Abstract
Background
Buprenorphine is an FDA-approved maintenance therapy for opioid use disorders and is increasingly being used in pregnant women with opioid use disorders as an alternative to methadone. Dosing of buprenorphine in pregnant women is based on the regimen recommended for non-pregnant females and males. Limited data are available defining the pharmacokinetic (PK) properties of sublingual (SL) buprenorphine administered during pregnancy.
Objective
This study evaluated the impact of physiological changes associated with pregnancy on the PK of sublingual buprenorphine during and after pregnancy.
Study Design
Pregnant women (N=13), between 18 0/7 and 37 6/7 weeks’ singleton gestation, receiving sublingual buprenorphine twice daily for opioid use disorders were studied. PK-2 studies were performed between 18–25 weeks (N=7), PK-3 studies were performed between 31–37 weeks (N=11), and PK-P was performed 4–18 weeks postpartum (N=10). On the day of study, blood was withdrawn prior to the daily morning dose of buprenorphine and at 0.25, 0.5, 0.75, 1, 1.5, 2, 4, 8 and 12h post-dose. Buprenorphine plasma concentrations were analyzed by LCMS-MS. All PK parameters were observed or estimated using Microsoft Excel. Statistical analyses were performed to identify significant changes in study participants’ buprenorphine pharmacokinetic parameter estimates over the duration of the study. Univariate linear and generalized linear mixed models were employed to investigate changes in these measures over time, some of which were log transformed for normality.
Results
Dose-normalized (plasma concentration/dose) buprenorphine plasma concentrations were significantly lower during pregnancy (PK-2 + PK-3) than during the postpartum period (PK-P). Specific PK parameters (and level of significance) were as follows: the area under the BUP plasma concentration-time curves (AUC0→12, p<0.003), maximum BUP concentrations (Cmax, p<0.018), average BUP concentrations (Cavg, p<0.003), BUP concentrations at 0h (C0, p<0.002) and BUP concentrations at 12h (C12, p<0.001). None of these parameters differed significantly during pregnancy (ie PK-2 vs PK-3). The time to maximum BUP concentrations (Tmax) did not differ significantly between groups.
Conclusion
The dose-normalized plasma concentrations during a dosing interval and the overall exposure of BUP (AUC0→12) are lower throughout pregnancy compared to the postpartum period. This indicates an increase in apparent clearance of BUP during pregnancy. These data suggest that pregnant women may need a higher dose of sublingual buprenorphine compared to postpartum individuals. The dose of buprenorphine should be assessed after delivery to maintain similar buprenorphine exposure during the postpartum period.
Keywords: buprenorphine, plasma concentration, exposure, pregnancy, pharmacokinetics
Introduction
Opioid use disorders in America have increased at an alarming rate during the past decade. Among pregnant women aged 15–44, 5.4% admitted to currently using illicit drugs, with the highest rates during the first (9%) and second (4.8%) trimester compared with the third (2.4%) trimester.1 Untreated substance use during pregnancy not only increases maternal pregnancy complications, but also increases fetal risk. Specifically, untreated chronic heroin use is associated with an increased risk of pregnancy complications, such as fetal growth restriction, placental abruption, fetal death, preterm labor, 3rd trimester bleeding, fetal distress, meconium aspiration and puerperal morbidity.2, 3
Currently, methadone is most often prescribed as first-line pharmacotherapy for a pregnant woman with opioid use disorders, but buprenorphine use has increased,3 as recent evidence suggests comparable efficacy and less severe neonatal complications with buprenorphine compared to methadone.4 Dosing of buprenorphine is based mostly on expert panel recommendations and subjective data collected from the patient, and is dose-adjusted using patient symptoms of withdrawal. Despite such recommendations, there is a lack of consensus concerning appropriate induction and maintenance dosing, monitoring parameters, and duration of therapy due to a wide range of clinical and contextual factors and concerns about diversion.5 Buprenorphine is extremely lipophilic, highly bound to plasma proteins and mainly metabolized by CYP3A4 and UGT1A/2B.6 Pregnancy can substantially alter drug absorption, distribution, metabolism and/or elimination, possibly leading to changes in the most effective dose or dosing regimen that should be employed in this specific patient population.7
The current investigation examined the pharmacokinetics (PK) of buprenorphine administered sublingually during and after pregnancy in order to determine whether there are differences in the PK estimates during pregnancy, as well as between pregnancy and the postpartum period.
Materials and Methods
Participants
Women (N=17) were recruited from Magee-Womens Hospital and an outlying clinic. Participants were recruited and enrolled from June 1, 2014 through November 30, 2014. All participants were receiving twice-daily buprenorphine maintenance therapy, as prescribed for clinical purposes by their respective caregivers, and were expected to be at steady state on their current dose prior to each study visit. The protocol was approved by the University of Pittsburgh’s Institutional Review Board (IRB) and all participants underwent the informed consent process using IRB-approved consent documents.
Procedures
Demographic details, baseline laboratory parameters, medication and/or substance use history and obstetric history were collected for all participants. Eligibility criteria for these women included: (1) pregnant and on a stable, twice daily dose of buprenorphine for at least 7 days, (2) ≥18 years of age, (3) ability to willingly consent, and (4) willing to have urine samples screened for the presence of alcohol, barbiturates, opiates, cocaine (or metabolites), benzodiazepines, synthetic opioids, PCP and concurrent medications or substances. Exclusion criteria included: (1) hepatic or renal dysfunction, (2) sickle-cell disease and on active treatment, (3) HIV and on active treatment (due to potential drug interactions), (4) hypersensitivity to opioids, (5) co-morbid dependence on benzodiazepines, (6) concurrently taking monoamine oxidase inhibitors, neuroleptics or disulfiram, and (7) concurrently taking medications known to be CYP3A inducers (such as rifampin, phenobarbital, phenytoin, carbamazepine) or CYP3A inhibitors (such as azole antifungals, macrolide antibiotics or HIV protease inhibitors).
Up to three studies were performed in each participant. PK-2 studies were performed in the second trimester between 18–25 weeks (N=7), PK-3 studies were performed in the third trimester between 31–37 weeks (N=11), and PK-P was performed 4–18 weeks postpartum (N=10). All PK study visits were identical and conducted in the Clinical and Translational Research Center (CTRC) of the University of Pittsburgh Medical Center’s (UPMC) Montefiore Hospital. Participants arrived early in the morning after a ≥8h fast with their medication log, underwent a pre-dose blood draw (BD Vacutainer™ Glass Blood Collection Tubes with Sodium Heparin, BD Inc.) and oral fluid collection (Sarstedt Salivette Cotton Swab for Saliva Collection, Sarstedt Inc.) for trough buprenorphine concentrations and clinical laboratory parameters, provided a urine sample for laboratory tests and toxicology, had oral pH recorded (Hydrion™ Urine and Saliva pH Paper, Range 5.5–8.0, Micro Essential Lab), and then took their prescribed sublingual buprenorphine dose under direct supervision by study staff. Participants were instructed to allow the tablets to dissolve under their tongue, with minimal swallowing, until there was no visible residue remaining. Dissolution time was recorded after visual inspection of the sublingual area by study staff. Serial blood and oral fluid samples were subsequently collected over one dosing interval at 0 (pre-dose), 0.25, 0.5, 0.75, 1, 1.5, 2, 4, 8, 10 and 12h post-dose. All blood samples and oral fluid salivettes were centrifuged for 15m at 15,000 rpm to obtain plasma and oral fluid, respectively. Samples were immediately frozen at −80°C until analysis. All spontaneously voided urine was collected throughout the entire study period of 12h. Participants were not allowed to eat until 2h post-dose to allow for adequate drug absorption, and all food consumed during the study day was reported on a dietary log.
Assay Methodology
Buprenorphine (BUP) and its three active metabolites: norbuprenorphine (NBUP), buprenorphine glucuronide (BUPG) and norbuprenorphine glucuronide (NBUPG), along with the deuterated internal standards for BUP, NBUP and NBUPG (BUPG-was not available), were extracted from plasma samples by solid phase extraction methods. Plasma concentrations were determined using high performance liquid chromatography with tandem mass spectrometric detection (LCMS-MS). The peaks of interest were well separated and the overall run time for each sample was 7 minutes. Detection was accomplished utilizing ion spray tandem mass spectrometry in positive ion multiple reaction monitoring mode. The lower limit of quantification was 0.05 ng/mL for BUP and calibration curves were linear, ranging from 0.05–50 ng/mL for BUP with with coefficients of determination (r2) greater than 0.99. Both the intra-day and inter-day precisions were evaluated and the co-efficient of variation values were less than 15% for low, medium, and high controls. The accuracy as measured by bias was less than 5%. Cumulative urine and serial oral fluid samples were also collected and concentrations measured (data not shown).
Statistical Analysis
Non-compartmental analysis was performed and various PK parameters were calculated using Microsoft Excel. Maximum BUP plasma concentrations (Cmax), trough BUP concentrations at time zero (C0), BUP concentrations at twelve hours (C12), and time to maximum BUP concentrations (Tmax) were observed values using each participant’s plasma concentration-time profile. In each of the participants, the area under the BUP plasma concentration-time curve for the 12h dosing interval (AUC0→12) was calculated from time 0 to 12h using the trapezoidal rule. Mean values were then used to compare cohorts (see Table 2). Given the reportedly long half-life of buprenorphine (approximately 37h)7, and the shorter dosing interval (12h) used in the study participants, it was not possible to calculate terminal half-life.
Revised Table 2.
Buprenorphine pharmacokinetic parameters
| Parameter (untransformed raw data, Mean ± SD) | PK-2 (2nd Trimester) N = 7 |
PK-3 (3nd Trimester) N = 11 |
PK-P (Postpartum) N = 10 |
p valuei (PK-2:PK-3) | p valueii (½PK-2+½PK-3:PK-P) |
|---|---|---|---|---|---|
| Dose, mg | 8.0 ± 3.1 | 10.0 ± 3.7 | 8.0 ± 0.0 | 0.3333 | 0.0276 |
| Tmax, h | 1.6 ± 2.8 | 1.0 ± 1.1 | 1.9 ± 3.6 | 0.9661 | 0.8411 |
| Dose-normalized Cmax, ng/ml | 0.5 ± 0.1 | 0.5 ± 0.2 | 0.7 ± 0.3 | 0.9964 | 0.0365 |
| Dose-normalized C0, ng/ml | 0.1 ± 0.0 | 0.2 ± 0.2 | 0.3 ± 0.3 | 0.8959 | 0.0032 |
| Dose-normalized C12, ng/ml | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.3 ± 0.3 | 0.1260 | 0.0001 |
| Dose-normalized Cavg, ng/ml | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.2 | 0.7965 | 0.0068 |
| Dose-normalized AUC, ng* h/mL | 1.9 ± 1.4 | 2.2 ± 1.2 | 4.0 ± 2.5 | 0.7953 | 0.0068 |
Tmax, time to maximum concentration; Dose-norm, Dose-normalized or plasma concentration/dose; Cmax, maximum plasma concentration; C0, time zero trough plasma concentration; C12, twelve-hour plasma concentration; Cavg, average plasma concentration from time zero to twelve-hours; AUC0→12, area under the plasma concentration-time curve from time zero to twelve-hours
Data presented as mean ± sd (raw and untransformed)
P-value columns represent comparisons between:
2nd vs 3rd trimester: PK-2 vs PK-3
Pregnancy vs Postpartum: the average of PK-2 & PK-3 vs PK-P
Univariate descriptive statistics were used to summarize sample demographic and clinical characteristics at each study visit. Means and standard deviations were reported for continuous variables, and frequencies and percentages were reported for categorical variables. BUP pharmacokinetic data were similarly summarized descriptively at each study visit using means and standard deviations. Statistical analyses were then performed to identify significant differences in BUP PK parameters between (1) PK-2 and PK-3 and (2) pregnancy and postpartum, without focus on a specific trimester of pregnancy.
The distributions of sample PK data were investigated, and a natural logarithmic transformation was applied to each of the PK variables in order to produce approximately normally distributed data for statistical analysis. Univariate linear mixed models were then fit to the log-transformed PK data to make the two comparisons of interest, as such modeling techniques utilize data from every participant and do not require that participants have complete data at all three study visits. Time (PK-2, PK-3 and PK-P) was treated as a categorical fixed effect in all models, and a random effect was included in the models to account for repeated measures over time from the same participants. For the random effect of time, a variance component covariance structure was assumed. Models were fit through maximum likelihood estimation in SAS version 9.4 (SAS Institute, Cary, NC), and convergence criteria were met without problems.
The fitted models were then used to compare PK parameters between the 2nd and 3rd trimesters, as well as between pregnancy and postpartum, using t-tests. Specifically, the first comparison tested whether the log-transformed PK parameter differed significantly between PK-2 and PK-3. For the second comparison, a formal hypothesis test was performed to determine if the log-transformed mean at PK-P differed significantly from the average of the log-transformed means at PK-2 and PK-3. The two p-values were adjusted for multiple comparisons using the Sidak adjustment method. A p-value < 0.05 indicated a statistically significant difference.
Results
Seventeen participants provided informed consent for the study. Two participants were excluded after baseline assessment: one for a failed urine comprehensive drug screen and one for an ectopic pregnancy. One participant was lost to follow-up after consent and was never screened. Thus, data from 14 pregnant women were available for pharmacokinetic analysis and 6 of these 14 participants were studied at all three time points.
Among these 14 participants, a total of 35 pharmacokinetic studies were completed: nine in PK-2, thirteen in PK-3 and thirteen in PK-P. Of these 35 studies, 7 were deemed invalid: two participants’ data was excluded from the PK-2 analysis and two participants’ data was excluded from PK-3 analysis because the buprenorphine concentration at time 0 was significantly higher than the buprenorphine concentration at 12h, indicating participants had an atypical concentration-time profile and were likely not adherent to a 12h dosing interval. Data from two participants were excluded from the PK-P analysis since they were switched by their providers to Suboxone® film (buprenorphine/naloxone) at the time of study, which may have different pharmacokinetic characteristics than sublingual buprenorphine tablets. Data from one participant was also excluded from the PK-P analysis after her medication log showed once daily rather than twice daily buprenorphine dosing. This resulted in 28 studies with pharmacokinetic data being available for further analysis (18 from the 6 participants that were studied at all three time points): seven for PK-2, eleven for PK-3, and ten for PK-P.
The characteristics of the study participants are listed in Table 1 according to the time of study. The mean gestational ages were 22.0 weeks for PK-2 and 33.9 weeks for PK-3. The PK-P study was performed at a mean of 7.4 weeks post-delivery. All but one participant studied postpartum (PK-P) was also studied at least once in pregnancy (PK-2 or PK-3 or both).
Revised Table 1.
Characteristics of study participants
| Characteristic (Mean ± SD) | PK-2 (2nd Trimester) N = 7 |
PK-3 (3nd Trimester) N = 11 |
PK-P (Postpartum) N = 10 |
|---|---|---|---|
| Age, y | 27.3 ± 5.0 | 27.9 ± 5.2 | 28.6 ± 5.6 |
| Smoker, current | 5 (71.4%) | 8 (72.7%) | 7 (70.0%) |
| Parity, nulliparous | 2 (28.6%) | 3 (27.3%) | 2 (20.0%) |
| Twice daily dose, mg | 8.0 ± 3.1 Range: 2 – 12 |
10.0 ± 3.7 Range: 2 – 16 |
8.0 ± 0.0 |
| Gestational age, wk | 22.0 ± 2.5 | 34.0 ± 2.2 | -- |
| Postpartum, wk | -- | -- | 7.1 ± 4.2 |
| Bodyweight, kg | 73.7 ± 7.6 | 74.1 ± 8.3 | 66.5 ± 9.4 |
| BMI, kg/m2 | 28.5 ± 3.8 | 28.6 ± 3.9 | 25.3 ± 4.6 |
| Waist circumference, in | 39.3 ± 3.1 | 42.0 ± 3.5 | 34.6 ± 3.9 |
| Albumin, g/dL | 3.4 ± 0.3 | 3.2 ± 0.3 | 4.0 ± 0.2 |
| Total Protein, g/dL | 5.9 ± 0.4 | 6.0 ± 0.4 | 6.6 ± 0.6 |
BMI, body mass index
Data presented as mean ± sd or number (%), as appropriate
The PK parameter estimates are summarized in Table 2. During pregnancy (average of PK-2 + PK-3), the dose-normalized (plasma concentration/dose) area under the BUP plasma concentration-time curves (AUC0→12), maximum BUP concentrations (Cmax), as well as the BUP concentrations at 0 and 12h were significantly lower than during the postpartum period. None of these parameters differed significantly during pregnancy (ie PK-2 vs PK-3). The time to maximum BUP concentrations (Tmax) did not differ significantly between groups.
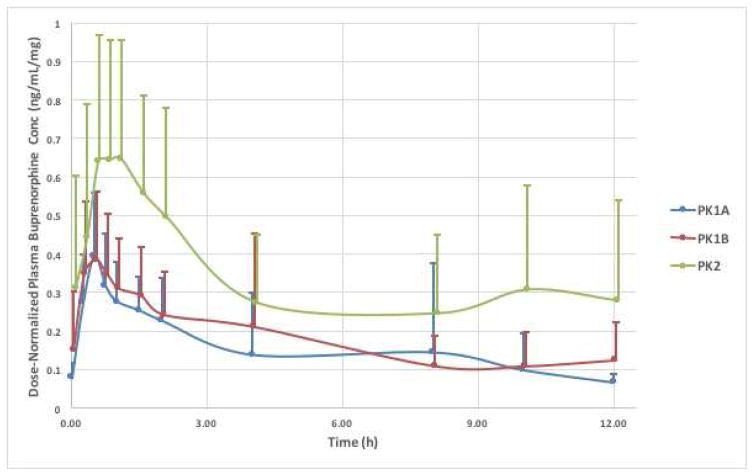
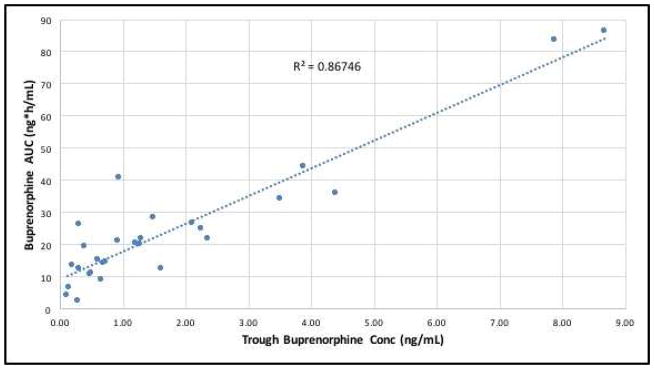
Figure 1 demonstrates the dose-normalized (plasma concentration/dose) average BUP plasma concentration-time curves (mean ± SD) over the 12h study in all participants (PK-2, n=7; PK-3, n=11; PK-P, n=10). Plasma concentrations rise rapidly in all study groups, start approaching trough (C0) concentrations by 4–6h, and nearly reach baseline values by 8h after drug administration. There is considerable variability in all groups, yet the mean buprenorphine plasma concentrations differed significantly between pregnancy and postpartum, as well as between third trimester and postpartum (ie PK-3 and PK-P) Figure 2 relates the raw trough plasma concentrations of buprenorphine to the area under the plasma-concentration time curves (AUC0→12) from all 28 studies. There is a strong correlation (coefficient of determination r2= 0.87) between the trough concentration just prior to a dose (C0) and the area under the plasma concentration time curve over the ensuing 12h. This relationship indicates that a single plasma sample concentration measurement prior to the next dose may be a surrogate marker of buprenorphine exposure in participants on a fixed dose and dosing regimen (the coefficient of determination is 0.57 when the 2 outliers are removed).
Figure 1. Dose-Normalized Buprenorphine Plasma Concentrations during Pregnancy and Postpartum.
The mean dose-normalized buprenorphine plasma concentration-time curves (±SD) during the 12h pharmacokinetic study visits: PK1a (n=7), PK1b (n=11) and PK2 (n=10). X axis = time in hours; Y axis = mean dosenormalized buprenorphine plasma concentrations in ng/mL per mg of buprenorphine.
Figure 2. Relationship between Trough Buprenorphine Plasma Concentrations (C0) and Area Under the Plasma Concentration-Time Curve (AUC0→12).
The relationship between trough buprenorphine plasma concentrations at time 0h (C0) and area under the plasma concentration-time curve (AUC0→12) during the 12h pharmacokinetic study visits (n=28). X axis = trough buprenorphine plasma concentrations at time 0h in ng/mL; Y axis = area under the buprenorphine plasma concentration-time curve during the 12h pharmacokinetic study visits in ng*h/mL
The average oral pH for all participants were as follows (mean ± SD): PK-2, 6.2 ± 0.44; PK-3, 6.4 ± 0.67; PK-P, 6.5 ± 0.71, as measured by salivary pH strips. There was no correlation between oral pH and dissolution time, AUC0→12, Cmax or Tmax in any of the three groups (data not shown).
Comment
This study demonstrates that dose-normalized exposure (AUC0→12) to buprenorphine following sublingual administration was approximately 50% lower during pregnancy compared with the postpartum period. These findings are consistent with the study of Concheiro et al who reported similar findings in their study of three pregnant women (1 with twin gestation).8 Our larger sample size and restriction to singleton gestation, however, allowed for more meaningful comparisons between pregnancy and the postpartum state.
Our findings are not unexpected given the physiological changes associated with pregnancy and the specific pharmacological characteristics of buprenorphine, which may impact the absorption, distribution, metabolism and/or elimination of this medication throughout gestation. The major contributor to the differences in buprenorphine exposure between pregnant and postpartum women is predicted to be changes in metabolism. Buprenorphine is cleared from the body through metabolism that involves CYP3A and UGT enzymes.9 The activity of CYP3A, the primary enzyme responsible for the metabolism of buprenorphine to norbuprenorphine, has been shown to be significantly increased during pregnancy. Buprenorphine and norbuprenorphine are conjugated to their respective glucuronide metabolites by UGT1A1, UGT1A3 and UGT2B7. Activity of glucuronide conjugating enzymes has also been shown to increase during pregnancy (specifically UGT1A and UGT2B enzymes).10
Buprenorphine is a small, very lipophilic compound (log P = 4.98) that is highly bound to plasma proteins (% bound = 98%).11 In pregnancy, protein levels decrease and maternal body fat increases leading to a larger volume of distribution for drugs like buprenorphine.12 This is consistent with our observation of a significant decrease in Cmax during both PK1a and PK1b as compared to PK2, as well as significant differences in protein levels and body weights of the study participants in each cohort.
The absorption and dissolution of sublingual buprenorphine could be affected by salivary pH, which generally decreases in pregnancy.13 A low salivary pH can reduce absorption, as less of the drug would be unionized, and this could contribute to a smaller AUC during pregnancy. In our population, salivary pH did not differ between groups, and therefore, was not correlated with changes in AUC0→12, Cmax, Tmax nor dissolution time. Absorption may also be influenced by pill size. Many participants in the current study chose to break up the sublingual buprenorphine tablet(s) into smaller pieces for convenience, comfortable placement in the sublingual area, as well as for taste-masking purposes to limit nausea while allowing the tablet(s) to dissolve. We did not control for this at the time of the study, which may account for some of the variation in plasma concentrations. However, this is also unlikely to account for the changes observed, for a recent publication did not demonstrate significant differences in dissolution nor absorption time between crushed and whole buprenorphine tablets.14
The clinical implications of the pharmacological findings of this study relate primarily to dosing of buprenorphine during pregnancy. Dosing of buprenorphine during pregnancy is generally based on data from non-pregnant adults. This approach has repeatedly been shown to lead to errors in dosing pregnant women with a variety of medications.15 Depending on the specific pharmacokinetic properties of the drug in question, either plasma concentrations are too high leading to potential side effects or plasma concentrations are too low leading to possible treatment failure. Such considerations have not been adequately incorporated into the dosing of buprenorphine for pregnant women. The failure rate during the induction period with buprenorphine has been reported to be as high as 33% in pregnant participants,4 and it is conceivable that some of these failures are due to inadequate buprenorphine plasma concentrations. Indeed, our data indicate that buprenorphine is cleared more extensively by pregnant than postpartum women. An inadequate dose may contribute both to abandonment of buprenorphine during the induction phase and continued illegal substance use during the stabilization and maintenance phases.
The 2015 recommendations by the Substance Abuse and Mental Health Services Administration (SAMHSA) did not recommend specific dosing strategies for pregnant women but rather refer to the package insert to determine the dose of buprenorphine.16 Unfortunately, the FDA-approved monograph does not distinguish dosing for pregnant women from dosing for non-pregnant individuals. A total daily dose of 8 – 16 mg is suggested during induction. To minimize dropout, the package insert suggests that the dose be “rapidly titrated to achieve clinical effectiveness” during the stabilization phase. This strategy of dosing to response, which recent literature shows may be between 16 – 24 mg per day for clinical effectiveness,17 is reasonable for pregnancy but since higher doses may be needed in pregnant women, implementation of this strategy may lead to obstacles. First, some insurers will only reimburse for doses up to 16 mg daily without peer review by an insurance intermediary, usually a physician but not necessarily an expert in drug addiction or obstetrical pharmacokinetics. The patient’s care provider is then obligated to explain why a higher dose is needed and justification may prove challenging. These not so subtle policies serve as an impediment to optimal care during pregnancy, when the risk of neonatal abstinence syndrome (NAS) from continued use of illegal opioids far outweighs insurance limits. Secondly, a need for a higher dose also raises the question of diversion both in the minds of the care provider and the insurance representative, yet our data provide prescribers the support they need in order to “titrate to clinical effectiveness” without fear of legal repercussions. Hopefully our data will serve as evidence to policy makers, insurers, and physicians alike that pregnant women may need higher doses of buprenorphine compared to non-pregnant adults simply because of the pregnancy-associated physiological changes that directly affect the pharmacokinetic properties of buprenorphine.
It is not clear from the present study at what time during pregnancy the more extensive clearance of buprenorphine occurs. Since many pregnant women undergo conversion to buprenorphine in the first half of pregnancy, it is important to determine when and how often dosing adjustments should be made during and after pregnancy, for both the prescriber’s benefit and that of the maternal-fetal dyad.
Acknowledgments
Dr Bastian was a T-32 scholar at the University of Pittsburgh and was supported by a grant from the National Institutes of Health (NIH) Ruth Kirschstein T-32 training grant (HD 071859) and in part by Grant No. 2 P50 DA005605-19 A2 from the NIH National Institute on Drug Abuse (NIDA). Drug analysis was supported in part by Grant No. HD047905 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
Funding sources had no involvement in the study design; collection, analysis and interpretation of the data; in the writing of the report; nor the decision to submit the article for publication.
The study team acknowledges the help and support of Michael England, MD, Stephanie Bobby, RN, Dawn Fischer, RN, and Donna DeAngelis, RN in the recruitment and screening of the participants in this study. We appreciate the advice provided by Frank Kunkle, MD and for the opportunity to visit his Suboxone® clinic. We are deeply thankful for the efforts of all the CTRC research nurses, dieticians and technicians who facilitated all study visits and specimen collections on behalf of UPMC. We also appreciate the statistical analysis provided by Mei Yang, MSc/MBA, on the preliminary data.
Footnotes
Conflicts of Interest: Drs Bastian, Rothenberger, Tarter, English, Venkataramanan and Caritis have no conflicts to report. Ms. Chen and Zhang also report no conflicts of interest.
The contents of this article are the views of the authors and do not necessarily represent the official view of the National Institutes of Health or National Institute on Drug Abuse.
The findings of this article were presented at the 36th annual meeting – The Pregnancy Meeting, Society for Maternal-Fetal Medicine, Atlanta, GA, February 1–6th, 2016.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Center for Behavioral Health Statistics and Quality. Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health. 2015 (HHS Publication No. SMA 15-4927, NSDUH Series H-50). Available at: http://www.samhsa.gov/data/
- 2.Center for Substance Abuse Treatment. SAHMSA/CSAT treatment improvement protocols. Rockville (MD): Substance Abuse and Mental Health Services Administration; 2008. Medication-assisted treatment for opioid addiction during pregnancy. Available at: http://www.ncbi.nlm.nih.gov/books/NBK26113. [PubMed] [Google Scholar]
- 3.Minozzi S, et al. Maintenance agonist treatment for opiate dependent pregnant women (review) Cochrane Database of Systemic Reviews. 2013;(12) doi: 10.1002/14651858.CD006318.pub3. Art No.: CD006318. [DOI] [PubMed] [Google Scholar]
- 4.Jones HE, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. NEJM. 2010;363:2320–31. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farmer CM, et al. Practice guidance for buprenorphine for the treatment of opioid use disorders: results of an expert panel process. Subst Abuse. 2015;36(2):209–16. doi: 10.1080/08897077.2015.1012613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benckiser Reckitt. Buprenorphine sublingual tablets [prescribing information] Columbus, OH: Roxane; Feb, 2015. Available at: http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6483#synlist. [Google Scholar]
- 7.Mattison D, et al. Clinical pharmacology during pregnancy. Academic Press Publications, Elsevier Inc; San Francisco, CA: 2013. [Google Scholar]
- 8.Conchiero, et al. Preliminary buprenorphine sublingual tablet pharmacokinetic data in plasma, oral fluid and sweat during treatment of opioid-dependent pregnant women. Ther Drug Monit. 2011;33(5):619–626. doi: 10.1097/FTD.0b013e318228bb2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elkader A, Sproule B. Buprenorphine – Clinical pharmacokinetics in the treatment of opioid dependence. Clin Pharmacokinet. 2005;44(7):661–680. doi: 10.2165/00003088-200544070-00001. [DOI] [PubMed] [Google Scholar]
- 10.Anderson G. Pregnancy-induced changes in pharmacokinetics: a mechanistic based approach. Clin Pharmacokinet. 2005;44(10):989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- 11.Elkader A, Sproule B. Buprenorphine – Clinical pharmacokinetics in the treatment of opioid dependence. Clin Pharmacokinet. 2005;44(7):661–680. doi: 10.2165/00003088-200544070-00001. [DOI] [PubMed] [Google Scholar]
- 12.Feghali M, Venkataramanan R, Caritis SN. Pharmacokinetics of drugs in pregnancy. Semin Perinatol. 2015 Nov;39(7):512–9. doi: 10.1053/j.semperi.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain K, Harshaminder K. Prevalence of oral lesions and measurement of salivary pH in the different trimesters of pregnancy. Singapore Med J. 2015;56(1):53–57. doi: 10.11622/smedj.2015010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simojoki K, et al. Bioavailability of buprenorphine from crushed and whole buprenorphine (Subutex) tablets. Eur Addict Res. 2010;16(2):85–90. doi: 10.1159/000279766. [DOI] [PubMed] [Google Scholar]
- 15.Caritis SN, Venkataramanan R, Cotroneo M, Chiao JP. Pharmacokinetics of orally administered ritodrine. AJOG. 1989 Jul;161(1):32–5. doi: 10.1016/0002-9378(89)90225-1. [DOI] [PubMed] [Google Scholar]
- 16.Substance Abuse and Mental Health Services Administration. Federal Guidelines for Opioid Treatment Programs. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015. HHS Publication No. (SMA) PEP15-FEDGUIDEOTP. [Google Scholar]
- 17.Guidelines for the psychosocially assisted pharmacological treatment for opioid dependence. Geneva: World Health Organization (WHO); 2009. Available at: http://www.ncbi.nlm.nih.gov/books/NBK143185/ [PubMed] [Google Scholar]