Abstract
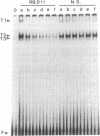
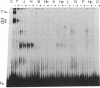
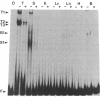
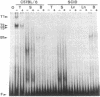
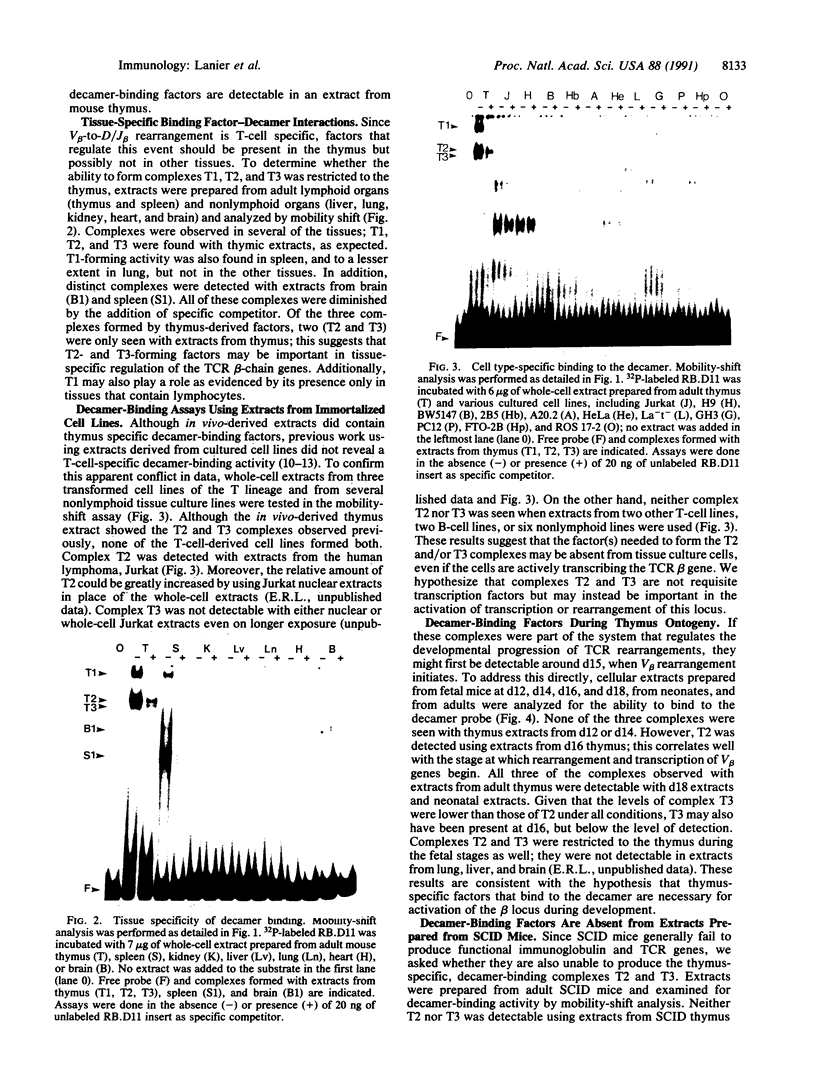
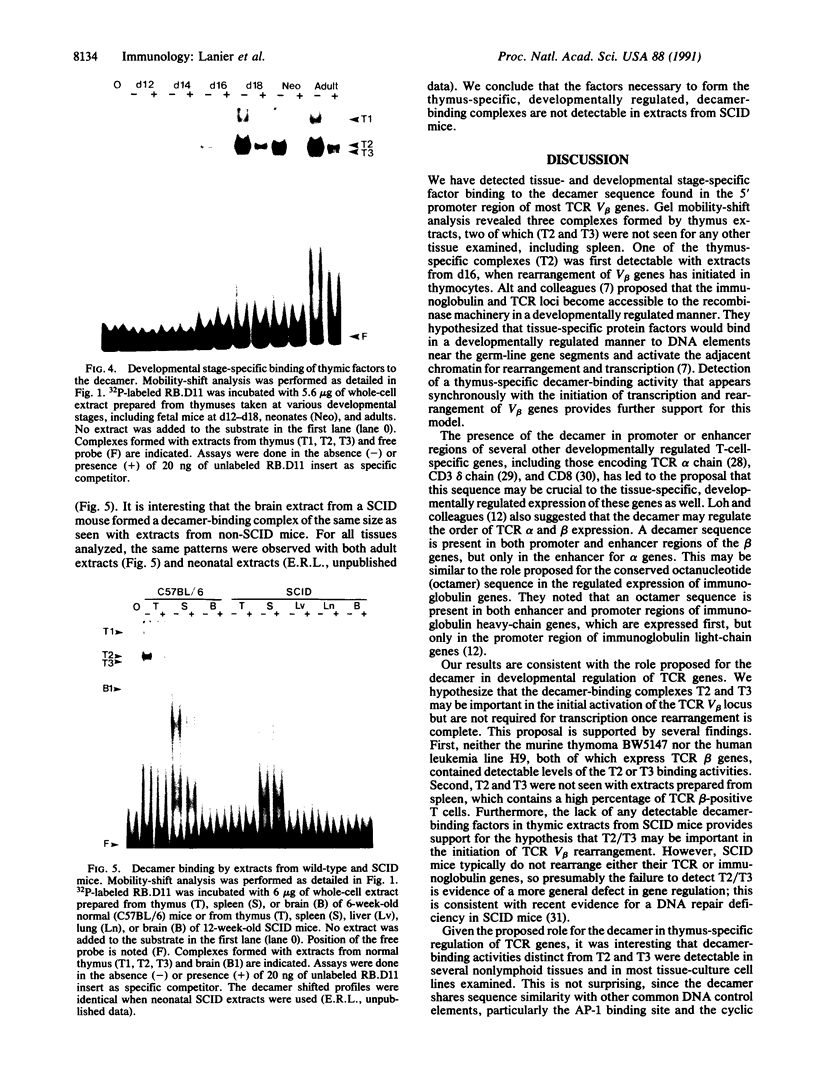
The gene segments encoding the beta chain of the T-cell antigen receptor undergo rearrangement in a precise developmental order: a D beta gene segment joins to a J beta gene segment prior to the rearrangement of a V beta gene segment to join the D/J beta fusion. Current evidence suggests that the rearrangement of V beta is restricted to T cells, whereas D-to-J beta rearrangements may occur in both B and T cells. Thus, the T-cell specificity seems to be regulated by the V beta coding region or its 5' flanking sequence. In support of this hypothesis, evidence is provided for thymus-specific factors that bind a highly conserved 10-base-pair (decamer) sequence that is an essential promoter element in mouse and human V beta genes. The presence of decamer-binding activities was assayed by gel mobility-shift analysis using protein extracts from thymus, spleen, and nonlymphoid organs of adult mice. Two shifted complexes, designated T2 and T3, were seen only when the decamer was incubated with extracts from thymus. When extracts from mice of various gestational ages were tested for decamer-binding activity, one of the thymus-specific complexes, T2, was first detected at day 16; this coincides with the time of initial activation of the V beta locus. No decamer-binding activity was detected in extracts prepared from the thymuses of SCID (severe combined immunodeficiency) mice, which characteristically fail to rearrange these genes. Moreover, neither T2 nor T3 was detectable with extracts from spleen or from two T-cell lines that express the beta chain; this suggests that the presence of these two complexes is not absolutely required for transcription of the T-cell receptor beta locus. We conclude that there are tissue-specific and developmentally regulated factors that form complexes with the decamer sequence 5' of V beta; these may represent initiation factors that control the activation of germ-line T-cell receptor V beta genes for transcription and/or rearrangement.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. J., Chou H. S., Loh D. Y. A conserved sequence in the T-cell receptor beta-chain promoter region. Proc Natl Acad Sci U S A. 1988 May;85(10):3551–3554. doi: 10.1073/pnas.85.10.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S. J., Miyake S., Loh D. Y. Transcription from a murine T-cell receptor V beta promoter depends on a conserved decamer motif similar to the cyclic AMP response element. Mol Cell Biol. 1989 Nov;9(11):4835–4845. doi: 10.1128/mcb.9.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Hattori K., Smeal T., Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988 Dec 2;55(5):875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Moore M. W., Yancopoulos G. D., Suh H., Lutzker S., Selsing E., Alt F. W. Recombination between immunoglobulin variable region gene segments is enhanced by transcription. Nature. 1986 Dec 11;324(6097):585–589. doi: 10.1038/324585a0. [DOI] [PubMed] [Google Scholar]
- Bosma M. J., Carroll A. M. The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- Cao Z. D., Barron E. A., Sharp Z. D. Prolactin upstream factor I mediates cell-specific transcription. Mol Cell Biol. 1988 Dec;8(12):5432–5438. doi: 10.1128/mcb.8.12.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M. F. Actin: protein structure and filament dynamics. J Biol Chem. 1991 Jan 5;266(1):1–4. [PubMed] [Google Scholar]
- Davis M. M. Molecular genetics of the T cell-receptor beta chain. Annu Rev Immunol. 1985;3:537–560. doi: 10.1146/annurev.iy.03.040185.002541. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier P., Krippl B., Blackwell T. K., Furley A. J., Suh H., Winoto A., Cook W. D., Hood L., Costantini F., Alt F. W. Separate elements control DJ and VDJ rearrangement in a transgenic recombination substrate. EMBO J. 1990 Jan;9(1):117–125. doi: 10.1002/j.1460-2075.1990.tb08087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos K., Galson D., Terhorst C. Tissue-specific nuclear factors mediate expression of the CD3 delta gene during T cell development. EMBO J. 1990 Jan;9(1):109–115. doi: 10.1002/j.1460-2075.1990.tb08086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk L. R., Leiden J. M. Identification and functional characterization of the human T-cell receptor beta gene transcriptional enhancer: common nuclear proteins interact with the transcriptional regulatory elements of the T-cell receptor alpha and beta genes. Mol Cell Biol. 1990 Oct;10(10):5486–5495. doi: 10.1128/mcb.10.10.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haars R., Kronenberg M., Gallatin W. M., Weissman I. L., Owen F. L., Hood L. Rearrangement and expression of T cell antigen receptor and gamma genes during thymic development. J Exp Med. 1986 Jul 1;164(1):1–24. doi: 10.1084/jem.164.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson E. A., Qin X. Q., Bump E. A., Schatz D. G., Oettinger M., Weaver D. T. A link between double-strand break-related repair and V(D)J recombination: the scid mutation. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4061–4065. doi: 10.1073/pnas.88.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killary A. M., Lugo T. G., Fournier R. E. Isolation of thymidine kinase-deficient rat hepatoma cells by selection with bromodeoxyuridine, Hoechst 33258, and visible light. Biochem Genet. 1984 Apr;22(3-4):201–213. doi: 10.1007/BF00484224. [DOI] [PubMed] [Google Scholar]
- Kim K. J., Kanellopoulos-Langevin C., Merwin R. M., Sachs D. H., Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979 Feb;122(2):549–554. [PubMed] [Google Scholar]
- Kronenberg M., Goverman J., Haars R., Malissen M., Kraig E., Phillips L., Delovitch T., Suciu-Foca N., Hood L. Rearrangement and transcription of the beta-chain genes of the T-cell antigen receptor in different types of murine lymphocytes. Nature. 1985 Feb 21;313(6004):647–653. doi: 10.1038/313647a0. [DOI] [PubMed] [Google Scholar]
- Liu J. L., Chiles T. C., Sen R. J., Rothstein T. L. Inducible nuclear expression of NF-kappa B in primary B cells stimulated through the surface Ig receptor. J Immunol. 1991 Mar 1;146(5):1685–1691. [PubMed] [Google Scholar]
- Majeska R. J., Rodan S. B., Rodan G. A. Parathyroid hormone-responsive clonal cell lines from rat osteosarcoma. Endocrinology. 1980 Nov;107(5):1494–1503. doi: 10.1210/endo-107-5-1494. [DOI] [PubMed] [Google Scholar]
- McDougall S., Peterson C. L., Calame K. A transcriptional enhancer 3' of C beta 2 in the T cell receptor beta locus. Science. 1988 Jul 8;241(4862):205–208. doi: 10.1126/science.2968651. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakauchi H., Tagawa M., Nolan G. P., Herzenberg L. A. Isolation and characterization of the gene for the murine T cell differentiation antigen and immunoglobulin-related molecule, Lyt-2. Nucleic Acids Res. 1987 May 26;15(10):4337–4347. doi: 10.1093/nar/15.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Read-Connole E., Gallo R. C. T4 positive human neoplastic cell lines susceptible to and permissive for HTLV-III. Lancet. 1984 Dec 22;2(8417-8418):1472–1473. doi: 10.1016/s0140-6736(84)91666-0. [DOI] [PubMed] [Google Scholar]
- Revzin A. Gel electrophoresis assays for DNA-protein interactions. Biotechniques. 1989 Apr;7(4):346–355. [PubMed] [Google Scholar]
- Royer H. D., Reinherz E. L. Multiple nuclear proteins bind upstream sequences in the promotor region of a T-cell receptor beta-chain variable-region gene: evidence for tissue specificity. Proc Natl Acad Sci U S A. 1987 Jan;84(1):232–236. doi: 10.1073/pnas.84.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlissel M. S., Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 1989 Sep 8;58(5):1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- Takeda J., Cheng A., Mauxion F., Nelson C. A., Newberry R. D., Sha W. C., Sen R., Loh D. Y. Functional analysis of the murine T-cell receptor beta enhancer and characteristics of its DNA-binding proteins. Mol Cell Biol. 1990 Oct;10(10):5027–5035. doi: 10.1128/mcb.10.10.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winoto A., Baltimore D. A novel, inducible and T cell-specific enhancer located at the 3' end of the T cell receptor alpha locus. EMBO J. 1989 Mar;8(3):729–733. doi: 10.1002/j.1460-2075.1989.tb03432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985 Feb;40(2):271–281. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Blackwell T. K., Suh H., Hood L., Alt F. W. Introduced T cell receptor variable region gene segments recombine in pre-B cells: evidence that B and T cells use a common recombinase. Cell. 1986 Jan 31;44(2):251–259. doi: 10.1016/0092-8674(86)90759-2. [DOI] [PubMed] [Google Scholar]