictor of MR, in contrast to ejection fraction or dP/dt16. Hence tethering (i.e. geometry) sets the stage for secondary MR, while the closing force merely modulates its severity. More recent studies have confirmed that not left ventricular dimensions per se, but rather the position and dynamics of the papillary muscles are major determinants of secondary MR18–20. In a normal-sized left ventricle with regional wall motion abnormalities causing one or both papillary muscles to be displaced, to move asymmetrically18, 19 or dyssynchronous20, 21, tethering forces increase, disrupting the force balance and causing secondary MR.
Figure 2. Secondary mitral regurgitation results from functional disruption in the force balance between tethering and closing forces, inhibiting normal mitral leaflet coaptation.
Figure 3. The acute impact of cardiac resynchronization therapy (CRT) on secondary mitral valve regurgitation (MR) at rest.
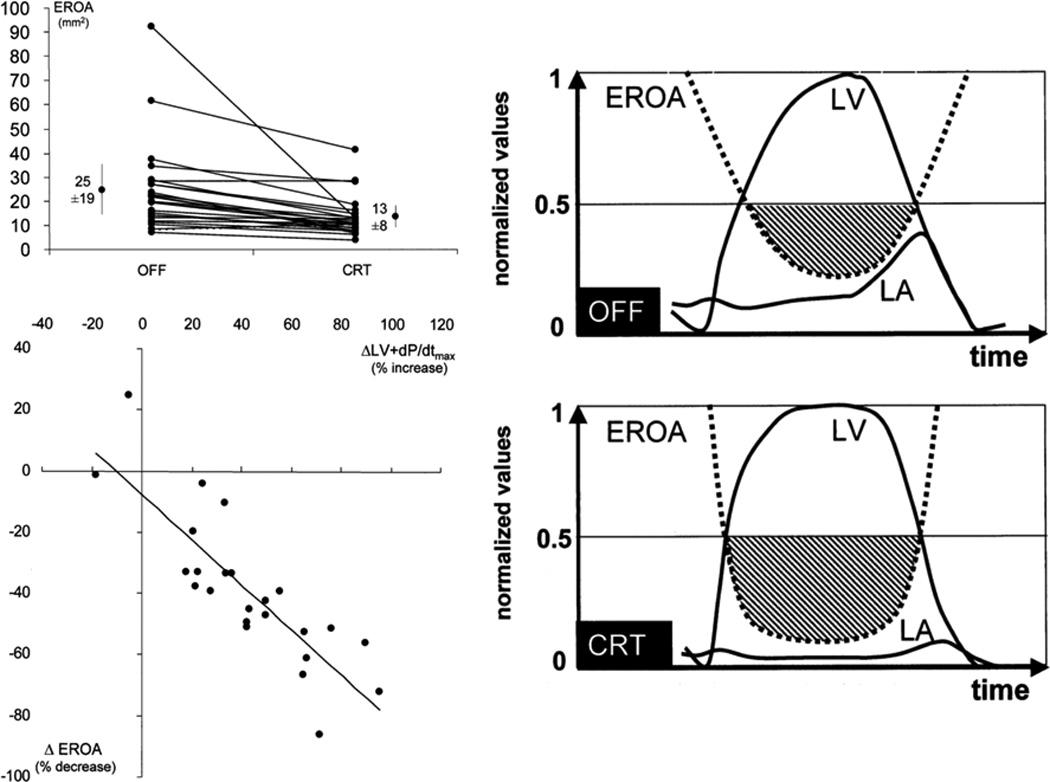
An immediately decreased effective regurgitant orifice (EROA) relates to the increase in closing force (dP/dt) and longer relative duration of maximal closing force. Adapted from Breithart et al14, with permission.
A dilated mitral annulus further augments these effects and increases MR18, although concomitant subvalvular tethering is usually needed to cause more than moderate MR22. Isolated annular dilation in patients with normal left ventricular dimensions and function, however, is an increasingly important cause of moderate (or more) secondary MR, particularly in patients with (long-standing) persistent or permanent atrial fibrillation23, 24. Such “atrial” functional MR has shown to be potentially reversible with rhythm control strategies (e.g. atrial fibrillation ablation)23, and typically responds well to annular reduction strategies25.
Of note, unlike the classic belief, the mitral valve leaflets are not innocent ‘healthy’ bystanders, but play an active role in the pathophysiology of secondary MR by undergoing significant leaflet tissue changes and adaptation26, 27. In reaction to the mechanical stretch, active leaflet growth and leaflet enlargement have been demonstrated28–30, sometimes (e.g. in chronic aortic regurgitation) even matching the severe chamber dilation, hence preventing secondary MR31. Conversely, in case of insufficient leaflet adaptation and/or adverse fibrotic leaflet changes, the leaflets’ maladaptive response might even contribute to the development and severity of secondary MR32.
Finally, inter-individual differences in mitral valve geometry, such as the inborn relative position of basal chordae tendineae insertion sites, modulate the individual susceptibility to development of (severe) secondary MR33.
The normal cardiovascular response to exercise
During exercise, the cardiovascular system adapts to meet increased oxygen demands of peripheral muscles34. Stimulation of the sympathetic system and withdrawal of the vagal tone result in increasing heart rate and myocardial contractility, and a decrease in systemic vascular resistance, thus increasing cardiac output35. The dynamic response of the left ventricle depends on the type of exercise performed, which can be either dynamic (isotonic), static (isometric), or a combination of both. In dynamic exercises (e.g. running, cycling, swimming), multiple muscle groups contract in order to achieve joint movement. Such activity is associated with profound vasodilation in the muscular vasculature causing a significant reduction in systemic vascular resistance to almost half its value at rest34, and a substantial increase in venous return due to the skeletal muscle pump. The decreased afterload, increased contractility, and increased venous return result in a significant increase in stroke volume. The rise in cardiac output is therefore mediated by an increase in stroke volume, with heart rate becoming the dominant determinant as stroke volume reaches its plateau35. In static exercise on the other hand (e.g. weightlifting, handgrip), systemic vascular resistance is not decreased and might even be increased. In the absence of afterload reduction, the stroke volume remains largely unchanged and the rise in cardiac output is determined mainly by the increase in heart rate. Hence, static exercise imposes a pressure load on the left ventricle, whereas dynamic exercise constitutes a volume load35. These differential loading conditions translate into different left ventricular geometric changes during exercise, as has been studied with radionuclide angiography36, 37, echocardiography38, 39 and cardiac magnetic resonance imaging40. In healthy subjects, dynamic exercise is associated with no increase (or a transient increase at mild-to-moderate exercise38) of the end-diastolic volume (EDV), while a concomitant decrease in end-systolic volume (ESV) is observed. This results in a substantially increased stroke volume and ejection fraction. In contrast, during static exercise, no significant changes in either ESV or EDV occur41, 42.
The pulmonary circulation adapts to the increased cardiac output and (mildly) increased left atrial pressures during exercise by recruiting and distending pulmonary arterial vessels, hence decreasing the pulmonary vascular resistance and blunting the rise in pulmonary pressures in response to a significant increase in flow and volume43, 44. In healthy individuals, the increase in mean pulmonary pressure with respect to cardiac output is not expected to surpass 3.0mmHg/L/min43. As a rule of thumb, a mean pulmonary pressure during exercise >30mmHg at cardiac output <10L/min is considered abnormal (however in trained athletes values up to a mean pulmonary pressure of 50 mmHg have been reported)45.
Secondary MR during exercise
Left ventricular geometry
In patients with ischemic or idiopathic cardiomyopathy the left ventricular volumetric response to dynamic or static exercise may differ significantly from that observed in normal subjects37, 46, 47. In general, both volume loading in dynamic exercise and pressure loading in static exercise result in exercise-induced ventricular dilation (EDV and ESV both tend to increase), while ejection fraction typically remains unchanged. The increase in left ventricular EDV during exercise is reported to be greater in ischemic (than in idiopathic dilated) cardiomyopathy46, which may be directly related to the extent of myocardial scar48, a finding of particular importance for the exercise dynamics of secondary MR. In addition, exercise may result in dyssynchronous contraction of the left ventricle in HF with (rate-dependent) conduction delay49. While the acute rise in blood pressure during static exercise increases the mitral closing force, this is completely outweighed by increased mitral tethering resulting from the impact of the rise in afterload on left ventricular geometry. Consequently, significant increases in secondary MR have been observed during static exercise47. Significant increases in systemic venous resistance are generally not observed during dynamic exercise in patients with cardiomyopathy. However, failure of adequate arterial dilatation during exercise occurs50 and, in the presence of an increased volume load, contributes to exercise-induced ventricular dilatation. Dynamic exercise studies therefore demonstrate increases in secondary MR in >75% of HF patients, both with ischemic51–55 and non-ischemic etiology54–59. Table 1 summarizes the current literature on determinants of increasing secondary MR during exercise in HF. Interestingly, neither left ventricular volume nor ejection fraction at rest or during exercise are reliable predictors of exercise-induced MR deterioration. In fact, with respect to exercise-induced left ventricular dilatation, changes in shape (local remodeling) may count more than changes in the actual volume51. An increase in left ventricular sphericity, which correlates with greater papillary muscle distance, may have a pronounced effect on mitral geometry even without a significant change in volume. On the other hand, an increase in left ventricular volume caused by apical dilatation of the cavity (which does not involve the mitral valve apparatus) will have no effect on mitral valve geometry. It is therefore not surprising that, when looking at variables at rest that can predict worsening of MR during exercise, left ventricular sphericity and papillary muscle dyssynchrony are more useful along with mitral valve tenting, which reveals an already strained balance between closing and tethering forces. In addition, progressive annular dilation during exercise may be a contributor to exercise-induced MR in a majority of cases and is most predictive in non-ischemic cardiomyopathy55.
Table 1.
Geometric determinants of increasing secondary mitral valve regurgitation during exercise.
| Author, Year, Ref. |
n | IMR | Regression | LVEDD | LVEF | LV sphericity | LV dyssynchrony | Annular dilation | Tenting area | Coaptation depth | WMSI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rest | Δ | rest | Δ | rest | Δ | rest | Δ | rest | Δ | rest | Δ | rest | Δ | rest | Δ | ||||
| Keren et al., 198947 |
17 | 50% | UV | - | |||||||||||||||
| Lapu-Bula et al., 200254 |
25 | 50% | UV | - | + | - | - | ||||||||||||
| Lancellotti et al., 200351 |
70 | 100% | UV MV |
- | - | + + |
+ +† |
+ +* |
+† +† |
||||||||||
| Giga et al., 200552 | 40 | 100% | UV MV |
- | - | - | - | - | + | - | + - |
- | + + |
- | + + |
- | + - |
||
| Lancellotti et al., 200553 |
35 | 100% | UV MV |
- | - | + + |
+ + |
||||||||||||
| Ennezat et al., 200655 |
70 | 50% | UV MV |
+ - |
- - |
+ + |
+ + |
||||||||||||
| Takano et al., 200657 |
17 | 5/17 | UV | - | |||||||||||||||
| D’Andrea et al., 200756 |
60 | 0% | UV MV |
- | - | + + |
+ + |
||||||||||||
| Yamano et al., 200859 |
32 | 0% | UV |
- | - | - | - | + | - | - | - | + | + | - | + | ||||
| Izumo et al., 200958 |
50 | 16/50 | UV MV |
- | - | - | - | + | + + |
- | + + |
- | + - |
- | + - |
- | + + |
||
The symbol ‘+’ indicates an association between the geometric determinant (or change in determinant) and the severity of secondary MR during exercise was found, in univariate (UV) and/or multivariate (MV) analysis. The symbol ‘–‘ indicates no correlation was found in UV and/or MV analysis. Only studies without inducible ischemia were included. Δ = changes during exercise; LV, left ventricle; LVEDD, left ventricular end diastolic dimension, LVEF, left ventricular ejection fraction; WMSI, wall motion score index; IMR, ischemic mitral regurgitation.
anterior myocardial infarction only.
inferior myocardial infarction only;
Left ventricular scar
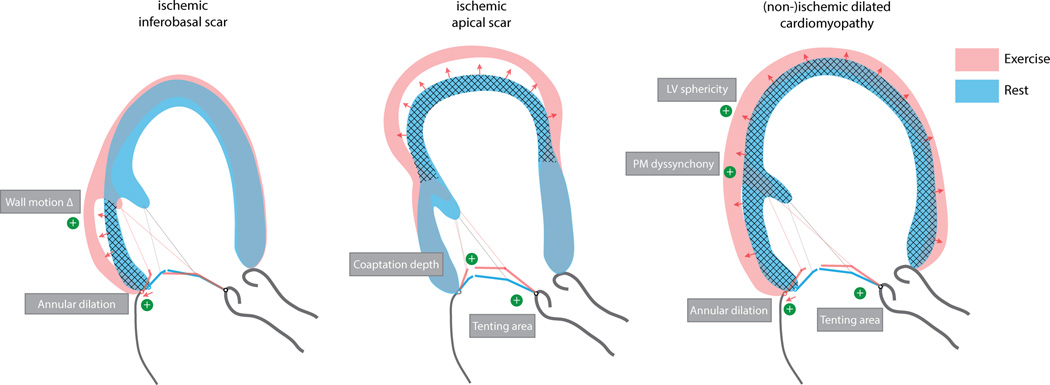
Especially in ischemic cardiomyopathy, the extent and localization of scar plays an important mechanistic role in dynamic MR deterioration (Figure 4)48. Following an anterior wall myocardial infarction extending to the apical segments of the inferior wall, patients show more apically tethered mitral leaflets, with coaptation depth being a determinant of exercise-induced MR deterioration51, 52. In patients with inferior wall infarction on the other hand, regional wall motion abnormalities during exercise tether the mitral valve more posteriorly, frequently restricting in particular the posterior leaflet, and increasing the annular dimension (in particular around P2–P3), thereby aggravating MR in a somewhat different way. Interestingly, significant contractile reserve of the inferoposterior wall in the latter group is related to an exercise-induced decrease in secondary MR, as tethering forces are reduced and the mitral annulus less distorted51. Large infarctions (which are frequently more anterior) or multiple infarctions leading to severe remodeling lead to more spherical ventricles with greater papillary muscle separation exerting traction on the mitral valve from opposing ends.
Figure 4. The differential left ventricular response to exercise in cardiomyopathy based on the underlying etiology and location of the scar.
Dominant determinants of worsening secondary MR during exercise are highlighted for each situation.
Closing versus tethering forces during exercise
Intriguingly, predictors of increasing MR during exercise (Table 1) are all related to left ventricular geometry51, 52, indicating that in the failing ventricle increases in tethering during exercise outweigh by far increases in closing force. This is supported by the observation that although onset of CRT causes an early decrease in secondary MR at rest (due to an immediate increase in closing forces14), this effect is not maintained during exercise. Indeed, early after CRT, secondary MR often deteriorates to pre-CRT severity during dynamic exercise, despite an acute reduction in resting MR60. Only after reverse left ventricular remodeling has taken place (resulting in reduced tethering forces), significant reductions in exercise-induced MR are observed (Figure 5)60.
Figure 5. Impact of cardiac resynchronization therapy (CRT) on secondary mitral valve regurgitation (MR) during exercise.
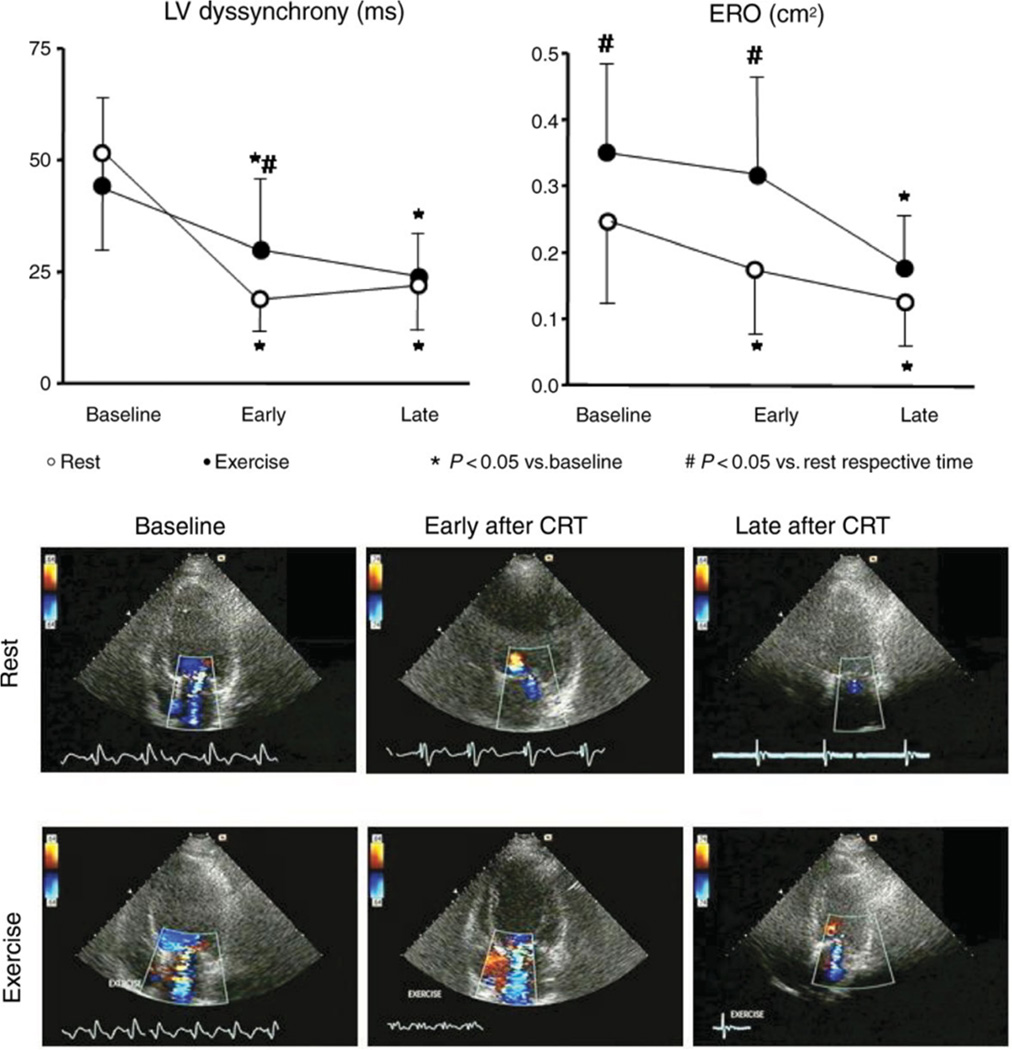
In contrast to the resting situation (Figure 4), during exercise immediately after CRT implantation, no decrease in EROA is observed. Only after 3 months when reverse remodeling has occurred, a significant reduction in exercise-induced MR is observed. Adapted with permission from Madaric et al60.
Of note, induction of myocardial ischemia during exercise in coronary artery disease associated with reversible wall motion abnormalities and a decrease in ejection fraction can also result in a subsequent increase in MR61. This type of MR, however, is not discussed here, since the underlying mechanisms (inducible reversible ischemia) and treatment approaches (revascularization) are different from the topic of this review. The term ‘ischemic MR’ in this review refers exclusively to post-myocardial infarction MR in patients with stable coronary heart disease. In these patients, it is the scar tissue and ventricular remodeling that truly determine the dynamic deterioration of MR during exercise62.
Prognostic and functional impact of the dynamic lesion of mitral valve regurgitation
Exercise-induced increase in secondary MR is associated with poor exercise capacity54, 59, 63 and outcomes9, 64–67 (Figure 6). In a mixed cohort of ischemic and non-ischemic cardiomyopathy, Lapu-Bula et al54 were the first to demonstrate a significant negative impact of exercise-induced MR deterioration on exercise capacity, even though MR was only mild-to-moderate at rest. The mechanism by which dynamic MR impairs exercise capacity is (1) an inhibition of the expected increase in exercise forward stroke volume, and (2) an exaggerated increase in exercise left atrial and pulmonary pressure54. The backward hemodynamic cascade of increased left atrial pressures and decreased left atrial compliance causes backward pulmonary venous congestion, gradually initiating pathologic changes in the pulmonary vasculature, thus increasing pulmonary vascular resistance and pulmonary arterial pressure68. Moreover, systolic pulmonary artery pressure during exercise is associated with dynamic increase in effective regurgitant orifice area (EROA)64, 69, 70, and is a particularly relevant hemodynamic (echocardiographic) parameter to assess during exercise in the evaluation and prognostication of secondary MR69, 71.
Figure 6. Prognostic impact of secondary mitral valve regurgitation (MR) at rest and during exercise.
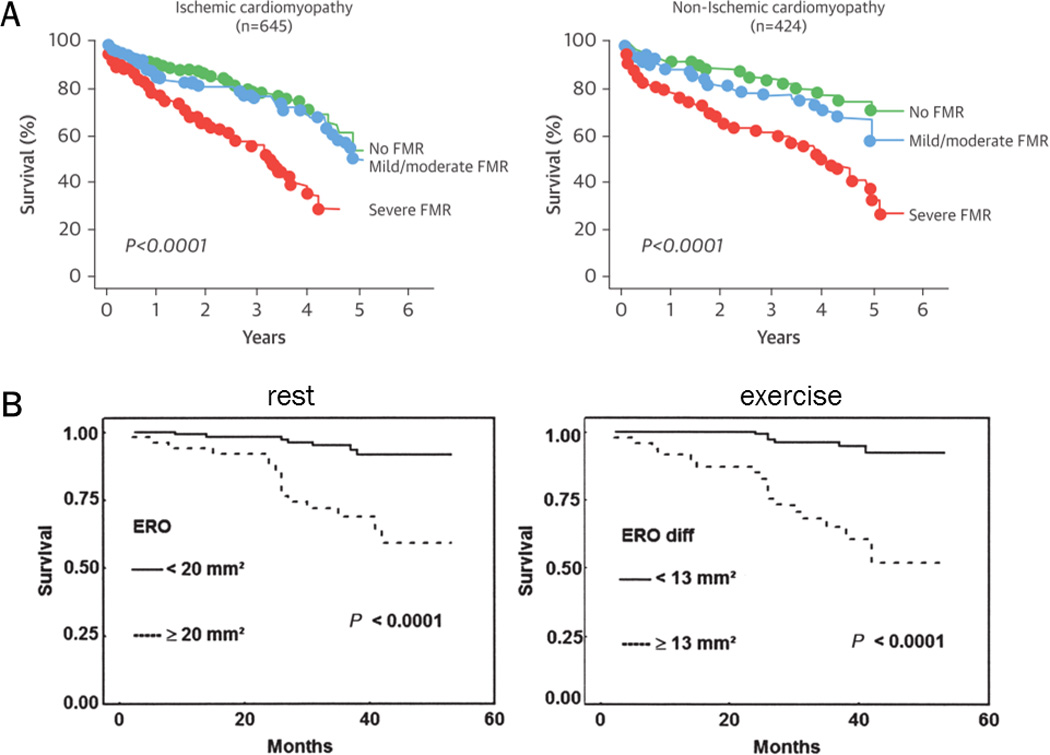
(A) Mortality in heart failure patients with secondary MR at rest follows a graded relationship with respect to MR severity. Severe MR (EROA>20mm2) already implies worse outcome for both ischemic and non-ischemic cardiomyopathy. Adapted from Rossi et al66 and Asgar et al67, with permission. (B) Mortality in patients with deteriorating secondary MR during exercise (EROA increase >13mm2), is increased and similar to patients with severe secondary MR at rest. Reproduced from Lancellotti et al9, with permission.
Piérard and Lancellotti72 further showed that exercise-induced MR deterioration, by augmenting backward flow and pulmonary vascular congestion may contribute to the development of acute pulmonary edema. Importantly, a significant increase in EROA during exercise (defined as an increase >13mm2) is observed in 30% of HF patients and associated with increased mortality as well as readmissions9, 64. Furthermore, all-cause mortality in HF patients with secondary MR follows a graded relationship with respect to MR severity, with EROA >20mm2 already implying poor outcome8, 66. This contrasts to the 40mm2 cut-off that is traditionally used in primary MR. Although occurrence of secondary MR is also a marker of underlying heart disease, the lower EROA threshold in secondary MR could also be rooted in the typically dynamic nature of secondary MR, which is frequently underestimated at rest. Therapies for secondary MR aiming at improved exercise capacity and outcomes should therefore not only focus on resting status, but also reduce the dynamic component of MR.
Impact of treatment approaches for secondary MR on exercise dynamics
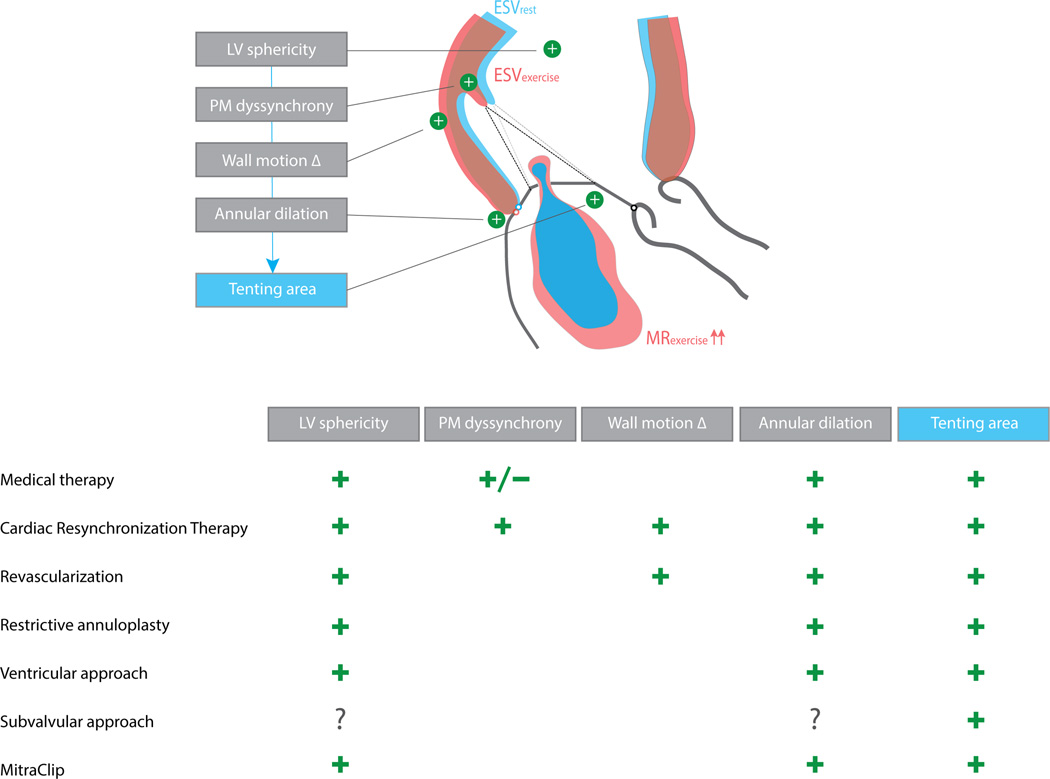
A complete and detailed overview of the evidence for the various therapeutic approaches for secondary MR, comprising guideline-directed HF medication, CRT, revascularization when appropriate, as well as mitral valve surgery and transcatheter interventions67, is beyond the scope of this review. Instead, a critical evaluation of the (potential) impact of these therapies on the dynamic lesion in secondary MR is made based on the underlying determinants causing exercise-induced MR aggravation (Figure 7).
Figure 7. Overview of geometric determinants of exercise-induced secondary mitral regurgitation and the respective impact of current treatment approaches.
‘+’ indicates favorable impact on the determinant, i.e. inhibition of its exercise-aggravation.
Medical therapy
Medical therapy remains a cornerstone in the treatment of chronic HF and secondary MR. Adequate decongestion should be pursued at all times and patients should take the maximally tolerated guideline-recommended73 target doses of neurohumoral blocker therapies. Adherence to neurohumoral blocker therapy reverses adverse left ventricular remodeling74, 75, although a reduction in secondary MR has only been reported in non-randomized small-sample series76.
In order to understand the potential effect of a drug on the severity of secondary MR it is helpful to view it in terms of the underlying lesion mechanism: the tug of war between mitral closing and tethering forces. Table 2 summarizes the effects of the most frequently used HF medications4. Preload-reducing medications, such as diuretics and nitrates, may significantly decrease the tethering forces, thus reducing EROA. These medications may also enhance mitral closing forces, as they tend to decrease left atrial pressure (reduce the size of V-waves) more than systolic left ventricular pressure, in particular in the presence of high left ventricular end-diastolic pressures. Since the failing heart operates on the flat portion of the cardiac function curve a significant reduction in left ventricular end-diastolic pressure will not affect cardiac output and systolic blood pressure significantly. Afterload-reducing drugs can also relieve tethering significantly in left ventricular dysfunction by decreasing left ventricular size and improving its shape.
Table 2.
Potential effects of common HF drugs on the balance of forces acting on the mitral valve lesion in secondary MR
| Drug | Preload | Afterload | Closing Force (LV-LA Pressure) |
Tethering Force (PM-Displacement) |
Net Effect (EROA) |
|---|---|---|---|---|---|
| Diuretic | ↓↓ | ↔ | ↑ | ↓ | ↓↓ |
| Nitrate | ↓↓ | ↓ | ↑ | ↓ | ↓↓ |
| ACE-I/ARB | ↓ | ↓↓ | ↑↔ | ↓ | ↓ |
| Hydralazine | ↔ | ↓↓↓ | ↑↔ | ↓ | ↓↓ |
| Chronic BB | ↓ | ↓ | ↑↔ | ↓ | ↓ |
ACE-I= ACE-inhibitor, ARB = Angiotensin Receptor Blocker, BB = Beta blocker.
Although arterial vasodilators reduce systolic ventricular pressure they may nevertheless increase closing force, if the reduction in MR they induce through a relief in tethering decreases left atrial pressure sufficiently. Please note that the assessment of the individual effects of hydralazine is theoretic insofar as the drug is typically administered together with diuretics and/or nitrates.
Whether such potential effects of medical therapy translate into attenuated exercise dynamics in secondary MR remains largely unknown. Most of the literature on this topic predates the era of neurohumoral blocker treatment in HF. Nevertheless, some interesting observations deserve attention: (1) nitrates (nitroglycerin, isosorbide dinitrate), mainly by reducing cardiac preload, offer a consistent and sustained reduction in left ventricular EDV as well as ESV at rest and during exercise in healthy subjects and patients with coronary artery disease77. Because they unload the heart, significant benefits on left ventricular performance (i.e., increased stroke volume and decreased pulmonary arterial wedge pressure) are observed during exercise in HF patients treated with digoxin and diuretics78. In this context, a significant reduction in dynamic secondary MR was also observed79; (2) combined pre- and afterload reduction with nitroprusside and diuretics significantly reduces left ventricular EDV during upright bicycle exercise, thereby significantly decreasing secondary MR and increasing forward stroke volume in advanced HF80; (3) arteriolar vasodilation (hydralazine) and venodilation (nitroglycerin) offer a synergistic reduction in pulmonary arterial wedge pressure and V-wave amplitude during static exercise in patients with chronic MR81; (4) intensive medical unloading of the heart in acute decompensation results in a significant decrease in intraventricular dyssynchrony, even in the absence of CRT82.
Cardiac Resynchronization Therapy
CRT reduces secondary MR83 and improves outcomes in patients with symptomatic HF with reduced ejection fraction and intraventricular conduction delay84. The mechanism by which CRT reduces secondary MR is three-fold, comprising both acute and chronic effects that optimize the force balance85: (1) an acute rise in - and longer duration of – the systolic closing force (dP/dt) immediately after onset of CRT14; (2) an immediately improved coordinated mechanical contraction of the papillary muscles, which reduces tethering86; and (3) long-term reverse left ventricular remodeling with significant reductions in EDV and ESV, which further lessens tethering forces83, 86, 87, and is probably the clinically most important effect.
The dynamic component of secondary MR during exercise is attenuated by CRT as well88, 89, resulting in increased forward stroke volume during exercise90 and improved exercise capacity91. However, dynamic worsening of MR during exercise is not entirely prevented by CRT88. The mechanism of MR reduction during exercise does not seem to be related to the acute effects, but probably relies on longer term reverse left ventricular remodeling and reduced dyssynchrony (Figure 5)60. Of note, the extent of reverse remodeling after CRT is most favorable in non-ischemic etiologies of HF83. The acute effects of CRT on the force-balance relationship are reversible, as cessation of biventricular pacing for 72h immediately causes a drop in dP/dt and an increase in secondary MR92. Furthermore, acute withdrawal of biventricular pacing after 6 months in patients with papillary muscle dyssynchrony results in recurrent dyssynchrony and abrupt reappearance of secondary MR93.
CRT is considered a valid and less invasive alternative to surgery in eligible patients with moderate-to-severe secondary MR and reduced ejection fraction as well as broad QRS complex, based on a long-term survival benefit in this particular population94. Currently, CRT is not indicated in HF patients with narrow QRS complex and it has been shown that worsening of left ventricular dyssynchrony may be associated with exercise-induced MR deterioration and worse outcomes56. Finally, even after CRT, 37–50% of patients show less than 1 grade improvement in MR86, 94. In such patients, additional interventions should be considered.
Revascularization
In patients with ischemic cardiomyopathy and (moderate) secondary MR, revascularization can recruit viable myocardial segments and enhance reverse left ventricular remodeling, thereby reducing the degree of secondary MR in a majority of patients95. Whether this strategy is effective in reducing the dynamic component of MR during exercise is unknown. Secondary MR improvement at 1 year after isolated coronary artery bypass grafting (CABG) was predicted by ≥5 viable segments of myocardium (16 segment model) and absence of papillary muscle dyssynchrony in the prospective study of Penicka et al96. This observation has important implications with respect to exercise: (1) viability post-infarction; i.e. preserved contractile reserve during exercise, has been shown to decrease exercise-induced MR51, 52, especially when inferior (basal) segments are involved; (2) papillary muscle dyssynchrony is implicated as an independent determinant of exercise-increased MR (Figure 7). Hence, the patients most likely to show improvement of secondary MR at rest after isolated revascularization (myocardial viability and/or papillary muscle synchronicity) are also the patients least likely to exhibit worsening of secondary MR during exercise preoperatively. Hypothetically, based on these findings, coronary artery disease patients with worsening of secondary MR during exercise (not associated with evidence of inducible ischemia) are less likely to show improvement of MR following isolated revascularization, and may therefore benefit from adjunctive therapy or intervention. This should certainly be subject to further study and exploration.
In the only prospectively randomized study to date that employed postoperative exercise echocardiography when comparing CABG versus CABG with mitral valve annuloplasty for moderate secondary MR, the dynamic (exercise-induced) MR component remained highly prevalent in the isolated CABG arm, but not after adjunctive mitral valve annuloplasty (Figure 8)97. Furthermore, in the Randomized Ischemic Mitral Evaluation (RIME) trial a better improvement in exercise capacity after 1 year was observed in the combined CABG with mitral valve annuloplasty arm than in the isolated CABG group, possibly due to improved dynamic MR during exercise98. In contrast, the moderate ischemic MR trial of the CardioThoracic Surgical trials Network (CTSN), comparing 151 patients with moderate ischemic MR undergoing CABG alone with 150 receiving concomitant mitral valve annuloplasty, did not demonstrate a difference in left ventricular remodeling (primary endpoint), functional capacity, or clinical outcome after 1 year95. However, in contrast to the RIME trial98 and the randomized trial by Fattouch et al97, exercise dynamics were not taken into account.
Figure 8. Impact of restrictive mitral valve annuloplasty (RMA) on the dynamic lesion in secondary mitral valve regurgitation (MR).
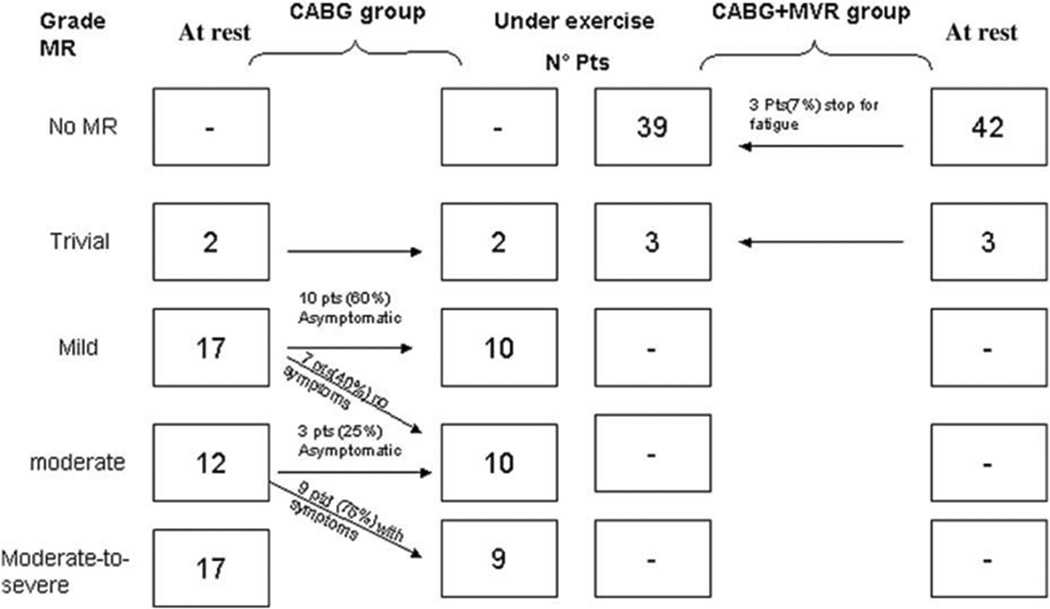
Sustained MR reduction during exercise in patients undergoing additional RMA for secondary MR is observed with exercise echocardiography, in contrast to the exercise-induced MR deterioration observed in coronary artery bypass grafting only patients. Reproduced from Fattouch et al97, with permission.
Mitral valve surgery
The surgical management of secondary MR remains challenging and controversial67. Not only the optimal surgical strategy but also indications for surgery remain highly debated, especially in case of moderate (possibly dynamic) secondary MR. Prospective randomized data to help decision-making are scarce, and the definition of moderate and severe secondary MR in the guidelines (severe = EROA ≥20mm2 instead of ≥40mm2) has been challenged99, 100. Inclusion criteria of all prospectively randomized clinical trials on mitral valve surgery for secondary MR are provided in Supplementary Table 1. It is unclear where mild-to-moderate secondary MR at rest that significantly increases during exercise can be situated, or whether alternative EROA thresholds for MR severity should apply for exercise-induced MR. Preoperative exercise echocardiography was performed only in the RIME trial. Based on the prognostic impact of exercise-induced secondary MR deterioration, the European (ESC) guidelines suggest mitral valve intervention in such patients when there is an indication for other cardiac surgery6. However, the impact of the available surgical approaches on the dynamic component of secondary MR and the respective exercise hemodynamics needs to be better understood.
Mitral valve annuloplasty
Mitral valve annuloplasty for secondary MR typically involves the insertion of an undersized prosthetic ring (‘restrictive’ annuloplasty), thereby reducing the anteroposterior dimensions of the mitral annulus, resulting in better apposition of the leaflets and increased coaptation (target coaptation length ≥8mm)101. The mechanism of action is two-fold: annular dimensions (typically dilated) are reduced, while the coaptation surface of the leaflets increases, thereby allowing more efficient use of closing forces during systole. At midterm follow-up, this procedure has shown to induce significant reverse left ventricular remodeling and improved systolic function, making it an effective strategy for patients with moderate to severe secondary MR98, 101, 102.
Secondary MR did not recur during exercise in patients treated with restrictive mitral valve annuloplasty and CABG in the randomized study of Fattouch et al (Figure 8)97. In another cohort study of 43 secondary MR patients, exercise echocardiography at 33±17 months after restrictive annuloplasty showed absence of more than mild MR at rest and during exercise in all but 4 subjects103. Hypothetically, inhibition of annular dilation during exercise, along with reverse left ventricular remodeling and optimization of the force balance, should be able to reduce the dynamic component of secondary MR, but not to abolish it, as tethering might still increase during exercise (Figure 7).
In addition, there is some concern that increased transmitral gradients after restrictive annuloplasty are associated with exercise-induced pulmonary hypertension and worsening functional capacity104. Although this was initially attributed to the small ring size, it was demonstrated recently that not the size of the prosthetic ring but rather the degree of subvalvular diastolic leaflet tethering is responsible for this functional stenosis after surgery. The effective mitral valve area at peak exercise was the strongest predictor of impaired exercise capacity and directly associated with all-cause mortality (Figure 9)103, 105. This should certainly be taken into account in the decision-making of patients with dynamic secondary MR, and adjunctive approaches that relieve diastolic tethering after surgery should be further explored.
Figure 9. Impact of restrictive mitral valve annuloplasty (RMA) on exercise hemodynamics and transmitral gradients in secondary mitral valve regurgitation (MR).
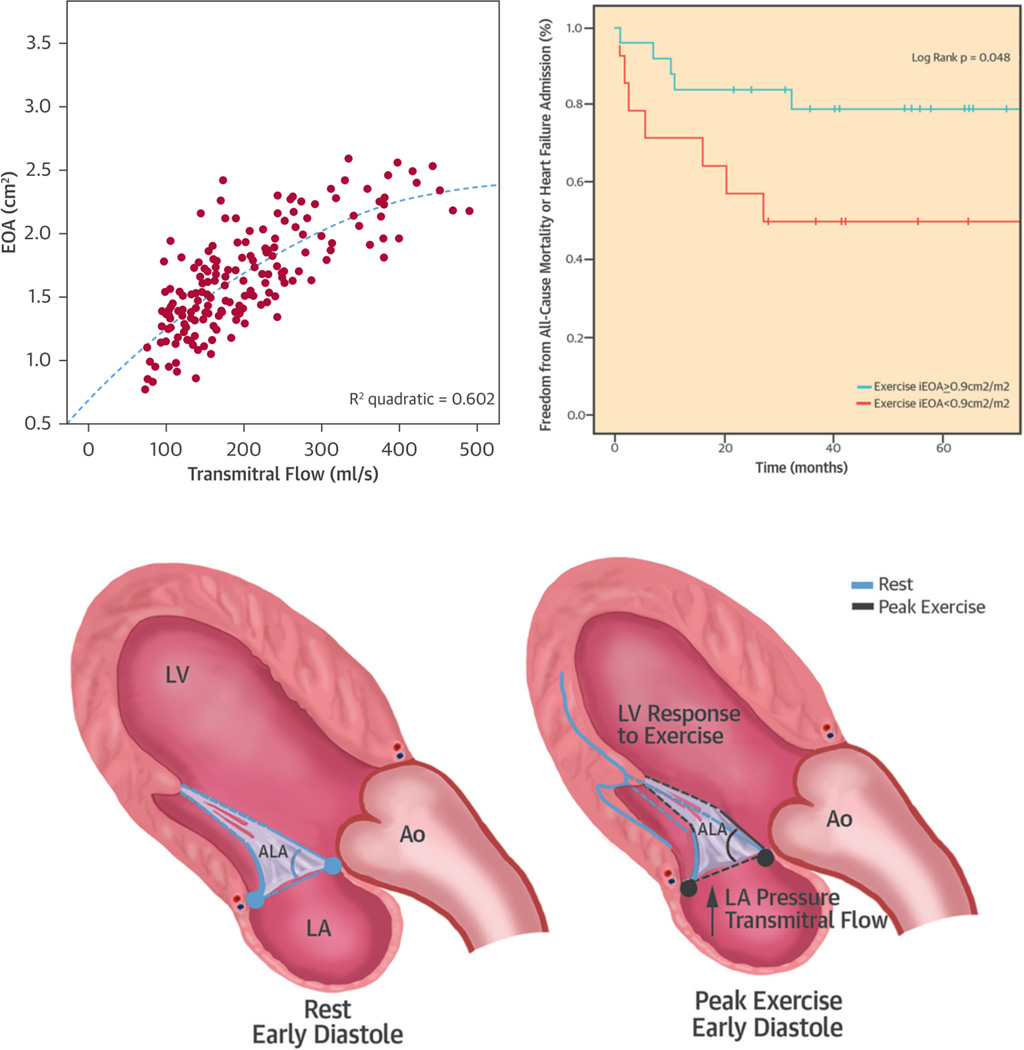
The mitral valve area after RMA increases during exercise because of an increasing anterior leaflet opening angle. This suggests that the functional stenosis after RMA relates to impaired diastolic anterior leaflet opening (i.e., ongoing tethering). Impaired effective orifice area at peak exercise is associated with worse outcomes. Adapted from Bertrand et al103 with permission.
Finally, in patients with severe secondary MR at rest, recurrence of moderate MR in up to 30% in the course of the first year after surgery is well recognized106, 107, with pathophysiological mechanisms mainly related to ongoing adverse remodeling and subvalvular tethering, usually in patients with already severely dilated left ventricles108. Importantly, preoperative presence of basal inferior wall aneurysm or dyskinesia was most predictive for MR recurrence in the annuloplasty group of the randomized severe MR CTSN trial109. In such patients, either adjunctive subvalvular/ventricular procedures to relieve tethering or mitral valve replacement should be considered.
Mitral valve replacement
Exercise-induced MR is definitively abolished after successful mitral valve replacement. However, similar concerns of transmitral gradients and exercise-induced pulmonary hypertension apply, especially in patients with some degree of prosthesis-patient mismatch110. In patients with secondary MR treated with mitral valve replacement, effective orifice area was in fact an important independent predictor of exercise-induced pulmonary hypertension and functional capacity111. Therefore, when opting for mitral valve replacement for treating dynamic secondary MR, careful selection of prosthesis type and size is warranted to maximize postoperative effective orifice area and avoid abnormally high transprosthetic gradients during exercise.
Ventricular and/or subvalvular approaches
Since tethering is the primary mechanism causing secondary MR and its exercise-induced deterioration, targeted ventricular or subvalvular procedures reducing the degree of mitral leaflet traction is a particularly promising approach (Figure 7). Furthermore, successful relief of tethering may also reduce the risk of functional mitral stenosis and thus improve exercise hemodynamics after restrictive annuloplasty103. However, ventricular reshaping strategies (aneurysmectomy, external constraint devices) have not been shown to be more effective in reducing MR than conventional mitral repair techniques (although they may be associated with independent survival benefits)112–114, and data regarding impact on exercise dynamics are lacking. A number of subvalvular approaches have been developed in animal studies and applied in small observational clinical series, with mixed levels of success115. Mitral leaflet augmentation, surgical papillary muscle relocation and/or approximation, or secondary chordal cutting have been proposed, as well as attempts to locally remodel the left ventricle e.g. by injecting a polymer in the ventricular wall, acting as a tissue-bulking agent reversing the ischemic distortion and limiting the outward displacement of the papillary muscle(s)116, 117. Despite promising results in small sample series or animal studies, none of these subvalvular or ventricular techniques have gained widespread clinical use or acceptance. Nevertheless, such lack of widespread use should not be seen as a refutation of the concept; it rather reflects the fact that some of the techniques are technically demanding. In fact, off-pump papillary muscle repositioning under echocardiographic guidance using a transventricular suture, combined with less restrictive annuloplasty using a partial ring (RING plus STRING technique), has been shown to produce an effective and lasting relief of tethering in the most challenging group patients: those with a tethering distance of ≥10 mm118.
Because the specific mechanisms of subvalvular tethering and secondary MR are heterogeneous and show large interindividual variations, the optimal subvalvular approach may require a personalized approach: targeting the individual patient’s geometry, including individually observed exercise-induced aggravation in tethering and MR
Transcatheter mitral valve interventions
Transcatheter edge-to-edge mitral valve repair using the MitraClip® device (Abbott Vascular, Menlo Park, California) involves a percutaneous transseptal left atrial approach emulating the Alfieri stitch by clipping the mitral valve leaflets together. Although randomized data from the Endovascular Valve Edge-to-Edge Repair Study (EVEREST II) mainly support MitraClip therapy in primary organic MR119 (for which the Alfieri stitch was originally intended) real-world registries show that it is predominantly used for secondary MR in clinical practice120. The rationale for MitraClip in secondary MR arises from the uncertain risk-benefit ratio of mitral valve surgery in patients with left ventricular dysfunction, in particular if there is no other indication for cardiac surgery67. The mechanism of action by which MitraClip may reduce secondary MR is (1) an acute increase in coaptation area improving closing force efficiency121; and (2) a slight decrease in annular (antero-posterior) dimensions122 relieving tethering. Tenting area is generally not reduced in the acute phase121, but may reduce over time in patients with significant reverse left ventricular remodeling post-procedure123.
To investigate whether these beneficial effects on remodeling translate into reduction of dynamic secondary MR a prospective multicenter study was performed in three Belgian centers (substudy of the Belgian MitraClip Registry, NCT02506387) comparing exercise echocardiography before and 6 months after MitraClip. In 31 secondary MR patients a significant reduction in secondary MR at peak exercise after 6 months, along with increased cardiac output and decreased pulmonary arterial pressures (Figure 10)124. However, a potential downside of the therapy is the reduction in mitral valve area caused by clipping the large mitral orifice into a double orifice of smaller size, causing increased transmitral pressure gradients at rest and during exercise125. The prospective Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT) trial is currently recruiting secondary MR patients to compare MitraClip therapy with standard medical care. In a subset of patients, exercise testing will be performed (NCT01626079)126. These data may provide important insight into the impact of MitraClip therapy on exercise hemodynamics and outcomes.
Figure 10. Impact of percutaneous mitral valve repair on mitral regurgitation (MR) severity and exercise hemodynamics in secondary MR patients.
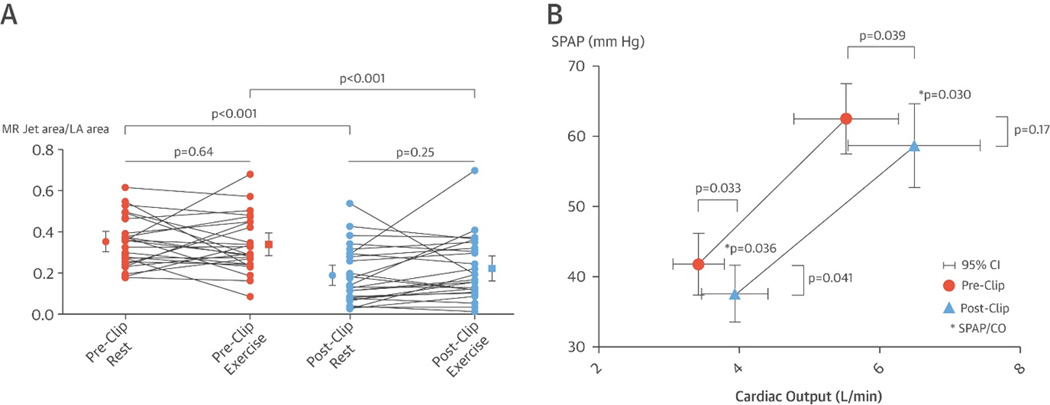
(A) Mitral regurgitation severity at rest and during peak exercise before and 6 months after percutaneous repair therapy. (B) Pulmonary pressures and cardiac output at rest and peak exercise are improved at 6 months after the therapy. CI, confidence interval; CO, cardiac output; LA, left atrial; MR, mitral regurgitation; SPAP, systolic pulmonary artery pressure. Reproduced from Van de Heyning et al124 with permission.
Numerous other percutaneous devices for mitral replacement and/or repair are currently under development in preclinical or phase I/II studies. Targets for repair include the mitral leaflets, chordae, annulus or left ventricle127.
Future research
Due to the paucity of data on exercise MR the approach to exercise-induced MR must, for the time being, rely heavily on pathophysiological insights gained from resting studies. At this point, we cannot offer definitive answers, only more precise questions, the most important of which may be: Can reduction of the dynamic component of secondary MR independently improve outcome? Is there an EROA threshold for secondary MR at peak exercise beyond which intervention is warranted even if resting MR is relatively mild? Is there a place for more aggressive ‘unloading’ of the heart using arterial and venous vasodilators on top of current guideline-recommended neurohormonal blockade in these patients?
Phenotyping secondary MR patients using exercise echocardiography should be incorporated in the protocol of prospective randomized studies assessing mitral interventions to help answer these questions. Finally, a patient-specific analysis of the three-dimensional geometry and dynamics causing mitral valve dysfunction is likely to improve our insights and therapeutic efficiency for secondary MR. Current advances in three-dimensional cardiac imaging and computational models already provide the ability to simulate mitral valve dynamics during physiological loading conditions, and virtually perform a therapy while evaluating its impact on the patients-specific mitral valve function and dynamics128. Such innovative technology holds great potential to tailor treatments to the individual and unique patient.
Summary
The dynamics of secondary MR are governed by the balance between mitral closing and tethering forces. Exercise-induced changes mainly result from the impact of hemodynamics on left ventricular geometry, specifically global and local deformation, dyssynchrony, and annular dilatation – all of which critically affect tethering. Exercise-induced worsening of secondary MR through the final common pathway of more tethering is associated with impaired exercise capacity and increased mortality. Therapies for secondary MR should focus not only on resting status, but also aim at reducing the dynamic component of the valvular lesion. Individualized targeting of underlying geometric culprits, aiming at inhibiting increases in tethering by improving left ventricular size, shape and function during exercise are key elements for success.
Supplementary Material
Acknowledgments
Sources of Funding
Funding in part provided by the Research Foundation – Flanders (FWO), 11N7214N (P.B.B.), and by grant R01 HL109506, National Institutes of Health, Bethesda, MD (R.A.L.) and grant 07CVD04 of the Leducq Foundation, Paris, France.
Footnotes
Disclosures
None.
References
- 1.Lamas GA, Mitchell GF, Flaker GC, Smith SC, Jr, Gersh BJ, Basta L, Moye L, Braunwald E, Pfeffer MA. Clinical significance of mitral regurgitation after acute myocardial infarction. Survival and Ventricular Enlargement Investigators. Circulation. 1997;96:827–833. doi: 10.1161/01.cir.96.3.827. [DOI] [PubMed] [Google Scholar]
- 2.Bursi F, Enriquez-Sarano M, Jacobsen SJ, Roger VL. Mitral regurgitation after myocardial infarction: a review. Am J Med. 2006;119:103–112. doi: 10.1016/j.amjmed.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 3.Trichon BH, Felker GM, Shaw LK, Cabell CH, O’Connor CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. The American Journal of Cardiology. 2003;91:538–543. doi: 10.1016/s0002-9149(02)03301-5. [DOI] [PubMed] [Google Scholar]
- 4.Levine RA, Schwammenthal E. Ischemic mitral regurgitation on the threshold of a solution: from paradoxes to unifying concepts. Circulation. 2005;112:745–758. doi: 10.1161/CIRCULATIONAHA.104.486720. [DOI] [PubMed] [Google Scholar]
- 5.Koelling TM, Aaronson KD, Cody RJ, Bach DS, Armstrong WF. Prognostic significance of mitral regurgitation and tricuspid regurgitation in patients with left ventricular systolic dysfunction. American Heart Journal. 2002;144:524–529. doi: 10.1067/mhj.2002.123575. [DOI] [PubMed] [Google Scholar]
- 6.Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schafers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M. Guidelines on the management of valvular heart disease (version 2012) Eur Heart J. 2012;33:2451–2496. doi: 10.1093/eurheartj/ehs109. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, 3rd, Thomas JD. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–e643. doi: 10.1161/CIR.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 8.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–1764. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 9.Lancellotti P, Gerard PL, Pierard LA. Long-term outcome of patients with heart failure and dynamic functional mitral regurgitation. Eur Heart J. 2005;26:1528–1532. doi: 10.1093/eurheartj/ehi189. [DOI] [PubMed] [Google Scholar]
- 10.Levine RA, Hung J. Ischemic mitral regurgitation, the dynamic lesion: clues to the cure. J Am Coll Cardiol. 2003;42:1929–1932. doi: 10.1016/j.jacc.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Dal-Bianco JP, Beaudoin J, Handschumacher MD, Levine RA. Basic mechanisms of mitral regurgitation. Can J Cardiol. 2014;30:971–981. doi: 10.1016/j.cjca.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwammenthal E, Chen C, Benning F, Block M, Breithardt G, Levine RA. Dynamics of mitral regurgitant flow and orifice area. Physiologic application of the proximal flow convergence method: clinical data and experimental testing. Circulation. 1994;90:307–322. doi: 10.1161/01.cir.90.1.307. [DOI] [PubMed] [Google Scholar]
- 13.Hung J, Otsuji Y, Handschumacher MD, Schwammenthal E, Levine RA. Mechanism of dynamic regurgitant orifice area variation in functional mitral regurgitation: physiologic insights from the proximal flow convergence technique. J Am Coll Cardiol. 1999;33:538–545. doi: 10.1016/s0735-1097(98)00570-1. [DOI] [PubMed] [Google Scholar]
- 14.Breithardt OA, Sinha AM, Schwammenthal E, Bidaoui N, Markus KU, Franke A, Stellbrink C. Acute effects of cardiac resynchronization therapy on functional mitral regurgitation in advanced systolic heart failure. Journal of the American College of Cardiology. 2003;41:765–770. doi: 10.1016/s0735-1097(02)02937-6. [DOI] [PubMed] [Google Scholar]
- 15.He S, Fontaine AA, Schwammenthal E, Yoganathan AP, Levine RA. Integrated mechanism for functional mitral regurgitation: leaflet restriction versus coapting force: in vitro studies. Circulation. 1997;96:1826–1834. doi: 10.1161/01.cir.96.6.1826. [DOI] [PubMed] [Google Scholar]
- 16.Otsuji Y, Handschumacher MD, Schwammenthal E, Jiang L, Song JK, Guerrero JL, Vlahakes GJ, Levine RA. Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: direct in vivo demonstration of altered leaflet tethering geometry. Circulation. 1997;96:1999–2008. doi: 10.1161/01.cir.96.6.1999. [DOI] [PubMed] [Google Scholar]
- 17.Kono T, Sabbah HN, Rosman H, Alam M, Jafri S, Goldstein S. Left ventricular shape is the primary determinant of functional mitral regurgitation in heart failure. J Am Coll Cardiol. 1992;20:1594–1598. doi: 10.1016/0735-1097(92)90455-v. [DOI] [PubMed] [Google Scholar]
- 18.Kalra K, Wang Q, McIver BV, Shi W, Guyton RA, Sun W, Sarin EL, Thourani VH, Padala M. Temporal changes in interpapillary muscle dynamics as an active indicator of mitral valve and left ventricular interaction in ischemic mitral regurgitation. J Am Coll Cardiol. 2014;64:1867–1879. doi: 10.1016/j.jacc.2014.07.988. [DOI] [PubMed] [Google Scholar]
- 19.Topilsky Y, Vaturi O, Watanabe N, Bichara V, Nkomo VT, Michelena H, Le Tourneau T, Mankad SV, Park S, Capps MA, Suri R, Pislaru SV, Maalouf J, Yoshida K, Enriquez-Sarano M. Real-time 3-dimensional dynamics of functional mitral regurgitation: a prospective quantitative and mechanistic study. J Am Heart Assoc. 2013;2:e000039. doi: 10.1161/JAHA.113.000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tigen K, Karaahmet T, Dundar C, Guler A, Cevik C, Basaran O, Kirma C, Basaran Y. The importance of papillary muscle dyssynchrony in predicting the severity of functional mitral regurgitation in patients with non-ischaemic dilated cardiomyopathy: a two-dimensional speckle-tracking echocardiography study. Eur J Echocardiogr. 2010;11:671–676. doi: 10.1093/ejechocard/jeq040. [DOI] [PubMed] [Google Scholar]
- 21.Liang YJ, Zhang Q, Fang F, Lee AP, Liu M, Yan BP, Lam YY, Chan GC, Yu CM. Incremental value of global systolic dyssynchrony in determining the occurrence of functional mitral regurgitation in patients with left ventricular systolic dysfunction. Eur Heart J. 2013;34:767–774. doi: 10.1093/eurheartj/ehs078. [DOI] [PubMed] [Google Scholar]
- 22.Otsuji Y, Kumanohoso T, Yoshifuku S, Matsukida K, Koriyama C, Kisanuki A, Minagoe S, Levine RA, Tei C. Isolated annular dilation does not usually cause important functional mitral regurgitation: comparison between patients with lone atrial fibrillation and those with idiopathic or ischemic cardiomyopathy. J Am Coll Cardiol. 2002;39:1651–1656. doi: 10.1016/s0735-1097(02)01838-7. [DOI] [PubMed] [Google Scholar]
- 23.Gertz ZM, Raina A, Saghy L, Zado ES, Callans DJ, Marchlinski FE, Keane MG, Silvestry FE. Evidence of atrial functional mitral regurgitation due to atrial fibrillation: reversal with arrhythmia control. J Am Coll Cardiol. 2011;58:1474–1481. doi: 10.1016/j.jacc.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 24.van Rosendael PJ, Katsanos S, Kamperidis V, Roos CJ, Scholte AJ, Schalij MJ, Ajmone Marsan N, Bax JJ, Delgado V. New insights on Carpentier I mitral regurgitation from multidetector row computed tomography. Am J Cardiol. 2014;114:763–768. doi: 10.1016/j.amjcard.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi Y, Abe Y, Sasaki Y, Bito Y, Morisaki A, Nishimura S, Shibata T. Mitral valve repair for atrial functional mitral regurgitation in patients with chronic atrial fibrillation. Interact Cardiovasc Thorac Surg. 2015;21:163–168. doi: 10.1093/icvts/ivv119. [DOI] [PubMed] [Google Scholar]
- 26.Timek TA, Lai DT, Dagum P, Liang D, Daughters GT, Ingels NB, Jr., Miller DC. Mitral leaflet remodeling in dilated cardiomyopathy. Circulation. 2006;114:I518–I523. doi: 10.1161/CIRCULATIONAHA.105.000554. [DOI] [PubMed] [Google Scholar]
- 27.Dal-Bianco JP, Aikawa E, Bischoff J, Guerrero JL, Handschumacher MD, Sullivan S, Johnson B, Titus JS, Iwamoto Y, Wylie-Sears J, Levine RA, Carpentier A. Active adaptation of the tethered mitral valve: insights into a compensatory mechanism for functional mitral regurgitation. Circulation. 2009;120:334–342. doi: 10.1161/CIRCULATIONAHA.108.846782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaput M, Handschumacher MD, Guerrero JL, Holmvang G, Dal-Bianco JP, Sullivan S, Vlahakes GJ, Hung J, Levine RA Leducq Foundation MTN. Mitral leaflet adaptation to ventricular remodeling: prospective changes in a model of ischemic mitral regurgitation. Circulation. 2009;120:S99–S103. doi: 10.1161/CIRCULATIONAHA.109.844019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debonnaire P, Al Amri I, Leong DP, Joyce E, Katsanos S, Kamperidis V, Schalij MJ, Bax JJ, Marsan NA, Delgado V. Leaflet remodelling in functional mitral valve regurgitation: characteristics, determinants, and relation to regurgitation severity. Eur Heart J Cardiovasc Imaging. 2015;16:290–299. doi: 10.1093/ehjci/jeu216. [DOI] [PubMed] [Google Scholar]
- 30.Saito K, Okura H, Watanabe N, Obase K, Tamada T, Koyama T, Hayashida A, Neishi Y, Kawamoto T, Yoshida K. Influence of chronic tethering of the mitral valve on mitral leaflet size and coaptation in functional mitral regurgitation. JACC Cardiovasc Imaging. 2012;5:337–345. doi: 10.1016/j.jcmg.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Beaudoin J, Handschumacher MD, Zeng X, Hung J, Morris EL, Levine RA, Schwammenthal E. Mitral valve enlargement in chronic aortic regurgitation as a compensatory mechanism to prevent functional mitral regurgitation in the dilated left ventricle. J Am Coll Cardiol. 2013;61:1809–1816. doi: 10.1016/j.jacc.2013.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grande-Allen KJ, Barber JE, Klatka KM, Houghtaling PL, Vesely I, Moravec CS, McCarthy PM. Mitral valve stiffening in end-stage heart failure: evidence of an organic contribution to functional mitral regurgitation. J Thorac Cardiovasc Surg. 2005;130:783–790. doi: 10.1016/j.jtcvs.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 33.Song JM, Kim JJ, Ha TY, Lee JW, Jung SH, Hwang IS, Lee I, Sun BJ, Kim DH, Kang DH, Song JK. Basal chordae sites on the mitral valve determine the severity of secondary mitral regurgitation. Heart. 2015;101:1024–1031. doi: 10.1136/heartjnl-2014-306854. [DOI] [PubMed] [Google Scholar]
- 34.Laughlin MH. Cardiovascular response to exercise. Am J Physiol. 1999;277:S244–S259. doi: 10.1152/advances.1999.277.6.S244. [DOI] [PubMed] [Google Scholar]
- 35.Manou-Stathopoulou V, Goodwin CD, Patterson T, Redwood SR, Marber MS, Williams RP. The effects of cold and exercise on the cardiovascular system. Heart. 2015;101:808–820. doi: 10.1136/heartjnl-2014-306276. [DOI] [PubMed] [Google Scholar]
- 36.Slutsky R, Karliner J, Ricci D, Schuler G, Pfisterer M, Peterson K, Ashburn W. Response of left ventricular volume to exercise in man assessed by radionuclide equilibrium angiography. Circulation. 1979;60:565–571. doi: 10.1161/01.cir.60.3.565. [DOI] [PubMed] [Google Scholar]
- 37.Tomai F, Ciavolella M, Crea F, Gaspardone A, Versaci F, Giannitti C, Scali D, Chiariello L, Gioffre PA. Left ventricular volumes during exercise in normal subjects and patients with dilated cardiomyopathy assessed by first-pass radionuclide angiography. Am J Cardiol. 1993;72:1167–1171. doi: 10.1016/0002-9149(93)90988-o. [DOI] [PubMed] [Google Scholar]
- 38.Furukawa K, Nishida K, Yamada C, Niki S, Sugihara H, Kohno Y, Katsume H, Ijichi H, Kitamura H, Kunishige H. Left ventricular size and performance during graded supine exercise in normal subjects. Jpn Heart J. 1983;24:503–514. doi: 10.1536/ihj.24.503. [DOI] [PubMed] [Google Scholar]
- 39.Crawford MH, Petru MA, Rabinowitz C. Effect of isotonic exercise training on left ventricular volume during upright exercise. Circulation. 1985;72:1237–1243. doi: 10.1161/01.cir.72.6.1237. [DOI] [PubMed] [Google Scholar]
- 40.La Gerche A, Claessen G, Van de Bruaene A, Pattyn N, Van Cleemput J, Gewillig M, Bogaert J, Dymarkowski S, Claus P, Heidbuchel H. Cardiac MRI: a new gold standard for ventricular volume quantification during high-intensity exercise. Circ Cardiovasc Imaging. 2013;6:329–338. doi: 10.1161/CIRCIMAGING.112.980037. [DOI] [PubMed] [Google Scholar]
- 41.Paulsen WJ, Boughner DR, Friesen A, Persaud JA. Ventricular response to isometric and isotonic exercise. Echocardiographic assessment. Br Heart J. 1979;42:521–527. doi: 10.1136/hrt.42.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stefadouros MA, Grossman W, el-Shahawy M, Witham C. The effect of isometric exercise on the left ventricular volume in normal man. Circulation. 1974;49:1185–1189. doi: 10.1161/01.cir.49.6.1185. [DOI] [PubMed] [Google Scholar]
- 43.Naeije R, Vanderpool R, Dhakal BP, Saggar R, Saggar R, Vachiery JL, Lewis GD. Exercise-induced pulmonary hypertension: physiological basis and methodological concerns. Am J Respir Crit Care Med. 2013;187:576–583. doi: 10.1164/rccm.201211-2090CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis GD, Bossone E, Naeije R, Grunig E, Saggar R, Lancellotti P, Ghio S, Varga J, Rajagopalan S, Oudiz R, Rubenfire M. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation. 2013;128:1470–1479. doi: 10.1161/CIRCULATIONAHA.112.000667. [DOI] [PubMed] [Google Scholar]
- 45.Bossone E, Rubenfire M, Bach DS, Ricciardi M, Armstrong WF. Range of tricuspid regurgitation velocity at rest and during exercise in normal adult men: implications for the diagnosis of pulmonary hypertension. J Am Coll Cardiol. 1999;33:1662–1666. doi: 10.1016/s0735-1097(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 46.Shen WF, Roubin GS, Hirasawa K, Choong CY, Hutton BF, Harris PJ, Fletcher PJ, Kelly DT. Left ventricular volume and ejection fraction response to exercise in chronic congestive heart failure: difference between dilated cardiomyopathy and previous myocardial infarction. Am J Cardiol. 1985;55:1027–1031. doi: 10.1016/0002-9149(85)90740-4. [DOI] [PubMed] [Google Scholar]
- 47.Keren G, Katz S, Gage J, Strom J, Sonnenblick EH, LeJemtel TH. Effect of isometric exercise on cardiac performance and mitral regurgitation in patients with severe congestive heart failure. Am Heart J. 1989;118:973–979. doi: 10.1016/0002-8703(89)90232-9. [DOI] [PubMed] [Google Scholar]
- 48.Mann DL, Scharf J, Ahnve S, Gilpin E. Left ventricular volume during supine exercise: importance of myocardial scar in patients with coronary heart disease. J Am Coll Cardiol. 1987;9:26–34. doi: 10.1016/s0735-1097(87)80077-3. [DOI] [PubMed] [Google Scholar]
- 49.Lafitte S, Bordachar P, Lafitte M, Garrigue S, Reuter S, Reant P, Serri K, Lebouffos V, Berrhouet M, Jais P, Haissaguerre M, Clementy J, Roudaut R, DeMaria AN. Dynamic ventricular dyssynchrony: an exercise-echocardiography study. J Am Coll Cardiol. 2006;47:2253–2259. doi: 10.1016/j.jacc.2005.11.087. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan MJ, Knight JD, Higginbotham MB, Cobb FR. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation. 1989;80:769–781. doi: 10.1161/01.cir.80.4.769. [DOI] [PubMed] [Google Scholar]
- 51.Lancellotti P, Lebrun F, Pierard LA. Determinants of exercise-induced changes in mitral regurgitation in patients with coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol. 2003;42:1921–1928. doi: 10.1016/j.jacc.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Giga V, Ostojic M, Vujisic-Tesic B, Djordjevic-Dikic A, Stepanovic J, Beleslin B, Petrovic M, Nedeljkovic M, Nedeljkovic I, Milic N. Exercise-induced changes in mitral regurgitation in patients with prior myocardial infarction and left ventricular dysfunction: relation to mitral deformation and left ventricular function and shape. Eur Heart J. 2005;26:1860–1865. doi: 10.1093/eurheartj/ehi431. [DOI] [PubMed] [Google Scholar]
- 53.Lancellotti P, Stainier PY, Lebois F, Pierard LA. Effect of dynamic left ventricular dyssynchrony on dynamic mitral regurgitation in patients with heart failure due to coronary artery disease. Am J Cardiol. 2005;96:1304–1307. doi: 10.1016/j.amjcard.2005.06.077. [DOI] [PubMed] [Google Scholar]
- 54.Lapu-Bula R. Contribution of Exercise-Induced Mitral Regurgitation to Exercise Stroke Volume and Exercise Capacity in Patients With Left Ventricular Systolic Dysfunction. Circulation. 2002;106:1342–1348. doi: 10.1161/01.cir.0000028812.98083.d9. [DOI] [PubMed] [Google Scholar]
- 55.Ennezat PV, Marechaux S, Le Tourneau T, Lamblin N, Bauters C, Van Belle E, Gal B, Kacet S, Asseman P, Deklunder G, LeJemtel TH, de Groote P. Myocardial asynchronism is a determinant of changes in functional mitral regurgitation severity during dynamic exercise in patients with chronic heart failure due to severe left ventricular systolic dysfunction. Eur Heart J. 2006;27:679–683. doi: 10.1093/eurheartj/ehi682. [DOI] [PubMed] [Google Scholar]
- 56.D'Andrea A, Caso P, Cuomo S, Scarafile R, Salerno G, Limongelli G, Di Salvo G, Severino S, Ascione L, Calabro P, Romano M, Romano G, Santangelo L, Maiello C, Cotrufo M, Calabro R. Effect of dynamic myocardial dyssynchrony on mitral regurgitation during supine bicycle exercise stress echocardiography in patients with idiopathic dilated cardiomyopathy and 'narrow' QRS. Eur Heart J. 2007;28:1004–1011. doi: 10.1093/eurheartj/ehm021. [DOI] [PubMed] [Google Scholar]
- 57.Takano H, Adachi H, Ohshima S, Taniguchi K, Kurabayashi M. Functional mitral regurgitation during exercise in patients with heart failure. Circ J. 2006;70:1563–1567. doi: 10.1253/circj.70.1563. [DOI] [PubMed] [Google Scholar]
- 58.Izumo M, Lancellotti P, Suzuki K, Kou S, Shimozato T, Hayashi A, Akashi YJ, Osada N, Omiya K, Nobuoka S, Ohtaki E, Miyake F. Three-dimensional echocardiographic assessments of exercise-induced changes in left ventricular shape and dyssynchrony in patients with dynamic functional mitral regurgitation. Eur J Echocardiogr. 2009;10:961–967. doi: 10.1093/ejechocard/jep114. [DOI] [PubMed] [Google Scholar]
- 59.Yamano T, Nakatani S, Kanzaki H, Toh N, Amaki M, Tanaka J, Abe H, Hasegawa T, Sawada T, Matsubara H, Kitakaze M. Exercise-induced changes of functional mitral regurgitation in asymptomatic or mildly symptomatic patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2008;102:481–485. doi: 10.1016/j.amjcard.2008.03.086. [DOI] [PubMed] [Google Scholar]
- 60.Madaric J, Vanderheyden M, Van Laethem C, Verhamme K, Feys A, Goethals M, Verstreken S, Geelen P, Penicka M, De Bruyne B, Bartunek J. Early and late effects of cardiac resynchronization therapy on exercise-induced mitral regurgitation: relationship with left ventricular dyssynchrony, remodelling and cardiopulmonary performance. Eur Heart J. 2007;28:2134–2141. doi: 10.1093/eurheartj/ehm126. [DOI] [PubMed] [Google Scholar]
- 61.Peteiro J, Freire E, Montserrat L, Castro-Beiras A. The effect of exercise on ischemic mitral regurgitation. Chest. 1998;114:1075–1082. doi: 10.1378/chest.114.4.1075. [DOI] [PubMed] [Google Scholar]
- 62.Schwammenthal E, Levine RA. The non-ischaemic dynamics of ischaemic mitral regurgitation: solving the paradox. Eur Heart J. 2005;26:1454–1455. doi: 10.1093/eurheartj/ehi323. [DOI] [PubMed] [Google Scholar]
- 63.Izumo M, Suzuki K, Moonen M, Kou S, Shimozato T, Hayashi A, Akashi YJ, Osada N, Omiya K, Miyake F, Ohtaki E, Lancellotti P. Changes in mitral regurgitation and left ventricular geometry during exercise affect exercise capacity in patients with systolic heart failure. Eur J Echocardiogr. 2011;12:54–60. doi: 10.1093/ejechocard/jeq105. [DOI] [PubMed] [Google Scholar]
- 64.Lancellotti P, Troisfontaines P, Toussaint AC, Pierard LA. Prognostic importance of exercise-induced changes in mitral regurgitation in patients with chronic ischemic left ventricular dysfunction. Circulation. 2003;108:1713–1717. doi: 10.1161/01.CIR.0000087599.49332.05. [DOI] [PubMed] [Google Scholar]
- 65.Szymanski C, Levine RA, Tribouilloy C, Zheng H, Handschumacher MD, Tawakol A, Hung J. Impact of mitral regurgitation on exercise capacity and clinical outcomes in patients with ischemic left ventricular dysfunction. Am J Cardiol. 2011;108:1714–1720. doi: 10.1016/j.amjcard.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rossi A, Dini FL, Faggiano P, Agricola E, Cicoira M, Frattini S, Simioniuc A, Gullace M, Ghio S, Enriquez-Sarano M, Temporelli PL. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart. 2011;97:1675–1680. doi: 10.1136/hrt.2011.225789. [DOI] [PubMed] [Google Scholar]
- 67.Asgar AW, Mack MJ, Stone GW. Secondary Mitral Regurgitation in Heart Failure: Pathophysiology, Prognosis, and Therapeutic Considerations. J Am Coll Cardiol. 2015;65:1231–1248. doi: 10.1016/j.jacc.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 68.Magne J, Pibarot P, Sengupta PP, Donal E, Rosenhek R, Lancellotti P. Pulmonary hypertension in valvular disease: a comprehensive review on pathophysiology to therapy from the HAVEC Group. JACC Cardiovasc Imaging. 2015;8:83–99. doi: 10.1016/j.jcmg.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 69.Lancellotti P, Magne J, Dulgheru R, Ancion A, Martinez C, Pierard LA. Clinical Significance of Exercise Pulmonary Hypertension in Secondary Mitral Regurgitation. Am J Cardiol. 2015;115:1454–1461. doi: 10.1016/j.amjcard.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 70.Tumminello G, Lancellotti P, Lempereur M, D'Orio V, Pierard LA. Determinants of pulmonary artery hypertension at rest and during exercise in patients with heart failure. Eur Heart J. 2007;28:569–574. doi: 10.1093/eurheartj/ehl561. [DOI] [PubMed] [Google Scholar]
- 71.Claessen G, La Gerche A, Voigt JU, Dymarkowski S, Schnell F, Petit T, Willems R, Claus P, Delcroix M, Heidbuchel H. Accuracy of Echocardiography to Evaluate Pulmonary Vascular and RV Function During Exercise. JACC Cardiovasc Imaging. 2016;9:532–543. doi: 10.1016/j.jcmg.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 72.Pierard LA, Lancellotti P. The role of ischemic mitral regurgitation in the pathogenesis of acute pulmonary edema. N Engl J Med. 2004;351:1627–1634. doi: 10.1056/NEJMoa040532. [DOI] [PubMed] [Google Scholar]
- 73.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL American College of Cardiology Foundation/American Heart Association Task Force on Practice G. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 74.Greenberg B, Quinones MA, Koilpillai C, Limacher M, Shindler D, Benedict C, Shelton B. Effects of long-term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction. Results of the SOLVD echocardiography substudy. Circulation. 1995;91:2573–2581. doi: 10.1161/01.cir.91.10.2573. [DOI] [PubMed] [Google Scholar]
- 75.Doughty RN, Whalley GA, Walsh HA, Gamble GD, Lopez-Sendon J, Sharpe N Investigators CES. Effects of carvedilol on left ventricular remodeling after acute myocardial infarction: the CAPRICORN Echo Substudy. Circulation. 2004;109:201–206. doi: 10.1161/01.CIR.0000108928.25690.94. [DOI] [PubMed] [Google Scholar]
- 76.Capomolla S, Febo O, Gnemmi M, Riccardi G, Opasich C, Caporotondi A, Mortara A, Pinna GD, Cobelli F. Beta-blockade therapy in chronic heart failure: diastolic function and mitral regurgitation improvement by carvedilol. Am Heart J. 2000;139:596–608. doi: 10.1016/s0002-8703(00)90036-x. [DOI] [PubMed] [Google Scholar]
- 77.Slutsky R, Battler A, Gerber K, Gordon D, Froelicher V, Karliner J, Ashburn W. Effect of nitrates on left ventricular size and function during exercise: comparison of sublingual nitroglycerin and nitroglycerin paste. Am J Cardiol. 1980;45:831–840. doi: 10.1016/0002-9149(80)90129-0. [DOI] [PubMed] [Google Scholar]
- 78.Hecht HS, Karahalios SE, Schnugg SJ, Ormiston JA, Hopkins JM, Rose JG, Singh BN. Improvement in supine bicycle exercise performance in refractory congestive heart failure after isosorbide dinitrate: radionuclide and hemodynamic evaluation of acute effects. Am J Cardiol. 1982;49:133–140. doi: 10.1016/0002-9149(82)90287-9. [DOI] [PubMed] [Google Scholar]
- 79.Keren G, Katz S, Strom J, Sonnenblick EH, LeJemtel TH. Dynamic mitral regurgitation. An important determinant of the hemodynamic response to load alterations and inotropic therapy in severe heart failure. Circulation. 1989;80:306–313. doi: 10.1161/01.cir.80.2.306. [DOI] [PubMed] [Google Scholar]
- 80.Stevenson LW, Brunken RC, Belil D, Grover-McKay M, Schwaiger M, Schelbert HR, Tillisch JH. Afterload reduction with vasodilators and diuretics decreases mitral regurgitation during upright exercise in advanced heart failure. J Am Coll Cardiol. 1990;15:174–180. doi: 10.1016/0735-1097(90)90196-v. [DOI] [PubMed] [Google Scholar]
- 81.Roth A, Shotan A, Elkayam U. A randomized comparison between the hemodynamic effects of hydralazine and nitroglycerin alone and in combination at rest and during isometric exercise in patients with chronic mitral regurgitation. Am Heart J. 1993;125:155–163. doi: 10.1016/0002-8703(93)90069-l. [DOI] [PubMed] [Google Scholar]
- 82.Mullens W, Borowski AG, Curtin R, Grimm RA, Thomas JD, Tang WHW. Mechanical dyssynchrony in advanced decompensated heart failure: Relation to hemodynamic responses to intensive medical therapy. Heart Rhythm. 2008;5:1105–1110. doi: 10.1016/j.hrthm.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 83.St John Sutton MG, Plappert T, Hilpisch KE, Abraham WT, Hayes DL, Chinchoy E. Sustained reverse left ventricular structural remodeling with cardiac resynchronization at one year is a function of etiology: quantitative Doppler echocardiographic evidence from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) Circulation. 2006;113:266–272. doi: 10.1161/CIRCULATIONAHA.104.520817. [DOI] [PubMed] [Google Scholar]
- 84.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L Cardiac Resynchronization-Heart Failure Study I. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 85.Solis J, McCarty D, Levine RA, Handschumacher MD, Fernandez-Friera L, Chen-Tournoux A, Mont L, Vidal B, Singh JP, Brugada J, Picard MH, Sitges M, Hung J. Mechanism of decrease in mitral regurgitation after cardiac resynchronization therapy: optimization of the force-balance relationship. Circ Cardiovasc Imaging. 2009;2:444–450. doi: 10.1161/CIRCIMAGING.108.823732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ypenburg C, Lancellotti P, Tops LF, Boersma E, Bleeker GB, Holman ER, Thomas JD, Schalij MJ, Pierard LA, Bax JJ. Mechanism of improvement in mitral regurgitation after cardiac resynchronization therapy. Eur Heart J. 2008;29:757–765. doi: 10.1093/eurheartj/ehn063. [DOI] [PubMed] [Google Scholar]
- 87.Sitges M, Vidal B, Delgado V, Mont L, Garcia-Alvarez A, Tolosana JM, Castel A, Berruezo A, Azqueta M, Pare C, Brugada J. Long-term effect of cardiac resynchronization therapy on functional mitral valve regurgitation. Am J Cardiol. 2009;104:383–388. doi: 10.1016/j.amjcard.2009.03.060. [DOI] [PubMed] [Google Scholar]
- 88.Lancellotti P, Melon P, Sakalihasan N, Waleffe A, Dubois C, Bertholet M, Pierard LA. Effect of cardiac resynchronization therapy on functional mitral regurgitation in heart failure. Am J Cardiol. 2004;94:1462–1465. doi: 10.1016/j.amjcard.2004.07.154. [DOI] [PubMed] [Google Scholar]
- 89.Bordachar P, Lafitte S, Reuter S, Serri K, Garrigue S, Laborderie J, Reant P, Jais P, Haissaguerre M, Roudaut R, Clementy J. Echocardiographic assessment during exercise of heart failure patients with cardiac resynchronization therapy. Am J Cardiol. 2006;97:1622–1625. doi: 10.1016/j.amjcard.2005.12.055. [DOI] [PubMed] [Google Scholar]
- 90.Marechaux S, Pincon C, Gal B, Kouakam C, Marquie C, Lacroix D, de Groote P, Mouquet F, Le Tourneau T, Dennetiere S, Guyomar Y, Solal AC, Logeart D, Asseman P, Le Jemtel TH, Ennezat PV. Functional mitral regurgitation at rest determines the acute hemodynamic response to cardiac resynchronization therapy during exercise: an acute exercise echocardiographic study. J Am Soc Echocardiogr. 2009;22:464–471. doi: 10.1016/j.echo.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 91.Parthenakis FI, Patrianakos AP, Simantirakis EN, Vardas PE. CRT and exercise capacity in heart failure: the impact of mitral valve regurgitation. Europace. 2008;10(Suppl 3):iii96–iii100. doi: 10.1093/europace/eun232. [DOI] [PubMed] [Google Scholar]
- 92.Brandt RR, Reiner C, Arnold R, Sperzel J, Pitschner HF, Hamm CW. Contractile response and mitral regurgitation after temporary interruption of long-term cardiac resynchronization therapy. Eur Heart J. 2006;27:187–192. doi: 10.1093/eurheartj/ehi558. [DOI] [PubMed] [Google Scholar]
- 93.Ypenburg C, Lancellotti P, Tops LF, Bleeker GB, Holman ER, Pierard LA, Schalij MJ, Bax JJ. Acute effects of initiation and withdrawal of cardiac resynchronization therapy on papillary muscle dyssynchrony and mitral regurgitation. J Am Coll Cardiol. 2007;50:2071–2077. doi: 10.1016/j.jacc.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 94.van Bommel RJ, Marsan NA, Delgado V, Borleffs CJ, van Rijnsoever EP, Schalij MJ, Bax JJ. Cardiac resynchronization therapy as a therapeutic option in patients with moderate-severe functional mitral regurgitation and high operative risk. Circulation. 2011;124:912–919. doi: 10.1161/CIRCULATIONAHA.110.009803. [DOI] [PubMed] [Google Scholar]
- 95.Smith PK, Puskas JD, Ascheim DD, Voisine P, Gelijns AC, Moskowitz AJ, Hung JW, Parides MK, Ailawadi G, Perrault LP, Acker MA, Argenziano M, Thourani V, Gammie JS, Miller MA, Page P, Overbey JR, Bagiella E, Dagenais F, Blackstone EH, Kron IL, Goldstein DJ, Rose EA, Moquete EG, Jeffries N, Gardner TJ, O'Gara PT, Alexander JH, Michler RE Cardiothoracic Surgical Trials Network I. Surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med. 2014;371:2178–2188. doi: 10.1056/NEJMoa1410490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Penicka M, Linkova H, Lang O, Fojt R, Kocka V, Vanderheyden M, Bartunek J. Predictors of improvement of unrepaired moderate ischemic mitral regurgitation in patients undergoing elective isolated coronary artery bypass graft surgery. Circulation. 2009;120:1474–1481. doi: 10.1161/CIRCULATIONAHA.108.842104. [DOI] [PubMed] [Google Scholar]
- 97.Fattouch K, Guccione F, Sampognaro R, Panzarella G, Corrado E, Navarra E, Calvaruso D, Ruvolo G. POINT: Efficacy of adding mitral valve restrictive annuloplasty to coronary artery bypass grafting in patients with moderate ischemic mitral valve regurgitation: a randomized trial. J Thorac Cardiovasc Surg. 2009;138:278–285. doi: 10.1016/j.jtcvs.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 98.Chan KM, Punjabi PP, Flather M, Wage R, Symmonds K, Roussin I, Rahman-Haley S, Pennell DJ, Kilner PJ, Dreyfus GD, Pepper JR Investigators R. Coronary artery bypass surgery with or without mitral valve annuloplasty in moderate functional ischemic mitral regurgitation: final results of the Randomized Ischemic Mitral Evaluation (RIME) trial. Circulation. 2012;126:2502–2510. doi: 10.1161/CIRCULATIONAHA.112.143818. [DOI] [PubMed] [Google Scholar]
- 99.Grayburn PA, Carabello B, Hung J, Gillam LD, Liang D, Mack MJ, McCarthy PM, Miller DC, Trento A, Siegel RJ. Defining "severe" secondary mitral regurgitation: emphasizing an integrated approach. J Am Coll Cardiol. 2014;64:2792–2801. doi: 10.1016/j.jacc.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 100.Marwick TH, Zoghbi WA, Narula J. Redrawing the borders: considering guideline revision in functional mitral regurgitation. JACC Cardiovasc Imaging. 2014;7:333–335. doi: 10.1016/j.jcmg.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 101.Bax JJ, Braun J, Somer ST, Klautz R, Holman ER, Versteegh MI, Boersma E, Schalij MJ, van der Wall EE, Dion RA. Restrictive annuloplasty and coronary revascularization in ischemic mitral regurgitation results in reverse left ventricular remodeling. Circulation. 2004;110:II103–II108. doi: 10.1161/01.CIR.0000138196.06772.4e. [DOI] [PubMed] [Google Scholar]
- 102.Bolling SF, Pagani FD, Deeb GM, Bach DS. Intermediate-term outcome of mitral reconstruction in cardiomyopathy. J Thorac Cardiovasc Surg. 1998;115:381–386. doi: 10.1016/S0022-5223(98)70282-X. discussion 387–8. [DOI] [PubMed] [Google Scholar]
- 103.Bertrand PB, Verbrugge FH, Verhaert D, Smeets CJ, Grieten L, Mullens W, Gutermann H, Dion RA, Levine RA, Vandervoort PM. Mitral valve area during exercise after restrictive mitral valve annuloplasty: importance of diastolic anterior leaflet tethering. J Am Coll Cardiol. 2015;65:452–461. doi: 10.1016/j.jacc.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Magne J, Senechal M, Mathieu P, Dumesnil JG, Dagenais F, Pibarot P. Restrictive annuloplasty for ischemic mitral regurgitation may induce functional mitral stenosis. J Am Coll Cardiol. 2008;51:1692–1701. doi: 10.1016/j.jacc.2007.11.082. [DOI] [PubMed] [Google Scholar]
- 105.Fino C, Iacovoni A, Ferrero P, Merlo M, Bellavia D, D'Elia E, Miceli A, Senni M, Caputo M, Ferrazzi P, Galletti L, Magne J. Determinants of functional capacity after mitral valve annuloplasty or replacement for ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2015;149:1595–1603. doi: 10.1016/j.jtcvs.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 106.McGee EC, Gillinov AM, Blackstone EH, Rajeswaran J, Cohen G, Najam F, Shiota T, Sabik JF, Lytle BW, McCarthy PM, Cosgrove DM. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2004;128:916–924. doi: 10.1016/j.jtcvs.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 107.Acker MA, Parides MK, Perrault LP, Moskowitz AJ, Gelijns AC, Voisine P, Smith PK, Hung JW, Blackstone EH, Puskas JD, Argenziano M, Gammie JS, Mack M, Ascheim DD, Bagiella E, Moquete EG, Ferguson TB, Horvath KA, Geller NL, Miller MA, Woo YJ, D'Alessandro DA, Ailawadi G, Dagenais F, Gardner TJ, O'Gara PT, Michler RE, Kron IL. Mitral-Valve Repair versus Replacement for Severe Ischemic Mitral Regurgitation. N Engl J Med. 2014;370:23–32. doi: 10.1056/NEJMoa1312808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hung J, Papakostas L, Tahta SA, Hardy BG, Bollen BA, Duran CM, Levine RA. Mechanism of recurrent ischemic mitral regurgitation after annuloplasty: continued LV remodeling as a moving target. Circulation. 2004;110:II85–II90. doi: 10.1161/01.CIR.0000138192.65015.45. [DOI] [PubMed] [Google Scholar]
- 109.Kron IL, Hung J, Overbey JR, Bouchard D, Gelijns AC, Moskowitz AJ, Voisine P, O'Gara PT, Argenziano M, Michler RE, Gillinov M, Puskas JD, Gammie JS, Mack MJ, Smith PK, Sai-Sudhakar C, Gardner TJ, Ailawadi G, Zeng X, O'Sullivan K, Parides MK, Swayze R, Thourani V, Rose EA, Perrault LP, Acker MA Investigators C. Predicting recurrent mitral regurgitation after mitral valve repair for severe ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2015;149:752–761. e1. doi: 10.1016/j.jtcvs.2014.10.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li M, Dumesnil JG, Mathieu P, Pibarot P. Impact of valve prosthesis-patient mismatch on pulmonary arterial pressure after mitral valve replacement. J Am Coll Cardiol. 2005;45:1034–1040. doi: 10.1016/j.jacc.2004.10.073. [DOI] [PubMed] [Google Scholar]
- 111.Fino C, Iacovoni A, Ferrero P, Senni M, Merlo M, Cugola D, Ferrazzi P, Caputo M, Miceli A, Magne J. Restrictive mitral valve annuloplasty versus mitral valve replacement for functional ischemic mitral regurgitation: An exercise echocardiographic study. J Thorac Cardiovasc Surg. 2014;148:447–453. e2. doi: 10.1016/j.jtcvs.2013.05.053. [DOI] [PubMed] [Google Scholar]
- 112.Jones RH, Velazquez EJ, Michler RE, Sopko G, Oh JK, O'Connor CM, Hill JA, Menicanti L, Sadowski Z, Desvigne-Nickens P, Rouleau JL, Lee KL Investigators SH. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009;360:1705–1717. doi: 10.1056/NEJMoa0900559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Acker MA, Bolling S, Shemin R, Kirklin J, Oh JK, Mann DL, Jessup M, Sabbah HN, Starling RC, Kubo SH Acorn Trial Principal I and Study C. Mitral valve surgery in heart failure: insights from the Acorn Clinical Trial. J Thorac Cardiovasc Surg. 2006;132:568–577. 577, e1–e4. doi: 10.1016/j.jtcvs.2006.02.062. [DOI] [PubMed] [Google Scholar]
- 114.Grossi EA, Patel N, Woo YJ, Goldberg JD, Schwartz CF, Subramanian V, Feldman T, Bourge R, Baumgartner N, Genco C, Goldman S, Zenati M, Wolfe JA, Mishra YK, Trehan N, Mittal S, Shang S, Mortier TJ, Schweich CJ, Jr Group R-MS. Outcomes of the RESTOR-MV Trial (Randomized Evaluation of a Surgical Treatment for Off-Pump Repair of the Mitral Valve) J Am Coll Cardiol. 2010;56:1984–1993. doi: 10.1016/j.jacc.2010.06.051. [DOI] [PubMed] [Google Scholar]
- 115.Mihos CG, Santana O. Is an adjunctive subvalvular repair during mitral annuloplasty for secondary mitral regurgitation effective in preventing recurrent regurgitation? Interact Cardiovasc Thorac Surg. 2016;22:216–221. doi: 10.1093/icvts/ivv328. [DOI] [PubMed] [Google Scholar]
- 116.Hung J, Solis J, Guerrero JL, Braithwaite GJ, Muratoglu OK, Chaput M, Fernandez-Friera L, Handschumacher MD, Wedeen VJ, Houser S, Vlahakes GJ, Levine RA. A novel approach for reducing ischemic mitral regurgitation by injection of a polymer to reverse remodel and reposition displaced papillary muscles. Circulation. 2008;118:S263–S269. doi: 10.1161/CIRCULATIONAHA.107.756502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Solis J, Levine RA, Johnson B, Guerrero JL, Handschumacher MD, Sullivan S, Lam K, Berlin J, Braithwaite GJ, Muratoglu OK, Vlahakes GJ, Hung J. Polymer injection therapy to reverse remodel the papillary muscles: efficacy in reducing mitral regurgitation in a chronic ischemic model. Circ Cardiovasc Interv. 2010;3:499–505. doi: 10.1161/CIRCINTERVENTIONS.109.850255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Langer F, Kunihara T, Hell K, Schramm R, Schmidt KI, Aicher D, Kindermann M, Schafers HJ. RING+STRING: Successful repair technique for ischemic mitral regurgitation with severe leaflet tethering. Circulation. 2009;120:S85–S91. doi: 10.1161/CIRCULATIONAHA.108.840173. [DOI] [PubMed] [Google Scholar]
- 119.Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, Loghin C, Trento A, Skipper ER, Fudge T, Letsou GV, Massaro JM, Mauri L Investigators EI. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–1406. doi: 10.1056/NEJMoa1009355. [DOI] [PubMed] [Google Scholar]
- 120.Maisano F, Franzen O, Baldus S, Schafer U, Hausleiter J, Butter C, Ussia GP, Sievert H, Richardt G, Widder JD, Moccetti T, Schillinger W. Percutaneous mitral valve interventions in the real world: early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J Am Coll Cardiol. 2013;62:1052–1061. doi: 10.1016/j.jacc.2013.02.094. [DOI] [PubMed] [Google Scholar]
- 121.Al Amri I, Debonnaire P, Witkowski T, van der Kley F, Palmen M, de Weger A, Klautz RJ, Bax JJ, Schalij MJ, Ajmone Marsan N, Delgado V. Mitral valve geometry and hemodynamics after surgical mitral valve annuloplasty and implications for percutaneous treatment of patients with recurrent mitral regurgitation. Am J Cardiol. 2013;112:714–719. doi: 10.1016/j.amjcard.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 122.Schmidt FP, von Bardeleben RS, Nikolai P, Jabs A, Wunderlich N, Munzel T, Hink U, Warnholtz A. Immediate effect of the MitraClip procedure on mitral ring geometry in primary and secondary mitral regurgitation. Eur Heart J Cardiovasc Imaging. 2013;14:851–857. doi: 10.1093/ehjci/jes293. [DOI] [PubMed] [Google Scholar]
- 123.Grayburn PA, Foster E, Sangli C, Weissman NJ, Massaro J, Glower DG, Feldman T, Mauri L. Relationship between the magnitude of reduction in mitral regurgitation severity and left ventricular and left atrial reverse remodeling after MitraClip therapy. Circulation. 2013;128:1667–1674. doi: 10.1161/CIRCULATIONAHA.112.001039. [DOI] [PubMed] [Google Scholar]
- 124.Van De Heyning CM, Bertrand PB, Debonnaire P, De Maeyer C, Vandervoort PM, Coussement P, Paelinck BP, De Bock D, Vrints CJ, Claeys MJ. Mechanism of Symptomatic Improvement After Percutaneous Therapy for Secondary Mitral Regurgitation: Resting and Exercise Hemodynamics. J Am Coll Cardiol. 2016;68:128–129. doi: 10.1016/j.jacc.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 125.Boerlage-van Dijk K, van Riel AC, de Bruin-Bon RH, Wiegerinck EM, Koch KT, Vis MM, Meregalli PG, Bindraban NR, Mulder BJ, Piek JJ, Bouma BJ, Baan J., Jr Mitral inflow patterns after MitraClip implantation at rest and during exercise. J Am Soc Echocardiogr. 2014;27:24–31. e1. doi: 10.1016/j.echo.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 126. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); [2012 - [cited 2016 Dec 1]]. Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation. Available from http://clinicaltrials.gov/ct2/show/NCT01626079, NLM Identifier: NCT01626079. [Google Scholar]
- 127.Herrmann HC, Maisano F. Transcatheter therapy of mitral regurgitation. Circulation. 2014;130:1712–1722. doi: 10.1161/CIRCULATIONAHA.114.009881. [DOI] [PubMed] [Google Scholar]
- 128.Chandran KB, Kim H. Computational mitral valve evaluation and potential clinical applications. Ann Biomed Eng. 2015;43:1348–1362. doi: 10.1007/s10439-014-1094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.