Abstract
Background
Clostridium difficile infection (CDI) is characterized by high rates of recurrence, resulting in substantial health care costs. The aim of this study was to analyze the cost-effectiveness of treatments for the management of second recurrence of community-onset CDI in France.
Methods
We developed a decision-analytic simulation model to compare 5 treatments for the management of second recurrence of community-onset CDI: pulsed-tapered vancomycin, fidaxomicin, fecal microbiota transplantation (FMT) via colonoscopy, FMT via duodenal infusion, and FMT via enema. The model outcome was the incremental cost-effectiveness ratio (ICER), expressed as cost per quality-adjusted life year (QALY) among the 5 treatments. ICERs were interpreted using a willingness-to-pay threshold of €32,000/QALY. Uncertainty was evaluated through deterministic and probabilistic sensitivity analyses.
Results
Three strategies were on the efficiency frontier: pulsed-tapered vancomycin, FMT via enema, and FMT via colonoscopy, in order of increasing effectiveness. FMT via duodenal infusion and fidaxomicin were dominated (i.e. less effective and costlier) by FMT via colonoscopy and FMT via enema. FMT via enema compared with pulsed-tapered vancomycin had an ICER of €18,092/QALY. The ICER for FMT via colonoscopy versus FMT via enema was €73,653/QALY. Probabilistic sensitivity analysis with 10,000 Monte Carlo simulations showed that FMT via enema was the most cost-effective strategy in 58% of simulations and FMT via colonoscopy was favored in 19% at a willingness-to-pay threshold of €32,000/QALY.
Conclusions
FMT via enema is the most cost-effective initial strategy for the management of second recurrence of community-onset CDI at a willingness-to-pay threshold of €32,000/QALY.
Introduction
Clostridium difficile infection (CDI) is the leading cause of healthcare associated diarrhea, presenting a significant burden to global healthcare systems [1]. In recent years, there has been an increase of incidence and severity of CDI in North America and Europe. Rates of community-acquired CDI have also increased and community-associated CDI is estimated to be responsible for more than one third of all CDI cases [2,3]. The main problem in CDI is symptomatic relapse after antimicrobial therapy completion. Moreover, the risk of recurrent CDI is increased in patients who have already had one recurrence, rising from 25% after an initial episode to 45% after a first recurrence and to 65% after two recurrences [4]. Recurrent CDI is associated with a diminished quality of life and increased morbidity. In addition, recurrent CDI also increases the risk of person-to-person transmission [4]. A recent study focusing on the economic consequences of recurrent CDI compared to patients with CDI who did not experience a recurrence showed that there were substantially higher pharmacological and hospitalization costs among the patients with recurrent CDI [5].
Treatment of multiple recurrent CDI remains challenging. In 2014, the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) provided an overview of currently available CDI treatments [6]. Vancomycin use is recommended for treatment of multiple recurrent CDI, administered as a pulsed or tapered regimen. Fidaxomicin is also recommended for the treatment of multiple recurrent CDI. Nevertheless, both of these drugs are moderately supported by the ESCMID guideline (grade B-II). Fecal microbiota transplantation (FMT) consists of transplanting a fecal suspension from a healthy donor into a patient’s gastrointestinal tract through duodenal infusion, enema, or colonoscopy. FMT has been a successful therapeutic approach to recurrent CDI in numerous case series and in two randomized clinical trials [7,8]. The ESCMID endorses FMT as first-line therapy for patients who have had three or more CDI episodes with a strong recommendation (grade A-1).
Economic analyses compare different treatments in terms of clinical outcomes and costs [9]. To date, published cost-effectiveness analyses of CDI involving FMT have been performed in the USA and in Canada using cost data that may not apply to European countries [10–15]. The aim of this study was to analyze the cost-effectiveness of 5 strategies constructed from the ESCMID guideline for the management of multiple recurrence of CDI in adults where the first-line treatments were pulsed-tapered vancomycin, fidaxomicin, FMT via colonoscopy, FMT via duodenal infusion, and FMT via enema.
Methods
Model structure
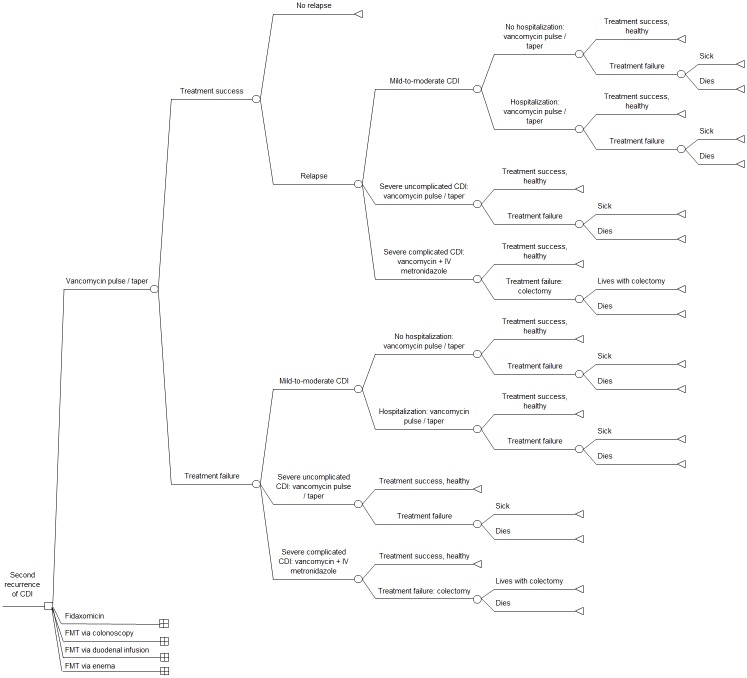
We conducted a decision-analytic model comparing 5 strategies for the management of second recurrence of CDI according to the ESCMID guideline (Fig 1). All analyses were performed using the TreePlan add-in (Decision Toolworks, San Francisco, USA) for Excel 2010 (Microsoft Corporation, USA) and PopTools [16]. Our model was based, in part, on previously published decision analytic models that investigated the potential value of FMT for CDI and cost-effectiveness analyses evaluating fidaxomicin versus vancomycin in CDI [10,12–15,17–20].
Fig 1. Decision tree comparing 5 strategies for the treatment of second recurrence of community-onset Clostridium difficile infection.
Note: expanded model details shown for vancomycin pulse/taper arm only. Abbreviations: CDI: Clostridium difficile infection; FMT: fecal microbiota transplantation.
As defined in European guidelines, we considered recurrent CDI to be an episode occurring within 8 weeks after the onset of a previous episode that resolved after completion of the initial treatment [6]. Multiple recurrences were defined as more than two recurrences (i.e. more than 3 episodes). Treatment failure was defined as a need for further CDI therapy.
The patient modeled in the study was an adult experiencing a second recurrence (i.e. third occurrence) of mild-to-moderate CDI diagnosed at an outpatient visit (Fig A-E in S1 File). Following European guidelines, the first-line therapies for the strategies were pulsed-tapered vancomycin, fidaxomicin, FMT via colonoscopy, FMT via duodenal infusion, and FMT via enema [6]. Following treatment, patients were considered cured or treatment failures. Patients who failed treatment either had mild-to-moderate CDI, severe uncomplicated CDI, or severe complicated CDI. It was assumed that one medical consultation was required for each patient with CDI and that severe CDI required hospitalization. Patients initially cured could develop a recurrence of mild-to-moderate CDI, severe uncomplicated CDI, or severe complicated CDI. On the basis of published data, we assumed that recurrence appeared 10 days after treatment by pulsed-tapered vancomycin, 31 days after treatment by fidaxomicin, and 32 days after treatment by FMT [7,8,21–26]. To evaluate each treatment separately, we assumed that patients who failed therapy and patients who had a relapse were treated with the same treatment as their previous episode if they had mild-to-moderate CDI or severe uncomplicated CDI. Patients with severe complicated CDI received oral vancomycin plus intravenous metronidazole, as recommended in European guidelines [6]. Following treatment, they could be either cured or treatment failures requiring colectomy. We considered the possibility of adverse events and death from FMT via colonoscopy and FMT via duodenal infusion. Adverse events and death from FMT via colonoscopy were assumed to be equivalent to those of a colonoscopy procedure [27–30]. Similarly, due to a lack of current data, adverse events and death from FMT via duodenal infusion were assumed to be equivalent to adverse events and death from upper gastrointestinal endoscopy [31]. We assumed that adverse events lasted one day. Adverse effects of vancomycin, fidaxomicin and FMT via enema were assumed to be negligible and were not included in the model [32,33].
The analysis was performed from a societal perspective. The time horizon was 78 days. This duration was determined based on the duration of adverse events, the duration of initial therapy for the relapse, the expected time to relapse, and the duration of treatment for another relapse. This time horizon was chosen to put all the treatment strategies on an equal footing for the evaluation. At the end of the 78 days, patients could be in one of 5 health conditions: healthy, mild-to-moderate CDI, severe uncomplicated CDI, postcolectomy, and death. According to Van Nood et al.’s protocol, FMT treatment included a 4-day course of oral vancomycin [7], one day of transplant delivery and 2 days to resolution of symptoms. Dose and duration of all treatments are detailed in Table 1 and are consistent with published guidelines.
Table 1. Base case estimates, range, and distribution for model variables.
| Variable | Base case value | Range | Distribution | Standard deviation | References |
|---|---|---|---|---|---|
| Probabilities | |||||
| Oral vancomycin pulse/taper—cure | 0.771 | 0.652–0.890 | Beta | 0.061 | [8,49] |
| Oral vancomycin pulse/taper—relapse | 0.568 | 0.408–0.727 | Beta | 0.081 | [8,49] |
| Fidaxomicin—cure | 0.812 | 0.719–0.904 | Beta | 0.047 | [21–25,50,51] |
| Fidaxomicin—relapse | 0.211 | 0.105–0.316 | Beta | 0.054 | [21–25,50,51] |
| FMT colonoscopy—cure | 0.894 | 0.852–0.937 | Beta | 0.022 | [8,52–58] |
| FMT colonoscopy—relapse | 0.022 | 0.001–0.043 | Beta | 0.011 | [8,52–58] |
| FMT colonoscopy after second FMT—cure | 0.563 | 0.319–0.806 | Beta | 0.124 | [8,52,55–57] |
| FMT duodenal infusion—cure | 0.795 | 0.723–0.867 | Beta | 0.037 | [7,26,59,60] |
| FMT duodenal infusion—relapse | 0.021 | 0.000–0.049 | Beta | 0.014 | [7,26,59,60] |
| FMT duodenal infusion after second FMT—cure | 0.750 | 0.326–1.174 | Beta | 0.217 | [7,26] |
| FMT enema—cure | 0.833 | 0.712–0.955 | Beta | 0.062 | [61–63] |
| FMT enema—relapse | 0.000 | 0.000–0.000 | Beta | 0.000 | [61–63] |
| FMT enema after second FMT—cure | 0.500 | 0.100–0.900 | Beta | 0.204 | [61,63] |
| Severe uncomplicated CDI | 0.180 | 0.115–0.246 | Beta | 0.033 | [64] |
| Severe complicated CDI | 0.012 | 0.011–0.013 | Beta | 0.001 | [36,64] |
| Colectomy | 0.318 | 0.293–0.344 | Beta | 0.013 | [36,37] |
| Postcolectomy mortality | 0.407 | 0.350–0.463 | Beta | 0.029 | [37,65–67] |
| Adverse events of FMT colonoscopy | 0.002 | 0.000–0.012 | Beta | 0.005 | [27–29] |
| Adverse events of FMT duodenal infusion | 0.0005 | 0.000–0.002 | Beta | 0.0007 | [31] |
| Mortality from FMT colonoscopy | 0.0003 | 0.0002–0.0003 | Beta | 0.00003 | [28–30,68] |
| Mortality from FMT duodenal infusion | 0.0002 | 0.000–0.0004 | Beta | 0.0001 | [31] |
| Hospitalization for mild CDI | 0.000 | 0.000–0.000 | Beta | 0.000 | Expert opinion |
| Mortality from mild CDI | 0.007 | 0.002–0.012 | Beta | 0.002 | [69,70] |
| Mortality from severe uncomplicated CDI | 0.339 | 0.221–0.457 | Beta | 0.060 | [71] |
| Costsa | |||||
| Oral vancomycin pulse/taperb | 58 | not varied | Local sources | ||
| Fidaxomicin (200 mg bid, 10 days) | 1416 | not varied | Local sources | ||
| Oral vancomycin (500 mg qid, 10 days) | 50 | not varied | Local sources | ||
| Intravenous metronidazole (500 mg tid, 10 days) | 11 | not varied | Local sources | ||
| Outpatient visit | 43 | not varied | Local sources | ||
| Donor and stool testing prior to FMT | 825 | not varied | NABM | ||
| Stool transplant preparation and traceability of samples | 154 | not varied | Local sources | ||
| Oral vancomycin (500 mg qid, 4 days) prior to FMT | 20 | not varied | Local sources | ||
| FMT delivery by colonoscopy | 289 | not varied | Local sources | ||
| FMT delivery by duodenal infusion | 76 | not varied | Local sources | ||
| FMT delivery by enema | 5 | not varied | Local sources | ||
| Follow-up outpatient visits | 86 | not varied | Local sources | ||
| Mean cost of hospitalization for mild-to-moderate CDI | 2190 | 2099–2280 | Gamma | 45 | [46] |
| Mean cost of hospitalization for severe CDI | 8412 | 7725–9098 | Gamma | 343 | [46] |
| Colectomy | 719 | not varied | CCAM | ||
| Adverse events of FMT colonoscopy | 283 | not varied | CCAM | ||
| Adverse events of FMT duodenal infusion | 229 | not varied | CCAM | ||
| Utilities | |||||
| Severe CDI (complicated or uncomplicated) | 0.600 | 0.505–0.695 | Beta | 0.156 | [47] |
| Mild-to-moderate CDI | 0.782 | 0.628–0.936 | Beta | 0.154 | [48] |
| Postcolectomy | 0.536 | 0.382–0.690 | Beta | 0.154 | [72] |
| Adverse events of FMT colonoscopy or FMT duodenal infusion | 0.770 | 0.670–0.920 | Beta | 0.154 | [73] |
| Healthy | 1 | ||||
| Death | 0 |
Abbreviations: bid: twice daily; CCAM: French Common Classification of Medical Procedures; CDI: Clostridium difficile infection; FMT: fecal microbiota transplantation; NABM: French Nomenclature of Procedures in Laboratory Medicine; IV: intravenous; od: once daily; qid: 4 times daily; tid: 3 times daily.
aCosts are reported as 2016 Euros.
bOral vancomycin pulse/taper: oral vancomycin at 125 mg qid for 10 days, then 500 mg od every 2 days for 21 days.
Model input parameters
Inputs for effectiveness data, costs, and utilities were pooled from published sources, which included clinical studies and systematic reviews. Additional input was sought from clinical experts for parameters for which data were limited, i.e. treatment pathway. Clinical experts were employees of the Lille University Hospital. All model variables are reported in Table 1.
Probabilities
We selected reports of treatments used to treat patients with multiple recurrent CDI (i.e. more than 2 episodes) from published sources. We used the protocol of pulsed-tapered vancomycin used in the only randomized controlled trial evaluating pulsed-tapered vancomycin published to date to define vancomycin cure and recurrence rates [8]. For treatment by fidaxomicin, reports were case series and retrospective cohort studies. We did not include randomized controlled trials evaluating the efficacy of fidaxomicin as patients with more than one occurrence of CDI within 3 months before the start of the trials were excluded from these studies [34,35]. For FMT, published studies including 2 randomized controlled trials were used [7,8]. We used cure rates of second-time FMT administration based on published studies. Because no data were available to define vancomycin and intravenous metronidazole cure, we used the probability of having a colectomy to define oral vancomycin plus intravenous metronidazole failure [36,37]. Incidence rates of severe CDI reported in the literature are often based on different definitions. We used the definition of severe CDI reported in European guidelines to define the probability of having severe uncomplicated CDI and severe complicated CDI [6]. Probability that a non-severe CDI was treated in hospital was assumed to be zero (infectious diseases physician input). Due to a paucity of data from published sources, it was assumed that treatments had the same probabilities of cure for mild and severe uncomplicated CDI.
Costs
All cost inputs are presented in 2016 Euro (€). Medication costs were obtained from prices negotiated by the largest central purchasing unit in the French public hospital sector. The cost of treatment with FMT included a single fecal transplantation procedure. Donor testing prior to FMT included routine laboratory screening, stool testing, and serologic testing. Donor testing was consistent with current guidelines (Table A in S1 File) [38]. The 2014 Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) was used to include rates of vancomycin-resistant Enterococci (VRE) and rates of carbapenem-resistant Enterobacteriaceae (CRE) in France [39].
Cost information for donor testing was obtained from the French Nomenclature of Procedures in Laboratory Medicine (NABM) [40]. The activity-based costing database for laboratories was used to value these costs. This database is reported by the French Directorate-General for Care Provision (DGOS) and provides a national benchmark of non-clinical cost data for hospitals, i.e. administrative, technical, and logistic data [41].
Costs of materials and technical time for stool transplant preparation and traceability of samples were provided by the department of Pharmacy of Lille University Hospital. Personnel costs were obtained using annual wage data reported by the French National Institute for Statistics and Economic Studies (INSEE) [42]. These costs were adjusted using the coefficient for calculating the total wage cost for each personnel category provided by the DGOS [43].
The cost of a colonoscopy procedure was provided by Lille University Hospital, according to their protocol. Cost of FMT via duodenal infusion included chest or abdominal radiograph to confirm correct placement of nasoduodenal tube. Cost of FMT via enema included the enema kit and the cost of the nurse performing the procedure. Cost of bowel preparation was included to all patients undergoing FMT, regardless of mode of delivery [44]. The cost of one outpatient visit was included in each treatment strategy. Cost of FMT included the procedure for donation. This included an outpatient visit for assessment of donor, an outpatient visit on the day of donation, a hospital letter to general practitioner, and a venipuncture for blood screening. FMT strategies also included two follow-up outpatient visits for recipients. Costs of visits for donors and recipients in FMT strategies were obtained from the French General Inspectorate of Social Affairs (IGAS) [45]. Hospital admission costs were obtained from the French National Costs Study (ENC) 2013 using the diagnostic related group “Severe digestive system diseases” [46].
Utilities
Given that utilities for patients with CDI are not currently available in the literature, we derived our quality-of-life estimate for CDI from utility of non-infectious diarrhea using European Quality of Life-5 Dimensions (EQ-5D) questionnaires [47,48]. Patients who were cured by pulsed-tapered vancomycin and fidaxomicin were assumed to spend 10 days in a state of mild-to-moderate or severe disease, and the subsequent duration of treatment in the healthy state, whereas patients who were cured by FMT were assumed to spend 7 days in disease state, i.e. the duration of treatment by FMT. Patients who were treatment failures remained in the initial disease state through the course of treatment.
Analysis
Parameters were assumed to be independent. In addition to base case analysis, deterministic univariate sensitivity analyses were performed to investigate the impact of model uncertainties and robustness of our analysis. As FMT costs were based on standard costs in one hospital, ranges for these costs were not available. To account for uncertainty regarding these costs, a threshold analysis was performed to determine the variation in common costs of FMT that would change the strategies lying on the efficiency frontier.
In addition, because of the current debate regarding the superiority of the upper gastrointestinal route [74–76], we examined a scenario where cure and recurrence rates of FMT via duodenal infusion were the same as the ones of FMT via colonoscopy.
Finally, we conducted a probabilistic sensitivity analysis using 10,000 Monte Carlo simulations to simultaneously assess the effect of uncertainty in all parameters on model conclusions. Parameters used in sensitivity analyses appear in Table 1.
Model outcome
The model outcome was the incremental cost-effectiveness ratio (ICER) expressed as cost per quality-adjusted life year (QALY) among the 5 strategies. No threshold is defined by French Health Authorities. Following the WHO’s Commission on Macroeconomics and Health [77], the GDP per capita (€32,000 in 2015 [78]) was used for interpreting the ICER. QALYs were obtained by multiplying the utility weight of a state by the time spent in that state. The study was designed, conducted, and reported in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement [79].
Results
Base case analysis
Base case analysis, using the mean value of each parameter, showed that FMT via enema was costlier and more effective than pulsed-tapered vancomycin, yielding an ICER of €18,092/QALY (Table 2). FMT via colonoscopy was costlier and more effective than FMT via enema, yielding an ICER of €73,653/QALY. Fidaxomicin and FMT via duodenal infusion were dominated by FMT via colonoscopy and FMT via enema: they were both more expensive and less effective than FMT via colonoscopy and FMT via enema.
Table 2. Base case analysis of competing strategies for the management of second recurrence of community-onset Clostridium difficile infection.
| Treatment | Cost (€) | QALY | ICER |
|---|---|---|---|
| Vancomycin pulse/taper | 1235 | 0.1812 | |
| Fidaxomicin | 2464 | 0.1988 | (Dominated) |
| FMT via duodenal infusion | 1834 | 0.2013 | (Dominated) |
| FMT via enema | 1610 | 0.2019 | 18,092a |
| FMT via colonoscopy | 1816 | 0.2047 | 73,653b |
Abbreviations: FMT: fecal microbiota transplantation; ICER: incremental cost-effectiveness ratio; QALY: quality-adjusted life year. Costs values are reported as 2016 Euros.
aICER calculated for FMT via enema relative to pulsed-tapered vancomycin.
bICER calculated for FMT via colonoscopy relative to FMT via enema.
Sensitivity analyses
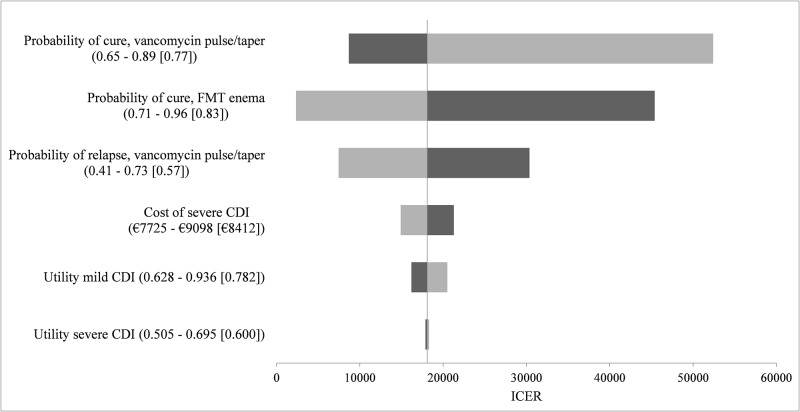
Deterministic sensitivity analyses were used to explore the potential impact of six factors on the base case results: probabilities of cure and probability of relapse of vancomycin, probability of cure of FMT via enema, cost of severe CDI, and utilities of mild and severe CDI. Probability of cure and of relapse of pulsed-tapered vancomycin and probability of cure of FMT via enema, and to a lesser extent cost of severe CDI and utility of mild CDI, had an influence on the model (Fig 2). The model was sensitive to variation in probability of cure of FMT via enema. Indeed, varying this parameter within its stated range led to a change of sign in the ICER when considering FMT via enema versus FMT via colonoscopy: FMT via enema became either less effective and costlier that FMT via colonoscopy, or more effective and less costly than FMT via colonoscopy.
Fig 2. Tornado diagram, FMT via enema versus pulsed-tapered vancomycin.
Name of the variable (lower bound of the parameter—higher bound of the parameter [base case]). The ICER corresponding to the lower parameter bound is shown in black, while the ICER corresponding to the higher parameter bound is shown in grey. This figure represents the impact of the uncertainty of six parameters on the base case results. Abbreviations: CDI: Clostridium difficile infection; FMT: fecal microbiota transplantation; ICER: incremental cost-effectiveness ratio.
In addition, a threshold analysis was performed to find the variation in common costs of FMT that would change the strategies lying on the efficiency frontier. When common costs of FMT were increased by 81% of the baseline estimate, fidaxomicin (€2464, 0.199 QALY) was on the efficiency frontier, with an ICER of €69,890/QALY compared with pulsed-tapered vancomycin (€1235, 0.181 QALY), and FMT via enema (€2690, 0.202 QALY), compared with fidaxomicin, had an ICER of €72,212/QALY.
When considering a scenario where cure and recurrence rates of FMT via duodenal infusion were the same as the ones of FMT via colonoscopy, FMT via duodenal infusion (€1581, 0.2048 QALY) was on the efficiency frontier, with an ICER of €14,678/QALY compared with pulsed-tapered vancomycin, and both other routes of FMT administration were dominated.
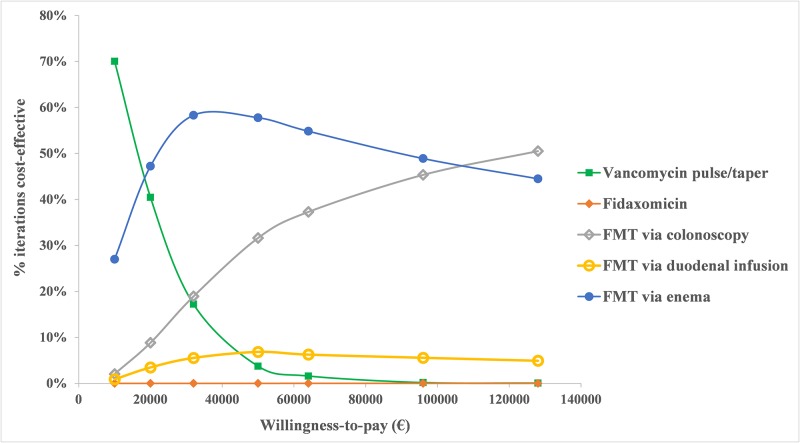
Probabilistic sensitivity analysis with 10,000 Monte Carlo simulations demonstrated that FMT via enema was the most cost-effective strategy in 58% of simulations and FMT via colonoscopy was favored in 19% at a willingness-to-pay threshold of €32,000/QALY. The cost-effectiveness acceptability curve displayed in Fig 3 shows the proportion of the time each treatment was cost-effective at various willingness-to-pay thresholds.
Fig 3. Acceptability curve of treatments of second recurrence of Clostridium difficile infection.
This figure illustrates the proportion of the time each treatment was cost-effective at different willingness-to-pay thresholds. Abbreviations: FMT: fecal microbiota transplantation.
Discussion
Our decision model indicated that the current standard approach using pulsed-tapered vancomycin is less costly than FMT, but FMT is more effective regardless of mode of delivery. The extra cost associated with FMT via enema for this increased effectiveness compared with vancomycin was €18,092/QALY. Thus, FMT via enema appears to be the most cost-effective strategy at a willingness-to-pay threshold of €32,000/QALY. The base case analysis showed that FMT via duodenal infusion and fidaxomicin were dominated by FMT via colonoscopy and FMT via enema. Fidaxomicin was on the efficiency frontier if common costs of FMT increased by 81%. However, such an increase in FMT costs is unlikely to occur.
Similar results have been demonstrated by other economic analyses evaluating FMT for treatment of recurrent CDI [10,14,15]. A cost-effectiveness analysis of strategies for treatment of recurrent CDI performed from a societal perspective in the USA concluded that FMT via colonoscopy was cost-effective with an ICER of $17,016 compared with vancomycin at a willingness-to-pay threshold of $50,000/QALY [10]. A recent study performed in Canada compared 6 strategies to treat recurrent CDI using the perspective of the Ontario Ministry of Health and Long-Term Care. The authors concluded that FMT via colonoscopy dominated all other strategies in the base case at a willingness-to-pay threshold of $50,000/QALY [15]. However, route of FMT often depends on the delivery method which is considered the safest for the patient [8]. Therefore, it appeared important to incorporate adverse events from colonoscopy and upper gastrointestinal endoscopy in our analysis. Our model also accounted for the utilities weights of these adverse events and for their respective associated costs. Another study by Varier et al. compared vancomycin with FMT for recurrent CDI from a third-party payer perspective using U.S. data. Even though the authors accounted for adverse events of colonoscopy, they found that FMT administered via colonoscopy was both less costly and more effective than prolonged oral vancomycin taper at all willingness-to-pay thresholds based on the probabilistic sensitivity analysis [14].
To our knowledge, this study is the first cost-effectiveness analysis investigating the use of treatment strategies including FMT for the treatment of second recurrence of community-onset CDI in France. Our analysis contains several limitations. First, we assumed that patients entering the model received outpatient treatment and we did not incorporate hospitalizations. However, it should be noted that patients with multiple comorbidities can be hospitalized for moderate CDI or can develop CDI while hospitalized, which was not captured by our model. Second, our conclusions are limited by the quality of the studies included. The lack of a standardized protocol for FMT administration leads to difficulties in comparison of efficacy across studies. For instance, we included studies which used less than 50 g of stool, although it is recommended that a large volume of stool be attempted [80]. This may have overestimated the recurrence rates of treatment with FMT. Third, we did not have a range for the majority of the costs included as these costs are not currently available at a national level and may also vary throughout countries. The Fidaxomicin/Vancomycin cost ratio is very high in our study compared to other cost-effectiveness studies, especially in those conducted in the USA [10,12]. Additionally, because only one study has stratified FMT results to Clostridium difficile ribotype 027 strain, we did not account for potential differences in treatment efficacy between NAP1/BI/027 and non-NAP1/BI/027 strains [56].
We could have added in our model a treatment by FMT as an alternative to colectomy for patients with severe complicated CDI, as it has been shown that FMT was also effective for these patients [81]. However, as this alternative is not mentioned in current guidelines, we did not consider it in our model. It should be noted that fidaxomicin could also be considered for treatment before FMT instead of vancomycin, although it would increase costs of FMT strategies. Indeed, fidaxomicin causes less disruption of the anaerobic microbiota than vancomycin, within the limitation of the method used to explore the intestinal microbiota [35,82]. Published studies used for this analysis were limited to immunocompetent patients. However, FMT has been shown to be safe, well tolerated, and effective also in immunocompromised patients [83,84]. Two recent randomized controlled trials have shown similar cure rates between frozen FMT and fresh FMT in treating recurrent CDI [74,85]. Using frozen FMT would reduce costs associated with donor screening frequency, provide immediate availability of the FMT, and enable delivery of the treatment to hospitals without on-site laboratory facilities [85].
In conclusion, this study, performed from a societal perspective, may give insights to healthcare decision makers when considering treatment for second recurrence of community-onset CDI.
Supporting Information
(DOCX)
Acknowledgments
The authors acknowledge the contribution of Dr Rodrigue Dessein, Dr Emilie Frealle, and Dr Anny Dewilde, Lille University Hospital, in providing information about donor screening prior to FMT. We are grateful to Jean-Louis Mouton, health manager at the Ambulatory Surgery Center, Lille University Hospital, for his contribution in determining the cost of colonoscopy.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Knight DR, Elliott B, Chang BJ, Perkins TT, Riley TV. Diversity and Evolution in the Genome of Clostridium difficile. Clin Microbiol Rev. 2015. July;28(3):721–41. 10.1128/CMR.00127-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eyre DW, Tracey L, Elliott B, Slimings C, Huntington PG, Stuart RL, et al. Emergence and spread of predominantly community-onset Clostridium difficile PCR ribotype 244 infection in Australia, 2010 to 2012. Euro Surveill. 2015;20(10):21059 [DOI] [PubMed] [Google Scholar]
- 3.Desai K, Gupta SB, Dubberke ER, Prabhu VS, Browne C, Mast TC. Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach. BMC Infect Dis. 2016;16:303 10.1186/s12879-016-1610-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012. December;18 Suppl 6:21–7. [DOI] [PubMed] [Google Scholar]
- 5.Shah DN, Aitken SL, Barragan LF, Bozorgui S, Goddu S, Navarro ME, et al. Economic burden of primary compared with recurrent Clostridium difficile infection in hospitalized patients: a prospective cohort study. J Hosp Infect. 2016. July;93(3):286–9. 10.1016/j.jhin.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 6.Debast SB, Bauer MP, Kuijper EJ, European Society of Clinical Microbiology and Infectious Diseases. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014. March;20 Suppl 2:1–26. [DOI] [PubMed] [Google Scholar]
- 7.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013. January 31;368(5):407–15. 10.1056/NEJMoa1205037 [DOI] [PubMed] [Google Scholar]
- 8.Cammarota G, Masucci L, Ianiro G, Bibbò S, Dinoi G, Costamagna G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015. May;41(9):835–43. 10.1111/apt.13144 [DOI] [PubMed] [Google Scholar]
- 9.Henrikson NB, Skelly AC. Economic studies part I: basics and terms. Evid-Based Spine-Care J. 2012. November;3(4):7–11. 10.1055/s-0032-1328137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konijeti GG, Sauk J, Shrime MG, Gupta M, Ananthakrishnan AN. Cost-effectiveness of competing strategies for management of recurrent Clostridium difficile infection: a decision analysis. Clin Infect Dis. 2014. June;58(11):1507–14. 10.1093/cid/ciu128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartsch SM, Umscheid CA, Fishman N, Lee BY. Is fidaxomicin worth the cost? An economic analysis. Clin Infect Dis. 2013. August;57(4):555–61. 10.1093/cid/cit346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stranges PM, Hutton DW, Collins CD. Cost-effectiveness analysis evaluating fidaxomicin versus oral vancomycin for the treatment of Clostridium difficile infection in the United States. Value Health J Int Soc Pharmacoeconomics Outcomes Res. 2013. April;16(2):297–304. [DOI] [PubMed] [Google Scholar]
- 13.Varier RU, Biltaji E, Smith KJ, Roberts MS, Jensen MK, LaFleur J, et al. Cost-effectiveness analysis of treatment strategies for initial Clostridium difficile infection. Clin Microbiol Infect. 2014. December;20(12):1343–51. 10.1111/1469-0691.12805 [DOI] [PubMed] [Google Scholar]
- 14.Varier RU, Biltaji E, Smith KJ, Roberts MS, Kyle Jensen M, LaFleur J, et al. Cost-effectiveness analysis of fecal microbiota transplantation for recurrent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2015. April;36(4):438–44. 10.1017/ice.2014.80 [DOI] [PubMed] [Google Scholar]
- 15.Lapointe-Shaw L, Tran KL, Coyte PC, Hancock-Howard RL, Powis J, Poutanen SM, et al. Cost-Effectiveness Analysis of Six Strategies to Treat Recurrent Clostridium difficile Infection. PloS One. 2016;11(2):e0149521 10.1371/journal.pone.0149521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hood GM. PopTools version 3.2.5. 2010. http://www.poptools.org
- 17.Nathwani D, Cornely OA, Van Engen AK, Odufowora-Sita O, Retsa P, Odeyemi IAO. Cost-effectiveness analysis of fidaxomicin versus vancomycin in Clostridium difficile infection. J Antimicrob Chemother. 2014. November;69(11):2901–12. 10.1093/jac/dku257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zowall H, Brewer C, Deutsch A. Cost-Effectiveness of Fecal Microbiota Transplant in Treating Clostridium Difficile Infection in Canada. Value Health. 2014. November 1;17(7):A676. [DOI] [PubMed] [Google Scholar]
- 19.Ghantoji SS, Sail K, Lairson DR, DuPont HL, Garey KW. Economic healthcare costs of Clostridium difficile infection: a systematic review. J Hosp Infect. 2010. April;74(4):309–18. 10.1016/j.jhin.2009.10.016 [DOI] [PubMed] [Google Scholar]
- 20.Wagner M, Lavoie L, Goetghebeur M. Clinical and economic consequences of vancomycin and fidaxomicin for the treatment of Clostridium difficile infection in Canada. Can J Infect Dis Med Microbiol. 2014;25(2):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orenstein R. Fidaxomicin failures in recurrent Clostridium difficile infection: a problem of timing. Clin Infect Dis. 2012. August;55(4):613–4. 10.1093/cid/cis495 [DOI] [PubMed] [Google Scholar]
- 22.Johnson S, Gerding DN. Fidaxomicin “chaser” regimen following vancomycin for patients with multiple Clostridium difficile recurrences. Clin Infect Dis. 2013. January;56(2):309–10. 10.1093/cid/cis833 [DOI] [PubMed] [Google Scholar]
- 23.Soriano MM, Danziger LH, Gerding DN, Johnson S. Novel Fidaxomicin Treatment Regimens for Patients With Multiple Clostridium difficile Infection Recurrences That Are Refractory to Standard Therapies. Open Forum Infect Dis. 2014. September;1(2):ofu069 10.1093/ofid/ofu069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eiland EH, Sawyer AJ, Massie NL. Fidaxomicin Use and Clinical Outcomes for Clostridium difficile-Associated Diarrhea. Infect Dis Clin Pract Baltim Md. 2015. January;23(1):32–5. 10.1097/IPC.0000000000000181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vargo CA, Bauer KA, Mangino JE, Johnston JEW, Goff DA. An antimicrobial stewardship program’s real-world experience with fidaxomicin for treatment of Clostridium difficile infection: a case series. Pharmacotherapy. 2014. September;34(9):901–9. 10.1002/phar.1451 [DOI] [PubMed] [Google Scholar]
- 26.MacConnachie AA, Fox R, Kennedy DR, Seaton RA. Faecal transplant for recurrent Clostridium difficile-associated diarrhoea: a UK case series. QJM Mon J Assoc Physicians. 2009. November;102(11):781–4. [DOI] [PubMed] [Google Scholar]
- 27.Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008. November 4;149(9):638–58. [DOI] [PubMed] [Google Scholar]
- 28.Lüning TH, Keemers-Gels ME, Barendregt WB, Tan ACITL, Rosman C. Colonoscopic perforations: a review of 30,366 patients. Surg Endosc. 2007. June;21(6):994–7. 10.1007/s00464-007-9251-7 [DOI] [PubMed] [Google Scholar]
- 29.Hagel AF, Boxberger F, Dauth W, Kessler HP, Neurath MF, Raithel M. Colonoscopy-associated perforation: a 7-year survey of in-hospital frequency, treatment and outcome in a German university hospital. Colorectal Dis. 2012. September;14(9):1121–5. 10.1111/j.1463-1318.2011.02899.x [DOI] [PubMed] [Google Scholar]
- 30.Pignone M, Saha S, Hoerger T, Mandelblatt J. Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2002. July 16;137(2):96–104. [DOI] [PubMed] [Google Scholar]
- 31.Chan MF. Complications of upper gastrointestinal endoscopy. Gastrointest Endosc Clin N Am. 1996. April;6(2):287–303. [PubMed] [Google Scholar]
- 32.Borody TJ, Paramsothy S, Agrawal G. Fecal Microbiota Transplantation: Indications, Methods, Evidence, and Future Directions. Curr Gastroenterol Rep. 2013;15(8). Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3742951/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee CH, Belanger JE, Kassam Z, Smieja M, Higgins D, Broukhanski G, et al. The outcome and long-term follow-up of 94 patients with recurrent and refractory Clostridium difficile infection using single to multiple fecal microbiota transplantation via retention enema. Eur J Clin Microbiol Infect Dis. 2014. August;33(8):1425–8. 10.1007/s10096-014-2088-9 [DOI] [PubMed] [Google Scholar]
- 34.Cornely OA, Crook DW, Esposito R, Poirier A, Somero MS, Weiss K, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012. April;12(4):281–9. 10.1016/S1473-3099(11)70374-7 [DOI] [PubMed] [Google Scholar]
- 35.Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011. February 3;364(5):422–31. 10.1056/NEJMoa0910812 [DOI] [PubMed] [Google Scholar]
- 36.Bhangu A, Nepogodiev D, Gupta A, Torrance A, Singh P, West Midlands Research Collaborative. Systematic review and meta-analysis of outcomes following emergency surgery for Clostridium difficile colitis. Br J Surg. 2012. November;99(11):1501–13. 10.1002/bjs.8868 [DOI] [PubMed] [Google Scholar]
- 37.Stewart DB, Hollenbeak CS, Wilson MZ. Is colectomy for fulminant Clostridium difficile colitis life saving? A systematic review. Colorectal Dis. 2013. July;15(7):798–804. 10.1111/codi.12134 [DOI] [PubMed] [Google Scholar]
- 38.Sokol H, Galperine T, Kapel N, Bourlioux P, Seksik P, Barbut F, et al. Faecal microbiota transplantation in recurrent Clostridium difficile infection: Recommendations from the French Group of Faecal microbiota Transplantation. Dig Liver Dis. 2015. September 7; [DOI] [PubMed] [Google Scholar]
- 39.European Antimicrobial Resistance Surveillance Network (EARS-Net). http://ecdc.europa.eu/en/activities/surveillance/EARS-Net/Pages/index.aspx
- 40.Caisse nationale de l’Assurance maladie. Nomenclature des actes de biologie médicale. 2014. http://www.codage.ext.cnamts.fr/codif/nabm/index_presentation.php?p_site=AMELI
- 41.Direction générale de l’offre de soins. Calcul des coûts par activité, données 2013. 2013; http://social-sante.gouv.fr/IMG/pdf/Rapport_Base_d_Angers_-_Donnees_2013.pdf
- 42.Insee—Tableaux de l’Économie Française - Édition 2014. http://www.insee.fr/fr/publications-et-services/sommaire.asp?ref_id=TEF14
- 43.Direction générale de l’offre de soins. Guide pour le suivi de la masse salariale. Ministère chargé de la santé; 2014. http://social-sante.gouv.fr/IMG/pdf/Guide_suivi_masse_salariale_23-03-15.pdf
- 44.Aroniadis OC, Brandt LJ. Fecal microbiota transplantation: past, present and future. Curr Opin Gastroenterol. 2013. January;29(1):79–84. 10.1097/MOG.0b013e32835a4b3e [DOI] [PubMed] [Google Scholar]
- 45.Inspection Générale des Affaires Sociales. Evaluation de la tarification des soins hospitaliers et des actes médicaux. 2012 Apr. Report No.: 2011-NaN-056–01.
- 46.Référentiel de coûts MCO 2013 | Stats ATIH. http://www.scansante.fr/r%C3%A9f%C3%A9rentiel-de-co%C3%BBts-mco-2013
- 47.Stouthard M, Essink-Bot M, Bonsel G, Barendregt J, Kramers P. Disability Weights for Diseases in The Netherlands Department of Public Health. Erasmus University Rotterdam; 1997. [Google Scholar]
- 48.Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology. 2015. July;149(1):102–109.e6. 10.1053/j.gastro.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 49.McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002. July;97(7):1769–75. 10.1111/j.1572-0241.2002.05839.x [DOI] [PubMed] [Google Scholar]
- 50.Bono BR, Culshaw DL, Mitropoulos IF, Chaudhry SB. Patients With Recalcitrant Clostridium difficile–Associated Diarrhea Treated Successfully With Fidaxomicin: A Case Series. Infect Dis Clin Pract. 2014. March;22(2):92–5. [Google Scholar]
- 51.Penziner S, Dubrovskaya Y, Press R, Safdar A. Fidaxomicin therapy in critically ill patients with Clostridium difficile infection. Antimicrob Agents Chemother. 2015. March;59(3):1776–81. 10.1128/AAC.04268-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khan MA, Sofi AA, Ahmad U, Alaradi O, Khan AR, Hammad T, et al. Efficacy and safety of, and patient satisfaction with, colonoscopic-administered fecal microbiota transplantation in relapsing and refractory community- and hospital-acquired Clostridium difficile infection. Can J Gastroenterol Hepatol. 2014. September;28(8):434–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cammarota G, Ianiro G, Gasbarrini A, Masucci L, Sanguinetti M. Faecal transplantation for Clostridium difficile infection. Three cases treated in Italy. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2014. May;46(5):475. [DOI] [PubMed] [Google Scholar]
- 54.Pathak R, Enuh HA, Patel A, Wickremesinghe P. Treatment of relapsing Clostridium difficile infection using fecal microbiota transplantation. Clin Exp Gastroenterol. 2013;7:1–6. 10.2147/CEG.S53410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012. May;107(5):761–7. 10.1038/ajg.2011.482 [DOI] [PubMed] [Google Scholar]
- 56.Mattila E, Uusitalo-Seppälä R, Wuorela M, Lehtola L, Nurmi H, Ristikankare M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology. 2012. March;142(3):490–6. 10.1053/j.gastro.2011.11.037 [DOI] [PubMed] [Google Scholar]
- 57.Rohlke F, Surawicz CM, Stollman N. Fecal flora reconstitution for recurrent Clostridium difficile infection: results and methodology. J Clin Gastroenterol. 2010. September;44(8):567–70. 10.1097/MCG.0b013e3181dadb10 [DOI] [PubMed] [Google Scholar]
- 58.Yoon SS, Brandt LJ. Treatment of refractory/recurrent C. difficile-associated disease by donated stool transplanted via colonoscopy: a case series of 12 patients. J Clin Gastroenterol. 2010. September;44(8):562–6. 10.1097/MCG.0b013e3181dac035 [DOI] [PubMed] [Google Scholar]
- 59.Rubin TA, Gessert CE, Aas J, Bakken JS. Fecal microbiome transplantation for recurrent Clostridium difficile infection: report on a case series. Anaerobe. 2013. February;19:22–6. 10.1016/j.anaerobe.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 60.Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003. March 1;36(5):580–5. 10.1086/367657 [DOI] [PubMed] [Google Scholar]
- 61.Kassam Z, Hundal R, Marshall JK, Lee CH. Fecal transplant via retention enema for refractory or recurrent Clostridium difficile infection. Arch Intern Med. 2012. January 23;172(2):191–3. 10.1001/archinte.172.2.191 [DOI] [PubMed] [Google Scholar]
- 62.Silverman MS, Davis I, Pillai DR. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol. 2010. May;8(5):471–3. 10.1016/j.cgh.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 63.Tvede M, Rask-Madsen J. Bacteriotherapy for chronic relapsing Clostridium difficile diarrhoea in six patients. Lancet Lond Engl. 1989. May 27;1(8648):1156–60. [DOI] [PubMed] [Google Scholar]
- 64.Wenisch JM, Schmid D, Kuo H-W, Simons E, Allerberger F, Michl V, et al. Hospital-acquired Clostridium difficile infection: determinants for severe disease. Eur J Clin Microbiol Infect Dis. 2012. August;31(8):1923–30. 10.1007/s10096-011-1522-5 [DOI] [PubMed] [Google Scholar]
- 65.Synnott K, Mealy K, Merry C, Kyne L, Keane C, Quill R. Timing of surgery for fulminating pseudomembranous colitis. Br J Surg. 1998. February;85(2):229–31. 10.1046/j.1365-2168.1998.00519.x [DOI] [PubMed] [Google Scholar]
- 66.Perera AD, Akbari RP, Cowher MS, Read TE, McCormick JT, Medich DS, et al. Colectomy for fulminant Clostridium difficile colitis: predictors of mortality. Am Surg. 2010. April;76(4):418–21. [DOI] [PubMed] [Google Scholar]
- 67.Byrn JC, Maun DC, Gingold DS, Baril DT, Ozao JJ, Divino CM. Predictors of mortality after colectomy for fulminant Clostridium difficile colitis. Arch Surg Chic Ill 1960. 2008. February;143(2):150–154; discussion 155. [DOI] [PubMed] [Google Scholar]
- 68.ASGE Standards of Practice Committee, Fisher DA, Maple JT, Ben-Menachem T, Cash BD, Decker GA, et al. Complications of colonoscopy. Gastrointest Endosc. 2011. October;74(4):745–52. 10.1016/j.gie.2011.07.025 [DOI] [PubMed] [Google Scholar]
- 69.Olson MM, Shanholtzer CJ, Lee JT, Gerding DN. Ten years of prospective Clostridium difficile-associated disease surveillance and treatment at the Minneapolis VA Medical Center, 1982–1991. Infect Control Hosp Epidemiol. 1994. June;15(6):371–81. [DOI] [PubMed] [Google Scholar]
- 70.Miller MA, Hyland M, Ofner-Agostini M, Gourdeau M, Ishak M, Canadian Hospital Epidemiology Committee. Canadian Nosocomial Infection Surveillance Program. Morbidity, mortality, and healthcare burden of nosocomial Clostridium difficile-associated diarrhea in Canadian hospitals. Infect Control Hosp Epidemiol. 2002. March;23(3):137–40. 10.1086/502023 [DOI] [PubMed] [Google Scholar]
- 71.Karas JA, Enoch DA, Aliyu SH. A review of mortality due to Clostridium difficile infection. J Infect. 2010. July;61(1):1–8. 10.1016/j.jinf.2010.03.025 [DOI] [PubMed] [Google Scholar]
- 72.Hayes JL, Hansen P. Is laparoscopic colectomy for cancer cost-effective relative to open colectomy? ANZ J Surg. 2007. September;77(9):782–6. 10.1111/j.1445-2197.2007.04226.x [DOI] [PubMed] [Google Scholar]
- 73.Vermeulen J, Gosselink MP, Busschbach JJV, Lange JF. Avoiding or reversing Hartmann’s procedure provides improved quality of life after perforated diverticulitis. J Gastrointest Surg. 2010. April;14(4):651–7. 10.1007/s11605-010-1155-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Youngster I, Sauk J, Pindar C, Wilson RG, Kaplan JL, Smith MB, et al. Fecal Microbiota Transplant for Relapsing Clostridium difficile Infection Using a Frozen Inoculum From Unrelated Donors: A Randomized, Open-Label, Controlled Pilot Study. Clin Infect Dis. 2014. June 1;58(11):1515–22. 10.1093/cid/ciu135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Link A, Lachmund T, Schulz C, Weigt J, Malfertheiner P. Endoscopic peroral jejunal fecal microbiota transplantation. Dig Liver Dis. 2016. August 12; [DOI] [PubMed] [Google Scholar]
- 76.Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013. April;108(4):500–8. 10.1038/ajg.2013.59 [DOI] [PubMed] [Google Scholar]
- 77.WHO Commission on Macroeconomics and Health. Macroeconomics and health: investing in health for economic development Report of the Commission on Macroeconomics and Health. Geneva: World Health Organization; 2001. [Google Scholar]
- 78.Insee—Tableau—1.115—Produit intérieur brut et revenu national brut par habitant (Milliers de personnes, Milliards d’euros et Euros par personne). http://www.insee.fr/fr/themes/comptes-nationaux/tableau.asp?sous_theme=1&xml=t_1115
- 79.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health J Int Soc Pharmacoeconomics Outcomes Res. 2013. April;16(2):e1–5. [DOI] [PubMed] [Google Scholar]
- 80.Dodin M, Katz DE. Faecal microbiota transplantation for Clostridium difficile infection. Int J Clin Pract. 2014. March;68(3):363–8. 10.1111/ijcp.12320 [DOI] [PubMed] [Google Scholar]
- 81.Aroniadis OC, Brandt LJ. Intestinal Microbiota and the Efficacy of Fecal Microbiota Transplantation in Gastrointestinal Disease. Gastroenterol Hepatol. 2014. April;10(4):230–7. [PMC free article] [PubMed] [Google Scholar]
- 82.Tannock GW, Munro K, Taylor C, Lawley B, Young W, Byrne B, et al. A new macrocyclic antibiotic, fidaxomicin (OPT-80), causes less alteration to the bowel microbiota of Clostridium difficile-infected patients than does vancomycin. Microbiol Read Engl. 2010. November;156(Pt 11):3354–9. [DOI] [PubMed] [Google Scholar]
- 83.Di Bella S, Gouliouris T, Petrosillo N. Fecal microbiota transplantation (FMT) for Clostridium difficile infection: focus on immunocompromised patients. J Infect Chemother. 2015. April;21(4):230–7. 10.1016/j.jiac.2015.01.011 [DOI] [PubMed] [Google Scholar]
- 84.Kelly CR, Ihunnah C, Fischer M, Khoruts A, Surawicz C, Afzali A, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014. July;109(7):1065–71. 10.1038/ajg.2014.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee CH, Steiner T, Petrof EO, Smieja M, Roscoe D, Nematallah A, et al. Frozen vs Fresh Fecal Microbiota Transplantation and Clinical Resolution of Diarrhea in Patients With Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA. 2016. January 12;315(2):142–9. 10.1001/jama.2015.18098 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.