Abstract
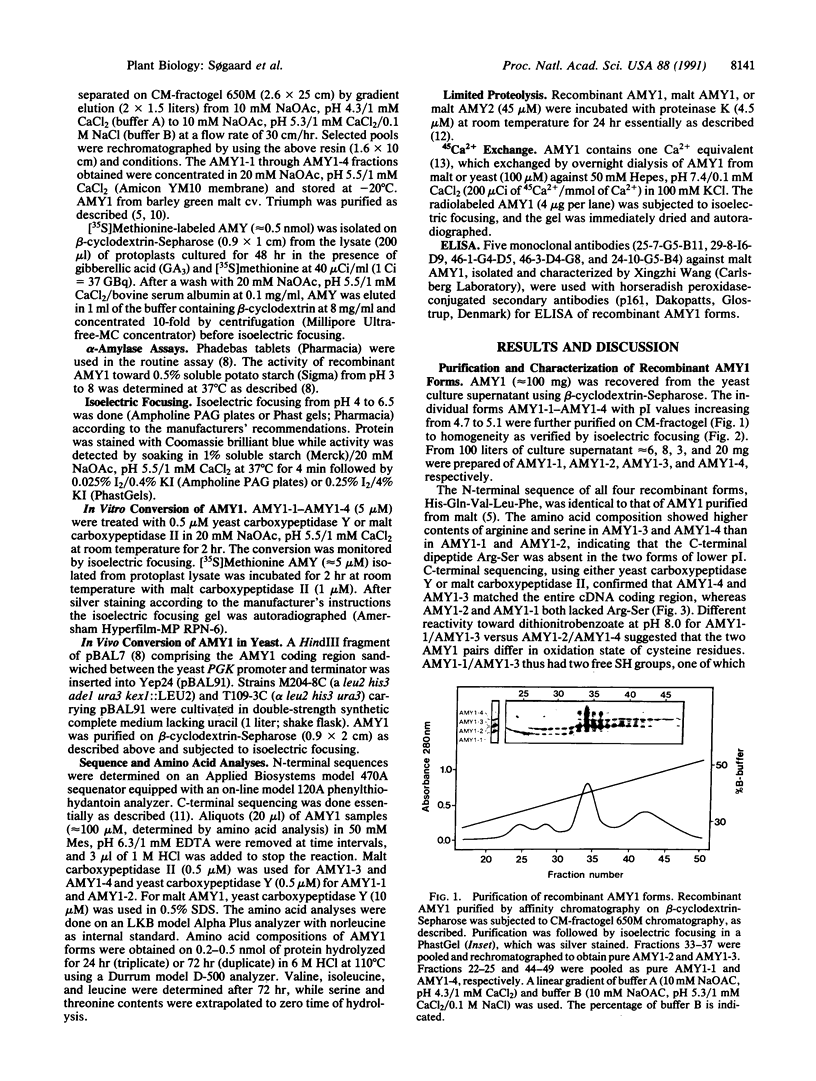
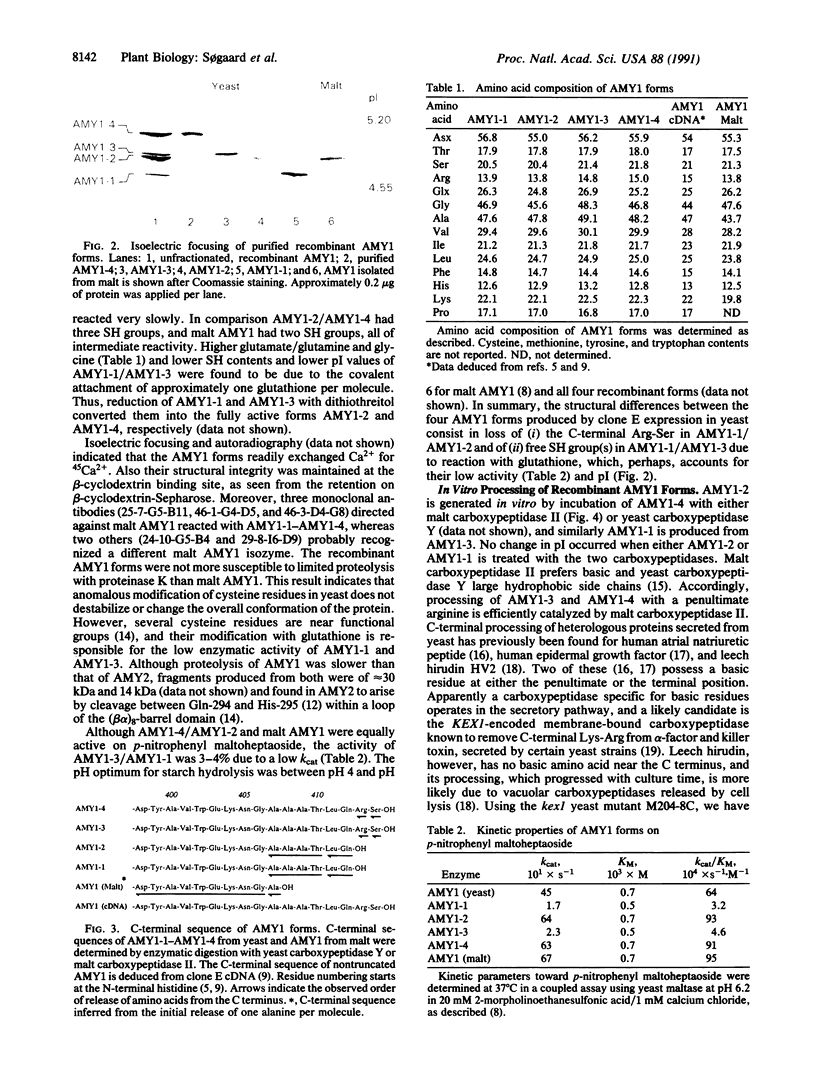
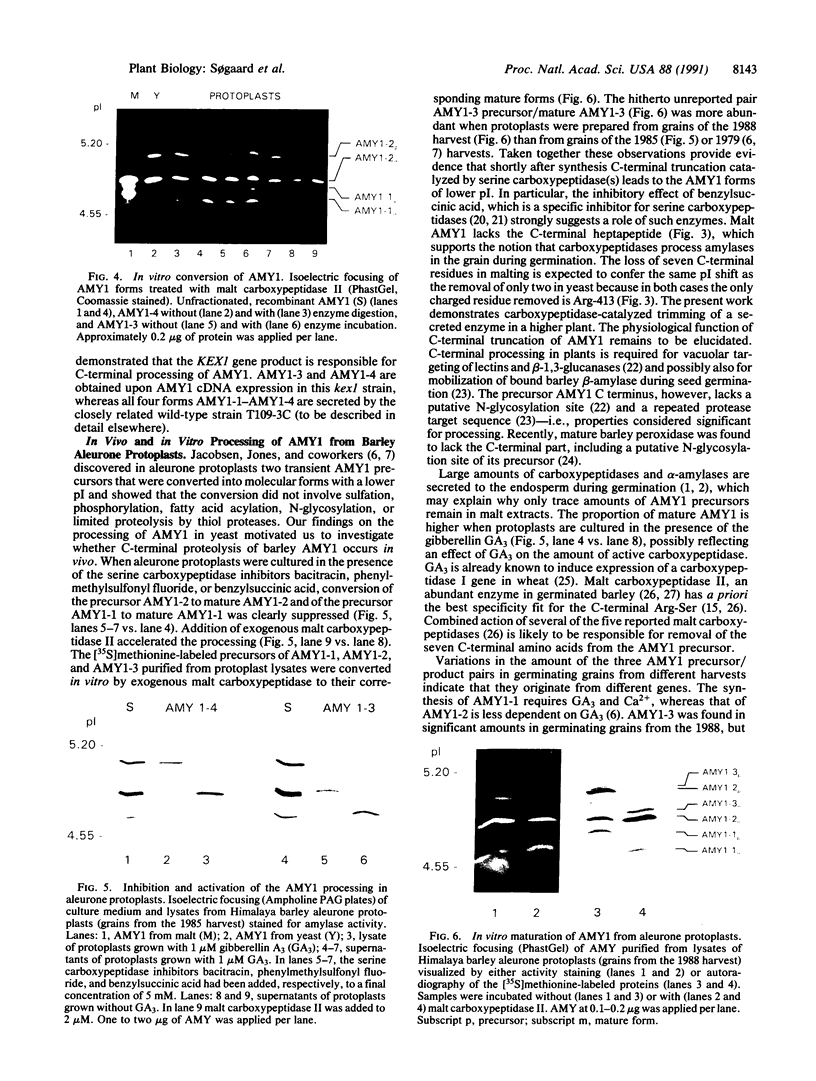
C-terminal processing of low pI barley alpha-amylase (AMY1) results in multiple forms in malt, aleurone protoplasts, and transformed yeast. Expression of an AMY1 cDNA in yeast thus leads to four secreted forms with distinct pI values between 4.7 and 5.1 and essentially identical Mr. AMY1-1 and AMY1-2 lacking the C-terminal Arg-Ser are generated by carboxypeptidase in vitro from AMY1-3 and AMY1-4, respectively. In vivo processing is due to the KEX1-encoded yeast carboxypeptidase. AMY1-2 and AMY1-4 are fully active, whereas AMY+-1 and AMY1-3 retain 3-4% activity toward p-nitrophenyl maltoheptaoside and have one fewer SH group, due to reaction with glutathione. AMY1-1-AMY1-4 are indistinguishable from malt AMY1 with respect to Ca(2+)-, substrate-, and beta-cyclodextrin-binding as well as recognition by three monoclonal antibodies and limited proteolysis by proteinase K. Transient AMY1 precursors present in barley aleurone protoplasts were trapped by addition of serine carboxypeptidase inhibitors, indicating that endogenous carboxypeptidase participates in the maturation of AMY1 during germination. Three pairs of precursor/mature AMY1 forms are recognized, presumably corresponding to the three genes encoding AMY1. Malt carboxypeptidase II can convert in vitro the precursors isolated from protoplasts into processed enzyme, and AMY1 from malt accordingly lacks the C-terminal heptapeptide. This report thus demonstrates posttranslational protein modification by carboxypeptidase in higher plants.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baulcombe D. C., Barker R. F., Jarvis M. G. A gibberellin responsive wheat gene has homology to yeast carboxypeptidase Y. J Biol Chem. 1987 Oct 5;262(28):13726–13735. [PubMed] [Google Scholar]
- Bednarek S. Y., Wilkins T. A., Dombrowski J. E., Raikhel N. V. A carboxyl-terminal propeptide is necessary for proper sorting of barley lectin to vacuoles of tobacco. Plant Cell. 1990 Dec;2(12):1145–1155. doi: 10.1105/tpc.2.12.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D. S., Sticher L., van Huystee R., Wagner D., Jones R. L. The calcium requirement for stability and enzymatic activity of two isoforms of barley aleurone alpha-amylase. J Biol Chem. 1989 Nov 15;264(32):19392–19398. [PubMed] [Google Scholar]
- Dmochowska A., Dignard D., Henning D., Thomas D. Y., Bussey H. Yeast KEX1 gene encodes a putative protease with a carboxypeptidase B-like function involved in killer toxin and alpha-factor precursor processing. Cell. 1987 Aug 14;50(4):573–584. doi: 10.1016/0092-8674(87)90030-4. [DOI] [PubMed] [Google Scholar]
- George-Nascimento C., Gyenes A., Halloran S. M., Merryweather J., Valenzuela P., Steimer K. S., Masiarz F. R., Randolph A. Characterization of recombinant human epidermal growth factor produced in yeast. Biochemistry. 1988 Jan 26;27(2):797–802. doi: 10.1021/bi00402a046. [DOI] [PubMed] [Google Scholar]
- Jacobsen J. V., Bush D. S., Sticher L., Jones R. L. Evidence for precursor forms of the low isoelectric point alpha-amylase isozymes secreted by barley aleurone cells. Plant Physiol. 1988 Dec;88(4):1168–1174. doi: 10.1104/pp.88.4.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. L., Jacobsen J. V. Regulation of synthesis and transport of secreted proteins in cereal aleurone. Int Rev Cytol. 1991;126:49–88. doi: 10.1016/s0074-7696(08)60682-8. [DOI] [PubMed] [Google Scholar]
- Khursheed B., Rogers J. C. Barley alpha-amylase genes. Quantitative comparison of steady-state mRNA levels from individual members of the two different families expressed in aleurone cells. J Biol Chem. 1988 Dec 15;263(35):18953–18960. [PubMed] [Google Scholar]
- MacGregor E. A. Alpha-amylase structure and activity. J Protein Chem. 1988 Aug;7(4):399–415. doi: 10.1007/BF01024888. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan S., Gill B. S., Swegle M., Chandra G. R. Structural genes for alpha-amylases are located on barley chromosomes 1 and 6. J Biol Chem. 1984 Nov 25;259(22):13637–13639. [PubMed] [Google Scholar]
- Rasmussen S. K., Welinder K. G., Hejgaard J. cDNA cloning, characterization and expression of an endosperm-specific barley peroxidase. Plant Mol Biol. 1991 Feb;16(2):317–327. doi: 10.1007/BF00020562. [DOI] [PubMed] [Google Scholar]
- Riehl-Bellon N., Carvallo D., Acker M., Van Dorsselaer A., Marquet M., Loison G., Lemoine Y., Brown S. W., Courtney M., Roitsch C. Purification and biochemical characterization of recombinant hirudin produced by Saccharomyces cerevisiae. Biochemistry. 1989 Apr 4;28(7):2941–2949. doi: 10.1021/bi00433a030. [DOI] [PubMed] [Google Scholar]
- Rogers J. C., Milliman C. Isolation and sequence analysis of a barley alpha-amylase cDNA clone. J Biol Chem. 1983 Jul 10;258(13):8169–8174. [PubMed] [Google Scholar]
- Silvanovich M. P., Hill R. D. Affinity chromatography of cereal alpha-amylase. Anal Biochem. 1976 Jun;73(2):430–433. doi: 10.1016/0003-2697(76)90191-3. [DOI] [PubMed] [Google Scholar]
- Søgaard M., Svensson B. Expression of cDNAs encoding barley alpha-amylase 1 and 2 in yeast and characterization of the secreted proteins. Gene. 1990 Oct 15;94(2):173–179. doi: 10.1016/0378-1119(90)90384-4. [DOI] [PubMed] [Google Scholar]
- Vlasuk G. P., Bencen G. H., Scarborough R. M., Tsai P. K., Whang J. L., Maack T., Camargo M. J., Kirsher S. W., Abraham J. A. Expression and secretion of biologically active human atrial natriuretic peptide in Saccharomyces cerevisiae. J Biol Chem. 1986 Apr 15;261(11):4789–4796. [PubMed] [Google Scholar]








