Abstract
Proteins are the major constituents of muscle and are key molecules regulating the metabolic changes during conversion of muscle to meat. Brazil is one of the largest exporters of beef and most Brazilian cattle are composed by zebu (Nellore) genotype. Bos indicus beef is generally leaner and tougher than Bos taurus such as Angus. The aim of this study was to compare the muscle proteomic and phosphoproteomic profile of Angus and Nellore. Seven animals of each breed previously subjected the same growth management were confined for 84 days. Proteins were extracted from Longissimus lumborum samples collected immediately after slaughter and separated by two-dimensional electrophoresis. Pro-Q Diamond stain was used in phosphoproteomics. Proteins identification was performed using matrix assisted laser desorption/ionization time-of-flight mass spectrometry. Tropomyosin alpha-1 chain, troponin-T, myosin light chain-1 fragment, cytoplasmic malate dehydrogenase, alpha-enolase and 78 kDa glucose-regulated protein were more abundant in Nellore, while myosin light chain 3, prohibitin, mitochondrial stress-70 protein and heat shock 70 kDa protein 6 were more abundant in Angus (P<0.05). Nellore had higher phosphorylation of myosin regulatory light chain-2, alpha actin-1, triosephosphate isomerase and 14-3-3 protein epsilon. However, Angus had greater phosphorylation of phosphoglucomutase-1 and troponin-T (P<0.05). Therefore, proteins involved in contraction and muscle organization, myofilaments expressed in fast or slow-twitch fibers and heat shock proteins localized in mitochondria or sarcoplasmic reticulum and involved in cell flux of calcium and apoptosis might be associated with differences in beef quality between Angus and Nellore. Furthermore, prohibitin appears to be a potential biomarker of intramuscular fat in cattle. Additionally, differences in phosphorylation of myofilaments and glycolytic enzymes could be involved with differences in muscle contraction force, susceptibility to calpain, apoptosis and postmortem glycolysis, which might also be related to differences in beef quality among Angus and Nellore.
Introduction
Brazil is the second largest producer and one of the largest beef exporters in the world [1]. The majority of the Brazilian herd is composed of Zebu cattle (Bos taurus indicus), mainly the Nellore breed. Zebu beef is usually less tender and has less marbling than that of taurine cattle (Bos taurus taurus), particularly the Angus breed [2, 3]. This reduces attractiveness of zebu beef, because tenderness and marbling are considered the main palatability characteristics by consumers [4].
There is an increasing number of studies aiming to understand the molecular mechanisms related to the differences in beef quality between zebu and taurine cattle genotypes [5, 6]. These studies look for biomarkers that might be used in livestock breeding programs. Moreover, they may provide scientific support for the meat industry in the development of strategies to improve meat quality [7, 8, 9].
Proteomics has been widely used for identification of proteins related to meat quality features, because proteins are the major constituent of muscle tissue and also responsible for the regulation of metabolic routes involved in the conversion of muscle to meat [10, 11]. Furthermore, proteomics can be used to study post-translational modifications, which may modify structure and, consequently, protein activity. Phosphorylation stands out among the main post-translational modifications, and phosphoproteomics is a useful technique to study phosphorylated proteins. In muscle tissue, phosphorylation could modulate the interaction among myofilaments and the activity of metabolic enzymes [12, 13, 14].
Proteomic studies comparing fresh muscle or beef from cattle breeds with different beef quality merits were able to identify differentially abundant proteins related to beef sensory attributes [15, 16, 17]. However, proteomics and phosphoproteomics studies comparing muscle or beef of zebu and taurine have not been conducted. Thus, the aim of this study was to compare the muscle proteomic and phosphoproteomic profile of Angus and Nellore cattle.
Materials and Methods
Ethical approval
All animal procedures were approved by the Animal Care and Use Committee of the Universidade Federal de Lavras, Brazil, protocol number 048/12.
Animal handling, slaughter and muscle sampling
Seven Nellore (BW = 375 ± 13 kg) and seven Angus bulls (BW = 383 ± 16 kg), with approximately 20 months of age and previously subjected the same growth management under grazing were confined ad libitum for 84 days with a standard feedlot diet used in Brazil based on corn silage and a corn-soybean meal concentrate with a roughage to concentrate ratio of 30:70. The animals were confined in covered individual stalls with concrete floor and equipped with drinking and feeding troughs. Detailed information about the diet and its chemical composition were previously published [6].
The slaughter was preceded by cerebral concussion followed by exsanguination. There was no electrical stimulation of carcasses. Immediately after exsanguinations, samples were collected from the Longissimus lumborum muscle between the 12th and 13th ribs, via incision through hide, and frozen in liquid nitrogen. Samples were then pulverized in liquid nitrogen and stored at -80°C until protein extraction.
Protein extraction and quantification
Approximately 100 mg of frozen muscle was added to a microtube containing 1 mL of extraction solution [(7 M) urea, (2 M) thiourea, (4% w/v) CHAPS, (1% w/v) dithiothreitol, (2% v/v) immobilized pH gradient (IPG) buffer, pH 4 to 7, (0.5 mM) benzamidine hydrochloride hydrate and (0.5 mM) phenylmethanesulfonyl fluoride]. Muscle sample and extraction solution were homogenized using LabGEN 125 Homogenizer (Cole-Parmer, Bunker Hill, IL, USA) at 9,500 rpm, twice for 15 seconds, with an interval of 30 seconds on ice. Subsequently, the homogenate was centrifuged at 20,200 g at 4°C for 30 minutes. The supernatant was collected and frozen at -80°C. Protein quantification was performed using the Bradford Protein Assay (BioRad, Hercules, CA, USA).
Two-dimensional electrophoresis
The first dimension or isoelectric focusing (IEF) was performed in 24 cm pH 4–7 IPG strips (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Initially, the strips were rehydrated for 16 hours in 450 μL rehydration solution (extraction solution containing 1,200 μg of protein, DeStreak Rehydration Solution (GE Healthcare Bio-Sciences), and 2% pH 4–7 IPG-buffer). The IEF was performed using Ettan IPGphor III System (GE Healthcare Bio-Sciences) at 20°C through the following program: step and hold until 200 V (2 h), step and hold until 500 V (1 h), gradient mode at 1,000 V (800 V/h), gradient mode at 10,000 V (16,500 V/h), and step and hold until 10,000 V (27,500 V/h). The current limit was 50 μA per strip.
For the second dimension, the strips were initially equilibrated in two successive steps of 20 minutes each, first in 5 mL of equilibration solution (6 M urea, 30% glycerol, 2% SDS, 0.002% bromophenol blue and 50 mM Tris- HCl pH 8.8) containing 1% DTT (reduction step), and then, in 5 mL of equilibration solution containing 2.5% iodoacetamide (alkylating step). Subsequently, the strips were placed on top of 1 mm thick 12.5% sodium dodecyl sulfate polyacrylamide gels and the proteins were separated using Ethan DALTsix (GE Healthcare Bio-Sciences) at 8°C. Electrophoresis was performed with 20 mA per gel for 40 minutes, followed by 40 mA per gel until the end of the run. The Low Molecular Weight Calibration Kit was used (GE Healthcare Bio-Sciences). Seven gels for each breed were made, one for each animal.
Gel-staining and image analysis
After two-dimensional electrophoresis (2DE), the gels were immersed in fixing solution [10% acetic acid (v/v) and 50% methanol (v/v)] for 12 hours under constant shaking. Subsequently, the gels were stained with the specific fluorescent dye for phosphoproteins Pro-Q Diamond (Invitrogen Molecular Probes, Eugene, OR, USA). All procedures for phosphoproteins staining followed the optimized method described in previously suggested protocol [18]. Images of the gels with phosphoproteins were obtained using Fuji Film 5100 FLA Fluorescence Imaging System Scanner (Fuji Medical Systems, Hanover Park, IL, USA) in fluorescent scanning mode, resolution of 300 dpi, excitation filter of 532 nm and emission filter of 580 nm.
Immediately after obtaining the images of phosphorylated proteins, the gels were stained for total protein with a solution containing 8% ammonium sulfate (w/v), 0.8% phosphoric acid (v/v), 0.08% coomassie blue G-250 (v/v) and 20% methanol (v/v) [19] for 72 h and then washed with 1% acetic acid (v/v) until complete removal of excess dye. Gels were scanned using ImageScanner III (GE Healthcare Bio-Sciences) at 300 dpi and subsequently stored in 2% acetic acid (v/v) at 20°C until extraction and spots digestion.
Spots detection and quantification were performed with Image Master 2D Platinum version 7.0 software (GE Healthcare Bio-Sciences). The volume of each spot (optical density x area) was normalized to the total volume of spots detected on each gel for comparison between breeds. Differences were considered significant when P-value was lower than 5% by ANOVA. The comparison between breeds for each spot made by Image Master was confirmed manually. Due to the high background, the phosphoprotein image of one Nellore was not used for comparisons.
Spots digestion and protein identification
Images of the gels stained with Pro-Q Diamond and coomassie blue G-250 were overlaid using Adobe Photoshop CC 2015.0 (Adobe Systems, San José, CA, USA) to facilitate excision of differentially abundant spots in the phosphoproteomics analysis (S1 Fig). The spots were excised manually and subjected to trypsinization [20].
Peptide mass spectra (MS and MS/MS) was obtained using matrix assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF/TOF). A matrix α-cyano-4-hydroxycinnamic was used. MALDI analysis was performed using Ultraflex III MALDI-TOF/TOF system (Bruker Daltonics, Bremen, Germany). The MS analyzes were performed with reflective positive peptide method, while the MS/MS analyses were performed using the LIFT positive method.
Protein identification was made using the MASCOT version 2.2 software (Matrix Science, Boston, MA, USA) at the MS/MS ion search mode, with the following parameters: tryptic specificity, one missed cleavage and a mass measurement tolerance of 0.2 Da for MS and 0.5 Da for MS/MS mode. Cysteine carbamidomethylation was used as fixed modification, while methionine oxidation was used as variable modification. The database used was the Bovidae deposited in UniProt. The proteins identified in MASCOT were validated by SCAFFOLD version 3.6.4 software (Proteome Software, Portland, OR, USA). The criteria used for the validation was of at least one peptide, with a probability score greater than or equal to 90% for both peptides and proteins.
Protein-protein interaction network
Differentially abundant proteins and phosphoproteins between Angus and Nellore muscle were loaded together in the String 10.0 bioinformatics software (available online: http://string-db.org/) to generate protein-protein interaction networks between the proteins identified in our study and between them and other proteins not identified here. Access numbers for each protein generated by UniProt were loaded into the software, which was set to search the Bos taurus database. The minimum required interaction score was set to 0.900 (highest confidence) and no more than 20 interactions were allowed in the first shell.
Results and Discussion
A total of 423 matches ID were detected in the analysis of total protein and 1,093 in the analysis of phosphorylated proteins, of which 38 and 55 differed (P<0.05) between breeds, respectively. Excision of differentially abundant spots was only performed for clearly visible and separable spots on gels (36 in the proteomic analysis and 23 in the phosphoproteomic study). Due to the 2DE/MS limitations for identification of low abundance proteins, it was not possible to identify all spots that were excised.
Differentially abundant proteins
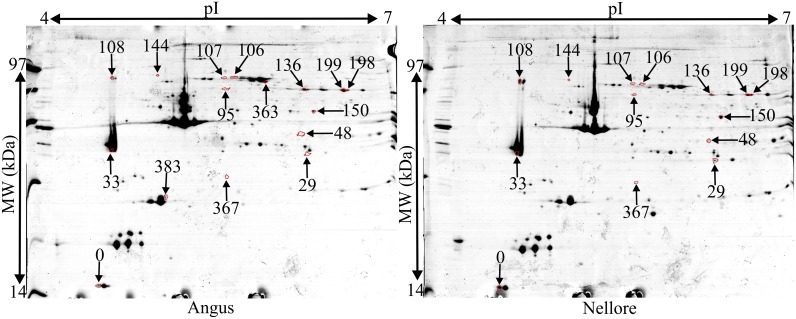
The proteomic analysis identified sixteen differentially abundant spots (P<0.05). Seven spots were more abundant in Angus and nine were more abundant in Nellore (Fig 1 and Table 1). Nellore had greater abundance of tropomyosin alpha-1 chain (TPM1, two spots), troponin T (TNNT3), myosin light chain 1 fragment (MYL1), cytoplasmic malate dehydrogenase (MDH1) and alpha-enolase (ENO1). However, Angus had greater abundance of prohibitin (PHB). Furthermore, a spot identified in Angus as myosin light chain 3 (MYL3) was not detected in Nellore (S2 Fig). Four spots were identified as phosphoglucomutase 1 (PGM1), two of them more abundant in Nellore and two in Angus. In addition, three proteins belonging to the heat shock proteins (HSPs) family were identified, two with greater abundance in Angus, mitochondrial stress-70 protein (HSPA9, two spots) and heat shock 70 kDa protein 6 (HSPA6), and one with greater abundance in Nellore, 78 kDa glucose-regulated protein (HSPA5). HSPA6 had confirmation with the realization of a blast in UniProt. Interestingly, HSPA6 was detected in only one of Nellore cattle (S3 Fig).
Fig 1. Differentially abundant proteins in the Longissimus lumborum muscle of Angus and Nellore bulls.
Numbers found in the figure correspond to the Match ID shown in Table 1.
Table 1. Differentially abundant proteins in the Longissimus lumborum muscle of Angus and Nellore bulls.
| Match IDa | Protein ID | ABVb | UniProtaccession number | Mascotscorec | Foldchange | P-valued | % Protein identification probabilitye | Identified peptidesf | % Proteincoverageg | pI observed/expected | MW kDa observed/expected |
|---|---|---|---|---|---|---|---|---|---|---|---|
| More abundant in Angus | |||||||||||
| 383 | Myosin light chain 3 | MYL3 | P85100 | 188 | NDh in Nellore | 0.0007 | 100 | 2 | 13 | 5.12/5.00 | 25.7/22.1 |
| 136 | Phosphoglucomutase-1 | PGM1 | Q08DP0 | 233 | 2.83 | 0.0031 | 100 | 3 | 8 | 6.30/6.36 | 63.1/61.8 |
| 199 | Phosphoglucomutase-1 | PGM1 | Q08DP0 | 933 | 2 | 0.0079 | 100 | 11 | 24 | 6.64/6.36 | 62.6/61.8 |
| 106 | Stress-70 protein, mitochondrial | HSPA9 | Q3ZCH0 | 86 | 1.64 | 0.0111 | 99 | 1 | 2 | 5.72/5.97 | 75.8/74.0 |
| 107 | Stress-70 protein, mitochondrial | HSPA9 | Q3ZCH0 | 264 | 2.03 | 0.0183 | 100 | 3 | 6 | 5.63/5.97 | 75.9/74.0 |
| 363 | Uncharacterized protein | HSPA6 | F1MWU9 | 90 | 4.6 | 0.0349 | 99 | 1 | 2 | 5.98/5.74 | 68.3/71.4 |
| 367 | Prohibitin | PHB | Q3T165 | 255 | 2.15 | 0.0238 | 100 | 3 | 13 | 5.65/5.57 | 29.7/29.8 |
| More abundant in Nellore | |||||||||||
| 0 | Myosin light chain 1 (fragment) | MYL1 | Q08E10 | 209 | 1.89 | 0.0140 | 100 | 3 | 18 | 4.50/4.73 | 14.4/19.7 |
| 108 | Tropomyosin alpha-1 chain | TPM1 | Q5KR49 | 635 | 2.93 | 0.0197 | 100 | 7 | 25 | 4.66/4.69 | 75.3/32.7 |
| 33 | Tropomyosin alpha-1 chain | TPM1 | Q5KR49 | 337 | 1.72 | 0.0422 | 100 | 5 | 15 | 4.63/4.69 | 35.9/32.7 |
| 48 | Troponin T | TNNT3 | Q8MKI3 | 91 | 1.96 | 0.0084 | 99 | 1 | 5 | 6.30/5.99 | 40.3/32.1 |
| 29 | Malate dehydrogenase, cytoplasmic | MDH1 | Q3T145 | 57 | 2.06 | 0.0009 | 95 | 1 | 4 | 6.34/6.16 | 35.2/36.7 |
| 150 | Alpha-enolase | ENO1 | Q9XSJ4 | 861 | 2.04 | 0.0152 | 100 | 11 | 29 | 6.39/6.37 | 49.0/47.6 |
| 198 | Phosphoglucomutase-1 | PGM1 | Q08DP0 | 711 | 3.28 | 0.0079 | 100 | 8 | 19 | 6.67/6.36 | 63.4/61.8 |
| 95 | Phosphoglucomutase-1 | PGM1 | A6QPB5 | 157 | 1.92 | 0.0160 | 100 | 1 | 3 | 5.64/5.48 | 65.2/62.5 |
| 144 | 78 kDa glucose-regulated protein | HSPA5 | Q0VCX2 | 303 | 2 | 0.0131 | 100 | 4 | 7 | 5.07/5.07 | 78.7/72.4 |
a Match ID correspond to the numbers shown in Fig 1
b Protein abbreviation is in accordance with gene abbreviation in UniProt
c The Mascot baseline significant score is 31
d P-value obtained by ANOVA comparing spots abundance between Angus and Nellore muscle
e Probability for validation by Scaffold software of the proteins identified by Mascot
f Number of peptides identified in Mascot and validated by Scaffold. The sequences of the peptides are found in S1 Table
g Protein coverage calculated by Scaffold (identified amino acids / total amino acids)
h ND spot not detected
Differentially abundant phosphoproteins
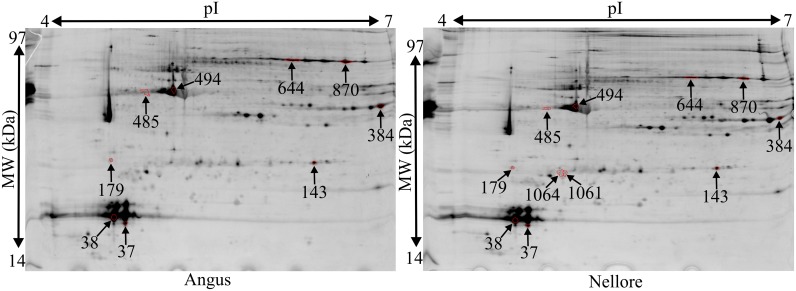
The phosphoproteomic approach identified eleven differentially phosphorylated spots (P<0.05), three more abundant in Angus and eight more abundant in Nellore (Fig 2 and Table 2). There were two spots of myosin light chain 1/3 (MYL1) that were detected only in Nellore (S4 Fig). Additionally, Nellore had higher phosphorylation of myosin regulatory light chain 2 (MYLPF, two spots), alpha actin 1 (ACTA1, two spots), triosephosphate isomerase (TPI1) and 14-3-3 protein epsilon (YWHAE). However, Angus had greater phosphorylation of PGM 1 (two spots) and TNNT3.
Fig 2. Differentially abundant phosphoproteins in the Longissimus lumborum muscle of Angus and Nellore bulls.
Numbers found in the figure correspond to the Match ID shown in Table 2.
Table 2. Differentially abundant phosphoproteins in the Longissimus lumborum muscle of Angus and Nellore bulls.
| Match IDa | Protein ID | ABVb | Uniprotaccession number | Mascotscorec | Foldchange | P-valued | % Protein identification probabilitye | Identified peptidesf | % Proteincoverageg | pI observed/expected | MW kDa observed/expected |
|---|---|---|---|---|---|---|---|---|---|---|---|
| More abundant in Angus | |||||||||||
| 384 | Troponin T | TNNT3 | Q8MKI4 | 103 | 3.56 | 0.0411 | 100 | 2 | 10 | 6.91/5.93 | 39.8/32.1 |
| 644 | Phosphoglucomutase-1 | PGM1 | Q08DP0 | 199 | 1.65 | 0.0065 | 100 | 3 | 8 | 6.19/6.36 | 64.9/61.6 |
| 870 | Phosphoglucomutase-1 | PGM1 | Q08DP0 | 377 | 1.95 | 0.0481 | 100 | 6 | 10 | 6.63/6.36 | 63.6/61.8 |
| More abundant in Nellore | |||||||||||
| 1061 | Myosin light chain 1/3 | MYL1 | A0JNJ5 | 412 | NDh in Angus | 0.0001 | 100 | 5 | 34 | 5.12/4.96 | 24.8/21.0 |
| 1064 | Myosin light chain 1/3 | MYL1 | A0JNJ5 | 80 | NDh in Angus | 0.0001 | 99 | 1 | 5 | 5.07/4.96 | 25.0/21.0 |
| 38 | Myosin regulatory light chain 2 | MYLPF | Q0P571 | 212 | 1.45 | 0.0063 | 100 | 3 | 24 | 4.69/4.91 | 18.1/19.0 |
| 37 | Myosin regulatory light chain 2 | MYLPF | Q0P571 | 113 | 1.26 | 0.0213 | 100 | 1 | 7 | 4.79/4.91 | 17.6/19.0 |
| 485 | Alpha actin 1 | ACTA1 | A4IFM8 | 465 | 1.82 | 0.0290 | 100 | 6 | 22 | 4.96/5.23 | 43.2/42.3 |
| 494 | Alpha actin 1 | ACTA1 | A4IFM8 | 213 | 1.29 | 0.0470 | 100 | 2 | 6 | 5.22/5.23 | 43.9/42.3 |
| 143 | Triosephosphate isomerase | TPI1 | Q5E956 | 550 | 1.31 | 0.0113 | 100 | 6 | 34 | 6.40/6.45 | 26.4/26.9 |
| 179 | 14-3-3 protein épsilon | YWHAE | P62261 | 219 | 1.25 | 0.0228 | 100 | 3 | 12 | 4.67/4.63 | 26.8/29.3 |
a Match ID correspond to the numbers shown in Fig 2
b Protein abbreviation is in accordance with gene abbreviation in UniProt
c The Mascot baseline significant score is 31
d P-value obtained by ANOVA comparing spots abundance between Angus and Nellore muscle
e Probability for validation by Scaffold software of the proteins identified by Mascot
f Number of peptides identified by Mascot and validated by Scaffold. The sequences of the peptides are found in S2 Table
g Protein coverage calculated by Scaffold (identified amino acids / total amino acids)
h ND spot not detected
Only one differentially phosphorylated spot was also differentially abundant in proteomics analysis. It was the spot 870 (PGM1), which was more abundant in Angus and corresponded to spots 198 and 199 (PGM1) in proteomics analysis, which were more abundant in Nellore and Angus, respectively. The other differentially abundant spots in phosphoproteomics study showed no significant difference in proteomic analysis among Angus and Nellore. Thus, the differences in phosphoproteins observed could be attributed to differences in phosphorylation level instead of amount in total protein.
Biological processes related to identified proteins and phosphoproteins and protein-protein interaction network
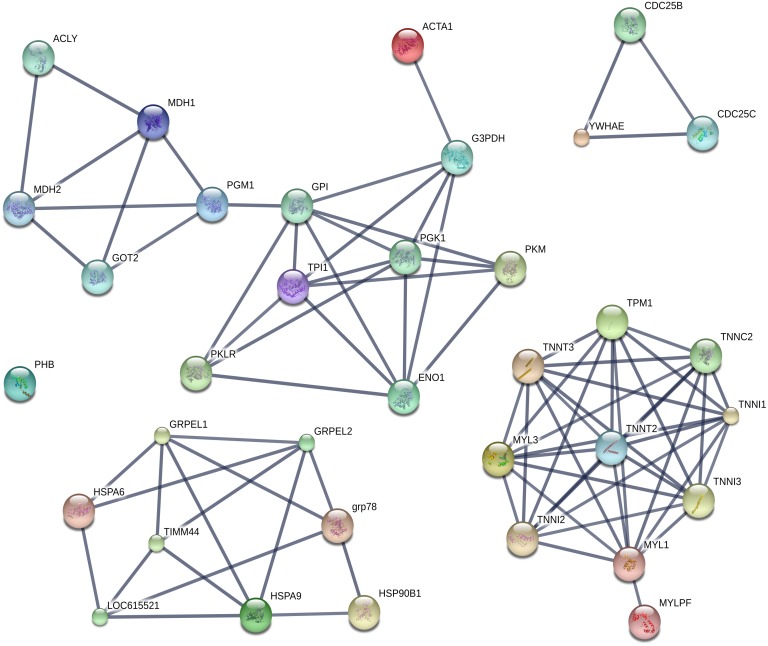
The main biological processes related to proteins and phosphoproteins identified in this study are summarized in Table 3. In addition, the protein-protein interaction map obtained through differentially abundant proteins and phosphoproteins between Angus and Nellore muscle using String 10.0 is shown in Fig 3. Proteins and phosphoproteins were loaded together in the analysis to obtain a more robust interaction network. Highly reliable relationships between the proteins identified in this study and among them and other unidentified proteins were obtained using this bioinformatics tool.
Table 3. Biological processes related to differentially abundant proteins and phosphoproteins in the Longissimus lumborum muscle of Angus and Nellore bulls.
| Protein ID | ABVa | Biological process described in UniProtb |
|---|---|---|
| 14-3-3 protein épsilon | YWHAE | Negative regulation of peptidyl-serine dephosphorylation; Protein targeting; Regulation of membrane repolarization; Regulation of potassium ion transmembrane transporter activity |
| Prohibitin | PHB | DNA biosynthetic process; Mitochondrion organization; Negative regulation of protein catabolic process; Negative regulation of transcription by competitive promoter binding; Protein stabilization |
| Stress-70 protein, mitochondrial | HSPA9 | Protein export from nucleus; Protein folding |
| Uncharacterized protein | HSPA6 | Cellular response to heat; Protein refolding |
| 78 kDa glucose-regulated protein | HSPA5 | Maintenance of protein localization in endoplasmic reticulum |
| Malate dehydrogenase, cytoplasmic | MDH1 | Carbohydrate metabolic process; Malate metabolic process; NADH metabolic process; Oxaloacetate metabolic process; Tricarboxylic acid cycle |
| Alpha-enolase | ENO1 | Glycolytic process |
| Phosphoglucomutase-1 | PGM1 | Glucose metabolic process; Glycogen biosynthetic process |
| Triosephosphate isomerase | TPI1 | Gluconeogenesis; Glyceraldehyde-3-phosphate biosynthetic process; Glycerol catabolic process; Glycolytic process; Pentose-phosphate shunt |
| Myosin light chain 3 | MYL3 | Regulation of striated muscle contraction |
| Myosin light chain 1/3 | MYL1 | Muscle contraction |
| Myosin regulatory light chain 2 | MYLPF | Skeletal muscle tissue development |
| Alpha actin 1 | ACTA1 | Positive regulation of gene expression; Skeletal muscle fiber development; Skeletal muscle thin filament assembly |
| Tropomyosin alpha-1 chain | TPM1 | Regulation of striated muscle contraction; |
| Troponin T | TNNT3 | Regulation of ATPase activity; Regulation of striated muscle contraction; Skeletal muscle contraction |
Fig 3. Protein—protein interaction analysis of differentially abundant proteins and phosphoproteins between Angus and Nellore muscle.
Data have been elaborated and graphed through String 10.0. ATP-citrate synthase (ACLY), Mitochondrial malate dehydrogenase (MDH2), Cytoplasmic malate dehydrogenase (MDH1), Mitochondrial aspartate aminotransferase (GOT2), Phosphoglucomutase-1 (PGM1), Glucose-6-phosphate isomerase (GPI), Triosephosphate isomerase (TPI1), Pyruvate kinase (PKLR), Alpha-enolase (ENO1), M1-type pyruvate kinase (PKM), Phosphoglycerate kinase 1 (PGK1), Glyceraldehyde-3-phosphate dehydrogenase (G3PDH), Alpha skeletal muscle actin (ACTA1), Uncharacterized protein (CDC25B), M-phase inducer phosphatase 3 (CDC25C), 14-3-3 protein epsilon (YWHAE), Myosin regulatory light chain 2 (MYLPF), Myosin light chain 1/3 (MYL1), Cardiac troponin I (TNNI3), Uncharacterized protein (TNNI1), Fast troponin C type 2 (TNNC2), Tropomyosin alpha-1 chain (TPM1), Troponin T fast skeletal muscle type (TNNT3), Myosin light chain 3 (MYL3), TNNI2 protein (TNNI2), Cardiac troponin T (TNNT2), Mitochondrial GrpE protein homolog 1 (GRPEL1), Mitochondrial GrpE protein homolog 2 (GRPEL2), 78 kDa glucose-regulated protein (GRP78), Endoplasmin (HSP90B1), Mitochondrial stress-70 protein (HSPA9), Mitochondrial import inner membrane translocase subunit TIM44 (TIMM44), LOC615521 protein (LOC615521), Uncharacterized protein (HSPA6).
Three major clusters were distinguished: Energy metabolism-related proteins (ACLY, MDH2, MDH1, GOT2, PGM1, GPI, TPI1, PKLR, ENO1, PKM, PGK1, G3PDH and ACTA1); proteins involved in the regulation of muscle contraction (MYLPF, MYL1, TNNI3, TNNI1, TNNC2, TPM1, TNNT3, MYL3, TNNI2 and TNNT2) and proteins related to protein folding, protein import into mitochondrial matrix and cellular stress response (GRPEL1, GRPEL2, GRP78, HSP90B1, HSPA9, TIMM44, LOC615521 and HSPA6). A small interaction network involving the phosphatases CDC25B and CDC25C and the adapter protein YWHAE was also obtained. PHB was the only protein identified in this study that did not show significant interactions. This result could be related to lack of information on PHB interactions in Bos taurus database and/or strict settings used in our analysis.
Differences related to meat quality
The Nellore and Angus cattle used in our study showed significant differences in the main characteristics related to meat quality, which were evaluated in parallel studies of our research group. Nellore had tougher beef as measured by Warner—Bratzler shear force (7.6 ± 0.33 vs. 6.9 ± 0.33 kgf) and lower myofibrillar fragmentation indices (MFI, 47.6 ± 4.28 vs. 69.2 ± 4.28%) [21]. Furthermore, Nellore had lower content of intramuscular fat (IMF, 2.7 ± 0.08 vs. 3.4 ± 0.08% ether extract). However, the content of total intramuscular collagen did not differ between Angus and Nellore [6].
Nellore had greater abundance of proteins involved in the regulation of muscle contraction, which are important substrates of proteolytic enzymes during the meat aging
TNNT3 [15] and TPM1 [22] have been more abundant in tough beef. These findings are in agreement with our results, because Nellore muscle showed greater abundance of TPM1 and TNNT3 compared to Angus. Tropomyosin (Tpm) and troponin T (TnT) are among the main substrates of the proteolytic enzymes related to meat tenderization [23], and TnT degradation during aging has been positively associated with beef tenderization [17, 24].
In addition, due to the role of TnT in the regulation of the complex that controls the interaction among actin and myosin filaments, it has been suggested that changes in the relation between them could occur in response to TnT degradation [25]. The degradation of TnT could also be related to the disruption of its interaction with other thin filaments and, consequently, with the breaking of thin filaments in the sarcomeric I-band, which might lead to fragmentation of myofibrils. This suggests that differences in the beef tenderness between Angus and Nellore could be partly related to differences in the muscle abundance of TNNT3 and TPM1, which are involved in regulating contraction and muscle structure organization [11].
Interestingly, the spot 108 that was identified as TPM1 had a molecular weight that was approximately twice the theoretical value (75.3 versus 32.7 kDa). Because TPM1 is a dimer of α-helices forming a coiled-coil [26], this result might indicate the presence of TPM1 dimer despite the denaturing conditions of electrophoresis. The presence of dimers in 2DE analysis has already been suggested [12].
Myofilaments expressed in fast or slow twitch fibers had different abundance between Angus and Nellore
MYL1 and MYL3 are the regulatory light chain of myosin. Nellore had increased abundance of MYL1, which is found in fast twitch fibers, while MYL3, which is found in slow twitch fibers, was only detected in Angus. Although we have not evaluated the proportion of muscle fiber types between breeds, the differences in the abundance of myosins expressed in fast or slow twitch fibers could suggest that muscle fiber type might have differed between Angus and Nellore. Likewise, TNNT3, an isoform also expressed in fast twitch fibers, was most abundant in Nellore. This is in line with a prior study, that observed lower proportion of fast twitch oxidative glycolytic fibers and higher of slow twitch oxidative fibers in the Longissimus dorsi muscle of crossbred Angus×Nellore compared to Nellore cattle [27]. In addition, Angus is considered to have a higher proportion of oxidative muscle fibers [28]. Muscle fiber type has been associated with meat quality [29]. However, more studies are needed to assess whether muscle fiber type is related to differences in beef quality between Angus and Nellore.
Similar to what was observed in our study, crossbred Angus×Holstein Friesian had lower abundance of MYL1 in Longissimus lumborum muscle than crossbred Belgian Blue×Holstein Friesian cattle, which were characterized by later body maturity and leaner carcasses [16]. In addition, Large White pigs (leaner carcass) had greater MYL1 abundance than Casertana pigs (fatter carcass) [30], and pigs with higher IMF content had lower abundance of fast twitch myofilaments and greater abundance of slow twitch myofilaments [31]. Furthermore, our results were also consistent with those obtained in a similar study, in which Podolian cattle had higher abundance of TnT and MYL1, tougher beef and lower MFI and IMF content than crossbreed Romagnola×Podolian and Friesian cattle [17]. Likewise, MYL1 was more abundant in Chianina cattle classified as tough beef [13].
Differences in abundance of enzymes involved in muscle energy metabolism between Angus and Nellore
Nellore had greater ENO1 abundance, a glycolytic enzyme that catalyzes the conversion of 2-phosphoglycerate to phosphoenolpyruvate. This result is in agreement with the higher abundance in Nellore of myofilaments expressed in fast twitch glycolytic fibers. Similarly, ENO1 was more abundant in steers compared to bulls and in Semitendinosus compared to Longissimus thoracis muscle, and this was consistent with the greater proportion of fast twitch glycolytic fibers reported for steers and Semitendinosus muscle [32].
Two spots identified as PGM1 were more abundant in Nellore, while two other spots, also identified as PGM1 were more abundant in Angus. PGM1 is involved in glycolysis and glycogenesis reversibly catalyzing the conversion of glucose 1-phosphate to glucose 6-phosphate. This could be related to metabolic differences in muscle tissue between breeds before slaughter. Glycogen synthesis and glucose degradation are both intense metabolic activities expected in muscles with a greater amount of fast twitch myofilaments [33], as it was observed in Nellore. Likewise, as the catalysis of glucose is the major metabolic pathway for the supply of carbon and reduced cofactor to the synthesis of intramuscular fat [16, 30, 34], it was also expected great abundance of PGM1 in Angus, because they had higher IMF content. Similarly, cattle with greater genomic estimated breeding value for IMF had higher expression of PGM1 [35].
Furthermore, Nellore had higher abundance of MDH1, an enzyme important in gluconeogenesis that catalyzes the oxidation of malate to oxaloacetate, which may then be used as precursor for glucose synthesis. This result is in line with what has already been discussed for ENO1 and PGM1.
The activity of enzymes involved in energy metabolism is of great importance for the meat quality characteristics, because the formation of actin-myosin complex and pH drop are the main changes responsible for the conversion of muscle to meat, and they are strongly influenced by postmortem energy metabolism [10, 25]. However, the relation between abundance of energy metabolism enzymes and meat tenderness has been a controversial topic [10, 15, 32]. This discrepancy could be related to differences in enzyme activity, that may be altered by post-translational modifications such as phosphorylation [7, 12, 13].
Angus and Nellore differed in the abundance of heat shock proteins located in the mitochondria or sarcoplasmic reticulum that are involved in Ca2+cellular traffic and apoptosis
Several studies have found a relationship between meat tenderness and HSPs abundance [22, 36, 37]. Due to the highly conserved chaperone capacity of proteins belonging to the HSPs family, many research groups have discussed the involvement of HSPs in the meat tenderizing process, without considering their singularities. In our study, four spots belonging to the HSPs family were identified, three more abundant in Angus and one in Nellore. Because HSPs have different cell locations, respond to different stimuli, and have different activities, we will discuss them separately to give greater biological significance to our results.
HSPA9 also known as mortalin and 75 kDa glucose-regulated protein (GRP-75) is the main mitochondrial HSP. It plays a key role in the translocation system that imports and exports protein across the mitochondrial membrane [38]. Despite belonging to the HSPs family, the expression of HSPA9 does not increase in response to heat stress. However, it is stimulated by glucose deprivation, Ca2+ influx and some cytotoxins [39]. Although HSPA9 is associated with anti-apoptotic processes due to its chaperone activity and inhibition of pro-apoptotic factors, it has also been proposed that under conditions of excessive stress, HSPA9 could not be able to prevent cell death and would change the mitochondrial functions leading to apoptosis [39].
HSPA9 abundance in Longissimus thoraci [40] and Semitendinosus [41] muscle immediately after slaughter had positive correlation with beef tenderness. These findings were in agreement with our result, as Angus had higher beef tenderness and greater HSPA9 abundance. This effect of HSPA9 on meat tenderness might be related to its anti-apoptotic effect, preventing the formation of protein aggregates, and might also be related to its role in triggering apoptosis. After slaughter and exsanguinations, muscle cells are subjected to various conditions of acute stress, such as interruption of the blood supply of glucose and oxygen, pH drop due to lactic acid accumulation, and increased Ca2+concentration in sarcoplasm and mitochondria [10]. All these conditions may induce apoptosis, which has been described as one of the main mechanism responsible for the meat tenderization through caspase proteolytic system [42].
Several mechanisms may involve HSPA9 in the triggering of apoptosis. For example, HSPA9 connects the inositol 1,4,5-trisphosphate receptor of the sarcoplasmic reticulum to the voltage-dependent anion channels of the mitochondria, facilitating the transfer of Ca2+ from the sarcoplasmic reticulum into the mitochondria. Overloading of Ca2+ in the mitochondria could lead to depolarization of the inner mitochondrial permeability transition pore and trigger apoptosis [43]. Additionally, the release of Ca2+ into the sarcoplasm leads to mitochondrial uptake of Ca2+ that cause conformational changes in the outer membrane-bound mitochondrial m-calpain large subunit, which leads to its binding to calpain small subunit and HSPA9. The formation of this complex allows the translocation of mitochondrial m-calpain from the outer membrane into the intermembrane space, where it is activated by a further increase of mitochondrial Ca2+ level triggering apoptosis [44].
Unlike what was observed for HSPA9, Nellore had greater abundance of HSPA5. This is the main HSP located in the sarcoplasmic reticulum, where is essential for the transport of newly synthesized polypeptides, for the folding and assembly of proteins, and for Ca2+ homeostasis [45]. HSPA5 has chaperone activity stimulated by its binding to Ca2+ and participates in the Ca2+ buffering in the sarcoplasmic reticulum. Ca2+ connected to HSPA5 corresponds to 25% of the Ca2+ reserves in the sarcoplasmic reticulum [46]. When Ca2+ reserves decrease or are depleted, there is a greater amount of unfolded proteins in the sarcoplasmic reticulum, which leads to increased expression of HSPA5 [47]. HSPA5 has been mainly related to inhibition of sarcoplasmic reticulum stress-related apoptosis [45]. To the best of our knowledge, HSPA5 has not been previously associated with meat tenderness differences.
The divergence in the abundance of HSPA9 and HSPA5 between Angus and Nellore was intriguing, as both proteins are related to cell flow of Ca2+. After slaughter, Ca2+ retained in the sarcoplasmic reticulum is released into the sarcoplasm stimulating the rigor mortis and the calpain activity, which is considered one of the main responsible for myofibrillar degradation and meat tenderization during aging [23, 25]. In addition, the output of Ca2+ from the sarcoplasmic reticulum to other cell compartments such as mitochondria triggers apoptosis [48].
A model has been proposed to explain how the Ca2+ flow could integrate the sarcoplasmic reticulum with the mitochondrial function [43]. In this model, HSPA5 is involved keeping Ca2+ within the sarcoplasmic reticulum, while HSPA9 is involved in the communication of sarcoplasmic reticulum and mitochondria that directs the Ca2+ flow from the former to the latter. Furthermore, it was suggested that the balance between the Ca2+ amount in the mitochondria and sarcoplasmic reticulum would be determinant to the decision between cell survival or death, wherein the Ca2+ overload within the mitochondria would direct to apoptosis. The massive Ca2+ influx into the matrix leads to mitochondria fission and accelerates the release of cytochrome c amplifying apoptosis via activation of caspases [8].
In this way, we could hypothesize that the greater abundance of HSPA5 in Nellore would delay, while the higher abundance of HSPA9 in Angus would accelerate apoptosis, rigor mortis, and beef tenderization. In agreement with this, HSPA9 level in muscle after slaughter had negative correlation with both pH at 3 hours and ultimate pH in the cattle carcass, and it was proposed that this result would be associated with an increased release of Ca2+ from the sarcoplasmic reticulum and, consequently, with higher enzyme activity and rigor mortis [49]. A positive relation found between μ-calpain and HSPA9 through correlation networks among protein biomarkers of beef tenderness also support our hypothesis [50]. Furthermore, it has been suggested that calcium-binding proteins, such as HSPA5, could contribute to the lower amount of free calcium after slaughter and, consequently, in reduced calpain activity [10].
Intriguingly, the calpastatin gene knockdown in bovine muscle satellite cells significantly increased the mRNA expression of μ-calpain, caspases and heat shock proteins, suggesting that they are involved in apoptosis during the calpastatin gene silencing [51]. Additionally, it has been reported that caspase-3, an effector enzyme of apoptosis, could inhibit the calpastatin activity, which is the calpain inhibitor [11]. The high calpastatin activity has been considered one of the main factors related to lower zebu beef tenderness compared to taurine [52]. Differences in the abundance of calpastatin, calpain and caspases were not detected in our study. This result may be attributed to limitations of 2DE to detect differences in low-abundance proteins [12]. However, in a parallel study with the same animals used here, there was a higher calpastatin activity in Nellore beef [21]. In view of this, we could suggest that the calpain/calpastatin proteolytic system and caspase-dependent apoptosis together would be related to differences in beef tenderness between Angus and Nellore. Furthermore, a greater susceptibility to caspase-dependent apoptosis would be related to lower calpastatin activity and greater MFI in Angus. However, more investigations are needed to evaluate this hypothesis.
As HSPA5 and HSPA9 are mainly located within the sarcoplasmic reticulum and mitochondria, respectively, the higher abundance of HSPA5 in Nellore and the greater abundance of HSPA9 in Angus would also be related to differences in muscle fiber types among them, since fast twitch glycolytic fibers have higher volume of sarcoplasmic reticulum, while slow twitch oxidative fibers have greater mitochondrial volume and abundance [53].
Another HSP that differed between Angus and Nellore was HSPA6, which was more abundant in Angus and was detected only in one of Nellore cattle. HSPA6 expression has been reported to be strictly stimulated by heat in fibroblasts [54]. In another study, HSPA6 expression was strongly induced by heat, but it had no significant effect on protection of HEK-293 cells against heat-induced cell death [55]. These findings are interesting because in a parallel study evaluating the same animals used in the current study, there was higher metabolic heat production and higher body temperatures in Angus [56].
Prohibitin seems to be a potential biomarker of intramuscular fat content in cattle
PHB are part of a group of proteins highly conserved and ubiquitously expressed in different cell tissues, being mainly located in the mitochondria, nucleus and plasma membrane [57]. Due to its location in several cellular compartments, translocation and interaction capacity with many transcription factors and proteins, PHB is involved in regulation of cell survival, apoptosis, metabolism and inflammation [58]. It may be upregulated under conditions of extreme stress and lead to apoptosis by modulating transcription factors and pro-apoptotic genes increasing caspases activity [59, 60].
In our study, PHB was more abundant in Angus. A greater abundance of PHB has already been described in bovine muscle classified as tender beef [61]. Furthermore, it was also observed by these authors higher abundance of other proteins of the inner and outer mitochondrial membranes, such as HSPA9, in the muscle of tender beef, which would be related to apoptosis. These findings support our suggestion that the difference in beef tenderness between Angus and Nellore would be partially explained by differences in apoptosis.
In addition to its relation with differences in meat tenderness, PHB could also be involved with differences in the IMF content between Angus and Nellore. It has been proposed that PHB would regulate adipocyte differentiation by modulating the insulin signaling pathway and mitochondrial biogenesis. Moreover, PHB also would regulate lipogenesis by modulating the pyruvate carboxylase and mitochondrial function [62]. PHB upregulation resulted in adipocyte hypertrophy associated with increase of white adipose tissue in mice [63]. Treatment of fibroblasts with insulin or peroxisome proliferator-activated receptor gamma (PPAR-γ) caused PHB upregulation and induced adipogenesis with increased expression of PPAR-γ [62]. These findings are interesting, because in a parallel study, there was greater abundance of PPAR-γ in Angus muscle [6]. In addition, higher PHB abundance in IMF compared to subcutaneous, perirenal, and intermuscular fat has been observed in pigs [64]. These findings together with our results suggest that differences in PHB abundance might partially explain the lower deposition of IMF in Nellore compared to Angus. Additionally, PHB could be considered as a potential biomarker of IMF in cattle.
Angus and Nellore differed in phosphorylation of myofilaments, which is related to affect muscle contraction strength and susceptibility to calpain and apoptosis
Nellore presented higher phosphorylation of MYLPF, MYL1 and ACTA1, while Angus had only increased TNNT3 phosphorylation. MYLPF phosphorylation might alter the structure and motor function of the myosin to increase the sensitivity of the contractile apparatus to Ca2+ [65]. Furthermore, MYLPF phosphorylation increased the contraction force in fast twitch skeletal muscle [66]. In addition, it has been suggested that phosphorylation of MYLPF might work as a kind of memory to enhance muscle contraction strength [67]. This hypothesis has been considered in an attempt to explain the relationship between tough meat and MYLPF phosphorylation [11]. These findings and hypotheses are consistent with our results, as Nellore had greater phosphorylation of MYLPF and tougher beef. A similar result was observed in a study with sheep, in which there was higher MYLPF phosphorylation in the group of animals classified as tough meat [14]. Moreover, greater phosphorylated MYLPF abundance has been reported in dark firm dry beef [68].
The phosphorylated MYLPF is expressed in fast twitch fibers. Since there was no difference in abundance of this protein in proteomic analysis, we suggest that the difference found would be related to differences in phosphorylation and or MYLPF dephosphorylation more than a possible difference in the fast and slow twitch fibers composition between Angus and Nellore. MYLPF is phosphorylated by Ca2+/calmodulin-dependent myosin light chain kinase and is dephosphorylated by protein phosphatase 1 [69]. Despite the involvement of Ca2+ in MYLPF phosphorylation mechanism, this process does not require high Ca2+ concentrations to occur. Other factors such as myosin light chain kinase:protein phosphatase-1 ratio appear to be important to affect the MYLPF phosphorylation [67]. Additionally, it was demonstrated that myosin light chain kinase rather than calmodulin is limiting to the phosphorylation of MYLPF [66].
Although Angus had lower TNNT3 abundance in proteomic study, they showed greater phosphorylation of another TNNT3 isoform. Phosphorylation of skeletal troponins increased their susceptibility to degradation by calpain possibly due to dissociation from the native complex [70]. In addition, it has been suggested that TnT would undergo cut-off in phosphorylated sites during post-rigor stage [71]. TnT is one of the main substrates for calpain and its degradation is related to the meat tenderization during aging [23]. Therefore, we could suggest that the greater phosphorylation of TNNT3 in Angus would partly explain its higher MFI and beef more tender compared to Nellore. Additionally, the lower TNNT3 phosphorylation in Nellore would be partially explained by greater TPM1 abundance observed in these animals, as was shown in proteomics analysis, because skeletal Tpm may inhibit the phosphorylation of skeletal TnT due to the strong interaction between them, reducing the exposure of TnT phosphorylation sites [72].
ACTA1 was other myofilament that had different phosphorylation level between Angus and Nellore. Likewise to what was observed in our study, higher level of ACTA1 phosphorylation was found in tough beef [13]. It has been suggested that phosphorylation of ACTA1 could prevent the onset of apoptosis and would be positively correlated with the meat toughness [11]. Our result and these findings give further support to our hypothesis that apoptosis would be involved in the difference of beef tenderness between Angus and Nellore.
Two enzymes involved in glucose metabolism had opposite levels of phosphorylation in Angus and Nellore
Angus and Nellore had greater abundance of PGM1 isoforms in the proteomics analysis, but the phosphoproteomics revealed higher PGM1 phosphorylation only in Angus. PGM1 is more active when phosphorylated due to a conformational change that exposes its active site in response to phosphorylation [73]. As previously discussed, this protein catalyzes reactions that drive glucose into glycolysis or glycogenesis. As muscle does not receive more nutrients after slaughter and as glycolysis becomes the major source of energy to the muscle cells, we could suggest that greater PGM1 phosphorylation would contribute to a faster glycolysis in Angus. Supporting this hypothesis, it was proposed that phosphorylation of PGM1 is related to faster rates of glycolysis and pH drop in postmortem muscle [74]. Furthermore, an increase in the phosphorylated PGM1 abundance was observed in cattle muscle from 0 to 1 day after slaughter, which would be related to an increase in glycogenolysis and glycolysis due to increased anaerobic postmortem muscle metabolism [75].
On the other hand, TPI1 phosphorylation was higher in Nellore. Similarly to our study, TPI1 was more phosphorylated in tough beef [12]. TPI1 is a glycolytic enzyme that catalyzes the reversible conversion of D-glyceraldehyde 3-phosphate from glycerone phosphate. The phosphorylation of TPI1 decreased its activity in HeLa cells [76]. In addition, it was reported a higher abundance of phosphorylated TPI1 in the pigs muscle with slow pH decline compared to fast pH decline group [77]. A moderate rate of pH decline in cattle muscle could be beneficial to meat tenderness due to the lower risk of cold shortening and influence on the activity of proteolytic enzymes [25].
YWHAE phosphorylation might also be involved with differences in force of muscle contraction and apoptosis between Angus and Nellore
YWHAE belongs to 14-3-3 protein family working as adapters in the regulation of several signaling pathways due to their abilities to bind to a large number of proteins. It has been suggested that phosphorylation of 14-3-3 proteins might result in dimer formation or dissociation, and it might also cause changes in their binding sites, which would modulate their interaction with target proteins [78]. Specifically, we did not find studies about the effect of phosphorylation on the regulation of YWHAE. Anyway, it has been well documented that YWHAE negatively regulates apoptosis [79]. In addition, it was proposed that 14-3-3 proteins might bind to phosphorylated myosin light chain kinase and this could influence its binding to myosin [80].
Because Nellore had greater phosphorylation of YWHAE and tougher beef, we could hypothesize that YWHAE phosphorylation would affect beef tenderness preventing apoptosis and enhancing the strength of muscle contraction. Additionally, this result might be involved with the difference in phosphorylation of MYLPF through its probable effect on the myosin light chain kinase activity. Other studies had already suggested the involvement of 14-3-3 proteins with the meat tenderness due to their likely involvement in apoptosis and muscle contraction force [15, 30].
Conclusions
We can conclude that differences in proteins involved with contraction and muscle organization, myofilaments expressed in fast or slow-twitch fibers and heat shock proteins localized in mitochondria or sarcoplasmic reticulum and involved in cell flux of calcium and apoptosis might be associated with differences in beef quality between Angus and Nellore. Furthermore, prohibitin appears to be a potential biomarker of intramuscular fat content in cattle. In addition, differences in phosphorylation of myofilaments and glycolytic enzymes could be involved in differences in muscle contraction force, susceptibility to calpain, apoptosis and postmortem glycolysis, which might also be related to differences in beef quality among Angus and Nellore.
This was the first proteomic and phosphoproteomic approach comparing taurine and zebu muscle and, among the new findings, we could highlight the possible importance of apoptosis for differences in beef tenderness between Angus and Nellore. Thus, we could suggest further studies to evaluate if a possible difference in apoptosis susceptibility among taurine and zebu muscle would be related to the difference in the calpain/calpastatin system, which is currently considered the main cause of difference in beef tenderness between them.
Supporting Information
The image obtained with Pro-Q Diamond was colored in blue and the image obtained with Coomassie blue G-250 was colored in red. The overlap of spots in the two images produced brown color.
(TIF)
Spots highlighted in the green square (match ID 383) and only detected in Angus muscle.
(TIF)
Spots highlighted in the green square (match ID 363) and only detected in the muscle of one of Nellore cattle.
(TIF)
Spots highlighted in the green square (match IDs 1064 and 1061) and only detected in Nellore muscle.
(TIF)
Sequence of the peptides identified in Mascot and validated by the Scaffold.
(DOCX)
Sequence of the peptides identified in Mascot and validated by the Scaffold.
(DOCX)
Acknowledgments
We thank the Núcleo de Análise de Biomoléculas (NUBIOMOL) and Laboratório de Biotecnologia Animal (LABTEC) of the Universidade Federal de Viçosa for providing the facilities.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the financial support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.United States Department of Agriculture. Livestock and poultry: world markets and trade [Internet]. USDA; [updated 2016 Apr; cited 2016 May 10]. http://apps.fas.usda.gov/psdonline/circulars/livestock_poultry.pdf
- 2.Lage JF, Paulino PVR, Valadares Filho SC, Souza EJO, Duarte MS, Benedeti PDB, et al. Influence of genetic type and level of concentrate in the finishing diet on carcass and meat quality traits in beef heifers. Meat Sci. 2012;90:770–74. 10.1016/j.meatsci.2011.11.012 [DOI] [PubMed] [Google Scholar]
- 3.Pereira ASC, Baldi F, Sainz RD, Utembergue BL, Chiaia HLJ, Magnabosco CU, et al. Growth performance, and carcass and meat quality traits in progeny of Poll Nellore, Angus and Brahman sires under tropical conditions. Anim Prod Sci. 2015;55:1295–02. [Google Scholar]
- 4.Font-i-Furnols M, Guerrero L. Consumer preference, behavior and perception about meat and meat products: An overview. Meat Sci. 2014;98:361–71. 10.1016/j.meatsci.2014.06.025 [DOI] [PubMed] [Google Scholar]
- 5.Giusti J, Castan E, Dal Pai M, Arrigoni MDB, Baldin SR, De Oliveira HN. Expression of genes related to quality of Longissimus dorsi muscle meat in Nellore (Bos indicus) and Canchim (5/8 Bos taurus× 3/8 Bos indicus) cattle. Meat Sci. 2013;94:247–52. 10.1016/j.meatsci.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 6.Martins TS, Sanglard LMP, Silva W, Chizzotti ML, Rennó LN, Serão NVL, et al. Molecular factors underlying the deposition of intramuscular fat and collagen in skeletal muscle of Nellore and Angus cattle. PLoS One. 2015;10(10):e0139943 10.1371/journal.pone.0139943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paredi G, Raboni S, Bendixen E, De Almeida AM, Mozzarelli A. “Muscle to meat” molecular events and technological transformations: the proteomics insight. J Proteomics. 2012;75:4275–89. 10.1016/j.jprot.2012.04.011 [DOI] [PubMed] [Google Scholar]
- 8.Ouali A, Gagaoua M, Boudida Y, Becila S, Boudjellal A, Herrera-Mendez CH, et al. Biomarkers of meat tenderness: present knowledge and perspectives in regards to our current understanding of the mechanisms involved. Meat Sci. 2013;95:854–70. 10.1016/j.meatsci.2013.05.010 [DOI] [PubMed] [Google Scholar]
- 9.Picard B, Lebret B, Cassar-Malek I, Liaubet L, Berri C, Le Bihan-Duval E, et al. Recent advances in omic technologies for meat quality management. Meat Sci. 2015;109:18–26. 10.1016/j.meatsci.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 10.D'Alessandro A, Zolla L. Meat science: From proteomics to integrated omics towards system biology. J Proteomics. 2013;78:558–77. 10.1016/j.jprot.2012.10.023 [DOI] [PubMed] [Google Scholar]
- 11.Lana A, Zolla L. Proteolysis in meat tenderization from the point of view of each single protein: a proteomic perspective. J Proteomics. 2016. May 10. Epub 2016 Feb 17. [DOI] [PubMed] [Google Scholar]
- 12.D'Alessandro A, Rinalducci S, Marrocco C, Zolla V, Napolitano F, Zolla L. Love me tender: An Omics window on the bovine meat tenderness network. J Proteomics. 2012a;75:4360–80. [DOI] [PubMed] [Google Scholar]
- 13.D'Alessandro A, Marrocco C, Rinalducci S, Mirasole C, Failla S, Zolla L. Chianina beef tenderness investigated through integrated Omics. J Proteomics. 2012b;75:4381–98. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Li X, Ni N, Liu Y, Chen L, Wang Z, et al. Phosphorylation of myofibrillar proteins in post-mortem ovine muscle with different tenderness. J Sci Food Agric. 2016;96:1474–83. 10.1002/jsfa.7244 [DOI] [PubMed] [Google Scholar]
- 15.Chaze T, Hocquette JF, Meunier B, Renand G, Jurie C, Chambon C, et al. Biological markers for meat tenderness of the three main French beef breeds using 2-DE and MS approach In: Toldra F, Nollet LML, editors. Proteomics in Foods. New York: Springer; 2013. p. 127–46. [Google Scholar]
- 16.Keady SM, Kenny DA, Ohlendieck K, Doyle S, Keane MG, Waters SM. Proteomic profiling of bovine M. longissimus lumborum from Crossbred Aberdeen Angus and Belgian Blue sired steers varying in genetic merit for carcass weight. J Anim Sci. 2013;91: 654–65. 10.2527/jas.2012-5850 [DOI] [PubMed] [Google Scholar]
- 17.Marino R, Albenzio M, Della Malva A, Santillo A, Loizzo P, Sevi A. Proteolytic pattern of myofibrillar protein and meat tenderness as affected by breed and aging time. Meat Sci. 2013;95:281–87. 10.1016/j.meatsci.2013.04.009 [DOI] [PubMed] [Google Scholar]
- 18.Agrawal GK, Thelen JJ. Development of a simplified, economical polyacrylamide gel staining protocol for phosphoproteins. Proteomics. 2005;5:4684–88. 10.1002/pmic.200500021 [DOI] [PubMed] [Google Scholar]
- 19.Neuhoff V, Stamm R, Eibl H. Clear background and highly sensitive protein staining with Coomassie Blue dyes in polyacrylamide gels: a systematic analysis. Electrophoresis. 1985;6:427–48. [Google Scholar]
- 20.Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nature. 2006;1:2856–60. [DOI] [PubMed] [Google Scholar]
- 21.Duarte MS, Martins TS, Paulino PVR, Chizotti ML, Ladeira MM, Filho RAT, et al. Calpastatin activity and beef tenderness of Nellore and Angus cattle fed two feeding strategies. Proceedings of the 59th International Congress of Meat Science and Technology; 2013 Aug 18–23; Izmir, Turkey. Izmir: Ege University, Food Engineering Development; 2013.
- 22.Carvalho ME, Gasparin G, Poleti MD, Rosa AF, Balieiro JCC, Labate CA, et al. Heat shock and structural proteins associated with meat tenderness in Nellore beef cattle, a Bos indicus breed. Meat Sci. 2014;96:1318–24. 10.1016/j.meatsci.2013.11.014 [DOI] [PubMed] [Google Scholar]
- 23.Kemp CM, Sensky PL, Bardsley RG, Buttery PJ, Parr T. Tenderness—An enzymatic view. Meat Sci. 2010;84:248–56. 10.1016/j.meatsci.2009.06.008 [DOI] [PubMed] [Google Scholar]
- 24.Cruzen SM, Paulino PVR, Lonergan SM, Huff-Lonergan E. Postmortem proteolysis in three muscles from growing and mature beef cattle. Meat Sci. 2014;96:854–61. 10.1016/j.meatsci.2013.09.021 [DOI] [PubMed] [Google Scholar]
- 25.Huff-Lonergan E, Zhang W, Lonergan SM. Biochemistry of postmortem muscle-Lessons on mechanisms of meat tenderization. Meat Sci. 2010;86:184–95. 10.1016/j.meatsci.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 26.Nevzorov IA, Levitsky DI. Tropomyosin: double helix from the protein world. Biochem. Mosc. 2011;76:1507–27. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira IM, Paulino PVR, Marcondes MI, Valadares Filho SC, Cavali J, Prados LF, et al. Beef quality traits of Nellore, F1 Simmental×Nellore and F1 Angus×Nellore steers fed at the maintenance level or ad libitum with two concentrate levels in the diet. R Bras Zootec. 2011;40:2894–02. [Google Scholar]
- 28.Cassar-Malek I, Picard B. Expression marker-based strategy to improve beef quality. Scientific World Journal, 2016. May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lefaucheur L. A second look into fibre typing—Relation to meat quality. Meat Sci. 2010;84:257–70. 10.1016/j.meatsci.2009.05.004 [DOI] [PubMed] [Google Scholar]
- 30.Murgiano L, D’Alessandro A, Egidi MG, Crisa A, Prosperini G, Timperio AM, et al. Proteomics and transcriptomics investigation on longissimus muscles in Large White and Casertana pig breeds. J Proteome Res. 2010;9:6450–66. 10.1021/pr100693h [DOI] [PubMed] [Google Scholar]
- 31.Ma X, Zheng C, Hu Y, Wang L, Yang X, Jiang Z. Dietary L-arginine supplementation affects the skeletal longissimus muscle proteome in finishing pigs. PLoS One. 2015;10(1):e0117294 10.1371/journal.pone.0117294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guillemin N, Jurie C, Cassar-Malek I, Hocquette JF, Renand G, Picard B. Variations in the abundance of 24 protein biomarkers of beef tenderness according to muscle and animal type. Animal. 2011a;5:885–94. [DOI] [PubMed] [Google Scholar]
- 33.Bouley J, Meunier B, Chambon C, De Smet S, Hocquette JF, Picard B. Proteomic analysis of bovine skeletal muscle hypertrophy. Proteomics. 2005;5:490–00. 10.1002/pmic.200400925 [DOI] [PubMed] [Google Scholar]
- 34.Smith SB, Crouse AD. Relative contributions of acetate, lactate, and glucose to lipogenesis in bovine intramuscular and subcutaneous adipose tissue. J Nutr. 1984;114:792–00. [DOI] [PubMed] [Google Scholar]
- 35.Cesar ASM, Regitano LCA, Koltes JE, Fritz-Waters ER, Lanna DPD, Gasparin G, et al. Putative regulatory factors associated with intramuscular fat content. PLoS One. 2015;10(6):e0128350 10.1371/journal.pone.0128350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim NK, Cho S, Lee SH, Park HR, Lee CS, Cho YM, et al. Proteins in longissimus muscle of Korean native cattle and their relationship to meat quality. Meat Sci. 2008;80:1068–73. 10.1016/j.meatsci.2008.04.027 [DOI] [PubMed] [Google Scholar]
- 37.Guillemin NP, Jurie C, Renand G, Hocquette JF, Micol D, Lepetit J, et al. Different phenotypic and proteomic markers explain variability of beef tenderness across muscles. International Journal of Biology. 2012;4:26–38. [Google Scholar]
- 38.Kaul SC, Deocaris CC, Wadhwa R. Three faces of mortalin: a housekeeper, guardian and killer. Exp Gerontol. 2007;42:263–74. 10.1016/j.exger.2006.10.020 [DOI] [PubMed] [Google Scholar]
- 39.Londono C, Osorio C, Gama V, Alzate O. Mortalin, apoptosis, and neurodegeneration. Biomolecules. 2012;2:143–64. 10.3390/biom2010143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guillemin N, Bonnet M, Jurie C, Picard B. Functional analysis of beef tenderness. J Proteomics. 2011b:75:352–65. [DOI] [PubMed] [Google Scholar]
- 41.Picard B, Gagaoua M, Micol D, Cassar-Malek I, Hocquette JF, Terlouw CEM. Inverse relationships between biomarkers and beef tenderness according to contractile and metabolic properties of the muscle. J Agric Food Chem. 2014;62:9808–18. 10.1021/jf501528s [DOI] [PubMed] [Google Scholar]
- 42.Ouali A, Herrera-Mendez CH, Coulis G, Becila S, Boudjellal A, Aubry L, et al. Revisiting the conversion of muscle into meat and the underlying mechanisms. Meat Sci. 2006:74:44–8. 10.1016/j.meatsci.2006.05.010 [DOI] [PubMed] [Google Scholar]
- 43.Kaufman RJ, Malhotra JD. Calcium trafficking integrates endoplasmic reticulum function with mitochondrial bioenergetics. Biochim Biophys Acta. 2014;1843:2233–39. 10.1016/j.bbamcr.2014.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ozaki T, Yamashita T, Ishiguro S. Ca2+-induced release of mitochondrial m-calpain from outer membrane with binding of calpain small subunit and Grp75. Arch Biochem Biophys. 2011;507:254–61. 10.1016/j.abb.2010.12.003 [DOI] [PubMed] [Google Scholar]
- 45.Wang M, Wey S, Zhang Y, Ye R, Lee AS. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal. 2009;11:2307–16. 10.1089/ARS.2009.2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lièvremont JP, Rizzuto R, Hendershot L, Meldolesi J. BiP, a major chaperone protein of the endoplasmic reticulum lumen, plays a direct and important role in the storage of the rapidly exchanging pool of Ca2+. J Biol Chem. 1997;272:30873–79. [DOI] [PubMed] [Google Scholar]
- 47.Coe H, Michalak M. Calcium binding chaperones of the endoplasmic reticulum. Gen Physiol Biophys. 2009;28:F96–F103. [PubMed] [Google Scholar]
- 48.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460–70. 10.1016/j.bbamcr.2013.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gagaoua M, Terlouw EMC, Micol D, Boudjellal A, Hocquette JF, Picard B. Understanding early post mortem biochemical processes underlying meat color and pH decline in the Longissimus thoracis muscle of young Blond D’Aquitaine bulls using protein biomarkers. J Agric Food Chem. 2015a;63:6799–09. [DOI] [PubMed] [Google Scholar]
- 50.Gagaoua M, Terlouw EMC, Boudjellal A, Picard B. Coherent correlation networks among protein biomarkers of beef tenderness: What they reveal. J Proteomics. 2015b;128:365–74. [DOI] [PubMed] [Google Scholar]
- 51.Van Ba H, Reddy BV, Hwang I. Role of calpastatin in the regulation of mRNA expression of calpain, caspase, and heat shock protein systems in bovine muscle satellite cells. In Vitro Cell Dev Biol Anim. 2015;51:447–54. 10.1007/s11626-014-9849-8 [DOI] [PubMed] [Google Scholar]
- 52.Whipple G, Koohmaraie M, Dikeman ME, Crouse JD, Hunt MC, Klemm RD. Evaluation of attributes that affect longissimus muscle tenderness in Bos taurus and Bos indicus cattle. J Anim Sci. 1990; 68:2716–28. [DOI] [PubMed] [Google Scholar]
- 53.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91:1447–31. 10.1152/physrev.00031.2010 [DOI] [PubMed] [Google Scholar]
- 54.Leung TKC, Rajendran MY, Monfries C, Hall C, Lim L. The human heat-shock protein family. Expression of a novel heat-inducible HSP70 (HSP70B′) and isolation of its cDNA and genomic DNA. Biochem J. 1990;267:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hageman J, Van Waarde MAWH, Zylicz A, Walerych D, Kampinga HH. The diverse members of the mammalian HSP70 machine show distinct chaperone-like activities. Biochem J. 2011;435:127–42. 10.1042/BJ20101247 [DOI] [PubMed] [Google Scholar]
- 56.Gomes RA, Busato KC, Ladeira MM, Johnson KA, Galvão MC, Rodrigues AC, et al. Technical note: Relationship between infrared thermography and heat production in young bulls. J Anim Sci. 2016;94:1105–09. 10.2527/jas.2015-0004 [DOI] [PubMed] [Google Scholar]
- 57.Peng YT, Chen P, Ouyang RY, Song L. Multifaceted role of prohibitin in cell survival and apoptosis. Apoptosis. 2015;20:1135–49. 10.1007/s10495-015-1143-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thuaud F, Ribeiro N, Nebigil CG, Désaubry L. Prohibitin ligands in cell death and survival: mode of action and therapeutic potential. Chembiol. 2013;20:316–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fusaro G, Dasgupta P, Rastogi S, Joshi B, Chellappan S. Prohibitin induces the transcriptional activity of p53 and is exported from the nucleus upon apoptotic signaling. J Biol Chem. 2003;278:47853–61. 10.1074/jbc.M305171200 [DOI] [PubMed] [Google Scholar]
- 60.Zhang L, Ji Q, Ni ZH, Sun J. Prohibitin induces apoptosis in BGC823 gastric cancer cells through the mitochondrial pathway. Asian Pac J Cancer Prev. 2012;13:3803–07. [DOI] [PubMed] [Google Scholar]
- 61.Laville E, Sayd T, Morzel M, Blinet S, Chambon C, Lepetit J, et al. Proteome changes during meat aging in tough and tender beef suggest the importance of apoptosis and protein solubility for beef aging and tenderization. J Agric Food Chem. 2009;57:10755–64. 10.1021/jf901949r [DOI] [PubMed] [Google Scholar]
- 62.Ande SR, Xu Z, Gu Y, Mishra S. Prohibitin has an important role in adipocyte differentiation. Int J Obes. 2012;36:1236–44. [DOI] [PubMed] [Google Scholar]
- 63.Ande SR, Nguyen KH, Padilla-Meier GP, Wahida W, Nyomba BLG, Mishra S. Prohibitin overexpression in adipocytes induces mitochondrial biogenesis, leads to obesity development, and affects glucose homeostasis in a sex-specific manner. Diabetes. 2014;63:3734–41. 10.2337/db13-1807 [DOI] [PubMed] [Google Scholar]
- 64.Gondret F, Guitton N, Guillerm-Regost C, Louveau I. Regional differences in porcine adipocytes isolated from skeletal muscle and adipose tissues as identified by a proteomic approach. J Anim Sci. 2008;86:2115–25. 10.2527/jas.2007-0750 [DOI] [PubMed] [Google Scholar]
- 65.Stull JT, Kamm KE, Vandenboom R. Myosin light chain kinase and the role of myosin light chain phosphorylation in skeletal muscle. Arch Biochem Biophys. 2011;510:120–28. 10.1016/j.abb.2011.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ryder JW, Lau KS, Kamm KE, Stull JT. Enhanced skeletal muscle contraction with myosin light chain phosphorylation by a calmodulin-sensing kinase. J Biol Chem. 2007;282:20447–54. 10.1074/jbc.M702927200 [DOI] [PubMed] [Google Scholar]
- 67.Sweeney HL, Bowman BF, Stull JT. Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am J Physiol. 1993;264:C1085–C95. [DOI] [PubMed] [Google Scholar]
- 68.Franco D, Mato A, Salgado FJ, López-Pedrouso M, Carrera M, Bravo S, et al. Tackling proteome changes in the longissimus thoracis bovine muscle in response to pre-slaughter stress. J Proteomics. 2015;122:73–5. 10.1016/j.jprot.2015.03.029 [DOI] [PubMed] [Google Scholar]
- 69.Gallagher PJ, Herring BP, Stull JT. Myosin light chain kinases. J Muscle Res Cell Motil. 1997;18:1–16. [DOI] [PubMed] [Google Scholar]
- 70.Di Lisa F, De Tullio R, Salamino F, Barbato R, Melloni E, Siliprandi N, et al. Specific degradation of troponin T and I by μ-calpain and its modulation by substrate phosphorylation. Biochem J. 1995;3081:57–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muroya S, Ohnishi-Kameyama M, Oe M, Nakajima I, Chikuni K. Postmortem changes in bovine troponin T isoforms on two-dimensional electrophoretic gel analyzed using mass spectrometry and western blotting: The limited fragmentation into basic polypeptides. Meat Sci. 2007;75:506–14. 10.1016/j.meatsci.2006.08.012 [DOI] [PubMed] [Google Scholar]
- 72.Mazzei GJ, Kuo JF. Phosphorylation of skeletal-muscle troponin I and troponin T by phospholipid-sensitive Ca2+-dependent protein kinase and its inhibition by troponin C and tropomyosin. Biochem J. 1984;218:361–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gururaj A, Barnes CJ, Vadlamudi RK, Kumar R. Regulation of phosphoglucomutase 1 phosphorylation and activity by a signaling kinase. Oncogene. 2004;23:8118–27. 10.1038/sj.onc.1207969 [DOI] [PubMed] [Google Scholar]
- 74.Anderson MJ, Lonergan SM, Huff-Lonergan E. Differences in phosphorylation of phosphoglucomutase 1 in beef steaks from the longissimus dorsi with high or low star probe values. Meat Sci. 2014;96:379–84. 10.1016/j.meatsci.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 75.Longo V, Lana A, Bottero MT, Zolla L. Apoptosis in muscle-to-meat aging process: The omic witness. J Proteomics. 2015;125:29–40. 10.1016/j.jprot.2015.04.023 [DOI] [PubMed] [Google Scholar]
- 76.Lee WH, Choi JS, Byun MR, Koo KT, Shin S, Lee SK, et al. Functional inactivation of triosephosphate isomerase through phosphorylation during etoposide-induced apoptosis in HeLa cells: Potential role of Cdk2. Toxicology. 2010;278:224–28. 10.1016/j.tox.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 77.Huang H, Larsen MR, Karlsson AH, Pomponio L, Costa LN, Lametsch R. Gel-based phosphoproteomics analysis of sarcoplasmic proteins in postmortem porcine muscle with pH decline rate and time differences. Proteomics. 2011;11:4063–76. 10.1002/pmic.201100173 [DOI] [PubMed] [Google Scholar]
- 78.Fu H, Subramanian RR, Masters SC. 14-3-3 proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol. 2000;40:617–47. 10.1146/annurev.pharmtox.40.1.617 [DOI] [PubMed] [Google Scholar]
- 79.Kim J, Parrish AB, Kurokawa M, Matsuura K, Freel CD, Andersen JL, et al. Rsk-mediated phosphorylation and 14-3-3ε binding of Apaf-1 suppresses cytochrome c-induced apoptosis. EMBO J. 2012;31:1279–92. 10.1038/emboj.2011.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haydon CE, Watt PW, Morrice N, Knebel A, Gaestel M, Cohen P. Identification of a phosphorylation site on skeletal muscle myosin light chain kinase that becomes phosphorylated during muscle contraction. Arch Biochem Biophys. 2002;397:224–31. 10.1006/abbi.2001.2625 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The image obtained with Pro-Q Diamond was colored in blue and the image obtained with Coomassie blue G-250 was colored in red. The overlap of spots in the two images produced brown color.
(TIF)
Spots highlighted in the green square (match ID 383) and only detected in Angus muscle.
(TIF)
Spots highlighted in the green square (match ID 363) and only detected in the muscle of one of Nellore cattle.
(TIF)
Spots highlighted in the green square (match IDs 1064 and 1061) and only detected in Nellore muscle.
(TIF)
Sequence of the peptides identified in Mascot and validated by the Scaffold.
(DOCX)
Sequence of the peptides identified in Mascot and validated by the Scaffold.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.