Abstract
Objective: The aim of this study was to determine characteristics of drug allergy alert overrides, assess how often they lead to preventable adverse drug events (ADEs), and suggest methods for improving the allergy-alerting system.
Design: Chart review was performed on a stratified random subset of all allergy alerts occurring during a 3-month period (August through October 2002) at a large academic hospital.
Measurements: Factors that were measured were drug/allergy combinations that triggered alerts, frequency of specific override reasons, characteristics of ADEs, and completeness of allergy documentation.
Results: A total of 6,182 (80%) of 7,761 alerts were overridden in 1,150 patients. In this sample, only 10% of alerts were triggered by an exact match between the drug ordered and allergy listed. Physicians' most common reasons for overriding alerts were “Aware/Will monitor” (55%), “Patient does not have this allergy/tolerates” (33%), and “Patient taking already” (10%). In a stratified random subset of 320 patients (28% of 1,150) on chart review, 19 (6%) experienced ADEs attributed to the overridden drug; of these, 9 (47%) were serious. None of the ADEs was considered preventable, because the overrides were deemed clinically justifiable. The degree of completeness of patients' allergy lists was highly variable and generally low in both paper charts and the CPOE system.
Conclusion: Overrides of drug-allergy alerts were common and about 1 in 20 resulted in ADEs, but all of the overrides resulting in ADEs appeared clinically justifiable. The high rate of alert overrides was attributable to frequent nonexact match alerts and infrequent updating of allergy lists. Based on these findings, we have made specific recommendations for increasing the specificity of alerting and thereby improving the clinical utility of the drug allergy alerting system.
Computerized physician order entry (CPOE) has gained recognition as a key tool that health care organizations can implement to improve patient safety.1,2,3 Increasing evidence shows that CPOE can reduce the frequency of medication errors in the inpatient setting.4,5,6,7,8,9,10 Many of the beneficial effects of CPOE result from integrated decision support tools, including suggestions regarding appropriate drug dosing, and real-time alerting for drug–drug interactions and drug allergies.11
Drug allergy alerting represents a particularly important part of decision support.5,12,13 Even though only about 20% of patients react when they receive medications to which they have “known allergies,” the reactions can be devastating when they occur.14 It should be possible to develop tools to warn providers reliably when the patient has had a prior reaction to a medication, whether an allergy (immune-mediated type I hypersensitivity reaction), or a sensitivity (adverse drug reaction that is non–immune-mediated, e.g., nausea or diarrhea). In CPOE applications that maintain patients' medication and allergy lists, ordered drugs can be checked against the patient's allergy list, and decision support can generate alerts that warn the physician of a possible allergy to the ordered drug. Physicians can either accept or override these alerts.
The challenge of ensuring that allergy alerting functions well stems from a number of factors. Maintaining accurate allergy lists can be difficult because there may not be clear distinctions between allergies and sensitivities, and there is no general consensus on whether both should be included in allergy lists. In addition, neither the specificity of alerting algorithms nor the relative effectiveness of different methods of alerting (e.g., alerts that interrupt workflow vs. those that display information, but do not interrupt) has been elucidated fully. A study by Abookire et al.16 found that nearly 80% of drug allergy alerts at Brigham and Women's Hospital were overridden by physicians, and Payne et al.17 found a 69% override rate at the Veterans Administration Puget Sound Health Care System in Seattle, WA. One reason cited for these high override rates was highly inclusive drug-class and drug cross-reactivity mapping, which generated a large number of allergy alerts for drugs with only slight potential to cause an allergic reaction (e.g., furosemide ordered in the context of an allergy to sulfa antibiotics). Policies requiring physicians to renew certain drugs for the same patient multiple times, causing many redundant alerts to be generated, also were cited. However, neither of these studies addressed the clinical consequences of allergy alerts that were overridden, such as adverse drug events (ADEs).
An excess of allergy alerts with low predictive value for allergic drug reactions and of alerts that are potentially inaccurate because of inconsistent information in the medical record, has the potential to erode physicians' faith in allergy alerts and to increase the likelihood that alerts containing important information will be ignored.15 However, failing to warn could also have deleterious consequences. Continuous quality improvement to ensure appropriate alerting is critical for continued efficacy and acceptance of CPOE decision support. Therefore, our goals were: (1) to characterize overridden drug allergy alerts in more detail; (2) to assess how often overriding allergy alerts leads to preventable ADEs in the inpatient setting; and (3) to suggest methods for improving drug allergy alerting systems.
Methods
Site
Brigham and Women's Hospital (BWH) is a 709-bed academic medical center that implemented CPOE beginning in 1993. At BWH, all inpatient orders are entered into the computer; approximately 85% are entered by physicians, and the remainder are entered by nurses, physician assistants, and students, and later cosigned by physicians.18 The CPOE application ensures the legibility and completeness of all medication orders by providing clinicians with a coded dictionary of medication names, default doses, and lists of commonly used doses. The CPOE application includes clinical decision-support features that generate alerts and reminders, including drug duplication checking, drug–drug interaction checking, drug–laboratory checking, renal dosing suggestions, and drug allergy checking.5,11,19
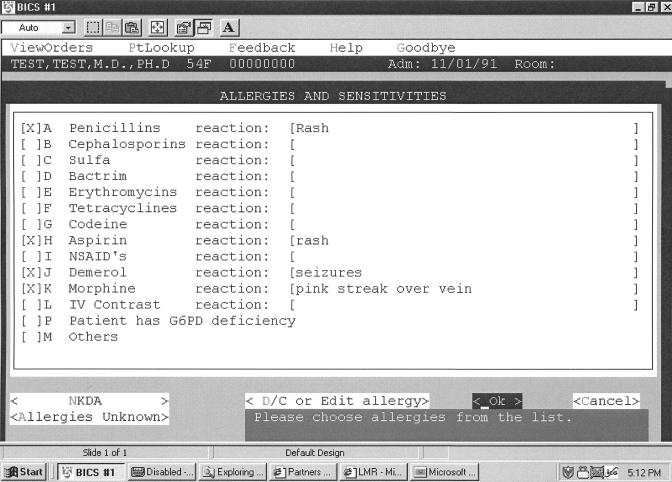
All patients admitted to BWH have drug allergies and sensitivities entered by the admitting physician into the CPOE database via standard admission order sets that require this information (▶). The allergy documentation screen consists of a list of the 15 most commonly occurring drug allergies, which can be selected by clicking a checkbox. If the clinician wishes to document an allergy for the patient that is not on the checkbox screen, a look-up function is provided that attempts to match the entered text with a coded list of medications, medication classes, and ingredients; free text may be entered if there is no coded match. Coded allergies are stored in the patient database as an ingredient or list of ingredients.15 The reaction (e.g., rash, anaphylaxis) is captured as free text.
Figure 1.
Allergy documentation screen.
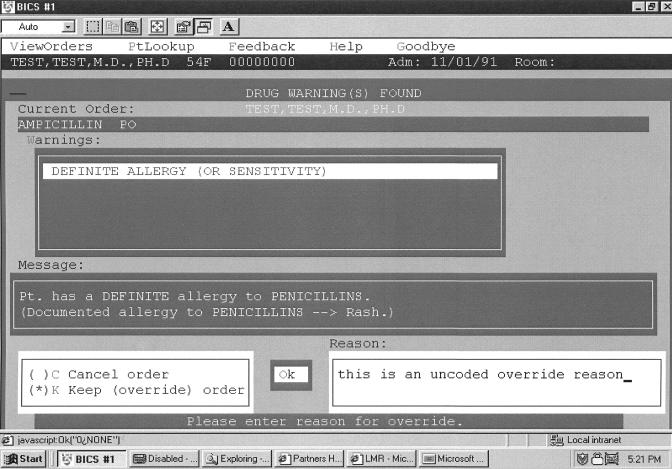
When medications are ordered by clinicians in the CPOE system, the orders are electronically compared with the patient's allergy list. A commercial knowledge base (First DataBank, San Bruno, CA) that uses class-based hierarchical ingredient knowledge (e.g., ampicillin is a penicillin) as well as cross-reactivity mapping (e.g., penicillins cross-react with cephalosporins) is checked to determine whether the drug ordered has a potential interaction with an item on the patient's allergy list. If so, the application generates an on-screen allergy alert (▶). The alert informs the clinician whether the alert is due to an exact or class-based match with the allergy list (“definite allergy”), or is the result of a cross-reaction (“possible allergy”) and displays the patient's previously documented reaction to the drug. The clinician can either discontinue the order or continue with the medication order by overriding the alert, which requires entering a free-text (uncoded) reason for the override action. The clinicians' reasons for overriding allergy alerts are attached to medication orders so that pharmacists and nurses can consider them when double-checking the orders for drug allergies and drug–drug interactions before dispensing and administration—important actions for ensuring medication safety. If a patient is readmitted to the hospital at a later date, the allergy list from any prior admission is displayed to the physician for reapproval.
Figure 2.
Example of an on-screen allergy alert.
Even though in the inpatient setting, allergies may be documented using CPOE applications, clinicians (especially nonphysicians) also document allergies in various locations in the patient's paper medical record. Examples of places in the paper record where allergies may be documented include admission notes, progress notes, the medication administration record, operative notes, nursing forms, consult sheets, and discharge documents.
Subjects
We identified all patients admitted to Brigham and Women's Hospital during a three-month period (August through October 2002) who had at least one drug-allergy alert during their admission. We analyzed the first overridden alert within each patient admission. Because a large fraction of alerts was found previously to be triggered by two categories of drugs (diuretics containing a sulfur moiety and narcotics),16 we selected a stratified random subset of patients for detailed analysis of consequences of overrides. Our goal was to analyze approximately 100 patients for each of three categories—sulfur-containing diuretics, narcotics, and all other drug classes combined. In the stratified sample, the first overridden medication that was administered to the patient was analyzed. The study was approved by the Institutional Review Board of Brigham and Women's Hospital.
Data Collection
For each of the overridden alerts, we used data from the computerized physician order entry application to determine the category of drug ordered, the category of override reason entered by the physician, and the combinations of drug ordered and drug on the allergy list (drug/allergy combinations) that triggered the alerts. Three conditions were considered “exact matches” between drug/allergy pairs: (1) the drug ordered and allergy listed were identical (e.g., aspirin ordered in the context of an allergy to aspirin); (2) the allergy listed was a drug category that included the ordered drug (e.g., ampicillin ordered in the context of an allergy to “penicillins”); or (3) the drug ordered and allergy listed shared common ingredients (e.g., acetaminophen+codeine ordered in the context of sensitivity to acetaminophen+oxycodone, since both of these medications contain acetaminophen). In the absence of common ingredients, a narcotic ordered in the context of a listed sensitivity to a different narcotic was considered a nonexact match.
For the stratified random sample, nurse reviewers performed complete chart reviews using a standardized data collection tool and reviewed progress notes, CPOE records, medication administration records, nursing notes/forms, and any other documentation. Information collected included: (1) details of the drug order, allergy alert, and override; (2) documentation of the patient's allergy history in the paper chart; (3) information about the drug (e.g., number of times the patient received the drug and whether the patient was taking the drug at home before the admission); and (4) whether there was any evidence that the patient experienced a possible ADE (defined as an injury resulting from medical intervention related to a drug20) related to administration of the alerted drug. Possible ADEs identified were classified as significant (such as rash or diarrhea), severe (such as gastrointestinal bleed), or life threatening (such as anaphylaxis).21
Both the order entry system and paper charts were used as data sources. Information on patient outcomes was collected from the date that the first overridden drug was administered through the fifth hospital day or until discharge, whichever came first.
Physicians' override reasons, entered as free text into the CPOE application at the time of order, were classified into categories (that captured similar override reasons into a single descriptive term) by the physicians on the research team (“Patient does not have this allergy/Tolerates,” “Aware/Will monitor,” “Patient taking already,” “Desensitization protocol,” or “Other”). When the override reason given was that the patient did not have the allergy or tolerated the drug, the reviewer documented whether the drug was subsequently removed from the allergy list in the patient's electronic medical record.
The 22 possible ADEs discovered by the nurse reviewers were evaluated by a panel of two physician reviewers who independently rated the degree of confidence that the event was a true ADE and that it was caused by the overridden drug.22 Confidence about the classification of events was rated on a six-point scale: (1) little or no evidence, (2) slight-to-modest evidence, (3) less than 50% evidence but close call, (4) more than 50% evidence but close call, (5) strong evidence, or (6) virtually certain evidence. Events with confidence scores of 4 or higher were considered ADEs related to the alerted drug. All such ADEs were rated on a previously described severity scoring system as “significant,” “serious,” “life threatening,” or “fatal.”20 Nausea and vomiting of greater than 2 days' duration was considered severe. Preventability of each ADE was rated on a four-point scale (definitely preventable, probably preventable, probably not preventable, definitely not preventable). Preventability ratings took into account whether the physician's override action appeared clinically justifiable based on information from chart review. Differences between the two reviewers' judgments about the classification of events were resolved by discussion. The kappa statistic for interrater agreement on the presence of an ADE was high (0.78).
Statistical Analysis
Student's t-test was used to make comparisons between normally distributed variables (age, number of allergies in paper chart, and number of medical problems in chart), and the χ2 test was used to compare categorical variables (sex, race/ethnic group, and whether the patient was already taking the alerted medication). All reported p-values were based on unpaired, two-tailed tests of significance. Analyses were performed using SAS statistical software23 and Microsoft Access 2000.
Results
Characteristics of the Study Population
There were 10,277 admissions to BWH during the three-month study period, and a total of 7,761 drug allergy alerts were triggered for 1,608 patients (16% of admissions). Alerts were overridden in 80% (6,182) of the orders in 1,150 patients (72%). From these patients, we selected a stratified random subset of 347 (30% of 1,150) patients for further evaluation. Three patients (1%) were excluded because the alerted medication was ordered as part of a drug-desensitization protocol, and 24 (7%) were excluded because they never actually received the overridden drug, leaving 320 patients (92% of 347) for full chart review. Characteristics of patients in the stratified random subset did not differ significantly from those of the full sample (▶).
Table 1.
Characteristics of Patients with Overridden Allergy Alerts
| Characteristic | Full Sample (n = 1,150) (%) | Subset (n = 320) (%) | No ADE (n = 301) (%) | ADE (n = 19) (%) | p Value |
|---|---|---|---|---|---|
| Age (yrs) | 0.005 | ||||
| Mean ± SD | 56 ± 17 | 59 ± 17 | 59 ± 17 | 48 ± 19 | |
| Range | 15-95 | 15-91 | 15-91 | 18-81 | |
| Sex | 0.059 | ||||
| Female | 785 (68) | 225 (70) | 208 (69) | 17 (89) | |
| Male | 365 (32) | 95 (30) | 93 (31) | 2 (11) | |
| Race or ethnic group | 0.382 | ||||
| White | 928 (81) | 268 (84) | 254 (84) | 14 (74) | |
| Black | 136 (12) | 32 (10) | 29 (10) | 3 (16) | |
| Hispanic | 36 (3) | 8 (2.5) | 7 (2.3) | 1 (5) | |
| Asian | 9 (1) | 2 (0.5) | 2 (0.7) | 0 (0) | |
| Other | 40 (3) | 10 (3) | 9 (3) | 1 (5) | |
| No. of Allergies in Paper Chart | 0.793 | ||||
| Mean ± SD | Not available* | 3.0 ± 1.9 | 3.0 ± 1.9 | 2.8 ± 1.6 | |
| Range | 1-9 | 1-9 | 1-8 | ||
| No. of Medical Problems in Chart | 0.121 | ||||
| Mean ± SD | Not available* | 8.2 ± 4.1 | 8.3 ± 4.2 | 6.8 ± 3.5 | |
| Range | 1-16 | 1-16 | 2-15 | ||
| Patients taking alerted medication at home before admission | Not available* | 107 (33) | 104 (35) | 3 (16) | 0.093 |
These values only calculated for the subset of charts undergoing full chart review.
Allergy Alerts and Override Reasons
In the full sample of 1,150 overridden alerts, the drug classes that most frequently generated alerts were narcotics (444, 39%) and cephalosporins (247, 21%). The remaining overrides were for sulfur-containing drugs (145, 13%), nonsteroidal anti-inflammatory drugs (NSAIDs) (125, 11%), other antibiotics (47, 4%), and other miscellaneous drugs (142, 12%).
Only 120 (10%) of the 1,150 overridden allergy alerts were triggered by an exact match between the ordered drug and the drug on the allergy list (▶). Thus, 90% of overridden alerts were triggered by nonexact drug/allergy matches, in which the drug and allergy had structural similarities or were in the same family but were not identical. For example, in the 444 orders for noncodeine narcotics, a listing of codeine in the patient's allergy list accounted for 194 (44%) of the alerts. A documented allergy to “penicillins,” or to a specific penicillin, was associated with 235 (95%) of 247 allergy alerts during orders for cephalosporin antibiotics.
Table 2.
Selected Drug/Allergy Pairs that Triggered Overridden Alerts
| Drug Ordered | Allergy Listed | No. of Alerts generated (%) | Exact Match? |
|---|---|---|---|
| Narcotics | |||
| Oxycodone (n = 162) | Codeine | 76 (47) | No |
| Morphine | 38 (23) | No | |
| Meperidine | 6 (4) | No | |
| Oxycodone | 6 (4) | Yes | |
| Other narcotics (individually named) | 15 (9) | No | |
| “Narcotics” (category) | 1 (0.6) | Yes | |
| Morphine (n = 99) | Codeine | 53 (54) | No |
| Oxycodone | 18 (18) | No | |
| Meperidine | 10 (10) | No | |
| Hydromorphone | 7 (7) | No | |
| Morphine | 3 (3) | Yes | |
| Other narcotics (individually named) | 8 (8) | No | |
| Cephalosporins | |||
| All cephalosporins (n = 247) | “Penicillins” (category) | 218 (88) | No |
| Various penicillins (individually named) | 17 (7) | No | |
| “Cephalosporins” (category) | 10 (4) | Yes | |
| Various cephalosporins (individually named) | 2 (1) | Yes | |
| Sulfur Drugs | |||
| Furosemide (n = 99) | “Sulfa” (category) | 74 (75) | No |
| Trimethoprim+sulfamethoxazole | 22 (22) | No | |
| Furosemide | 2 (2) | Yes | |
| Sulfisoxazole | 1 (1) | No | |
| Hydrochlorothiazide (n = 29) | “Sulfa” (category) | 20 (69) | No |
| Trimethoprim+sulfamethoxazole | 8 (28) | No | |
| Celecoxib | 1 (3) | No | |
| Trimethoprim+sulfamethoxazole (n = 11) | “Sulfa” (category) | 7 (64) | Yes |
| “G6PD deficiency” | 2 (18) | No | |
| Hydrochlorothiazide | 1 (9) | No | |
| Trimethoprim+sulfamethoxazole | 1 (9) | Yes |
The most frequent reasons given by physicians for overriding alerts in the full sample included “Aware/Will monitor” (55%), “Patient does not have this allergy/Tolerates” (33%), and “Patient taking already” (10%; ▶). The frequency of specific override reasons was similar in the stratified random subset.
Table 3.
Override Reasons versus Category of Drug Ordered: Full Sample (n = 1,150)
| Category of Override Reason Given by Physician | Narcotics | Cephalosporins | Sulfur Drugs | NSAIDs | Other Antibiotics | Other | Total (%) |
|---|---|---|---|---|---|---|---|
| Aware/Will monitor | 234 (53) | 131 (53) | 80 (55) | 73 (59) | 33 (70) | 77 (54) | 628 (55) |
| Patient does not have this allergy/Tolerates | 174 (39) | 104 (42) | 29 (20) | 30 (24) | 10 (21) | 29 (20) | 376 (33) |
| Patient taking already | 27 (6) | 8 (3) | 31 (21) | 15 (12) | 1 (2) | 31 (22) | 113 (10) |
| Other | 9 (2) | 4 (2) | 5 (3) | 7 (6) | 3 (6) | 5 (4) | 33 (3) |
| Total (%) | 444 (39) | 247 (21) | 145 (13) | 125 (11) | 47 (4) | 142 (12) | 1,150 (100%) |
Percentages in normal type are calculated within columns (drug category). Percentage in bold type reflect percent of full sample (n = 1,150)
Adverse Drug Events
Of the 320 patients' charts reviewed in detail, there were 22 possible ADEs related to the alerted drug identified by nurse reviewers. Of these, 19 (6% of 320) were confirmed by the two-physician panel as ADEs (▶). Ten (53%) of the ADEs were considered significant, and 9 (47%) were serious (Appendix 1). None of the events was life-threatening or fatal. Sixteen (84%) of the ADEs were due to narcotics, and there was one event (5%) each due to a sulfur-containing diuretic, a cephalosporin antibiotic, and insulin.
Table 4.
Rates of Adverse Drug Events Owing to Overridden Allergy Alerts
| Variable | Adverse Events (%) | Event Rate no./100 patients |
|---|---|---|
| Total adverse drug events | 19 | 5.9 |
| Severity | ||
| Significant | 10 (53) | 3.1 |
| Serious | 9 (47) | 2.8 |
| Life-threatening | 0 | — |
| Fatal | 0 | — |
| Drug category | ||
| Narcotics | 16 (84) | 5.1 |
| Sulfur diuretics | 1 (5) | 0.3 |
| Cephalosporins | 1 (5) | 0.3 |
| Insulin | 1 (5) | 0.3 |
| Alert based on nonexact match | 18 (95) | 5.6 |
| Override reason | ||
| Aware/will monitor | 8 (42) | 2.5 |
| Patient does not have this allergy/Tolerates | 11 (58) | 3.4 |
| ADE Type/Organ system affected | ||
| Gastrointestinal (nausea/vomiting) | 12 (63) | 3.8 |
| Allergic/cutaneous (hives, erythema, itching) | 3 (16) | 0.9 |
| Cardiovascular | 1 (5) | 0.3 |
| Metabolic | 1 (5) | 0.3 |
| Renal | 1 (5) | 0.3 |
| Other (jaw swelling) | 1 (5) | 0.3 |
Percentages may not sum to 100 because of rounding.
The majority (12, 63%) of ADEs were gastrointestinal, with symptoms of nausea or vomiting; these were all owing to narcotics. Three ADEs (16%) were allergic events with cutaneous manifestations (hives, erythema, itchiness); these were due to hydromorphone and morphine. Additionally, there was one case (5%) each of hypotension, elevated creatinine, jaw swelling, and hypoglycemia.
The majority of ADEs (18, 95%) resulted from override of a nonexact match alert. All 19 ADEs were deemed nonpreventable, because in each case, the reviewers agreed that it was clinically justifiable for the physician to have overridden the alert, because the clinical need for the drug outweighed the risk of a serious allergic reaction.
Univariate analyses of patient characteristics found that age was the only significant correlate of an ADE (p = 0.005; ▶). Patients with ADEs tended to be younger than those without ADEs, likely because patients on narcotics were younger, and narcotics were the most common class of medication involved in ADEs. There were trends toward lower risk of ADE in patients who were male (p = 0.06) and in patients who were already taking the alerted medication at home before admission (p = 0.09).
Allergy Documentation
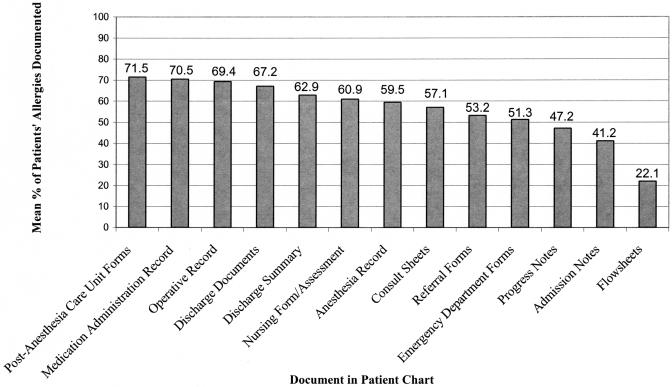
Using each patient's total number of unique drug allergies listed in the electronic and paper medical records as the denominator, we assessed the completeness of allergy documentation in various components of patients' paper charts by determining the average percentage of a patient's allergies documented on a particular type of form. Rates of drug allergy documentation varied widely by form, ranging from 72% to 22% (▶). The mean rate of allergy documentation across all forms was 56.5%.
Figure 3.
Allergy documentation in paper charts.
In 145 cases (45%) within the stratified random subset in which the physician's override reason was “Patient does not have an allergy/Tolerates” or “Patient taking already,” 12 (8%) were due to exact matches. In only two (17% of 12) of these cases did the physician subsequently update the allergy list by removing the drug that triggered the alert.
Discussion
The goals of this study were to evaluate the characteristics of overridden allergy alerts in the inpatient setting, analyze physicians' reasons for override, assess how often overrides lead to preventable ADEs, and use the lessons learned to recommend strategies to improve the clinical utility of drug allergy alerting.
The 80% override rate of drug allergy alerts in this study was similar to the override rate previously found in the same hospital and CPOE system by Abookire et al.16 and somewhat lower than the 91% override rate found at five adult primary care practices by Weingart et al.24 In both of those studies, the high override rates were partly attributed to alerting protocols that generated alerts as long as the ordered drug was in the same family as a drug on the patient's allergy list. We also found that the majority (90%) of overrides occurred when the two drugs belonged to the same family but were not identical (for example, codeine and hydromorphone).
The current design of our allergy-alerting protocol uses a commercial medication knowledge base to create groupings of medications that may have similar allergenic properties. In many cases, the clinical relevance of the relationships is inferred from pharmacologic or structural similarity and not from clinical data. In these instances, the likelihood of a patient's suffering from an adverse consequence from the administration of a related drug is low (e.g., if a patient with codeine sensitivity takes morphine). As more clinical data are gathered, this information should be incorporated into allergy checking rule bases, but in the absence of such data, health care institutions will need to make their own decisions about whether to alert for medications that are nonexact matches to the documented allergy.
In this study, we found that when “allergies” to specific narcotics were documented in the patient's allergy list, nausea and vomiting were the most common reactions noted. In fact, symptoms such as nausea, vomiting, respiratory depression, and constipation are not true allergic reactions, but rather direct side effects of, or sensitivities to, the narcotics' pharmacologic activity.25 Although allergic, anaphylactic, and anaphylactoid (pseudo-allergic, non–immune-mediated reaction resulting in basophil or mast cell activation and release of vasoactive mediators) reactions to narcotics have been reported, such reactions are rare.26,27 Furthermore, an individual patient's responses may be very different to various narcotics, and several different physiologic variables can affect a patient's sensitivity to narcotics at a given time.27 Therefore, instead of frequently alerting about possible “allergies” to ordered narcotics whenever nonexact drug/allergy matches arise, we would recommend generating an alert only when the ordered drug is an exact match to (i.e., is identical to) the drug on the allergy list, and in the case of nonexact matches, to simply display the previously documented reaction in an informative but noninterruptive manner. Such noninterruptive alerts could advise increased monitoring or early use of premedications to attempt to ameliorate symptoms when they occur. In addition, better ways to differentiate true allergies from sensitivities when documenting “allergy lists” in CPOE should be explored.
The protocol for sulfa-allergy alerting can also be improved substantially. We found that when sulfonamide-containing diuretics, such as furosemide, were ordered, most allergy alerts were triggered by a documented allergy to “sulfa”—a term that typically denotes sulfonamide antibiotic, such as sulfamethoxazole. However, the weight of evidence from chemical–structural and clinical studies strongly suggests that immunologic cross-reactivity between sulfonamide antibiotics and sulfonamide nonantibiotics (such as furosemide, hydrochlorothiazide, glyburide, and celecoxib) is unlikely and that patients with a history of allergic reaction to a sulfonamide antibiotic were no more likely to have a subsequent allergic reaction to a different sulfonamide than to a nonsulfonamide, such as penicillin.28,29,30,31,32 Thus, we recommend that no allergy alert should be generated when clinicians order sulfonamide nonantibiotics in the context of a documented “sulfa” (sulfonamide antibiotic) allergy. Alternatively, any alerts that are generated should be noninterruptive and simply warn the clinician that the patient may have a generally increased propensity to developing allergic reactions.
We also examined the reasons given by physicians for overriding drug allergy alerts and the clinical consequences of such override actions. The fact that half of the override reasons were “Aware/Will monitor” reflects the complex calculus involved in the risk–benefit analysis of therapeutic decision making, particularly with nonexact match allergy alerts. Such decisions must weigh the need to treat with a particular drug (e.g., need for pain relief) against the likelihood, type, and severity of a possible adverse reaction (e.g., life-threatening anaphylaxis vs. mild nausea).25
Another common reason given was “Patient does not have this allergy/Tolerates,” accounting for nearly a third of overrides. The high incidence of this override reason suggests that physicians may often be using the patient's self-report or other information at the time of visit to determine if a patient will tolerate the medication. Other significant contributing factors to the use of this override option may be the infrequent updating of patients' allergy lists, resulting in many lists' being out of date or inaccurate, and the numerous locations where allergies are documented in the chart, often with little agreement. Improving the maintenance of patients' allergy lists could be accomplished by having clinicians select override reasons from a menu of choices; selection of reasons such as “Patient does not have this allergy/Tolerates” or “Patient taking already” from the menu would automatically prompt the clinician to remove the drug from the allergy list. Electronic medical records linked to CPOE could create the ability to maintain a single allergy list, rather than the numerous ones that exist today.
We feel it is reasonable to require input of the override reason, which enables pharmacists and nurses downstream in the medication order process to see not only that the ordering physician has considered the risk of drug allergy but also understand the reason why the physician felt it was safe to override the alert. Furthermore, modifying the override reason field so that physicians pick from a menu of choices enables automatic updating of the patient's allergy history—if the reason chosen is, for example, that the patient tolerates the drug well or is already taking the medication at home. Finally, the override reasons provide information that help us better understand why certain alerts are not accepted, and therefore to potentially modify the alerting strategy. This study shows the importance of analyzing override reasons as a quality improvement tool to improve alerting strategies, and organizations should consider making this part of their routine improvement processes after implementing CPOE.
Of the overrides of alerts in the stratified random subset based on nonexact drug/allergy matches, only 6% resulted in an adverse drug event, and none of these ADEs was preventable, since all of the overrides appeared clinically justifiable. Thus, the high override rate appears to be attributable primarily to excessive and inappropriate alerting, rather than to poor clinical judgment or to clinician disregard for allergy alerting. Weingart et al.24 similarly found ADEs resulting from overridden alerts to be infrequent (none in a subset of 31 overridden alerts resulted in an ADE), and reviewers in that study agreed with the prescriber's override decision in almost every case (65 of 68 overrides). Results of these studies suggest that decreasing alerting of nonexact drug/allergy matches should be a safe strategy to reduce overalerting. The fact that the majority of ADEs in this study were narcotic-induced nausea or vomiting, which are not true allergic reactions, lends further support to this notion. Reducing overalerting is important because too-frequent false alerts to safety hazards not only waste clinicians' time, but also may cause truly important warnings to be overlooked or ignored. We have listed our recommended strategies for improving allergy alerting in ▶.
Table 5.
Specific Recommendations to Improve Allergy Alerting
| Allergies vs. Sensitivities—Data about reactions should be coded, not free text. Collect coded data about both allergies and sensitivities/intolerances and be able to differentiate between them. Subsequent allergy alerts should specify the nature of the previously noted reaction and indicate whether this was a sensitivity or a true allergic reaction. |
| Alerts—Display interruptive alerts only for true allergic reactions (not sensitivities) and make dangerous warnings (e.g., anaphylaxis) readily identifiable and easily distinguishable from less dangerous warnings (e.g., rash). |
| Narcotics—When a clinician orders a narcotic, an interruptive alert should be generated only when the ordered drug is an exact match to the drug on the allergy list. In the case of nonexact matches, the previously documented reaction should be displayed in an informative but noninterruptive manner. Noninterruptive alerts may recommend increased monitoring or early use of premedications to prevent or ameliorate adverse symptoms. |
| Sulfa Allergies—When a clinician orders a sulfonamide nonantibiotic in the context of a “sulfa” (sulfonamide antibiotic) allergy, either no alert should be generated or a noninterrruptive alert can be displayed warning the clinician that the patient may have an increased propensity to developing allergic reactions. However, the alert should not imply that there will be specific cross-reactivity between the sulfonamide antibiotic taken in the past and the sulfonamide nonantibiotic being ordered currently. |
| Override Reasons and Consequent Actions—Override reasons should be selected from a menu of choices rather than entered as free text, to facilitate data analysis and updating of patient allergy lists. Selecting the override reasons “Patient does not have this allergy/Tolerates” or “Patient taking already” should automatically take the clinician to a screen that allows him or her to remove the drug from the allergy list. Organizations should audit override reasons on a regular basis to identify alerts that are not useful. |
| Improving New Allergy Capture—Prompt clinicians to enter new allergies if it can be inferred that an allergic reaction may have occurred (e.g., certain instances when a new order for diphenhydramine has been written). |
| Electronic Medical Records—EMRs eventually will decrease the need for multiple allergy lists in multiple locations. The goal should be a single, universal allergy list for each patient. |
Limitations of this study include that the study period was short and the sample of medication orders analyzed was small compared with the total number entered into the CPOE system each year. In addition, the rate of ADEs owing to administering alerted drugs may be overestimated since we did not use a control group to measure the baseline rate of ADEs resulting from nonalerted drug orders. Given the complex medical regimens and types of diseases in our patient population, it was difficult to determine whether ADEs were caused by the overridden drugs or to other drugs or the patients' disease states. Furthermore, the accuracy of the two-physician panel's judgments as to the justifiability of the physicians' overrides was reliant on the thoroughness of documentation in the patients' charts. Finally, this study was conducted within a single CPOE system, so the rates of overrides and ADEs may not be generalizable, although we feel that many of the lessons learned will be applicable to allergy alerting in other systems.
We found that overrides of drug allergy alerts were common, many were clinically justifiable, and few resulted in ADEs. The high rate of overrides is due in part to the current design of the allergy-checking protocol, which generates an excess of alerts with low predictive value for true drug allergies as well as to infrequent updating of patients' allergy lists. Based on our findings, we have made a number of specific recommendations which should improve the clinical utility of the allergy alerting functionality of CPOE systems; the effects of these recommendations on rates of overrides and ADEs should subsequently be evaluated. Future research that is needed includes similar analysis and refinement of other types of medication-related decision support, such as drug–drug interaction and drug–laboratory checking. We have shown that analyzing drug/allergy combinations that trigger alerts, clinicians' override reasons, and ADEs resulting from administration of alerted drugs is useful for identifying ways in which drug allergy alerting functions of CPOE systems can be improved.
Appendix 1. Description of Adverse Drug Events
| Serious Adverse Drug Events (n = 9) |
| • A 41-year-old patient with a history of reaction meperidine (reaction: seizures) who was admitted for gastrointestinal bleeding had 4 days of nausea and vomiting after administration of hydromorphone patient-controlled analgesia (PCA). Diphenhydramine was administered starting on the first day of symptoms. |
| • A 33-year-old patient with a history of reaction to meperidine (rigors) had an episode of hypotension (systolic blood pressure 80 mm Hg) on the first day of placement of a hydromorphone epidural after gastric bypass surgery. |
| • A 28-year-old patient with a history of penicillin allergy, admitted for a normal delivery, had jaw swelling after administration of cefazolin. The drug was discontinued 8 hours later, and diphenhydramine was administered. |
| • An 18-year-old patient with a history of allergy to acetaminophen+oxycodone had itching for a three day period after hydromorphone PCA was placed postsurgery. Diphenhydramine was subsequently administered. |
| • A 70-year-old patient with a history of “sulfa” allergy who was admitted for treatment of pneumonia had an increase in serum creatinine (from 1.3 to 1.7 mg/dL) after administration of furosemide. The patient had been taking the medication before admission. |
| • A 24-year-old patient with a history of diabetes mellitus who had been on an insulin pump at home (set at 0.7 units per hour) had hypoglycemia after receiving insulin in the hospital at 1.0 U/hr. The dosing rate was subsequently decreased to 0.7 units per hour. |
| • A 57-year old patient with a history of allergy to morphine (local rash) had nausea and vomiting for 4 days while on a hydromorphone PCA after being admitted for severe constipation secondary to multiple sclerosis. |
| • A 24-year-old patient with a history of reaction to morphine (nausea) who was admitted for sickle-cell crisis experienced nausea and vomiting for 5 days while on oxycodone PCA. The patient was discharged on the medication. |
| • A 36-year-old patient with a history of allergy to acetaminophen+oxycodone (hives) had severe nausea and itching after administration of morphine. Symptoms were relieved with ondansetron and nalbuphine, and the morphine was discontinued after 4 days. |
| Significant Adverse Drug Events (n = 10) |
| • A 52-year-old patient with a history of reaction to codeine had nausea and vomiting after placement of hydromorphone PCA after surgery for thyroid cancer. |
| • A 57-year-old patient with a history of reaction to codeine had nausea and vomiting during a two-day placement of morphine PCA after surgery for pelvic osteoarthritis. |
| • A 31-year-old patient with a history of allergy to tramadol (hives) who was admitted for lumber disc herniation had nausea for two days after placement of morphine PCA. Symptoms subsided after PCA was discontinued. |
| • An 81-year-old patient with a documented reaction to acetaminophen+oxycodone (gastrointestinal upset) was given a hydromorphone PCA after colon surgery and had nausea, dry heaves, and dizziness until the PCA was discontinued the following day. |
| • A 59-year-old patient with a documented reaction to morphine [hallucinations] had nausea after placement of a hydromorphone PCA after surgery to repair a femoral neck fracture. No adverse symptoms were documented beyond the first day after an antagonist was administered intravenously concurrently with the medication. |
| • A 68-year-old patient with a history of codeine allergy (shortness of breath) who was admitted for a right lung nodule had two days of nausea after placement of a hydromorphone PCA. She was then switched to oral hydromorphone. |
| • A 45-year-old patient with a history of adverse reaction to codeine (gastrointestinal upset and angioedema) had nausea, vomiting, itching, and hives for two days while on hydromorphone PCA. |
| • A 67-year-old patient with a history of reaction to morphine (nausea) had nausea for three days after placement of hydromorphone PCA. Symptoms improved after administration of ondansetron. The nausea subsided 14 hours after the PCA was discontinued. |
| • A 54-year-old patient with a history of sensitivity to morphine (nausea and vomiting) had nausea and vomiting after administration of acetaminophen+codeine. The medication was discontinued. |
| • A 68-year-old patient with a history of adverse reaction to morphine (nausea) admitted for cholelithiasis had itching 3 days after placement of a hydromorphone PCA. The patient was discharged on the medication and diphenhydramine. |
Supported by a grant from the National Library of Medicine (R01 LM007203) and a student research grant from Harvard Medical School.
The authors thank Cathy A. Foskett, RN, and Katherine R. Zigmont, RN, for their assistance with chart review and data collection; Elisabeth Burdick, MS, and Brian Chan, BS, for their assistance with the statistical analysis; and Ashish K. Jha, MD, Thomas D. Sequist, MD, Edward Lowenstein, MD, Michael T. Bailin, MD, and Maura E. LeBaron for their support and thoughtful review of the manuscript.
References
- 1.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press, 2001. [PubMed]
- 2.Bates DW, Gawande AA. Improving safety with information technology. N Engl J Med. 2003;348:2526–34. [DOI] [PubMed] [Google Scholar]
- 3.Leapfrog Group. Computer physician order entry factsheet. Washington, DC: Leapfrog Group, April, 2003, Available at. http://www.leapfroggroup.org/safety.htm. Accessed July 13, 2004.
- 4.Evans RS, Pestotnik SL, Classen DC, et al. A computer-assisted management program for antibiotics and other antiinfective agents. N Engl J Med. 1998;338:232–8. [DOI] [PubMed] [Google Scholar]
- 5.Bates DW, Leape LL, Cullen DJ, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280:1311–6. [DOI] [PubMed] [Google Scholar]
- 6.Bates DW, Miller EB, Cullen DJ, et al. Patient risk factors for adverse drug events in hospitalized patients. Arch Intern Med. 1999;159:2553–60. [DOI] [PubMed] [Google Scholar]
- 7.Bates DW, Teich JM, Lee J, et al. The impact of computerized physician order entry on medication error prevention. J Am Med Inform Assoc. 1999;6:313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teich JM, Merchia PR, Schmiz JL, Kuperman GJ, Spurr C, Bates DW. Effects of computerized physician order entry in prescribing practices. Arch Intern Med. 2000;160:2741–7. [DOI] [PubMed] [Google Scholar]
- 9.Kaushal R, Shojania KG, Bates DW. Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch Intern Med. 2003;163:1409–16. [DOI] [PubMed] [Google Scholar]
- 10.Kuperman GJ, Gibson RF. Computer physician order entry: benefits, costs, and issues. Ann Intern Med. 2003;139:31–9. [DOI] [PubMed] [Google Scholar]
- 11.Kuperman GJ, Teich JM, Gandhi TK, et al. Patient safety and computerized medication ordering at Brigham and Women's Hospital. Jt Comm J Qual Improv. 2001;27:509–21. [DOI] [PubMed] [Google Scholar]
- 12.Leape LL, Bates DW, Cullen DJ, et al. Systems analysis of adverse drug events. ADE Prevention Study Group. JAMA. 1995;274:35–43. [PubMed] [Google Scholar]
- 13.Gandhi TK, Burstin HR, Cook EF, et al. Drug complications in outpatients. J Gen Intern Med. 2000;15:149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bates DW, Leape LL. Adverse drug reactions. In: Carruthers SG, Hoffman BB, Melmon KL, Neirenberg D (eds). Melmon and Morrelli's Clinical Pharmacology, (ed 4). New York, NY: McGraw-Hill/Appleton & Lange, 2000, pp 1223–56.
- 15.Kuperman GJ, Gandhi TK, Bates DW. Effective drug-allergy checking: methodological and operational issues. J Biomed Inform. 2003;36:70–9. [DOI] [PubMed] [Google Scholar]
- 16.Abookire SA, Teich JM, Sandige H, et al. Improving allergy alerting in a computerized physician order entry system. Proc AMIA Symp. 2000:2–6. [PMC free article] [PubMed]
- 17.Payne TH, Nichol WP, Hoey P, Savarino J. Characteristics and override rates of order checks in a practitioner order entry system. Proc AMIA Symp. 2002:602–6. [PMC free article] [PubMed]
- 18.Teich JM, Glaser JP, Beckley RF, et al. The Brigham integrated computing system (BICS): advanced clinical systems in an academic hospital environment. Int J Med Inf. 1999;54:197–208. [DOI] [PubMed] [Google Scholar]
- 19.Chertow GM, Lee J, Kuperman GJ, et al. Guided medication dosing for inpatients with renal insufficiency. JAMA. 2001;286:2839–44. [DOI] [PubMed] [Google Scholar]
- 20.Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA. 1995;274:29–34. [PubMed] [Google Scholar]
- 21.Folli HL, Poole RL, Benitz WE, Russo JC. Medication error prevention by clinical pharmacists in two children's hospitals. Pediatrics. 1987;79:718–22. [PubMed] [Google Scholar]
- 22.Hutchinson TA, Lane DA. Methods for causality assessment of suspected adverse drug reactions. J Clin Epidemiol. 1989;42:5–16. [DOI] [PubMed] [Google Scholar]
- 23.SAS Institute, Inc. Version 8e. Cary, NC: SAS Institute, Inc., 2001 1999.
- 24.Weingart SN, Toth M, Sands DZ, et al. Physicians' decisions to override computerized drug alerts in primary care. Arch Intern Med. 2003;163:2625–31. [DOI] [PubMed] [Google Scholar]
- 25.Edwards IR. Pharmacological basis of adverse drug reactions. In: Speight TM, Holford NHG (eds). Avery's Drug Treatment, (ed 4). Auckland, New Zealand: Adis International, 1997, pp 261–99.
- 26.Ledford DK. Allergy, anaphylaxis, and general anesthesia. Immunol and Allergy Clin North Am. 2001;21:795–812. [Google Scholar]
- 27.Hardman JG, Limbird LE (eds). Goodman and Gilman's The Pharmacological Basis of Therapeutics (ed 9). New York, NY: McGraw Hill, 1996.
- 28.Tilles SA. Practical issues in the management of hypersensitivity reactions: sulfonamides. South Med J. 2001;94:817–24. [PubMed] [Google Scholar]
- 29.Knowles S, Shapiro L, Shear NH. Should celecoxib be contraindicated in patients who are allergic to sulfonamides? Revisiting the meaning of ‘sulfa’ allergy. Drug Safety. 2001;24:239–47. [DOI] [PubMed] [Google Scholar]
- 30.Shepherd GM. Hypersensitivity reactions to drugs: evaluation and management. Mt Sinai J Med. 2003;70:113–25. [PubMed] [Google Scholar]
- 31.Cribb AE, Lee BL, Trepanier LA, Spielberg SP. Adverse reactions to sulphonamide and sulphonamide-trimethoprim antimicrobials: clinical syndromes and pathogenesis. Adverse Drug React Toxicol Rev. 1996;15:9–50. [PubMed] [Google Scholar]
- 32.Strom BL, Schinnar R, Apter AJ, et al. Absence of cross-reactivity between sulfonamide antibiotics and sulfonamide nonantibiotics. N Engl J Med. 2003;349:1628–35. [DOI] [PubMed] [Google Scholar]