Abstract
Understanding how stromal signals regulate the development of pancreatic ductal adenocarcinoma (PDAC) may suggest novel therapeutic interventions in this disease. In this study, we assessed the metastatic role of stromal signals suggested to be important in the PDAC microenvironment. Src and IGF-1R phosphorylated the pro-metastatic molecule Annexin A2 (AnxA2) at Y23 and Y333 in response to stromal signals HGF and IGF-1, respectively, and IGF-1 expression was regulated by the Sonic Hedgehog (Shh) pathway. Both Shh and HGF were heterogeneously expressed in PDAC stroma, and only dual inhibition of these pathways could significantly suppress AnxA2 phosphorylation, PDAC growth and metastasis. Taken together, our results illuminate tumor-stromal interactions which drive metastasis, and provide a mechanism-based rationale for a stroma-directed therapy for PDAC.
Keywords: Pancreatic ductal adenocarcinoma, Stroma, Tumor microenvironment, Cancer associated fibroblast, metastasis
INTRODUCTION
One of the hallmarks of pancreatic ductal adenocarcinoma (PDAC) is the presence of dense desmoplastic stroma, which makes up approximately 60–90% of the tumor volume (1). Emerging evidence suggests that the stroma develops concurrently with the neoplasm and likely plays a role in regulating the initiation and progression of PDAC (2,3). Hence, understanding how the stromal signals regulate PDAC growth, invasion and metastases could potentially lead to the development of novel targeted and more effective therapies for pancreatic cancer.
The hedgehog signaling (Hh) pathway has been shown to be a key regulator of PDAC stromal cell signaling (4,5) and a facilitator of PDAC metastases formation (6,7). Additionally, recent reports have shown that sonic hedgehog (Shh) is overexpressed in PDAC tumor cells, while its downstream signaling is confined to the stromal compartment, forming a paracrine signaling axis between neoplastic and stromal cells (8,9). However, the role of Hh signaling and stromal depletion in PDAC is debatable (10,11). Thus, this dispute has suggested the necessity for the molecular dissection of the complexity of stromal signals in PDAC.
The recent characterization of pancreatic-associated protein, Annexin A2(AnxA2), depicted a pro-metastatic mechanism in PDAC (12,13) for dissecting the stromal signaling in the tumor microenvironment. It was demonstrated that Tyrosine 23(Y-23) phosphorylation on AnxA2 is required for the cell surface translocation of AnxA2 and subsequent PDAC invasion and metastases formation (12,13). Insulin-like growth factor 1 receptor (IGF-1R) tyrosine kinase has been implicated in the phosphorylation of AnxA2(14). The IGF-1 ligand was postulated by others (8) to be secreted by stromal cells under the regulation of Hh signaling. More recently, it was shown that the oncogenic signaling is transmitted from tumor cells to the stromal cells through this paracrine Shh axis and subsequently receives the reciprocal signals from the stromal cells via an IGF-1R-mediated axis (9).
Additionally, AnxA2 has been characterized as an in vitro and in vivo substrate of the Src family of non-receptor tyrosine kinases (15,16). c-Met was shown to be upstream of Src and indirectly responsible for AnxA2 phosphorylation during cell scattering and branching morphogenesis (17). The role of the hepatocyte growth factor (HGF)/c-Met is also well documented in PDAC (18). In tumor cells, c-Met is often overexpressed, rather than mutated (19), which activates the pathway and results in excessive proliferation and tumor cell motility (20). HGF signals through its receptor c-Met, and is required for Src-induced AnxA2 phosphorylation in the epithelial to mesenchymal transition (EMT) during branching morphogenesis in early development (17).
Both IGF-1 and HGF are extracellular molecules that are present in PDAC stroma (21,22). We therefore hypothesized that stromal signals activate intracellular effectors in PDAC such as IGF-1R and Src that culminate in Y-23-AnxA2 phosphorylation. We specifically investigated two paracrine stromal-to-epithelial pathways: the Hh/IGF-1/IGF-1R pathway and the HGF/c-Met/Src pathway.
Materials and Methods
Cell lines and compounds
The human Panc10.05, Panc2.8 and the murine KPCA(A) and KPCA(Y23A) pancreatic tumor cell lines cell line and hCAFs were established in accordance with the Johns Hopkins Medical Institution Institutional Review Board (JHMI IRB)-approved protocols, and obtained between 2012 and 2014, and authenticated by DNA and gene expression profiling and cultured as previously described (13,23,24). hCAFs used were at passage 2 and cultured for 3–4 additional passages if necessary. The Panc02 cells were authenticated by DNA and gene expression profiling and cultured as previously described (25). Dasatinib, Tyrphostin AG490 and INCB28060 were obtained from Selleck Chemicals, Sigma Aldrich and AbMole, respectively. NVP-AEW541 and NVP-LDE225 were provided by Novartis.
Mouse studies
All animal experiments conformed to the guidelines of the Animal Care and Use Committee of the Johns Hopkins University. The animals were maintained in accordance with the guidelines of the American Association of Laboratory Animal Care. Procedures for the orthotopic model were described previously (12,13).
Western blotting, immunohistochemistry and ELISA
Elution of AnxA2 (26)and Western blot with rabbit anti-AnxA2, mouse anti-P-Y23-AnxA2, mouse anti-beta-actin, rabbit anti-HGF (all from Santa Cruz Biotechnology), rabbit anti-IGF-1(Abcam) or goat anti-Shh (R&D Systems) antibodies were described previously (12) Immunohistochemistry (IHC) staining for E-Cadherin and HGF was performed with rabbit anti-E-Cadherin (Abcam) and rabbit anti-HGF (Santa Cruz Biotechnology) antibodies using a standard protocol on an automated stainer from Leica Microsystems. IHC for SMA was performed as previously described (27). IHC for AnxA2 was conducted with mouse anti-AnxA2(Invitrogen), or mouse anti-P-Y23-AnxA2 antibodies, IHC for Shh with goat-anti-Shh (R&D), and IHC for IGF-1 with rabbit-anti-IGF-1(Abcam) antibodies as described previously (13). All IHC slides were analyzed and scored by a pathologist (A.L). The levels of secreted IGF-1 were measured by ELISA (R&D) following the manufacturer’s instructions.
Invasion assay
Invasion assays were performed using the Trevigen invasion assay kit according to the manufacturer’s instructions (Trevigen) with modifications as previously described (13). Cell invasion was measured using the CCK8 assay (Sigma).
Targeted LC/MS/MS MRM method
For mass spectrometry, multiple reaction monitoring (MRM) was carried out on a platform of mass spectrometer 5500 QTRAP (AB SCIEX) online coupled with Prominence HPLC system (SHIMADZU).
Quantitative real time RT-PCR
The RNeasy Micro Kit (Qiagen) was used to extract total RNA. Quantitative real-time RT-PCR (qPCR) was performed on the StepOnePlus Real Time PCR System (Life Technology) and analyzed by the StepOne software V2.1.
shRNA knockdown
The lentiviral plasmid encoding shRNAs were obtained from Dharmacon and used by following a previously described procedure (13).
Statistical analysis
Statistical analysis was performed using GraphPad Prism v6.0(GraphPad Software). The data are presented as the means ± standard error of the mean (SEM). For all analyses p value equal or less than 0.05 was considered statistically significant.
RESULTS
Src and IGF-1R kinases phosphorylate AnxA2 on Tyrosine 23 in PDAC
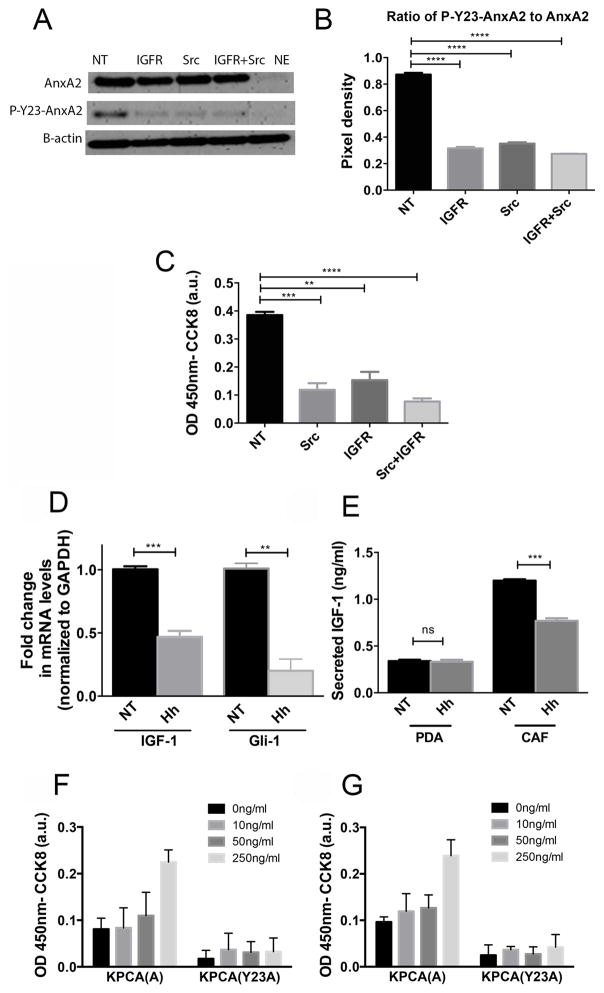
Src and IGF-1R kinases have been implicated in AnxA2 phosphorylation in a number of benign and malignant biologic processes (14,28–32). The role of IGF-1R and Src in AnxA2 phosphorylation however has not yet been established in PDAC; thus, were first examined. We used IGF-1R (NVP-AE541)(33) and Src (dasatinib)(34) inhibitors to examine if either IGF-1R and Src are responsible for phosphorylating AnxA2 at Y-23 in the Panc10.05 human pancreatic tumor cell line known to lack mutations in those pathways and express high levels of surface AnxA2(12,19). Dasatinib was shown to inhibit Src kinase activity but it was also found to be active against other tyrosine kinases (33). Nevertheless, we found that both Src and IGF-1R inhibitors decrease the amount of cell surface AnxA2 and most notably decrease the levels of phosphorylated (P)-Y23-AnxA2 in the human PDAC cell line in vitro (Fig. 1A,B; Supplementary Fig.S1A,B). A similar reduction in P-Y23-AnxA2 levels was observed in other PDAC cell lines including the Panc2.8 human PDAC cell line and the KPC tumor cell line derived from the Kras/p53 mutation conditional knock-in mice (KPC mice)(13,35) following the same inhibitor treatments (Supplementary Fig. S1C,D). By contrast, a decrease in AnxA2 phosphorylation was not observed with inhibition of other tyrosine kinases such as the epidermal growth factor receptor (EGFR) kinase by Tyrphostin AG490(36) (Supplementary Fig. S2). Moreover, the treatment of PDAC cells with Src and/or IGF-1R inhibitors decreased invasion when analyzed by the Boyden chamber invasion assay (Fig. 1C). Taken together, these data support IGF-1R and Src as two independent kinases that phosphorylate AnxA2 at Y-23 in PDAC.
Figure 1. Inhibition of Src and IGF-1R kinases results in decreased phosphorylation of AnxA2 at Tyrosine 23 on the cell surface of human PDAC cells and subsequent decrease in PDAC invasion while stromal factors, IGF-1 and HGF, upstream of IGF-1R and Src kinases, enhance invasion of tumor cells in PDAC cells.
A. Western blot of Panc10.05 human PDAC cells treated with IGF-1R inhibitor (1μM) and Src inhibitor (50 nM) for 60 minutes. AnxA2 was eluted off the surface of PDAC cells with EGTA as previously described (12,26). The levels of total surface AnxA2 and P-Y23-AnxA2 were quantified by Western blot. β-actin was used as a loading control. B. Quantification of relative expression of P-Y23-AnxA2 to total cell surface AnxA2(ratio) C. An invasion assay using PDAC cells treated with IGF-1R and/or Src inhibitors D. qRT-PCR analysis of IGF-1 and Gli-1(control) in hCAFs. The gene expression of IGF-1 and Gli1 was normalized to GAPDH and is shown as a fold change. E. IGF-1 secretion determined by ELISA from single cultures of PDACand hCAF, respectively. F and G. An invasion assay using murine KPCA (A) and KPCA (Y23A) PDAC cells was performed. Serum free media, rIGF-1(C) or rHGF (D) at depicted concentrations were added to the PDAC cell suspension prior to plating. Data are means ± SEM from three technical replicates and representative of at least duplicate experiments. NT-vehicle treatment, Hh-Hh inhibitor (NVP-LDE225 at 1μM) treatment, IGFR-NVP-AEW541 inhibitor, Src-dasatinib inhibitor. ns-not significant, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001(unpaired student’s t-test).
Stromal factors, IGF-1 and HGF, upstream of IGF-1R and Src kinases, enhance invasion of tumor cells in PDAC cells
Next, we evaluated the upstream signals leading to IGF-1R and Src-mediated phosphorylation of AnxA2, respectively. IGF-1 was postulated (8) to be secreted by stromal cells under the regulation of Hh signaling. We therefore assessed the expression and secretion of IGF-1 in human cancer-associated fibroblasts (hCAFs), which are the major component of PDAC stroma. Four different human fibroblasts and 3 different human PDAC cell lines were tested for the expression and/or secretion of IGF-1 and the data shown below are representatives of themNVP-LDE225(37) was used to target the Hh signaling pathway and determine the effect of Hh inhibition on IGF-1 expression and secretion. Our data show that Hh inhibition in hCAFs led to a decrease in IGF-1 expression as well as an anticipated decrease in Gli-1 expression (Fig. 1D). Moreover, inhibition of Hh pathway in hCAFs resulted in significant reduction of IGF-1 secretion from those cells. Conversely, tumor cells secrete minimal amounts of IGF-1 when compared to hCAFs and secretion of this protein from tumor cells is not affected by Hh pathway inhibition (Fig. 1E). Taken together, these results suggested that the stromal production of IGF-1 appears to be regulated by the Hh signaling.
In addition, as described above, the Src-mediated AnxA2 phosphorylation appears to be under the regulation of the HGF/c-Met pathway. It is known that HGF is also specifically secreted by the stromal fibroblast in PDAC (22) likely under autocrine regulation (38,39). However, whether tumor cells may also play a role in regulating the stromal production of HGF remains to be explored.
As the Y-23 phosphorylation of AnxA2 was shown to be critical for PDAC invasion, we sought to determine if IGF-1 and HGF, upstream of IGF-1R and c-Met/Src kinases, respectively, affect the invasion of tumor cells. We utilized AnxA2 knockout KPC murine PDAC cell lines, in which the wild type AnxA2 cDNA (designated “KPCA(A)”) or the mutated AnxA2 cDNA expressing an alanine instead of tyrosine at position 23(designated “KPCA (Y23A)”) were reintroduced. The change of tyrosine to alanine renders the protein unable to be phosphorylated at position 23(12,13). As shown in Fig. 1F,G, addition of exogenous recombinant murine IGF-1(rIGF-1) and HGF (rHGF) proteins into the culture media increases the invasive potential of murine PDAC cells expressing the wild-type AnxA2 cDNA in a dose dependent manner. Notably, the addition of rIGF-1 or rHGF showed no significant effect on KPCA (Y23A) invasion ability. This data supports the hypothesis that stromal factors, IGF-1 and HGF, are upstream of IGF-1R and c-Met/Src kinases that are responsible for Y-23 phosphorylation of AnxA2 and enhance invasion of PDAC cells likely through Y-23 phosphorylation.
Dual inhibition of HGF and IGF-1from hCAFs results in a reduction of PDAC cell invasion
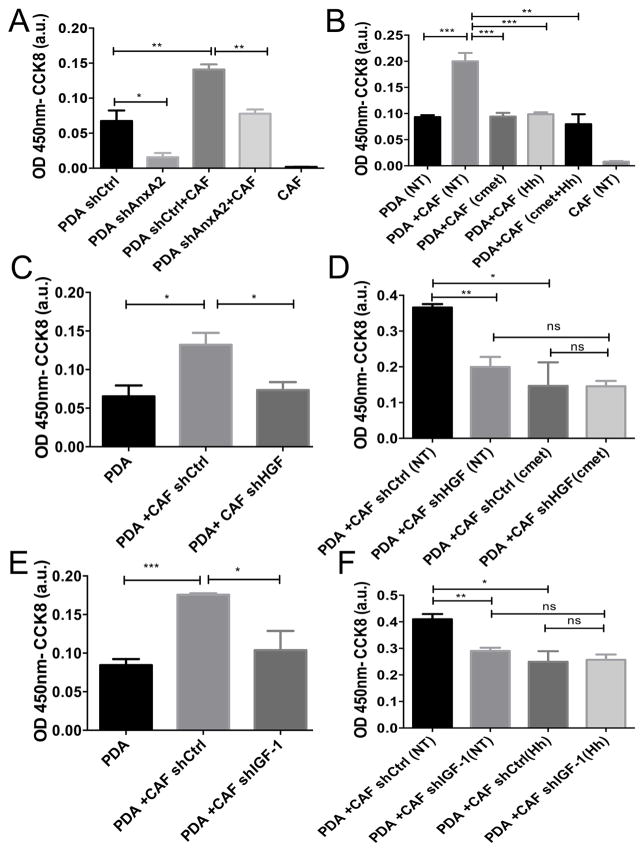
We next sought to determine if hCAFs as a source of HGF and IGF-1 could enhance the invasion of PDAC cells. Utilizing invasion assay we showed that co-culture of Panc10.05 with hCAFs significantly increases the invasive potential of the tumor cells comparing to the single culture of Panc10.05 (Fig. 2A). It should be noted that hCAFs are not invasive. However, when AnxA2 was knockdown in the Panc10.05 cells by the shRNA of AnxA2(shAnxA2; Supplementary Fig. S3A), Panc10.05 cells lost their invasive potential and their invasion was also not enhanced by hCAFs (Fig. 2A). Knockdown of AnxA2 did not affect the cells proliferation capability (12).
Figure 2. IGF-1 and HGF secreted by hCAFs regulate the invasion of human PDAC cells in an AnxA2 dependent manner.
A. Invasion assays of PDAC cells transfected with control (shCtrl) or AnxA2 targeting shRNA (shAnxA2) in single culture (PDAC cells only) or in co-culture with hCAFs. B. Invasion assays of PDAC cells co-cultured with hCAFs (2:1 here and below) and treated with vehicle (NT), c-Met inhibitor (at 10pM), or Hh inhibitor (at 1μM). C. Invasion assays of PDAC cells co-cultured with hCAFs transfected with control (shCtrl) or HGF targeting shRNA (shHGF). D. Invasion assays of PDAC cells co-cultured with hCAFs transfected with control (shCtrl) or HGF targeting shRNA (shHGF) and treated with vehicle (NT) or c-Met inhibitor. E. Invasion assays of PDAC cells co-cultured with hCAFs transfected with control (shCtrl) or IGF-1 targeting shRNA (shIGF-1). F. Invasion assays of PDAC co-cultured with hCAFs transfected with control (shCtrl) or IGF-1 targeting shRNA (shIGF-1) and treated with vehicle (NT) or Hh inhibitor. Data are means ± SEM from 3 technical replicates and representative of at least 3 experiments. ns-not significant, *p<0.05, **p<0.01, ***p<0.001(unpaired student’s t-test).
We then performed an invasion assay using co-culture of Panc10.05 and hCAF cells and inhibited HGF/c-Met and/or Hh/IGF-1 signaling using pharmacological agents under clinical development for intervening with these two signaling pathways by targeting HGF/c-Met (INC280)(40) and Hh (NVP-LDE225), respectively. Hh inhibitor did not inhibit the invasion of singly cultured Panc10.05 cells. c-Met inhibitor modestly, but not significantly inhibited the invasion of singly cultured Panc10.05 cells (Supplementary Fig. S4A), likely because c-Met is expressed on PDAC cells and can be activated by HGF in the media. By contrast, as shown in Fig. 2B, inhibition of either HGF/c-Met or Hh/IGF-1 results in a significant decrease in invasion of Panc10.05 cells co-cultured with hCAFs when compared to control. Moreover, there is a strong trend towards greater decrease in invasion upon dual signaling inhibition comparing to single inhibitions; though, it is not statistically significant, likely due to the fact that single inhibitions have already reduced the invasion of PDAC cells co-cultured with hCAFs close to the level of single PDAC cell culture, making an additional reduction in invasion difficult to be discerned by this assay.
To further confirm that the observed decrease in invasion in Fig. 2B is specifically mediated by HGF and IGF-1, we used lentiviral-shRNA to knockdown HGF and IGF-1 in hCAFs, respectively (Supplementary Fig. S3B, C). A significant decrease in invasion was observed when Panc10.05 cells were co-cultured with hCAFs following HGF knockdown (Fig. 2C) or IGF-1 knockdown (Fig. 2E), but not with control shRNA(shCtrl)-transfected hCAFs. Furthermore, the invasion capacity of co-cultured Panc10.05 cells returned to their baseline as single Panc10.05 culture when either HGF or IGF-1 was knockdown from hCAFs (Fig. 2C,E). Moreover, HGF/c-Met inhibitor treatment had no effect on the invasion of Panc10.05 cells co-cultured with hCAFs that had HGF knockdown (Fig.2D); nor did the Hh inhibitor treatment when hCAFs had IGF-1 knockdown (Fig. 2F). Treatment of Panc10.05 cells with the above-mentioned inhibitors nor knockdown of HGF or IGF-1 from hCAFs affected proliferation of those cells (Supplementary Fig. S4B, C). These results confirmed that HGF acts through the c-Met pathway and Hh acts through IGF-1 to enhance invasion.
IGF-1 and HGF secreted by hCAFs regulate AnxA2 phosphorylation of PDAC tumor cells
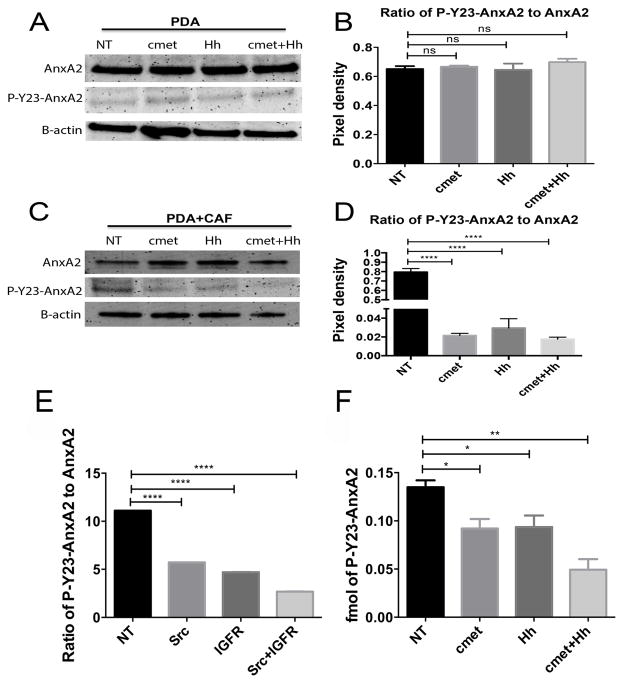
Next, we explored how the signaling mediated by IGF-1 and HGF is transmitted to the neoplastic cells in PDAC. We examined whether inhibiting the Hh/IGF-1/IGF-1R and the HGF/c-Met/Src signaling pathways via pharmacological agents can modulate AnxA2 tyrosine phosphorylation in PDAC cells co-cultured with stromal cells. After treating the Panc10.05/hCAF co-cultures with NVP-LDE225 and INC280, AnxA2 was eluted from the surface of the tumor cells into the EGTA containing buffer (26)and P-Y23-AnxA2 was evaluated by Western blot. As a control, single Panc10.05 cell cultures were also treated with the inhibitors to confirm that the signals that lead to IGF-1R and c-Met/Src activation originate from the stroma. Inhibition of Hh and HGF/c-Met in the 10% FBS media led to a modest decrease in P-Y23-AnxA levels in single Panc10.05 culture (Supplementary Fig. S5A–D), suggesting that factors present in serum (Supplementary Fig. S6) have a minor effect in activating the pathways under study leading to AnxA2 phosphorylation. To attenuate the activation of the pathways under study by factors present in the serum we used serum-reduced media (5% FBS), which contains decreased concentrations of HGF and IGF-1(Supplementary Fig. S6). As expected, neither the Hh inhibitor nor the HGF/c-Met inhibitor affected the levels of total AnxA2 or P-Y23-AnxA2 in Panc10.05 cells in the singly cultured serum-reduced media (Fig. 3A,B; Supplementary Fig. S5E,F). It is likely the concentration of HGF and IGF-1 in serum-reduced media is lower than the effective thresholds. However, because the treatment of Hh inhibitors had to be done in the medium with 10% FBS (Fig. 1), we continued to use the same medium with 10%FBS for the remaining experiments even through there is a minor effect from the HGF and IGF-1 in the serum. In Panc10.05 cells co-cultured with hCAFs in a no-contact fashion only the HGF/c-Met inhibitor decreased the total cell surface AnxA2 on Panc10.05 cells (Supplementary Fig. S5G). Importantly, each treatment significantly decreased the amount of P-Y23-AnxA2 on the cell surface of Panc10.05 cells co-cultured with hCAFs while dual inhibition of HGF/c-Met and Hh led to a significant decrease of P-Y23-AnxA2 when compared to single inhibitions (Fig. 3C,D; Supplementary Fig. S5H). When HGF/c-Met and Hh are inhibited in vitro, the degree of inhibition of P-Y23-AnxA2 on tumor cells is demonstrated more potently when the tumor cells are co-cultured with hCAFs compared to those grown in single culture in 10%FBS media. Thus, the effect of HGF/c-Met and Hh inhibition on P-Y23-AnxA in tumor cells co-cultured with hCAFs must have exerted primarily through hCAFs, but not the existing serum factors.
Figure 3. IGF-1 and HGF secreted by hCAFs regulate AnxA2 phosphorylation of human PDAC cells.
A. Western blot of AnxA2 and P-Y23-AnxA2 eluted from PDAC cells after 24 hours of treatment with the Hh inhibitor (at 1μM) and/or HGF/c-Met inhibitor (at 10pM) in reduced (5%) serum media. β-actin used as a loading control. B. Quantification of the Western blot from panel A and shown as the ratio of P-Y23-AnxA to AnxA2. C. Western blot of AnxA2 and P-Y23-AnxA2 eluted from the PDAC cells after co-culture with hCAFs in a contact independent manner using a transwell system. Cells were treated as in panel A but in full media. D. Quantification of the Western blot from panel C was performed as in panel B. Blots are representative of at least 3 experiments. E. Semi-quantitative analysis by mass spectrometry of P-Y23-AnxA2 in cell surface elution of single culture of PDAC cells after treatment with IGF-1R inhibitor and/or Src inhibitor in full serum media. Relative score of P-Y23-AnxA2(B) in total AnxA2 are shown. Note, AnxA2 and P-AnxA2 were scored separately with different units due to the different intensities of signals detected by mass spectrometry. F. Absolute-quantitative analysis of P-Y23-AnxA2 by mass spectrometry in cell surface elution of the PDAC cells after co-culture with hCAFs and treatment with Hh inhibitor and/or HGF/c-Met inhibitor. Data are means ± SEM from 3 technical replicates and representative of at least 2 experiments. NT-vehicle treatment, IGFR-NVP-AEW541 inhibitor, Src-dasatinib inhibitor, c-Met-INC280 treatment, Hh-NVP-LDE treatment. ns-not significant, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001(unpaired student’s t-test).
Because two independent signaling pathways that originate from the stroma (IGF-1 and HGF) play a role in AnxA2 phosphorylation, we next attempted to determine whether these two signaling pathways result in phosphorylation of AnxA2 at different sites, making it necessary for the dual stromal regulation of AnxA2. By using the mass spectrometry approach, we identified 4 phospho-tyrosine sites in addition to Y-23(Supplementary Fig. S7 and S8). We concentrated our studies on Y-333 since this site showed a significant decrease in phosphorylation after both Src and IGF-1R inhibitor treatments (Supplementary Fig. S8E,F). With this mass spectrometry approach, we confirmed that the inhibition of Src and IGF-1R resulted in a significant decrease in Y-23 phosphorylation (Fig. 3E; Supplementary Fig. S8A).
We next determined if the same stromal factors that play a role in Y-23 phosphorylation (Fig. 3C,D) would also regulate Y-333 phosphorylation by quantifying the absolute levels of phosphorylation on Y-333 and Y-23 with a targeted LC/MS/MS MRM method. We found that both stromal pathways affect the phosphorylation of Y-333 and Y-23 in a similar way since the fold changes in each of the treatment groups when normalized to vehicle control are similar (Fig. 3F; Supplementary Fig. S8G). Of note, the fold of decrease in Y-23 phosphorylation in Fig. 3F is not as significant as that seen in Fig. 3C,D, likely due to the differences in detection between Western blot and mass spectrometry (41). Although the existence of multiple phosphorylation sites does not explain why two stromal signals are needed for AnxA2 phosphorylation, these data on different tyrosine sites provide further confirmation that dual stromal signaling regulates the phosphorylation of AnxA2 in PDAC and have also suggested investigating other potential mechanisms at the stroma level.
In vivo targeting of stromal signals suppresses hCAF activation, AnxA2 phosphorylation, and epithelial to mesenchymal transition
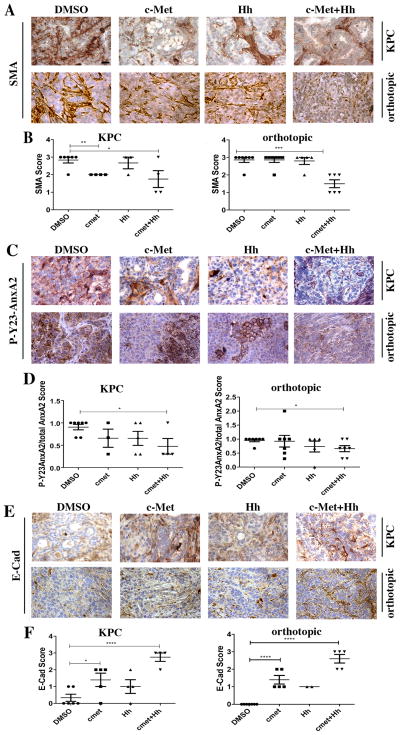
Next, we used two PDAC mouse models to evaluate the role of the Hh/IGF-1 and HGF/c-Met pathways on the regulation of AnxA2 in primary PDAC tumors in vivo. The first is KPC mice, which exhibits a multi-stage tumorigenesis that progresses from normal, through PanIN lesions, to invasive and metastatic PDAC (35). The second is an orthotopic implant model where tumors are grown subcutaneously from a cell line (the KPC cells) derived from KPC mice and are then implanted orthotopically into the pancreas of syngeneic mice (13). We chose to implant the subcutaneously grown tumor into the pancreas instead of injecting the tumor cell suspensions into the pancreas to avoid the spilling of tumor cells to the peritoneum. In addition, the tumors would be immediately established in the pancreas following the orthotopic implantation. First, we evaluated the effects of the Hh and HGF/c-Met inhibitor treatments on the activation of stroma of the PDAC tumors in these mice using IHC analysis to determine the expression of α-smooth muscle actin (SMA), a marker of activated pancreatic stellate (fibroblast) cells (42). As shown in both mouse models, dual targeting of Hh and HGF/c-Met resulted in a statistically significant and strong inhibition of SMA expression (Fig. 4A, B). By contrast, the Hh inhibitor only moderately suppressed the expression of SMA in KPC mice (Fig. 4B; Supplementary Fig. S9A) and had no effect on SMA in the orthotopic mouse model (Fig. 4B). Since the HGF/c-Met inhibitor targets the activation of c-Met on the tumor cell surface, it showed no effect on the expression of SMA.
Figure 4. In vivo targeting of stromal signals in KPC and orthotopic mouse models of PDAC suppresses hCAF activation, AnxA2 phosphorylation, and the epithelial to mesenchymal transition (EMT).
Panels A, D, G show representative IHC of SMA, P-Y23-AnxA and E-Cadherin (E-Cad) on primary tumors from both KPC transgenic mice and orthotopic model treated with stromal inhibitors or vehicle (DMSO). Panels B, E, H show semi-quantitative analysis scores of KPC tumors and panels C, F, I of orthotopic tumors harvested from this study. Protein expression was semi-quantified with a score from 0 to 3(0 representing no expression and 3 representing high expression). Mice with primary tumors confirmed by ultrasound were used for this study (at least 7 per group (KPC) and at least 9 per group (orthotopic)). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001(unpaired student’s t-test). Positive staining is shown in brown. Scale bar, 20 μm.
Next, we semi-quantified the expression level for the total cellular AnxA2 and the cell surface P-Y23-AnxA2 by IHC. The relative expression of P-Y23-AnxA2 to the total cellular AnxA2 as a ratio of P-Y23-AnxA2 to AnxA2 was calculated. The results show that in both the KPC and orthotropic implant models, dual inhibition of stromal signaling leads to a statistically significant decrease in the cell surface levels of P-Y23-AnxA2 on PDAC cells (Fig. 4C,D; Supplementary Fig. S9B). This data suggest that targeting both Hh and HGF signaling is required for strong inhibition of P-Y23-AnxA2 on the PDAC tumor cell surface.
We then assessed the effect of the dual inhibition of Hh and HGF/c-Met on EMT because P-Y23-AnxA2 in PDAC was previously shown to be responsible for inducing EMT (12). We performed IHC staining for E-cadherin, an epithelial marker, and semi-quantified the expression of E-cadherin on PDAC. As shown, the combination of HGF/c-Met and Hh inhibitors significantly and strongly increased the levels of E-Cadherin in both mouse models as compared to the vehicle treatments (Fig. 4E,F; Supplementary Fig. S9C). By contrast, single inhibition showed a moderate increase in the level of E-Cadherin in both models. These results suggest that dual inhibition of Hh and HGF/c-Met signaling has a stronger inhibitory effect on EMT in primary PDAC tumors.
Inhibition of stromal-neoplasm crosstalk leads to suppression of primary PDAC growth and decreased metastases formation in vivo
We next tested whether inhibition of the stromal signaling that leads to AnxA2 phosphorylation has an effect on primary tumor growth and metastases formation. We chose to examine this using the orthotropic implant model, which allowed us to establish baseline primary tumors almost identical in size in all mice. Tumor implanted mice develop metastases spontaneously at about the same rate. We first determined if Hh and HGF/c-Met inhibition could suppress primary PDAC growth. Tumors from each treatment group were examined by ultrasound at baseline and then again on the last day of treatment to assess the tumor volume. Single inhibition of Hh or HGF/c-Met signaling modestly decreased primary tumor volume (Fig. 5A). Importantly, only dual inhibition of Hh and HGF/c-Met signaling significantly decreased primary tumor volume (Fig. 5A). We previously reported that AnxA2 does not affect primary PDAC growth but only metastasis formation (12,13); therefore, the effect of dual stromal signaling inhibition may be mediated by other mechanisms, which remain to be explored.
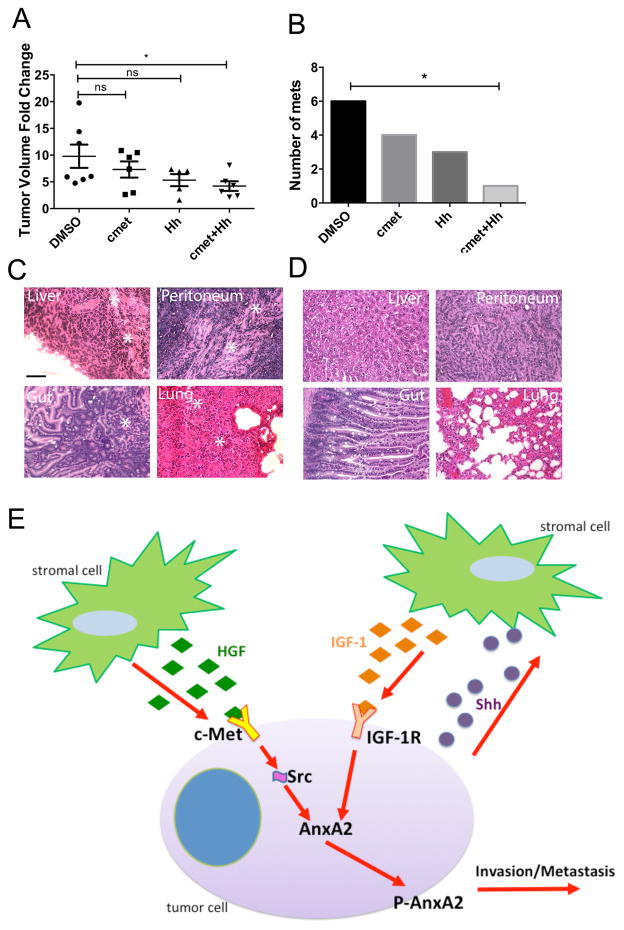
Figure 5. Inhibition of stromal-neoplasm crosstalk leads to a decrease in primary PDAC growth and metastases formation in vivo.
A. Fold change in primary tumor volume, calculated as end-point tumor volume/baseline tumor volume is shown. Mice were subjected to different treatments as indicated. DMSO-vehicle treatment. ns-not significant, *p<0.05, **p<0.01(unpaired student t-test). B. Summary of gross and histological quantification (combined) of all metastases (at least 7 mice per group). *p<0.05(Fisher’s exact test). C. Representative H&E images of metastases from the DMSO group were marked by *. D. Representative H&E images of normal tissues from the DMSO group. Scale bars for C and D, 200 μm. E. A working model showing dual stromal signaling pathways regulates a single pro-metastatic mechanism.
We next tested whether inhibition of the stromal signaling that leads to AnxA2 phosphorylation has an effect on metastases formation in vivo. No obvious toxicity was observed during the entire course of treatment with either single or dual inhibitors. No weight difference was observed among different treatment groups. Mice began dying or became morbid on day 17 following orthotopic pancreatic tumor implantation; therefore, all mice were euthanized and their pancreata, livers, lungs, peritoneum and gut were harvested to examine metastases formation grossly and histologically by the H&E staining. In addition, AnxA2 IHC staining was utilized to aide the detection of micrometastases. The results showed that single inhibition of Hh or HGF/c-Met shows a trend towards decrease in the numbers of metastases formed in comparison to the vehicle treatment (Fig. 5B). More importantly, our results showed that dual blockade of stromal signals resulted in statistically significant reduction of the total numbers of metastases formed compared to vehicle, suggesting that both Hh signaling and HGF/c-Met signaling in the stroma contribute to the development of PDAC metastases (Fig. 5B–E).
Heterogeneous expression of Shh and HGF in the PDAC TME in mice may explain the requirement of dual stromal signaling to activate AnxA2 effector functions
Next, we evaluated the biological significance of having dual stromal signaling in regulating the same intratumoral invasion pathway. First, we analyzed the spatial expression of the activating ligands under study, HGF, Shh and IGF-1 by IHC on primary tumors from KPC and orthotopic PDAC mice (Fig. 6) and investigated the intertumoral and intratumoral expression of these proteins in tumor tissues using sequential tissue slices. When the expression of either protein, Shh or HGF (Fig. 6A,B), is generally high in a tumor from one mouse, the expression of the other protein tends to be low in the tumor from the same mouse, suggesting that there is an intertumoral heterogeneity between different tumors regardless of whether they develop spontaneously as in the KPC mice or are orthotopically implanted. These data also suggest that stromal cells provided by the host mice may contribute to the heterogeneity of tumors. In addition, we determined the correlation in expression of Shh and IGF-1 in the primary tumors. In summary, IHC analysis revealed a significant positive correlation between Shh and IGF-1 and a negative correlation between Shh and HGF or between IGF-1 and HGF in the primary PDAC tumors (table 1; Supplementary Fig. S10).
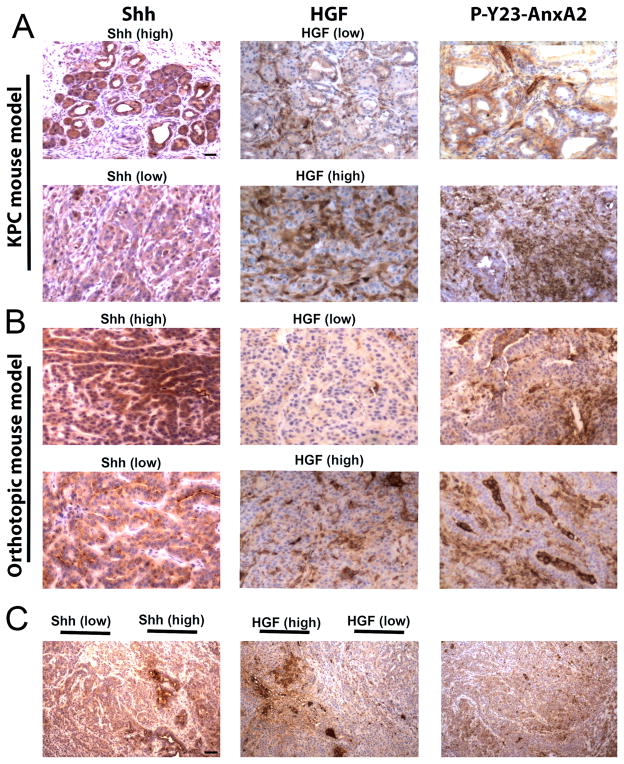
Figure 6. The stromal signals Shh and HGF are heterogeneously expressed in the tumor microenvironment of PDACs from different mouse models.
A and B. Representative IHC images of primary tumors stained for expression of Shh, HGF, P-Y23-AnxA2 show intertumoral heterogeneity in Shh and HGF expression in KPC (A) and orthotopic mouse models (B), respectively. C. Representative IHC images from the KPC mice show intratumoral heterogeneity in the expression of Shh and HGF. Scale bars, 20 μm for A, B and 200 μm for C.
Table 1.
Summary of the correlation analysis between IGF-1, Shh, HGF expression in KPC and orthotopic mouse models of PDAC.
| Mouse # | IGF-1 | Shh | HGF | |
|---|---|---|---|---|
| Orthotopic | 632 | Low | High | Low |
| 679 | Low | Low | High | |
| 633 | High | High | Low | |
| 678 | Low | High | High | |
| 638 | High | High | Low | |
| 634 | Low | Low | High | |
| 680 | High | High | Low | |
| KPC | 432 | High | High | Low |
| 7767 | Low | Low | High | |
| 1000 | Low | Low | High | |
| 9082 | Low | Low | High | |
| 0121 | Low | Low | High | |
| 7772 | Low | Low | High | |
| 9066 | High | High | Low |
Correlation between IGF-1/Shh (all mice) and Shh/HGF (all mice) of IHC scoring was performed and phi (ϕ) coefficients were calculated.IGF1/Shh (ϕ=0.7454 and **p<0.01) and Shh/HGF (ϕ=-0.8660 and **** p<0.0001).
As further support, we also found that there is intratumoral heterogeneity of both Shh and HGF. Specifically, whether the tumors are considered overall high Shh/low HGF or overall high HGF/low Shh, Shh and HGF have opposing expression patterns between different areas within the same tumor (Fig. 6C). Interestingly, the heterogeneity of Shh/IGF-1 and HGF does not correlate with the overall distribution of P-Y23-AnxA2 on epithelial tumor cells, which tends to be homogenous. These findings are consistent with the aforementioned results showing that both Hh/IGF-1 and HGF/c-Met signaling regulate tyrosine phosphorylation of AnxA2. Taken together, our data suggest the need for targeting both pathways to overcome the heterogeneity in expression of the stromal signals that lead to phosphorylation of AnxA2 and its consequent activation during the course of invasion and metastases.
Discussion
The role of IGF-1 and HGF in the invasion/metastases process in cancer has been suggested (43). Other intracellular pathways such as PRL-3, TRP and RasGRF1 were reported to be regulated by both IGF-1 and HGF signaling, respectively (44–46). We chose to dissect the stromal regulation of the pro-metastatic protein AnxA2, because of the promising pre-clinical data describing the role of AnxA2 in invasion/metastases in PDAC as well as the established methodology to study this protein (12,13,47). It remains to be explored whether other intracellular pathways are also subjected to the dual regulation of IGF-1 and HGF signaling in PDAC.
This study demonstrates for the first time the role of phosphorylation of AnxA2 in regulating invasion and metastases through stromal signaling within the pancreatic TME. Our results point to a dual process within the stroma that mediates AnxA2 phosphorylation, thereby facilitating an EMT tumor cell phenotype, tumor cell invasion in vitro, and metastases formation in vivo (Fig. 5E). Importantly, we show that strong inhibition of invasion and metastasis requires inhibition of both signaling pathways. After we identified a second phosphorylation site on AnxA2, we demonstrated that stromal signals interact similarly with both phosphorylation sites, suggesting that, despite the complexity of the stromal signaling, tumor cells are not plastic at this level of regulation. However, we found that the presence of intertumoral and intratumoral differential spatial expression of stromal signals likely contributes to tumor heterogeneity.
Dual regulation of a protein is common in other biologic processes (48,49). Phosphorylation regulation of proteins by two independent kinases has also been reported in non-diseased cells (49) and in cancer (50). The mechanism by which the two stromal signaling pathways in this study complement each other in vivo remains to be explored. We showed that both of these pathways regulate AnxA2 phosphorylation at different tyrosine sites. We cannot exclude that, in addition to Src and IGF-1R, other kinases may also participate in AnxA2 phosphorylation, nor can we rule out additional phosphorylation sites (51) that may be differentially regulated when compared with Y-23 and Y-333. Nevertheless, the similarity in the regulation between Y-23 and Y-333 suggested that tumor cells are more homogeneously regulated than we initially conceived. Moreover, the cellular heterogeneity of expression of stromal signals in the TME appears to better explain the need for dual stromal signaling culminating in AnxA2 phosphorylation regulation and subsequent invasion and metastases. Both IGF-1 and HGF are primarily secreted by cells of the mesenchymal origin, such as fibroblasts (52)although, we cannot exclude that other cell types present in the PDAC TME can contribute to the amount of those ligands present. Importantly, we showed that blockade of a single pathway is not effective in suppression of primary PDAC growth and metastases formation, since it most likely targets only tumor areas where the protein is expressed. Conversely, dual inhibition of these stromal signals is more effective in targeting heterogeneous tumors and larger tumor areas, which results in significant decrease in primary tumor volume and metastases.
Thus, our finding may provide an explanation for the ineffectiveness of single signal inhibition of the Hh and c-Met pathways (53). Controversial data have been reported on the role of Hh signaling and stromal depletion. Olive et al. and Provenzano et al. reported a beneficial effect of stromal targeting via Hh signaling inhibition or via enzymatic degradation of hyaluronan on the sensitivity to gemcitabine in transgenic mouse model of PDAC, respectively (54,55). Others reported that depletion of stromal fibroblasts in PDAC via Hh dependent or independent mechanisms accelerates progression of cancer and decreases survival (10,11). Our study suggests a possible mechanism for the observed discrepancy between the published findings by demonstrating the complexity and heterogeneity of the PDAC stromal cells and their signaling. Further investigation is warranted to uncover the complexity of stromal signals in PDAC, as well as determining if inhibition of pro-metastatic stromal signals culminates in AnxA2 phosphorylation resulting in a survival benefit.
Supplementary Material
Acknowledgments
Grant Support: This work was supported in part by NIH R01 CA169702(L. Zheng), NIH K23 CA148964-01 (L. Zheng), Viragh Foundation and the Skip Viragh Pancreatic Cancer Center at Johns Hopkins (E.M. Jaffee, L. Zheng), the NCI SPORE in Gastrointestinal Cancers P50 CA062924 (E.M. Jaffee, L. Zheng) and a Lustgarten Foundation (L. Zheng) grant.
We thank Dr. Goggins for providing us with the hCAF cells.
Footnotes
Disclosure of Potential Conflict of Interests: No relevant conflicts of interest to disclose.
References
- 1.Maitra A, Hruban RH. Pancreatic cancer. Annual review of pathology. 2008;3:157–88. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rucki AA, Zheng L. Pancreatic cancer stroma: understanding biology leads to new therapeutic strategies. World journal of gastroenterology : WJG. 2014;20(9):2237–46. doi: 10.3748/wjg.v20.i9.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu GC, Kimmelman AC, Hezel AF, DePinho RA. Stromal biology of pancreatic cancer. Journal of cellular biochemistry. 2007;101(4):887–907. doi: 10.1002/jcb.21209. [DOI] [PubMed] [Google Scholar]
- 4.Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425(6960):846–51. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 5.Shinozaki S, Ohnishi H, Hama K, Kita H, Yamamoto H, Osawa H, et al. Indian hedgehog promotes the migration of rat activated pancreatic stellate cells by increasing membrane type-1 matrix metalloproteinase on the plasma membrane. Journal of cellular physiology. 2008;216(1):38–46. doi: 10.1002/jcp.21372. [DOI] [PubMed] [Google Scholar]
- 6.Feldmann G, Dhara S, Fendrich V, Bedja D, Beaty R, Mullendore M, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer research. 2007;67(5):2187–96. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian H, Callahan CA, DuPree KJ, Darbonne WC, Ahn CP, Scales SJ, et al. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(11):4254–9. doi: 10.1073/pnas.0813203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends in pharmacological sciences. 2009;30(6):303–12. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Tape CJ, Ling S, Dimitriadi M, McMahon KM, Worboys JD, Leong HS, et al. Oncogenic KRAS Regulates Tumor Cell Signaling via Stromal Reciprocation. Cell. 2016;165(4):910–20. doi: 10.1016/j.cell.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25(6):719–34. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25(6):735–47. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng L, Foley K, Huang L, Leubner A, Mo G, Olino K, et al. Tyrosine 23 phosphorylation-dependent cell-surface localization of annexin A2 is required for invasion and metastases of pancreatic cancer. PloS one. 2011;6(4):e19390. doi: 10.1371/journal.pone.0019390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foley K, Rucki AA, Xiao Q, Zhou D, Leubner A, Mo G, et al. Semaphorin 3D autocrine signaling mediates the metastatic role of annexin A2 in pancreatic cancer. Science signaling. 2015;8(388):ra77. doi: 10.1126/scisignal.aaa5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao WQ, Chen GH, Chen H, Pascale A, Ravindranath L, Quon MJ, et al. Secretion of Annexin II via activation of insulin receptor and insulin-like growth factor receptor. The Journal of biological chemistry. 2003;278(6):4205–15. doi: 10.1074/jbc.M210545200. [DOI] [PubMed] [Google Scholar]
- 15.Isacke CM, Trowbridge IS, Hunter T. Modulation of p36 phosphorylation in human cells: studies using anti-p36 monoclonal antibodies. Molecular and cellular biology. 1986;6(7):2745–51. doi: 10.1128/mcb.6.7.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellagamba C, Hubaishy I, Bjorge JD, Fitzpatrick SL, Fujita DJ, Waisman DM. Tyrosine phosphorylation of annexin II tetramer is stimulated by membrane binding. The Journal of biological chemistry. 1997;272(6):3195–9. doi: 10.1074/jbc.272.6.3195. [DOI] [PubMed] [Google Scholar]
- 17.de Graauw M, Tijdens I, Smeets MB, Hensbergen PJ, Deelder AM, van de Water B. Annexin A2 phosphorylation mediates cell scattering and branching morphogenesis via cofilin Activation. Molecular and cellular biology. 2008;28(3):1029–40. doi: 10.1128/MCB.01247-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel MB, Pothula SP, Xu Z, Lee AK, Goldstein D, Pirola RC, et al. The role of the hepatocyte growth factor/c-MET pathway in pancreatic stellate cell-endothelial cell interactions: antiangiogenic implications in pancreatic cancer. Carcinogenesis. 2014;35(8):1891–900. doi: 10.1093/carcin/bgu122. [DOI] [PubMed] [Google Scholar]
- 19.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science (New York, NY) 2008;321(5897):1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delitto D, Vertes-George E, Hughes SJ, Behrns KE, Trevino JG. c-Met signaling in the development of tumorigenesis and chemoresistance: potential applications in pancreatic cancer. World journal of gastroenterology : WJG. 2014;20(26):8458–70. doi: 10.3748/wjg.v20.i26.8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergmann U, Funatomi H, Yokoyama M, Beger HG, Korc M. Insulin-like growth factor I overexpression in human pancreatic cancer: evidence for autocrine and paracrine roles. Cancer research. 1995;55(10):2007–11. [PubMed] [Google Scholar]
- 22.Ide T, Kitajima Y, Miyoshi A, Ohtsuka T, Mitsuno M, Ohtaka K, et al. Tumor-stromal cell interaction under hypoxia increases the invasiveness of pancreatic cancer cells through the hepatocyte growth factor/c-Met pathway. International journal of cancer Journal international du cancer. 2006;119(12):2750–9. doi: 10.1002/ijc.22178. [DOI] [PubMed] [Google Scholar]
- 23.Jaffee EM, Schutte M, Gossett J, Morsberger LA, Adler AJ, Thomas M, et al. Development and characterization of a cytokine-secreting pancreatic adenocarcinoma vaccine from primary tumors for use in clinical trials. Cancer J Sci Am. 1998;4(3):194–203. [PubMed] [Google Scholar]
- 24.Walter K, Omura N, Hong SM, Griffith M, Goggins M. Pancreatic cancer associated fibroblasts display normal allelotypes. Cancer biology & therapy. 2008;7(6):882–8. doi: 10.4161/cbt.7.6.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corbett TH, Roberts BJ, Leopold WR, Peckham JC, Wilkoff LJ, Griswold DP, Jr, et al. Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer research. 1984;44(2):717–26. [PubMed] [Google Scholar]
- 26.Deora AB, Kreitzer G, Jacovina AT, Hajjar KA. An annexin 2 phosphorylation switch mediates p11-dependent translocation of annexin 2 to the cell surface. The Journal of biological chemistry. 2004;279(42):43411–8. doi: 10.1074/jbc.M408078200. [DOI] [PubMed] [Google Scholar]
- 27.Bever KM, Sugar EA, Bigelow E, Sharma R, Laheru D, Wolfgang CL, et al. The prognostic value of stroma in pancreatic cancer in patients receiving adjuvant therapy. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2015;17(4):292–8. doi: 10.1111/hpb.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia P, Shoelson SE, Drew JS, Miller WT. Phosphopeptide occupancy and photoaffinity cross-linking of the v-Src SH2 domain attenuates tyrosine kinase activity. The Journal of biological chemistry. 1994;269(48):30574–9. [PubMed] [Google Scholar]
- 29.Spijkers-Hagelstein JA, Mimoso Pinhancos S, Schneider P, Pieters R, Stam RW. Src kinase-induced phosphorylation of annexin A2 mediates glucocorticoid resistance in MLL-rearranged infant acute lymphoblastic leukemia. Leukemia. 2013;27(5):1063–71. doi: 10.1038/leu.2012.372. [DOI] [PubMed] [Google Scholar]
- 30.Wang YQ, Zhang F, Tian R, Ji W, Zhou Y, Sun XM, et al. Tyrosine 23 Phosphorylation of Annexin A2 Promotes Proliferation, Invasion, and Stat3 Phosphorylation in the Nucleus of Human Breast Cancer SK-BR-3 Cells. Cancer biology & medicine. 2012;9(4):248–53. doi: 10.7497/j.issn.2095-3941.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang Y, Chan JL, Zong CS, Wang LH. Effect of tyrosine mutations on the kinase activity and transforming potential of an oncogenic human insulin-like growth factor I receptor. The Journal of biological chemistry. 1996;271(1):160–7. doi: 10.1074/jbc.271.1.160. [DOI] [PubMed] [Google Scholar]
- 32.Butler AA, Yakar S, Gewolb IH, Karas M, Okubo Y, LeRoith D. Insulin-like growth factor-I receptor signal transduction: at the interface between physiology and cell biology. Comparative biochemistry and physiology Part B, Biochemistry & molecular biology. 1998;121(1):19–26. doi: 10.1016/s0305-0491(98)10106-2. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Echeverria C, Pearson MA, Marti A, Meyer T, Mestan J, Zimmermann J, et al. In vivo antitumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell. 2004;5(3):231–9. doi: 10.1016/s1535-6108(04)00051-0. [DOI] [PubMed] [Google Scholar]
- 34.Steinberg M. Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia. Clinical therapeutics. 2007;29(11):2289–308. doi: 10.1016/j.clinthera.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7(5):469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 36.Caceres-Cortes JR. A potent anti-carcinoma and anti-acute myeloblastic leukemia agent, AG490. Anti-cancer agents in medicinal chemistry. 2008;8(7):717–22. doi: 10.2174/187152008785914752. [DOI] [PubMed] [Google Scholar]
- 37.Pan S, Wu X, Jiang J, Gao W, Wan Y, Cheng D, et al. Discovery of NVP-LDE225, a Potent and Selective Smoothened Antagonist. ACS medicinal chemistry letters. 2010;1(3):130–4. doi: 10.1021/ml1000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kochhar KS, Johnson ME, Volpert O, Iyer AP. Evidence for autocrine basis of transformation in NIH-3T3 cells transfected with met/HGF receptor gene. Growth factors (Chur, Switzerland) 1995;12(4):303–13. doi: 10.3109/08977199509028968. [DOI] [PubMed] [Google Scholar]
- 39.Johnson M, Koukoulis G, Kochhar K, Kubo C, Nakamura T, Iyer A. Selective tumorigenesis in non-parenchymal liver epithelial cell lines by hepatocyte growth factor transfection. Cancer letters. 1995;96(1):37–48. doi: 10.1016/0304-3835(95)03915-j. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Wang Q, Yang G, Marando C, Koblish HK, Hall LM, et al. A novel kinase inhibitor, INCB28060, blocks c-MET-dependent signaling, neoplastic activities, and cross-talk with EGFR and HER-3. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(22):7127–38. doi: 10.1158/1078-0432.CCR-11-1157. [DOI] [PubMed] [Google Scholar]
- 41.Aebersold R, Burlingame AL, Bradshaw RA. Western blots versus selected reaction monitoring assays: time to turn the tables? Molecular & cellular proteomics : MCP. 2013;12(9):2381–2. doi: 10.1074/mcp.E113.031658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer biology & therapy. 2006;5(12):1640–6. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- 43.Leopold PL, Vincent J, Wang H. A comparison of epithelial-to-mesenchymal transition and re-epithelialization. Seminars in cancer biology. 2012;22(5–6):471–83. doi: 10.1016/j.semcancer.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fagerli UM, Holt RU, Holien T, Vaatsveen TK, Zhan F, Egeberg KW, et al. Overexpression and involvement in migration by the metastasis-associated phosphatase PRL-3 in human myeloma cells. Blood. 2008;111(2):806–15. doi: 10.1182/blood-2007-07-101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gkika D, Prevarskaya N. Molecular mechanisms of TRP regulation in tumor growth and metastasis. Biochimica et biophysica acta. 2009;1793(6):953–8. doi: 10.1016/j.bbamcr.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 46.Tarnowski M, Schneider G, Amann G, Clark G, Houghton P, Barr FG, et al. RasGRF1 regulates proliferation and metastatic behavior of human alveolar rhabdomyosarcomas. International journal of oncology. 2012;41(3):995–1004. doi: 10.3892/ijo.2012.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng L, Jaffee EM. Annexin A2 is a new antigenic target for pancreatic cancer immunotherapy. Oncoimmunology. 2012;1(1):112–14. doi: 10.4161/onci.1.1.18017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ignar-Trowbridge DM, Nelson KG, Bidwell MC, Curtis SW, Washburn TF, McLachlan JA, et al. Coupling of dual signaling pathways: epidermal growth factor action involves the estrogen receptor. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(10):4658–62. doi: 10.1073/pnas.89.10.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Umata T, Hirata M, Takahashi T, Ryu F, Shida S, Takahashi Y, et al. A dual signaling cascade that regulates the ectodomain shedding of heparin-binding epidermal growth factor-like growth factor. The Journal of biological chemistry. 2001;276(32):30475–82. doi: 10.1074/jbc.M103673200. [DOI] [PubMed] [Google Scholar]
- 50.Che P, Yang Y, Han X, Hu M, Sellers JC, Londono-Joshi AI, et al. S100A4 promotes pancreatic cancer progression through a dual signaling pathway mediated by Src and focal adhesion kinase. Scientific reports. 2015;5:8453. doi: 10.1038/srep08453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xue Y, Ren J, Gao X, Jin C, Wen L, Yao X. GPS 2. 0, a tool to predict kinase-specific phosphorylation sites in hierarchy. Molecular & cellular proteomics : MCP. 2008;7(9):1598–608. doi: 10.1074/mcp.M700574-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487(7408):500–4. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kundranda M, Kachaamy T. Promising new therapies in advanced pancreatic adenocarcinomas. Future oncology (London, England) 2014;10(16):2629–41. doi: 10.2217/fon.14.197. [DOI] [PubMed] [Google Scholar]
- 54.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science (New York, NY) 2009;324(5933):1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer cell. 2012;21(3):418–29. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.