Abstract
Background
Intestinal barrier dysfunction has been implicated in necrotizing enterocolitis (NEC), but has not been directly measured in human NEC.
Methods
Small intestines removed during surgery were immediately mounted in an Ussing chamber. mRNA expression of tight junction (TJ) proteins was measured with RT-PCR.
Results
Fifteen infants were included, 5 with NEC and 10 with other diagnoses. Average transepithelial resistance (TER) was 11.61 ± 1.65 Ω/cm2 in NEC specimens, 23.36 ± 1.48 Ω/cm2 at resection margin, and 46.48 ± 5.65 Ω/cm2 in controls. Average flux of permeability marker mannitol was 0.23 ± 0.06 μMol/cm2 per h in NEC, 0.04 ± 0.01 μMol/cm2 per h at resection margin, and 0.017 ± 0.004 μMol/cm2 per h in control tissue (p < 0.05). RT-PCR analysis showed marked decrease in mRNA expression of a TJ protein occludin in NEC affected tissue (p < 0.03 vs. control). Additionally, mRNA expression of myosin light chain kinase (MLCK), an important regulator of TJ permeability, was increased in NEC specimens.
Conclusion
These studies show for the first time that NEC intestinal tissue have increased intestinal permeability, even at grossly healthy-appearing resection areas. The increase in intestinal permeability in NEC appeared to be related in part to a decrease in occludin and an increase in MLCK expression.
Level of evidence
Level 2.
Keywords: Necrotizing enterocolitis, Intestinal barrier function, Tight junction, Occludin
1. Necrotizing enterocolitis
Necrotizing enterocolitis (NEC) is an inflammatory disease of the intestine that affects 7–10% of low birth weight premature neonates and 5–10% of all neonates [1–3]. Term infants are less frequently affected and often have congenital abnormalities [4,5]. In the United States, NEC is responsible for a hospitalization rate of 110/100,000 of live births [6]. NEC can lead to intestinal perforation or necrosis, overwhelming systemic infection, multi-organ failure, and death. More than one third of infants with NEC require surgery and 20–40% of NEC infants die [7,8]. Survivors of NEC have a significant rate of neurodevelopmental impairment [9]. The estimated total annual cost to care for infants with NEC is approximately $1 billion dollars [10]. Unfortunately, there has been little improvement in the morbidity and mortality of NEC. Despite extensive research, the underlying etiology of NEC remains unknown [11]. A major predisposing factor to develop NEC is prematurity. Proposed contributors to the development of NEC include immaturity of gut motility and digestion, intestinal circulatory regulation, gut barrier function, and immune defense [12]. Formula feeding, hypoxia, disruption of commensal intestinal bacteria and a genetic predisposition have also been implicated [13–15].
1.1. Intestinal epithelial barrier
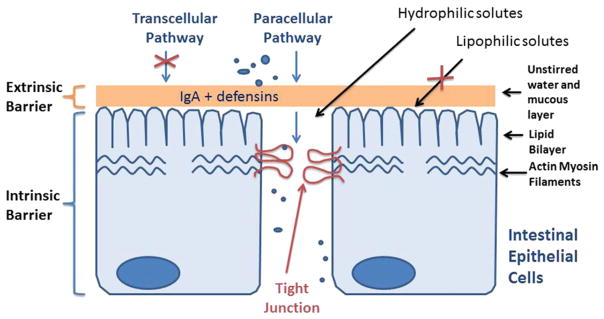
A major function of gastrointestinal epithelial cells throughout the GI tract is to provide a physical barrier against the penetration of noxious substances present in the lumen, including bacteria, bacterial antigens, and digestive enzymes and degraded food products. Intact intestinal epithelial barrier is essential in preventing intestinal penetration of luminal antigens [8]. The tight junctions (TJ) form an extracellular seal across the intercellular spaces between the adjacent cells and provide the gate function to the paracellular permeation of water-soluble compounds (Fig. 1). Tight junctions regulate the paracellular flux of water, nutrients, and ions. Intestinal TJ or paracellular permeability is a measure of “tightness or leakiness” of the intestinal TJ barrier [21,22]. The “leakiness” of the epithelial TJ barrier or intestinal permeability is actively regulated at the level of the TJ in both physiologic and pathologic states [22,23]. The disruption of the TJ barrier allows an increase in paracellular permeation of luminal antigens which in turn promote gastrointestinal mucosal injury and inflammation in various intestinal permeability disorders, including inflammatory bowel disease (ulcerative colitis and Crohn’s disease) and NEC [24].
Fig. 1.
Intestinal epithelial barrier. Tight junctions form an extracellular seal between adjacent cells and provide gate function to the paracellular permeation.
1.2. Intestinal epithelial tight junction proteins
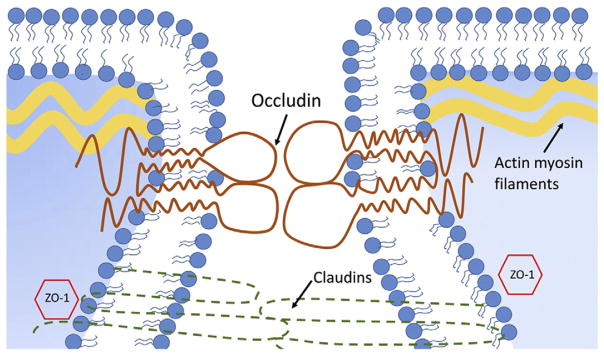
Tight junctions are composed of cytoplasmic and transmembrane TJ proteins (Fig. 2) [17,25]. The cytoplasmic TJ proteins include ZO-1, ZO-2, ZO-3 and cingulin; and transmembrane TJ proteins include occludin, claudin family of proteins, and junctional adhesion molecules [26]. Occludin and several claudin family of proteins have been shown to serve as the TJ sealing elements [27]. Occludin has been shown to play a critical role in the regulation of intestinal epithelial TJ barrier function. Previous studies have demonstrated that over-expressing occludin in enterocytes caused an enhancement in TJ barrier function [28]. Conversely, studies from our laboratory and others showed that knocking-down occludin expression or over-expressing mutant occludin resulted in a loss of TJ barrier function [29]. Together, these studies suggested the important role of occludin in the regulation of the tight junction permeability. TJ proteins also play a role in intracellular signaling, interactions with cellular cytoskeleton providing cellular motility, and intracellular trafficking, among other functions.
Fig. 2.
Tight junction proteins in intestinal epithelial cells. Cytoplasmic and transmembrane TJ proteins contribute to paracellular barrier function.
Recent studies have shown that myosin light chain kinase (MLCK) plays an important role in regulation of intestinal tight junction permeability [30]. The activation of MLCK induces a contraction of peri-junctional actin-myosin filaments and causes opening of the TJ barrier. This process allows paracellular permeation of luminal antigens that induce an inflammatory response and culminates in intestinal inflammation. In several recent studies, MLCK has been identified as a potential therapeutic target to induce tightening of the intestinal barrier in various inflammatory conditions, including inflammatory bowel disease [30,31].
Intestinal barrier dysfunction has been implicated in many diseases, including inflammatory bowel disease, NEC, and alcoholic hepatitis [16,18–20]. In human inflammatory bowel disease, tightening of the intestinal barrier has been shown to both prevent and ameliorate intestinal inflammation [16]. Similarly, in animal models of inflammatory bowel disease, the development of intestinal inflammation is preceded by a defect in intestinal barrier, as evidenced by an increase in permeability [32]. The enhancement or preservation of intestinal TJ barrier has consistently shown to prevent or ameliorate intestinal inflammation [33]. Thus, it is well-established that the preservation or enhancement of the intestinal TJ barrier has an important therapeutic effect in various inflammatory conditions of the gut.
1.3. Intestinal barrier function in human NEC
Although functional permeability studies have been carried out in animal models of NEC, no studies have directly examined intestinal barrier function or intestinal permeability in intestinal tissues of NEC patients. It is well-known that intestinal permeability is higher in preterm infants, and reduces with increasing infant age [34,35]. Following summarizes the human studies related to intestinal barrier and human NEC. Bergmann et al. performed immunohistochemistry on a small number of previously preserved intestinal specimens of NEC and found that claudin-2 expression was increased, particularly in the crypts in small intestine and colon [36]. No functional assessment of intestinal barrier permeability in humans was performed in this study. Sevastiadou et al. investigated the effect of oral glutamine supplementation in premature infants on intestinal permeability and the development of NEC in premature infants [37]. In these studies, intestinal barrier function was indirectly assessed by oral administration of permeability markers lactulose and mannitol, and subsequent recovery in the urine. They found that pre-term infants with increased intestinal permeability were more likely to develop NEC and that glutamine supplementation reduced the intestinal permeability and appeared to prevent NEC development, suggesting that the defective intestinal TJ barrier or a “leaky gut” plays a critical role in the pathogenesis of NEC. Another study examined intestinal permeability via a sugar-absorption test in postoperative infants who had undergone intestinal resection for NEC, and found that intestinal permeability was significantly increased post-operatively [38]. Thus there have not been any human studies which directly examined intestinal permeability in patients with NEC.
The aims of the present study were to measure intestinal barrier function in human NEC and characterize the expression of key intestinal TJ barrier regulatory proteins.
2. Methods and materials
2.1. Specimen collection
All the studies were in compliance with the University of New Mexico IRB protocol 18-358. Neonates with complicated NEC, NEC stomas and non-NEC intestinal conditions were selected for surgery by neonatologists and pediatric surgeons, per standard of care. Surgical resection of small intestines was performed per the standard of care of NEC and non-NEC intestinal conditions. Upon surgical resection, freshly resected small intestinal tissues were immediately subjected to functional studies of intestinal barrier. The collaborating pathologists selected intestinal specimens for clinical analysis, per standard of care. Tissues were processed for histologic examination. The pathologists released residual intestines for this research study. Specimens were excluded from the study if there are only enough tissue to perform pathologic evaluation and clinical diagnosis.
2.2. Ussing chamber experiments
Freshly resected intestinal tissues were immediately placed in oxygenated Krebs buffer, dissected to remove serosal and muscular layers, and mounted on 0.12-cm2-aperture Ussing chambers [39]. Tissues were bathed on the serosal and mucosal sides with Krebs buffer. The serosal bathing solution contained 10 mM glucose, which was osmotically balanced on the mucosal side with 10 mM mannitol. Bathing solutions were oxygenated (95% O2–5% CO2) and circulated in water-jacketed reservoirs maintained at 37 °C. The spontaneous potential difference (PD) was measured with Ringer-agar bridges, and the PD was short circuited through Ag-AgCl electrodes with a voltage clamp that corrected for fluid resistance. Transepithelial electrical resistance (TER, Ω·cm2) was calculated from the spontaneous PD and short-circuit current. All Ussing chamber experiments were initiated within minutes of surgical resection.
2.3. Paracellular fluxes of [3H]mannitol
To assess intestinal permeability of paracellular marker mannitol, mucosal-to-serosal flux of mannitol was assessed by adding 0.2 μCi/ml [3H]mannitol (paracellular marker) to the mucosal compartment of Ussing chamber-mounted tissues. After a 15-min equilibration period, standards were taken from the mucosal side of each chamber and mannitol flux was determined by taking 0.5-ml samples from the serosal compartment. The presence of 3H was established by measuring β-emission in a liquid scintillation counter (LS6000SC, Beckman). Unidirectional [3H]mannitol fluxes from mucosa-to-serosal compartment were evaluated by determining the amount of [3H]mannitol in the serosal compartment, on a chamber unit area basis, as previously described [39].
2.4. Quantification of occludin and MLCK mRNA expression using real-time PCR
Total RNA was isolated from intestinal mucosal scrapings using an RNeasy kit (Qiagen, Valencia, CA), quantified spectrophotometrically, and equal amounts of RNA were used for complementary DNA synthesis (QuantiTect Reverse Transcription Kit; Qiagen, Valencia, CA) as previously described by us [40]. The real time PCRs were performed by using StepOnePlus real time PCR system (Applied Biosystems) and standard PCR conditions (50 °C for 2 min, 95 °C for 10 min, 40 cycles of 95 °C for 15 s, and 60 °C for 1 min). Taqman gene expression assay for occludin gene (Hs00170162_m1), Taqman gene expression assay for MLCK gene (Hs00364926_m1) and TaqMan gene Expression Master Mix (Applied Biosystems) were used for quantification of occludin and MLCK mRNA expression. The relative expression of occludin and MLCK mRNA was normalized to glyceraldehyde-3-phosphate dehydrogenase mRNA expression, as previously described [40].
2.5. Statistical analysis
Data are reported as mean ± standard error. Whenever needed, data were analyzed by using an analysis of variance for repeated measures (SigmaStat; Systat Software, San Jose, CA). A Tukey’s test was used for post-hoc analysis between treatments after analysis of variance (P < 0.05).
3. Results
3.1. Patient data
A total of 15 patients were included in the study over a collection period from August 2013 to December 2014. Five were in the NEC group, and 10 were in the control group. Diagnoses requiring intestinal resection in the control group included intestinal atresia and intestinal stricture. Average age in the NEC group was 23 days (range 11–42 d), and average age in the control group was 28.1 days (range 1–197 d). Average gestational age at birth was 30.4 weeks in the NEC group (range 25–36 weeks), and 35.6 weeks in the control group (range 25–36 weeks). Corrected gestational age at time of surgery was 34.2 weeks in the NEC group (range 26–43 weeks) and 39.5 weeks in the control group (range 31–47 weeks). The control groups were then stratified by weight and corrected gestational age to create groups of lower birth-weight, more premature infants that more accurately reflected the NEC-vulnerable population (Table 1).
Table 1.
Patient characteristics. Five NEC patients and 10 control patients were included in the study. Control groups were divided by weight and corrected gestational age.
| NEC (n = 5) | Control (n = 10)
|
|||||
|---|---|---|---|---|---|---|
| All | <2800 g | >2800 g | 31–40 wk CGA | 41–50 wk CGA | ||
| Current age (d) | 23 (11–42) | 35.5 (1–197) | 14.8 (1–70) | 56.2 (3–197) | 6.4 (1–28) | 64.6 (49–197) |
| Gestational age at birth (wk) | 30.4 (25–36) | 35 (29–40) | 33.2 (30–37) | 36.8 (29–40) | 34.2 (31–37) | 35.8 (29–40) |
| Corrected gestational age (wk) | 34.2 (26–43) | 39.9 (31–50) | 37.2 (31–50) | 42.6 (39–47) | 35 (31–40) | 44.8 (41–50) |
| Weight (g) | 1830 (720–2910) | 3325 (1430–6130) | 2212 (1430–2700) | 4437 (3650–6130) | 2493.2 (1430–3921) | 4156 (2515–6130) |
| Sex (M:F) | 3 to 2 | 3 to 2 | 2 to 3 | 4 to 1 | 3 to 2 | 3 to 2 |
| Percent with enteral nutrition | 100 | 40 | 20 | 100 | 20 | 100 |
| Percent receiving breast milk | 20 | 80 | 20 | 60 | 0 | 80 |
| Antibiotic duration (d) | 6.6 (3–9) | 1.2 (0–10) | 0.8 (0–4) | 1.75 (0–10) | 1 (1–10) | 1.4 (0–4) |
3.2. Intestinal tissue trans-epithelial resistance is decreased in human NEC
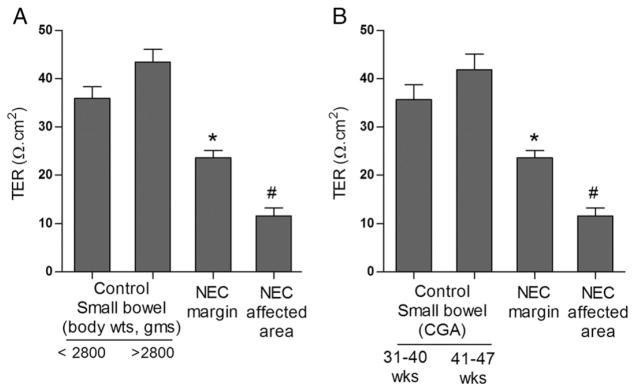
The intestinal barrier function in human NEC was determined by measuring trans-epithelial resistance (TER) after mounting the small intestinal tissue in Ussing chamber immediately after surgical resection. TER is a marker of intestinal TJ barrier function, with decreased TER correlating with a loss of intestinal TJ barrier function or increase in intestinal paracellular permeability. In NEC tissues, a marked decrease in TER was seen compared to control tissues. TER was also significantly decreased at resection margins of NEC tissues compared to control specimens. Average TER of NEC tissue at affected sites was 11.61 ± 1.65 Ω/cm2 and at resection margin was 23.36 ± 1.48 Ω/cm2. Average TER of small intestinal tissue from controls was 35.66 ± 3.05 Ω/cm2 for controls having corrected gestational age (CGA) of 31–40 weeks and 41.85 ± 3.9 Ω/cm2 for CGA of 41–47 weeks. In control infants with body weights below 2800 g, the mean intestinal tissue TER was 35.93 ± 2.38 Ω/cm2 and 43.45 ± 2.62 Ω/cm2 for infants with body weights more than 2800 g (Fig. 3).
Fig. 3.
Intestinal barrier function in NEC. The intestinal mucosal tissues were mounted on Ussing chambers as described in methods to measure transepithelial electrical resistance (TER). The NEC margin and NEC affected tissue had significantly lower TER compared to control tissue. Control groups were then stratified by weight (A) and corrected gestational age (B). Tissues from more preterm, lower birth-weight infants had lower TER compared to tissues from infants that were more mature (*, #, p < 0.01 vs. control, n ≥ 3).
3.3. Intestinal permeability of permeability probe [3H]mannitol is increased in human NEC
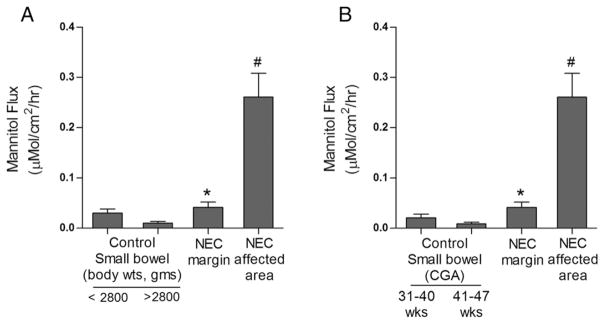
In this study, mucosal-to-serosal flux of paracellular marker [3H] mannitol was determined using an Ussing chamber setup. When compared to healthy control tissues, tissues from infants with NEC had a marked increased mucosal-to-serosal flux of mannitol. There was also a modest but significant increase in mannitol flux in intestinal tissues from the resection margins from infants with NEC compared to controls. Average flux of mannitol was 0.23 ± 0.06 μMol/cm2 per h in NEC affected tissues, and 0.04 ± 0.01 μMol/cm2 per h in resection areas. Average mucosal flux of mannitol in control small intestinal tissues was 0.020 ± 0.007 μMol/cm2 per h for CGA of 31–40 weeks group and 0.0085 ± 0.003 μMol/cm2 per h for CGA of 41–47 weeks group. In the control group with body weights below 2800 g, the average flux of mannitol in control small intestinal tissues was 0.030 ± 0.007 μMol/cm2 per h and 0.010 ± 0.003 μMol/cm2 per h in the group of body weights more than 2800 g (Fig. 4).
Fig. 4.
Intestinal paracellular permeability in NEC. The intestinal mucosal tissues were mounted on Ussing chambers as described in methods to measure paracellular flux of [3H]mannitol. The NEC margin and NEC affected tissue showed significantly increased [3H]mannitol flux compared to control tissues. When stratified by weight (A) and corrected gestational age (B), control tissues from more premature, lower birth-weight infants showed increased [3H]mannitol flux. (*, #, p < 0.01 vs. control, n ≥ 3).
3.4. Expression of occludin mRNA is decreased in human NEC compared to control tissues
Occludin is a TJ protein that provides a barrier function at the TJs. In the present study, occludin mRNA expression in intestinal tissue of patients with NEC was determined by real-time PCR. The occludin mRNA levels were markedly decreased in NEC tissue compared to control tissue. A modest decrease in occludin mRNA expression was also observed at the resection margins of infants with NEC (Fig. 5).
Fig. 5.
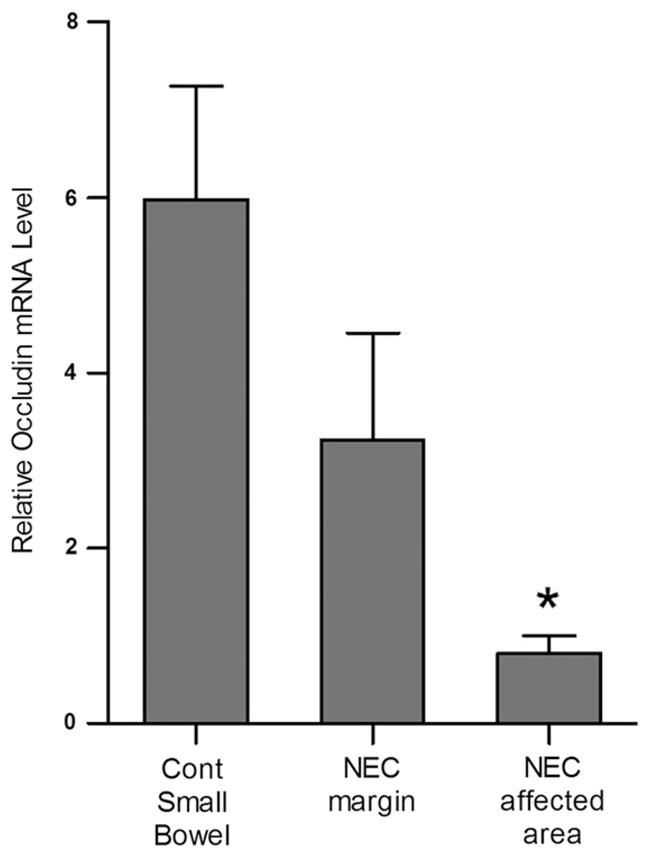
Occludin mRNA expression in NEC. RT-PCR analysis of intestinal mucosal tissues showed significantly decreased mRNA expression of occludin in NEC tissue compared to control tissue. The mRNA expression of occludin was normalized to mRNA expression of GAPDH (*, p < 0.01 vs. control, n ≥ 3).
3.5. MLCK mRNA expression is upregulated in human NEC
Myosin light chain kinase (MLCK) has been demonstrated to play a central role in regulation of intestinal barrier by causing an increase in intestinal TJ permeability in human intestinal diseases [30,31]. The MLCK mRNA expression was examined in intestinal tissue from NEC patients by RT-PCR analysis. There was an increase in MCLK mRNA in the intestinal tissues from infants with NEC compared to healthy control tissues. Similarly, there was also an increase in MLCK mRNA level at resection margins (Fig. 6).
Fig. 6.
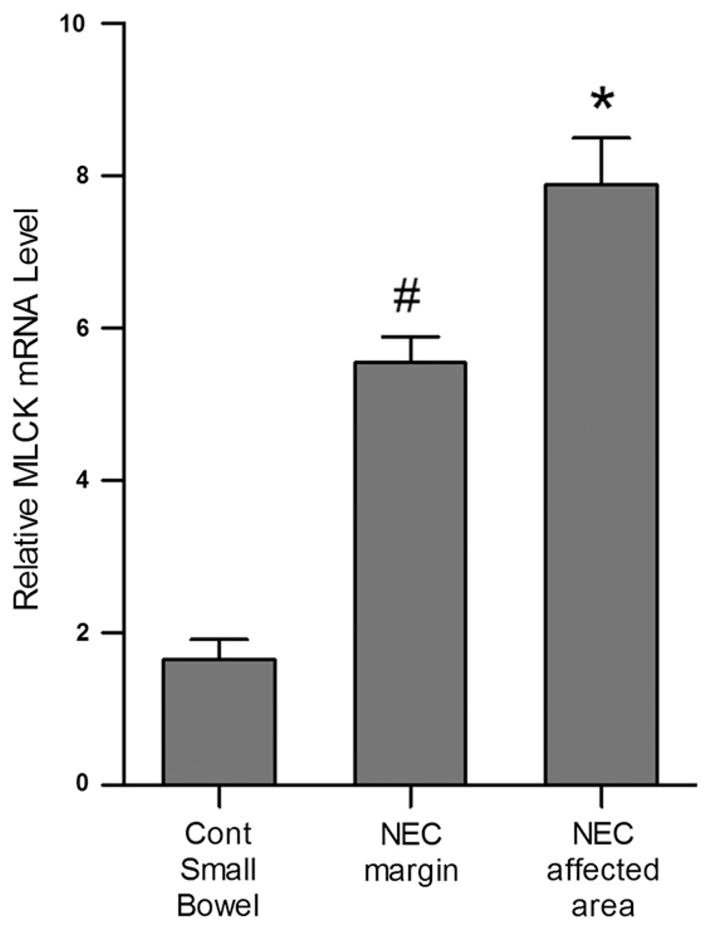
Myosin light chain kinase (MLCK) mRNA expression in NEC. RT-PCR analysis of intestinal mucosal tissues showed significantly increased mRNA expression of MLCK in NEC margin and NEC affected tissue compared to control tissue. The mRNA expression of MLCK was normalized to mRNA expression of GAPDH (#, *p < 0.01 vs control, n ≥ 3).
4. Discussion
Although the precise etiology of NEC remains unclear, several etiological factors such as prematurity implying immature gut barrier function and immune defense, formula feeding, mucosal ischemia, hypoxia and abnormal microbial colonization have been postulated to be involved in the development of NEC [13]. A defective intestinal epithelial TJ barrier has been demonstrated to be an important pathogenic factor of intestinal inflammation [16]. Growing evidence from human and animal studies suggests that an abnormal increase in intestinal permeability may be an important predisposing factor for NEC and inflammatory bowel disease [12]. Preterm neonates have been demonstrated to have increased intestinal permeability during the first several weeks of life [34]. This allows absorption of important macronutrients and growth factors from maternal milk, but also allows intestinal absorption of toxic luminal antigens, leaving them vulnerable to inflammatory disease processes such as NEC. There is also a paucity of information about the composition of the TJ in intestines of the healthy neonate and the neonate with NEC. Although the potential importance of defective intestinal barrier function as an etiologic factor of NEC is well-recognized, direct functional assessment of intestinal barrier in human NEC has not been previously conducted. There are also no reports of simultaneous electrophysiological measurements of the intestinal barrier function, intestinal TJ permeability, and the analysis of TJ barrier-regulating proteins in human NEC.
In this study, we demonstrate the presence of an intestinal barrier defect in human infants with NEC via multiple methods. The surgically resected intestinal tissues were mounted in Ussing chambers within 15 min of resection while being oxygenated. Our data showed that the intestinal barrier was markedly disrupted in NEC, as evidenced by a markedly decreased intestinal tissue TER and an increase in flux of paracellular marker (mannitol). Consistent with our present findings, in the previously published study which examined intestinal permeability in preterm infants, Sevastiadou et al. reported that preterm infants with increased intestinal permeability were at greatest risk to develop NEC. In this study, preterm infants underwent assessment of barrier function via urinary lactulose/mannitol recovery after treatment with glutamine, which enhanced intestinal barrier function. Infants with decreased intestinal barrier function had a higher incidence of NEC, suggesting that a pre-existing defect in intestinal barrier predisposes preterm infants to develop NEC. In our study, healthy margins were identified by the surgeons for the resection purpose based on the normal gross appearance of intestine from the serosal surface. The presence of intestinal barrier defects in these grossly healthy-appearing areas suggested that the intestinal barrier dysfunction was also present in normal-appearing intestinal tissues in NEC patients. These findings also suggest the possibility that a defect in intestinal barrier function may be pre-existing in NEC intestine and could contribute to the pathogenesis of NEC. It is also important to note that such intestinal barrier defects shown in normal-appearing areas may be a part of underlying diffuse inflammatory process seen in the NEC but not apparent in gross examination. A limitation of our NEC intestinal tissue experiments was that our studies were not designed to address whether the intestinal barrier defect was a pre-existing factor leading to the development of intestinal inflammation. In this regard, previous studies in pre-term infants have demonstrated that the infants with increased intestinal permeability were at greatest risk to develop NEC and also to have a persistent increase in intestinal permeability following surgical resection [37,38]. Nevertheless, our findings underscore the importance of intestinal barrier defect in the progression and severity of NEC. The findings in our study also provide a potential insight into the mechanisms that contribute to the intestinal barrier defect in NEC. Our results show for the first time that occludin mRNA expression is markedly decreased and MCLK mRNA is increased in intestinal tissues from both healthy appearing and diseased areas of NEC infants.
Tight junction proteins play a crucial role in the regulation of intestinal barrier function. Occludin, the first transmembrane TJ protein to be discovered, has been extensively studied. Targeted depletion of occludin causes a loss of intestinal TJ barrier function while an increase in occludin level results in an enhancement of TJ barrier, indicating that loss of occludin expression leads to an increase in paracellular permeability [26,28,29]. The studies from our laboratory and others have shown that occludin depletion causes a loss of intestinal epithelial barrier function while an increase in occludin levels in enterocytes causes an enhancement in TJ barrier [28,29]. However, a previous human study of intestinal NEC tissues did not show a change in occludin expression based on immunostaining [36]. Our results described herein show a marked decrease in occludin mRNA expression in NEC, suggesting that occludin depletion was likely to be an important factor contributing to the observed increase in intestinal permeability in NEC.
Previous studies from our laboratory and other groups have also demonstrated a central role of MLCK in modulating intestinal TJ barrier function or intestinal permeability [30,31,44–46]. These studies indicated that an increase in MLCK expression leads to MLCK-catalyzed phosphorylation of myosin light chain, contraction of peri-junctional actin/ myosin filaments, and contractile tension-generated mechanical opening of the TJ barrier and an increase in intestinal permeability [31]. Our data presented herein show that MLCK expression was greatly increased in NEC intestinal tissues. The change in MLCK mRNA expression was present at both affected tissues and grossly healthy resection margins in infants with NEC, suggesting that the observed increase in intestinal permeability in NEC was in part because of an increase in MLCK.
A number of animal studies have shown impaired intestinal barrier function in animal models of NEC [12]. Clark et al. demonstrated increased intestinal permeability of paracellular markers in a rat model of NEC [41]. Measurement of TJ protein expression in rat intestinal tissue revealed a decrease in occludin and an increase in claudin 3 in experimental NEC as compared to wild-type controls. Distribution of TJ proteins was also disrupted in intestinal epithelial cells in experimental NEC. Occludin was localized near the apical membrane in crypts and in the cytoplasm along the villi, as opposed to normal localization in the basolateral membrane in controls. These effects were blunted by treatment with EGF, which enhanced intestinal barrier function and prevented the development of NEC. Other animal studies have found similar findings in experimental NEC, with increased expression of claudin 3 and decreased expression of ZO-1, claudin 1, and occludin [42,43]. Bergmann et al. also used a mouse model of NEC to evaluate intestinal barrier function, and found that intestinal permeability increased before the onset of histologic evidence of NEC, supporting the hypothesis that impaired intestinal epithelial barrier precedes the development of NEC and may be a primary causative factor [44]. In this study, probiotic strains were used to enhance intestinal barrier function, which resulted in decreased rates of development of NEC. However, a potential concern with animal models of NEC is that experimental NEC is induced via hypoxia-ischemia, cold stress, formula feeding, live bacteria, formula feeding, or a combination of methods [12] and may not accurately recapitulate the complex nature of human NEC.
This study represents first report on the direct functional analysis of intestinal barrier function in freshly obtained human NEC tissues. Although major therapeutic advancement in prevention and treatment of NEC is lacking, administration of probiotics has been shown to be effective in improving intestinal barrier function in preterm infants in small clinical trials [47]; and represents a potential therapeutic strategy for tightening intestinal barrier and preventing the development of NEC [48,49]. Our data presented herein indicated that a defective intestinal barrier is present in both diseased and grossly normal-appearing areas of small intestine, supporting the hypothesis that a pre-existing defect in intestinal barrier may be present in NEC. Our data also suggested that a decrease in occludin and an increase in MLCK in intestinal tissues may be contributing factors responsible for the increase in intestinal permeability. Currently, our laboratory is examining the therapeutic effects of several TJ barrier-enhancing probiotic bacteria in prevention and therapy of NEC in both animal models of NEC and preterm infants at high risk for NEC.
Acknowledgments
Clifford Qualls, PhD, is acknowledged for his assistance with statistical analysis and study design. John Russell, MD, is acknowledged for salary support and encouragement related to this study.
Footnotes
This work was supported by Veterans Affairs (VA) Merit Review grant from the VA Research Service (TM), National Institute of Health/National Institute of Diabetes and Digestive and Kidney Diseases RO-1DK-64165-01 (TM) and KO1DK100562-02 (PN), and UNM School of Medicine Research Allocation Committee (CR).
References
- 1.Lemons JA, Bauer CR, Oh W, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January through December 1996. Pediatrics. 2001;107:E1. doi: 10.1542/peds.107.1.e1. [DOI] [PubMed] [Google Scholar]
- 2.Claud EC, Walker WA. Bacterial colonization, probiotic and necrotizing enterocolitis. J Clin Gastroenterol. 2008;42:S46–52. doi: 10.1097/MCG.0b013e31815a57a8. [DOI] [PubMed] [Google Scholar]
- 3.Guthrie SO, Gordon PV, Thomas V, et al. Necrotizing enterocolitis among infants in the United States. Perinatology. 2003;23:278–85. doi: 10.1038/sj.jp.7210892. [DOI] [PubMed] [Google Scholar]
- 4.Bolisetty S, Lui K, Oei J, et al. A regional study of underlying congenital diseases in term infants with necrotizing enterocolitis. Acta Pediatr. 2007;89:1226–30. doi: 10.1080/080352500750027619. [DOI] [PubMed] [Google Scholar]
- 5.McElhinney DB, Hindrick HL, Bush DM, et al. Necrotizing enterocolitis in neonates with congenital heart disease: risk factors and outcomes. Pediatrics. 2000;106:1080–7. doi: 10.1542/peds.106.5.1080. [DOI] [PubMed] [Google Scholar]
- 6.Holman RC, Stoll BJ, Curns AT, et al. Necrotizing enterocolitis hospitalizations among neonates in the United States. Paediatr Perinat Epidemiol. 2006;20:498–506. doi: 10.1111/j.1365-3016.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- 7.Henry MC, Moss RL. Necrotizing enterocolitis. Annu Rev Med. 2009;60:111–24. doi: 10.1146/annurev.med.60.050207.092824. [DOI] [PubMed] [Google Scholar]
- 8.Chan K, Ohlsson A, Synnes A, et al. Survival, morbidity, and resource use of infants of 25 weeks’ gestational age or less. Am J Obstet Gynecol. 2001;185:220–6. doi: 10.1067/mob.2001.115280. [DOI] [PubMed] [Google Scholar]
- 9.Hintz SR, Kendrick DE, Stoll BJ, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115:696–703. doi: 10.1542/peds.2004-0569. [DOI] [PubMed] [Google Scholar]
- 10.Neu J, Walk WA. Necrotizing enterocolitis. N Engl J Med. 2011;364:255–64. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obladen M. Necrotizing enterocolitis – 150 years of fruitless search for the cause. Neonatology. 2009;96:203–10. doi: 10.1159/000215590. [DOI] [PubMed] [Google Scholar]
- 12.Halpern MD, Denning PW. The role of intestinal epithelial barrier function in the development of NEC. Tissue Barriers. 2015;3(1–2):e1000707 1–10. doi: 10.1080/21688370.2014.1000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zani A, Pierro A. Necrotizing enterocolitis: controversies and challenges. F1000Res. 2015;4:1–10. doi: 10.12688/f1000research.6888.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel RM, Denning PW. Intestinal microbiota and its relationship with necrotizing enterocolitis. Pediatr Res. 2015;78(3):232–8. doi: 10.1038/pr.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sampath V. SIGIRR genetic variants in premature infants with necrotizing enterocolitis. Pediatrics. 2015;135(6):e1530–4. doi: 10.1542/peds.2014-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma TY, Anderson JM. Physiology of the gastrointestinal tract. Burlington, MA: Elsevier Academic Press; 2006. Tight junctions and the intestinal barrier. [Google Scholar]
- 17.Utech M, Brüwer M, Nusrat A. Tight junctions and cell–cell interactions. Methods Mol Biol. 2006;341:185–95. doi: 10.1385/1-59745-113-4:185. [DOI] [PubMed] [Google Scholar]
- 18.Hollander D. Intestinal permeability, leaky gut, and intestinal disorders. Curr Gastroenterol Rep. 1999;1:410–6. doi: 10.1007/s11894-999-0023-5. [DOI] [PubMed] [Google Scholar]
- 19.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 20.Linskens RK, Huijsdens XW, Savelkoul PH, et al. The bacterial flora in inflammatory bowel disease: current insights in pathogenesis and the influence of antibiotics and probiotics. Scand J Gastroenterol Suppl. 2001;(234):29–40. doi: 10.1080/003655201753265082. [DOI] [PubMed] [Google Scholar]
- 21.Madara JL, Parkos C, Colgan S, et al. The movement of solutes and cells across tight junctions. Ann N Y Acad Sci. 1992;664:47–60. doi: 10.1111/j.1749-6632.1992.tb39748.x. [DOI] [PubMed] [Google Scholar]
- 22.Madara JL, Pappenheimer JR. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol. 1987;100(2):149–64. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- 23.Shen L, Turner JR. Role of epithelial cells in initiation and propagation of intestinal inflammation. Eliminating the static: tight junction dynamics exposed. Am J Physiol Gastrointest Liver Physiol. 2006;290:G577–82. doi: 10.1152/ajpgi.00439.2005. [DOI] [PubMed] [Google Scholar]
- 24.Westerbeek EA, van den Berg A, Lafeber HN, et al. The intestinal bacterial colonisation in preterm infants: a review of the literature. Clin Nutr. 2006;25(3):361–8. doi: 10.1016/j.clnu.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 25.QH Y, Yang Q. Diversity of tight junctions (TJs) between gastrointestinal epithelial cells and their function in maintaining the mucosal barrier. Cell Biol Int. 2009;33:78–82. doi: 10.1016/j.cellbi.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Furuse M, Hirase T, Itoh M, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123(6 Pt 2):1777–88. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colegio OR, Van Itallie C, Rahner C, et al. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol. 2003;284:C1346–54. doi: 10.1152/ajpcell.00547.2002. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy KM, Skare IB, Stankewich MC, et al. Occludin is a functional component of the tight junction. J Cell Sci. 1996;109(Pt 9):2287–98. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- 29.Al-Sadi R, Khatib K, Guo S, et al. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2011;300(6):G1054–64. doi: 10.1152/ajpgi.00055.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Sadi R, Guo S, Dokladny K, et al. Mechanism of interleukin-1β induced-increase in mouse intestinal permeability in vivo. J Interferon Cytokine Res. 2012;32(10):474–84. doi: 10.1089/jir.2012.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye D, Ma TY. Cellular and molecular mechanisms that mediate basal and tumor necrosis factor-α-induced regulation of myosin light chain kinase gene activity. J Cell Mol Med. 2008;12(4):1331–46. doi: 10.1111/j.1582-4934.2008.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venkatraman A, Ramakrishna BS, Pulimood AB, et al. Increased permeability in dextran sulphate colitis in rats: time course of development and effect of butyrate. Scand J Gastroenterol. 2000;35(10):1053–9. doi: 10.1080/003655200451171. [DOI] [PubMed] [Google Scholar]
- 33.Sturm A, Dignass AU. Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol. 2008;14(3):348–53. doi: 10.3748/wjg.14.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weaver LT, Laker MF, Nelson R. Intestinal permeability in the newborn. Arch Dis Child. 1984;59(3):236–41. doi: 10.1136/adc.59.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Elburg RM, Fetter W, Bunkers CM, et al. Intestinal permeability in relation to birth weight and gestational and postnatal age. Arch Dis Child Fetal Neonatal Ed. 2003;88(1):F52–5. doi: 10.1136/fn.88.1.F52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergmann KR, Liu SX, Tian R, et al. Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am J Pathol. 2013;182(5):1595–606. doi: 10.1016/j.ajpath.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sevastiadou S, Malamitsi-Puchner A, Costalos C, et al. The impact of oral glutamine supplementation on the intestinal permeability and incidence of necrotizing enterocolitis/septicemia in premature neonates. J Matern Fetal Neonatal Med. 2011;24(10):1294–300. doi: 10.3109/14767058.2011.564240. [DOI] [PubMed] [Google Scholar]
- 38.Piena-Spoel M, Albers MJ, ten Kate J, et al. Intestinal permeability in newborns with necrotizing enterocolitis and controls: does the sugar absorption test provide guidelines for the time to (re-)introduce enteral nutrition? J Pediatr Surg. 2001;36(4):587–92. doi: 10.1053/jpsu.2001.22288. [DOI] [PubMed] [Google Scholar]
- 39.Nighot PK, Blikslager AT. ClC-2 regulates mucosal barrier function associated with structural changes to the villus and epithelial tight junction. Am J Physiol Gastrointest Liver Physiol. 2010;299:G449–56. doi: 10.1152/ajpgi.00520.2009. [DOI] [PubMed] [Google Scholar]
- 40.Ye D, Ma I, Ma TY. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2006;290:G496–504. doi: 10.1152/ajpgi.00318.2005. [DOI] [PubMed] [Google Scholar]
- 41.Clark JA, Doelle SM, Halpern MD, et al. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol. 2006;291(5):G938–49. doi: 10.1152/ajpgi.00090.2006. [DOI] [PubMed] [Google Scholar]
- 42.Shiou SR, Yu Y, Chen S, et al. Erythropoietin protects intestinal epithelial barrier function and lowers the incidence of experimental neonatal necrotizing enterocolitis. J Biol Chem. 2011;286(14):12123–32. doi: 10.1074/jbc.M110.154625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rentea RM, Liedel JL, Welak SR, et al. Intestinal alkaline phosphatase administration in newborns is protective of gut barrier function in a neonatal necrotizing enterocolitis rat model. J Pediatr Surg. 2012;47(6):1135–42. doi: 10.1016/j.jpedsurg.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 44.Ma TY, Boivin MA, Ye D, et al. Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005;288(3):G422–30. doi: 10.1152/ajpgi.00412.2004. [DOI] [PubMed] [Google Scholar]
- 45.Su L, Shen L, Clayburgh DR, et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 2009;136(2):551–63. doi: 10.1053/j.gastro.2008.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nighot P, Al-Sadi R, Rawat MT, et al. Matrix metalloproteinase 9-induced increase in intestinal epithelial tight junction permeability contributes to the severity of experimental DSS colitis. Am J Physiol Gastrointest Liver Physiol. 2015;309(12):G988–97. doi: 10.1152/ajpgi.00256.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stratiki Z, Costalos C, Sevastiadou S, et al. The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum Dev. 2007;83(9):575–9. doi: 10.1016/j.earlhumdev.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Alfaleh K, Bassler D. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2008;23(1):CD005496. doi: 10.1002/14651858.CD005496.pub2. [DOI] [PubMed] [Google Scholar]
- 49.Martin CR, Walker WA. Probiotics: role in pathophysiology and prevention in necrotizing enterocolitis. Semin Perinatol. 2008;32(2):127–37. doi: 10.1053/j.semperi.2008.01.006. [DOI] [PubMed] [Google Scholar]