Abstract
Some octopamine agonists were found to suppress the calling behavior of the stored product Indian meal moth, Plodia interpunctella. Compounds were screened using a calling behavior bioassay using female P. interpunctella. Four active derivatives, with inhibitory activity at the nanomolar range, were identified in order of decreasing activity: 2-(1-phenylethylamino)-2-oxazoline > 2-(2-ethyl,6-methylanilino)oxazolidine > 2-(2-methyl benzylamino)-2-thiazoline > 2-(2,6-diethylanilino)thiazolidine. Three-dimensional pharmacophore hypotheses were built from a set of 15 compounds. Among the ten common-featured models generated by the program Catalyst/HipHop, a hypothesis including a hydrogen-bond acceptor lipid, a hydrophobic aromatic and two hydrophobic aliphatic features was considered to be essential for inhibitory activity in the calling behavior. Active compounds mapped well onto all the hydrogen-bond acceptor lipid, hydrophobic aromatic and hydrophobic aliphatic features of the hypothesis. On the other hand, less active compounds were shown not to achieve the energetically favorable conformation that is found in the active molecules in order to fit the 3D common-feature pharmacophore models. The present studies demonstrate that inhibition of calling behavior is via an octopamine receptor.
| Abbreviation: | |
|---|---|
| AII | 2-(arylimino)imidazolidine |
| AIO | 2-(arylimino)oxazolidine |
| AIT | 2-(arylimino)thiazolidine |
| CBO | 2-(4-chlorobenzylamino)-2-(4-phenyl)oxazoline |
| CDM | chlordimeform |
| Confs | number of conformers |
| DIP | 2-(2,6-diethylphenylimino)piperidine |
| Features/Confs | total number of features divided by the number of conformers (summed over the entire family of conformers) |
| HBA | hydrogen-bond acceptor |
| HBAl | hydrogen-bond acceptor lipid |
| HBD | hydrogen-bond donor |
| Hp | hydrophobic |
| HpAl | hydrophobic aliphatic |
| HpAr | hydrophobic aromatic |
| mp | melting point |
| MTO | 2-(3-methyl benzylthio)-2-oxazoline |
| NI | negative ionizable |
| NIO | 2-(1-naphthylimino)oxazolidine |
| OA | octopamine |
| ODA | 2-phenyl-5,6-dihydro-4H-1,3,4-oxadiazine |
| ODO | 2-phenyl-5,6-dihydro-4H-1,3,4-oxadiazine-5(6H)-one |
| PBAN | pheromone biosynthesis activating neuropeptide |
| PEO | 2-(1-phenylethylamino)-2-oxazoline |
| PI | positive ionizable |
| PIT | 1-(2,6-dimethylphenyl)imidazolidine-2-thione |
| RA | ring aromatic |
| SBO | 2-(substituted benzylamino)-2-oxazoline |
| SBT | 2-(substituted benzylamino)-2-thiazoline |
| STO | 2-(substituted benzylthio)-2-oxazoline |
| ZETA | (Z,E)-9,12-tetradecadienyl acetate |
Keywords: Plodia interpunctella, calling behavior, HipHop, common-feature hypothesis, octopamine agonist
Introduction
The reproductive behavior in moths is dependent on chemical communication. In most species of moths, females produce and release an attractive blend of pheromone to which conspecific males orient. Production of the pheromone blend is under the regulation of a neuropeptide termed PBAN (Raina, 1993; Ma and Roelofs, 1995; Fabrias et al., 1995; Zhu et al., 1995; Fang et al., 1996; Foster and Roelofs, 1996; Jurenka, 1996; Rafaeli and Gileadi, 1997). The direct action of PBAN has been demonstrated by studies in vitro (Soroker and Rafaeli, 1989; Rafaeli et al., 1990; Arima et al., 1991; Jurenka et al., 1991; Fonagy et al., 1992; Matsumoto et al., 1995), that showed stimulation by isolated pheromone-gland tissue of pheromone production in the presence of synthetic PBAN. The exact tissue involved was determined to be the intersegmental membrane that is situated between the eighth and ninth abdominal segments (Rafaeli and Gileadi, 1995a, 1996). Female moths call conspecific males during specific periods when they emit their pheromone. The major pheromone component of the Indian meal moth Plodia interpunctella was identified as ZETA (Brady et al., 1971; Kuwahara et al., 1971; Zhu et al., 1999). The inhibitors of calling behavior and pheromone production have been reported (Hirashima et al., 2001). Biogenic amines, such as OA, play a key role as neurotransmitters, neurohormones and neuromodulators in invertebrate systems (Evans, 1980) with a physiological role analogous to adrenaline in vertebrates (Evans, 1980; Orchard, 1982; David and Coulon, 1985). In Helicoverpa armigera, it has been shown that OA and OA agonists significantly inhibit pheromone production caused by PBAN in intact and decapitated moths as well as in pheromone-gland incubations in vitro (Rafaeli and Gileadi, 1995a, 1996; Rafaeli et al., 1997, 1999; Hirashima et al., 1999a, 1999b). The pheromone inhibiting receptor, playing a neuromodulatory role, represents a novel type of octopaminergic receptor. Constant dark and light conditions significantly affected the pattern of change in levels of OA observed in the brain in a light:dark regime of male cabbage looper moths (Linn et al., 1996). Of particular interest, the pattern of decrease in OA levels correlated well with the pattern of response to sex pheromone during the dark period, supporting an earlier hypothesis that OA modulates neural pathways involved in perception of the odor signal.
In previous reports, we described the use of Catalyst/Hypo to derive a 4- and 5-feature hypothesis from a set of 17 OA antagonists (Pan et al., 1997) and 43 agonists (Hirashima et al., 1999c), respectively. Three-dimensional pharmacophore hypotheses were built from a set of 9 OA agonists responsible for the inhibition of in vitro sex-pheromone production in H. armigera (Hirashima et al., 1999b) and from 14 OA agonists in P. interpunctella (Hirashima et al., 2002a). These sets included a variety of types of molecules, covering 5 orders of magnitude in activity. For these types of training sets, the use of the hypothesis-generation tool was appropriate. This tool builds hypotheses (overlays of chemical features) for which the fit of individual molecules to a hypothesis can be correlated with the molecule's affinity. However, the high structural homology among the derivatives used in the current study combined with their smaller activity range makes this “quantitative” hypothesis generation method inappropriate. For this type of training set, the common-feature hypothesis generation, also called HipHop (Barnum et al., 1996), is more suitable. HipHop generates hypotheses consisting only of identification and overlay of common features (without the use of activity data). The aim of this work is to derive feature-based 3D models from a set of compounds using HipHop, as inhibitors in calling behavior in P. interpunctella in order to compare with the results of inhibitors in in vitro sex-pheromone production in P. interpunctella.
Materials and Methods
Synthesis of OA agonists
AIOs 1–24 (Fig. 1) were obtained by cyclodesulfurizing the corresponding N-arylthioureas with yellow mercuric oxide (Hirashima et al., 1996). AIIs 25–32 were prepared by refluxing the corresponding substituted anilines and 1-acetyl-2-imidazolidone in phosphoryl chloride followed by hydrolysis (Nathanson and Kaugars, 1989). AITs 33–40 and SBTs 41–44 were synthesized by cyclization of the corresponding N-arylthioureas with concentrated hydrogen chloride (Hirashima et al., 1994). STOs 45–47 were prepared from oxazolidine-2-thione and substituted benzylchloride in the presence of sodium hydride. SBOs 48–50 and CBO 51 were prepared by cyclodesulfurizing the corresponding N-arylthioureas with yellow mercuric oxide. from oxazolidine-2-thione and cinnamylamine in the presence of sodium hydride. DIP 53 was obtained by refluxing δ-valerolactam and the corresponding aniline in phosphoryl chloride. MTO 54 was prepared from oxazolidine-2-thione and m-methyl benzylchloride in the presence of sodium hydride. NIO 55 was obtained by cyclodesulfurizing the corresponding N-naphthylthiourea with yellow mercuric oxide. ODA 56 was obtained from benzoylhydrazine and dibromoethan in the presence of sodium hydroxide. ODO 57 was prepared from benzoylhydrazine and chloroacetyl chloride in the presence of pyridine. PEO 58 was obtained by cyclodesulfurizing the corresponding N-arylthiourea with yellow mercuric oxide. PIT 59 was synthesized by the cyclization of monoethanolamine hydrogen sulfate with 2,6-dimethylphenylisothiocyanate in the presence of sodium hydroxide as described in the previous report (Hirashima et al., 1998). All compounds were purified by either recrystallization or column chromatography on silica gel. The structures of the compounds thus synthesized were confirmed by 1H-, 13C-NMR (a JEOL JNM-EX400 spectrometer at 400 MHz, tetramethyl silane being used as an internal standard), mass spectra (a JEOL JMS-DX300 spectrometer connected to a JEOL-JMA3500 data processing system at an ionizing voltage of 30 eV) and elemental analysis, in which the differences between calculated and found C, H and N of all componds were less than 0.5% (data not shown), respectively. All meting points (mp, °C) were measured with Yanako MP-S3 micro-melting point apparatus (Yanako, Kyoto, Japan) and are uncorrected. CDM (96% pure) 52 was a gift from Nihon Nohyaku Co. Ltd. (Osaka, Japan) and used after purification by column chromatography on silica gel.
Figure 1.
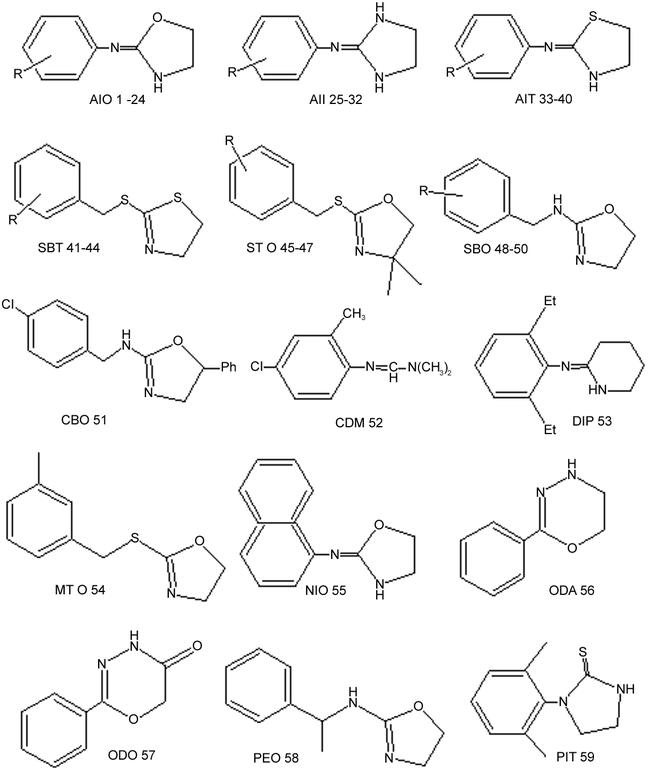
Structure of compounds used in this study.
Insects
The colony of P. interpunctella was raised on a diet of 80% ground rice, 10% glycerin, 5% brewer's yeast and 5% honey at 28°C, and 70% RH in a 14:10 (light : dark) photoperiod (Rafaeli and Gileadi, 1995b). Larvae of the wandering stage pupated between pieces of paper carton and male and female pupae were separated. Emerged virgin females were staged according to age.
Screening of calling-behavior inhibitors
Calling-behavior inhibitors were screened using five replicates of 10 adult female moths that were collected 24 h after emergence. Synthetic test compounds dissolved in 1 µl methanol were topically applied to the abdomen of moths at the onset of the first scotophase. Methanol alone (1 µl) was topically applied to control females. The treated females were isolated in test tubes for 3 h and their calling behavior was observed. The response was taken as quantal (calling/not calling). ID50s were calculated using a sigmoidal curve-fitting program designed for log dose-probit activity analyses using a Macintosh personal computer system.
Molecular modeling
All computational experiments were conducted on a Silicon Graphics O2, running under the IRIX 6.5 operating system. Hypotheses generation was applied against previously described data sets and their functionality is available as part of Catalyst/HipHop (version 4.0) modeling environment from Accelrys (San Diego, USA, http://www.accelrys.com/catalyst/). Molecules were edited using the Catalyst 2D/3D visualizer. Catalyst automatically generated conformational models for each compound using the Poling Algorithm (Smellie et al., 1995a, 1995b, 1995c). The number of conformations needed to produce a good representation of a compound's conformational space depends on the molecule. Conformation-generating algorithms were adjusted to produce a diverse set of conformations, avoiding repetitious groups of conformations all representing local minima. The conformations generated were used to align common molecular features and generate pharmacophoric hypotheses. HipHop used conformations generated to align chemically important functional groups common to the molecules in the study set. A pharmacophoric hypothesis then was generated from these aligned structures.
A preparative test was performed with HBA, HBAl, HBD, Hp, HpAr, HpAl, NI, RA and PI (Greene et al., 1994). NI and PI were used rather than negative charge and positive charge in order to broaden the search for deprotonated and protonated atoms or groups at physiological pH. The models emphasized a conformational diversity under the constraint of 20 kcal/mol energy threshold above the estimated global minimum based on use of the CHARMm force field (Brooks et al., 1983; Smellie et al., 1995a, 1995b, 1995c). Molecular flexibility was taken into account by considering each compound as a collection of conformers representing a different area of conformational space accessible to the molecule within a given energy range. Catalyst provides two types of conformational analysis: fast and best quality. Best option was used, specifying 250 as the maximum number of conformers. The molecules associated with their conformational models was submitted to Catalyst hypothesis generation. Hypotheses approximating the pharmacophore were described as a set of features distributed within a 3D space. This process only considered surface accessible functions such as HBA, HBAl, HBD, Hp, HpAr, HpAl, RA, NI and PI.
Results
Screening of calling-behavior inhibitors
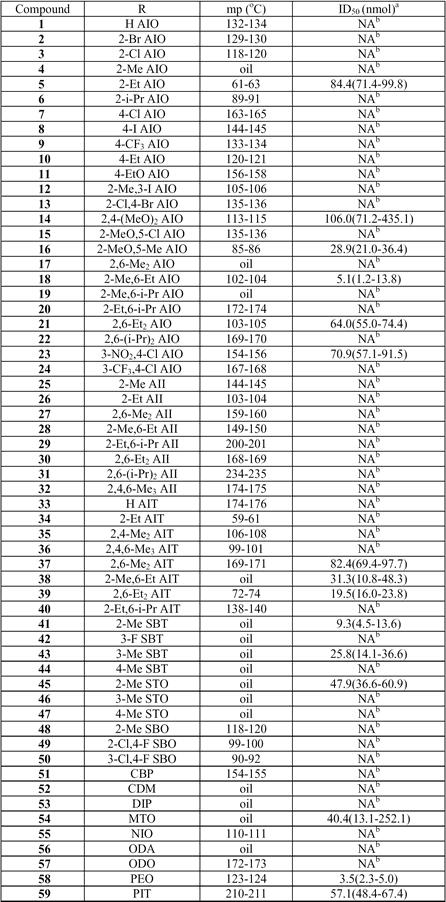
Initial screening of compounds (Table 1) was performed by topical application of various concentrations of 59 compounds in 1 µl methanol onto abdomens of females. All compounds showed the maximal inhibitory activity at 3 h after application, suggesting that the compounds get into the CNS in a more or less similar manner. Compound 58 (PEO) showed the highest inhibitory activity (ID50=4.4 nmol) and three other active derivatives, with activity at nanomolar range, were identified in order of decreasing activity: 2-(2-ethyl,6-methylanilino)oxazolidine 18 > 2-(2-methyl benzylamino)-2-thiazoline 41 > 2-(2,6-diethylanilino)thiazolidine 39.
Table 1.
Compounds used in this study
Assessment of 3D hypothesis
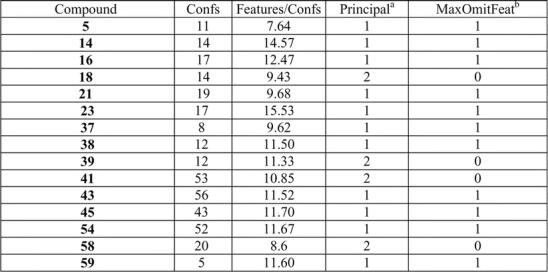
The activities of test compounds at several concentrations were examined. Activity was structure specific and hypotheses were generated to explain the specificity. A set of 15 molecules were selected as the target training set as shown in Table 2. Their experimental biological activities are listed in Table 1. Among the 15 molecules of the training set, compounds 18, 39, 41 and 58 were chosen as reference compounds (Principal 2 in Table 2), which were used to map all features, and 11 other molecules were used to map partially on the hypotheses for calling-behavior inhibitors (Principal 1 in Table 2). Except for this classification, the activities of the molecules were not used in the analysis. This tool builds hypotheses (overlays of common features) for which the fit of individual molecules to a hypothesis can be correlated with the molecule's activity. Potential hypothesis models were produced with the minimum permitted interfeature spacing of 2.00 Å generating alignments of common features (Barnum et al., 1996), which included the projected point of HBAl, HpAr and HpAl (Greene et al., 1994).
Table 2.
Characteristics for the common feature hypothesis run
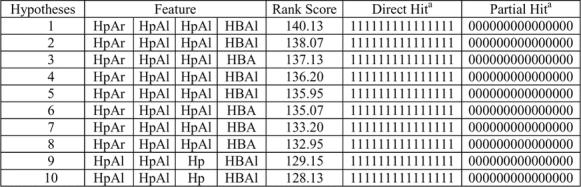
The characteristics of ten hypotheses are listed in Table 3. Hypotheses 1, 2, 4 and 5 consist of the same common-feature functions of an HpAr, two HpAl and an HBAl. The replacement of the HBAl in these hypotheses by an HBA leads to the second group, composed of hypotheses 3 and 6–8. Hypotheses 9 and 10 are obtained after replacing the HpAr in hypotheses 1, 2, 4 and 5. All the hypotheses contain 4 features with the ranking scores ranging from 140.13 to 128.13 (hypotheses 1–10) for calling-behavior inhibitors. The higher the ranking score, the less likely it is that the molecules in the training set fit the hypothesis by a chance correlation. The rank score range over the 10 generated hypotheses is 12.0 for calling-behavior inhibitors. The small rank score range observed here may be due to two factors, namely molecules in the training set are fairly rigid and have a high degree of structural homology. Due to the relatively small range and because of the placement of the identified hypotheses within this range, special care was taken to test for chance correlation. The small range of rank score suggests that these hypotheses were homogenous. Roughly speaking, hypotheses 1–10 have good similarity in 3D spatial shape and therefore these hypotheses are considered to be equivalent. The molecules map well onto all four features in a similar way in hypotheses 1–10 (data not shown) and therefore these hypotheses are considered to be equivalent. Roughly speaking, hypotheses 1–10 have good similarity in 3D spatial shape. Generally, more active molecules map well onto all the features of the hypothesis, and compounds that have low activity map poorly to the hypothesis. Other compounds in Table 1 with low activity also do not fit to these features.
Table 3.
Results of the common feature hypothesis run
Figs. 2–6 depict PEO (S)-58, the most active compound, followed by 41, 43, 44 and 18 mapped onto hypotheses 1, respectively. The molecule (S)-58 maps well onto the four features of hypotheses 1 (Fig. 2) and similarly, (R)-58 maps well onto the four features of hypotheses 1 (data not shown). The phenyl group of 58 overlaps with the HpAr feature of this hypothesis, whereas the nitrogen atom of oxazoline ring serves as an HBAl. The pair of HpAl features overlap neatly with the methyl and oxazoline groups. Thus, the most active OA agonist 58 in the training set maps closely with the statistically most significant hypothesis 1, which is characterized by four features (Fig. 2). Similarly, the phenyl group of SBT 41 overlaps with the HpAr feature of hypothesis 1, whereas the nitrogen atom of oxazoline ring serves as an HBAl (Fig. 3). The pair of HpAl features overlap neatly with the 2-methyl and thiazoline groups. The phenyl group of SBT 43 overlaps with the HpAr feature of hypothesis 1, whereas the nitrogen atom of oxazoline ring serves as an HBAl (Fig. 4) in a similar manner in case of 41. The pair of HpAl features overlap with the 3-methyl and thiazoline groups of 43, although it fits less (3.896) than 41 (4.000), leading to lower activity of 43 than 41. Meanwhile, the phenyl group of SBT 44 does not overlap with the HpAr feature of hypothesis 1 at all, although the nitrogen atom of oxazoline ring serves as an HBAl and the pair of HpAl features overlap neatly with the 4-methyl and thiazoline groups (Fig. 5). Thus, 44 is not active inhibitor at all. AIO with shorter bridge between phenyl and oxazoline rings is less suitable for the hypothesis than PEO 58 (Fig. 6). AIO 18 has a phenyl and oxazolidine ring directly connected by nitrogen and thus, is less flexible than 58, because 18 has less conformational flexibility than that of 58 (14 vs. 20 respectively). An HpAl feature overlaps neatly with the 2-methyl group and another HpAl is disposed over the oxazolidine ring of 18. The phenyl group of 18 overlaps with the HpAr feature of hypothesis 1, whereas neither the bridge nitrogen atom between a phenyl and oxazolidine ring, nor the oxygen atom of the oxazolidine ring serves as an HBAl, leading to lower activity of 18 than 41.
Figure 2.
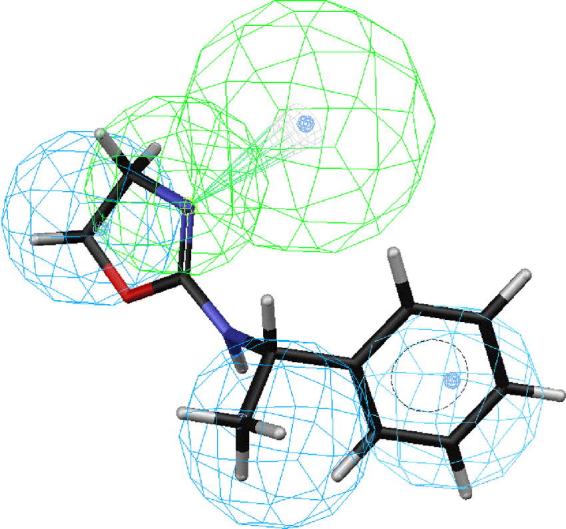
Mapping of (S)-58 2-(1-phenylethylamino)-2-oxazoline (PEO) onto hypothesis 1, which contains an HBAl (green), an HpAr (blue) and two HpAls (blue).
Figure 3.
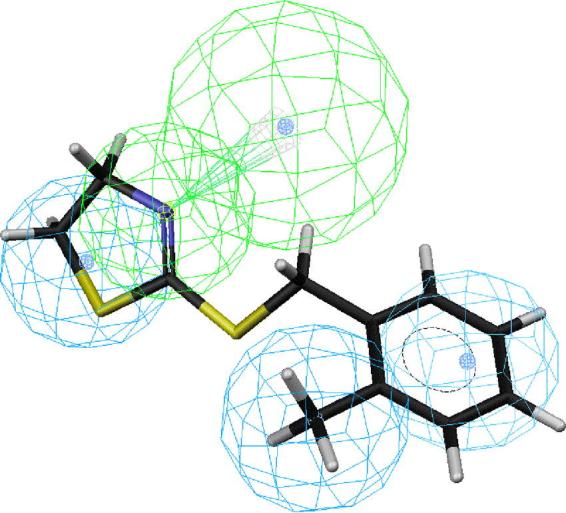
Mapping of 2-(2-methylbenzylamino)-2-thiazoline (41) onto hypothesis 1, which contains an HBAl (green), an HpAr (blue) and two HpAls (blue).
Figure 4.
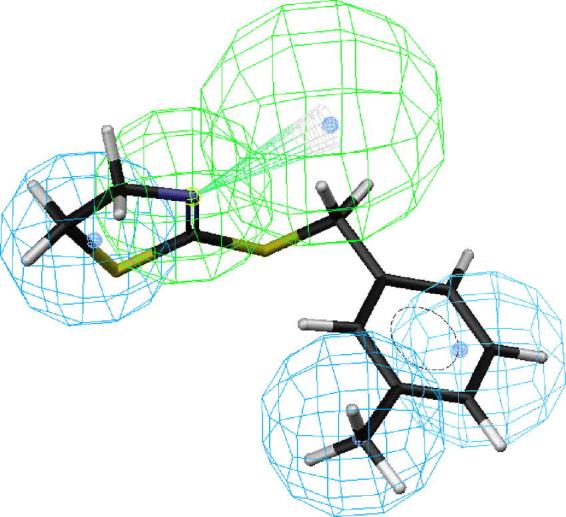
Mapping of 2-(3-methylbenzylamino)-2-thiazoline (43) onto hypothesis 1, which contains an HBAl (green), an HpAr (blue) and two HpAls (blue).
Figure 5.
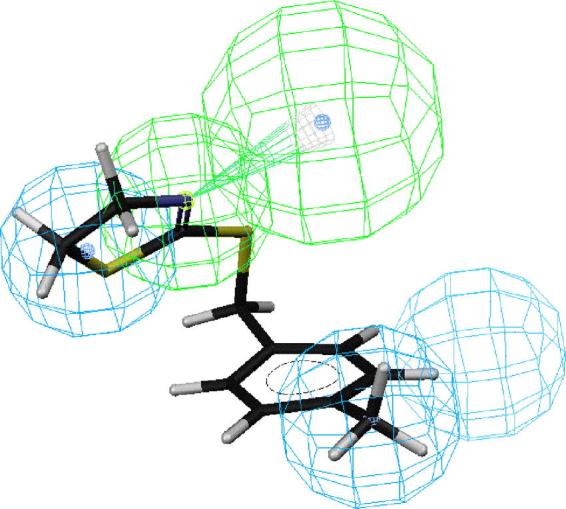
Mapping of 2-(4-methylbenzylamino)-2-thiazoline (44) onto hypothesis 1, which contains an HBAl (green), an HpAr (blue) and two HpAls (blue).
Figure 6.
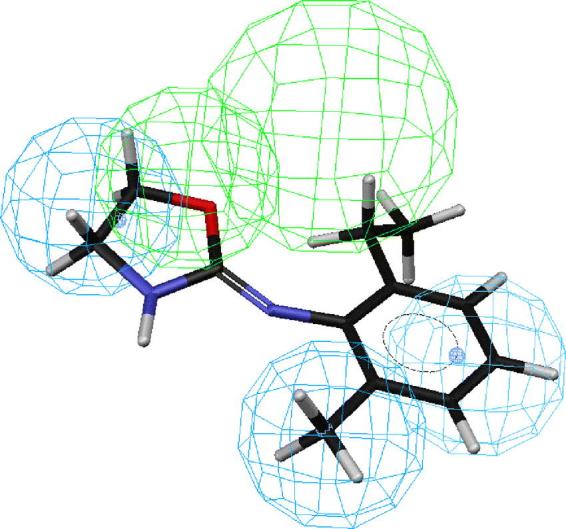
Mapping of 2-(2-ethyl,6-methylphenylimino)oxazolidine (18) onto hypothesis 1, which contains an HBAl (green), an HpAr (blue) and two HpAls (blue).
Discussion
The OA receptors have generated considerable pharmacological interest as targets for the identification of selective drugs/chemicals that may interact with specific receptor subtypes. To understand the basis of the increased susceptibility of Lepidoptera to OA or OA agonists, compared to other insect species, there is a need to characterize and compare OA receptor subtypes found in moths with those of other insect species. Identification of agonists with a high degree of selectivity enables the characterization and differentiation of OA receptor populations in various tissues and species will enable targeting potential insecticide activities. Potent and selective OA agonists could be useful toxins in invertebrates, particularly insects, with low toxicity in vertebrates (Nathanson, 1985). They are expected to inhibit pheromone biosynthesis in the pheromone gland (Hirashima et al., 2001). It is thus of critical importance to provide information on the pharmacological properties of OA receptor types and subtypes. This study was therefore designed to evaluate inhibitory activity of the OA agonists in calling behavior in P. interpunctella.
The major pheromone component of P. interpunctella was identified as ZETA (Brady et al., 1971; Kuwahara et al., 1971; Zhu et al., 1999). Recent research in P. interpunctella has advanced our understanding of the endogenous control of sex-pheromone production (Hirashima et al., 2001). Female moths attract or call conspecific males during specific periods when they emit their pheromone. In H. armigera, 14C from sodium acetate was incorporated into the pheromone component, (Z)-11-hexadecenol (Rafaeli and Soroker, 1989; Soroker and Rafaeli, 1989). The radioactivity was incorporated in the acetylation step of ZETA biosynthesis (Hirashima et al., 2001). However, no radioactivity significantly different from control was observed for (Z,E)-9,12-tetradecadien-1-ol, a precursor of biosynthesis to ZETA. Pheromone production by many moths is regulated by the timely release of PBAN. It was observed that OA and OA agonists inhibited the pheromone production activity of PBAN in H. armigera (Rafaeli and Gileadi, 1995a, 1996; Rafaeli et al, 1997; Hirashima et al., 1999a, 1999b). We have reported evidence of a successful short-term in vitro pheromonal bioassay technique, in which PBAN-stimulated incorporation of radioactivity was inhibited by OA agonists (Hirashima et al., 2001). Furthermore, OA antagonists, which have a selectivity for OA receptors (Evans, 1981), antagonised the inhibitory activity of OA, leading to recovery of pheromone biosynthesis in vitro (Hirashima et al., 2001). Thus, inhibition of PBAN-stimulated incorporation of radioactivity is via an OA receptor, in which an HBA, an HpAr, an HpAl and two Hps seem to be essential (Hirashima et al., 2002a).
Here we have further screened inhibitors of calling behavior. In this study, after screening 59 compounds, some of which have been reported as OA agonists (Hirashima et al., 1994, 1996, 2001, 2002a, 2002b, 2002c), four highly active compounds identified that inhibited calling behavior of female P. interpunctella. On the basis of dose-response studies a putative hypothetical model for active analogs was determined. Future design of new compounds will be directed at maximizing activity. OA is not likely to penetrate either the cuticle or the central nervous system of insects effectively, since it is fully ionized at physiological pH. Derivatization of the polar groups would be one possible solution to this problem in trying to develop potential pest-control agents. The present work shows how a set of activities of various compounds may be treated statisticaly to uncover the molecular characteristics that are essential for high inhibitory activity in calling behavior in P. interpunctella. These characteristics are expressed as common features disposed in three-dimensional space and are collectively termed a hypothesis. Hypotheses were obtained and applied to map the active or inactive compounds. Important features such as an HBAl, an HpAr and two HpAls of the surface-assessable models were found that are the minimum components of a hypothesis for effective compounds. Graphical examination of the ten hypotheses shows that there are three major families of models depending mainly on the location and the orientation of the projected point of the HBAl, HpAr and HpAls. It was found that more active compounds map well onto all the features of the hypotheses as was also shown in previous reports (Hirashima et al., 2002a, 2002b, 2002c). For some inactive compounds, their lack of affinity is primarily due to their inability to achieve an energetically favorable conformation shared by the active compounds. Taken together, an HBAl, an HpAr and two HpAls located on the molecule seem to be essential for high activity in inhibition of calling behavior, which corresponds to the reported results for OA agonists (Hirashima et al., 1999c) and inhibition of PBAN-stimulated incorporation of radioactivity (Hirashima et al., 2002a). Thus, inhibitions of calling behavior and PBAN-stimulated incorporation of radioactivity are via an OA receptor.
Acknowledgments
We thank Dr. Ada Rafaeli (Department of Stored Products, Pheromone Research Lab., Volcani Centre, Israel) for valuable suggestions in rearing P. interpunctella. This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan.
References
- Arima R, Takahara K, Kadoshima T, Numazaki F, Ando T, Uchiyama M, Nagasawa H, Kitamura A, Suzuki A. Hormonal regulation of pheromone biosynthesis in the silkworm moth Bombyx mori (Lepidoptera: Bombycidae) Appl Entomol Zool. 1991;26:137–148. [Google Scholar]
- Barnum D, Greene J, Smellie A, Sprague P. Identification of common functional configurations among molecules. J Chem Inf Comput Sci. 1996;36:563–571. doi: 10.1021/ci950273r. [DOI] [PubMed] [Google Scholar]
- Brady UE, Tumlinson JH, Brownlee RG, Silverstein RM. Sex stimulant and attractant in the Indian meal moth and in the almond moth. Science. 1971;171:802–804. doi: 10.1126/science.171.3973.802. [DOI] [PubMed] [Google Scholar]
- Brooks BR, Brucolleri RE, Olafson BD, States DJ, Swaminathan S, Karplus M. A program for macromolecular energy, minimization, and dynamics calculations. J Commput Chem. 1983;4:187–217. [Google Scholar]
- David JC, Coulon JF. Octopamine in invertebrates and vertebrates. A review. Prog Neurobiol. 1985;24:141–185. doi: 10.1016/0301-0082(85)90009-7. [DOI] [PubMed] [Google Scholar]
- Evans PD. Biogenic amines in the insect nervous system. Adv Insect Physiol. 1980;15:317–473. [Google Scholar]
- Evans PD. Multiple receptor types for octopamine in the locust. J Physiol (Lond) 1981;318:99–122. doi: 10.1113/jphysiol.1981.sp013853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrias G, Barrot M, Camps F. Control of the sex pheromone biosynthetic pathway in Thaumetopoea pityocampa by the pheromone biosynthesis activating neuropeptide. Insect Biochem Molec Biol. 1995;25:655–660. [Google Scholar]
- Fang NB, Teal PEA, Tumlinson JH. Effects of decapitation and PBAN injection on amounts of triacylglycerols in the sex pheromone gland of Manduca sexta (L) Archs Insect Biochem Physiol. 1996;32:249–260. [Google Scholar]
- Fonagy A, Matsumoto S, Schoofs L, De Loof A, Mitsui T. In vivo and in vitro pheromonotropic activity of two locustatachykinin peptides in Bombyx mori. Biosci Biotech Biochem. 1992;56:1692–1693. doi: 10.1271/bbb.56.1692. [DOI] [PubMed] [Google Scholar]
- Foster SP, Roelofs WL. Sex pheromone biosynthesis in the tortricid moth, Ctenopseustis herana (Felder and Rogenhofer) Archs Insect Biochem Physiol. 1996;32:135–147. [Google Scholar]
- Greene J, Kahn S, Savoj H, Sprague P, Teig S. Chemical function queries for 3D databse search. J Chem Inf Comput Sci. 1994;34:1297–1308. [Google Scholar]
- Hirashima A, Eiraku T, Shigeta Y, Kuwano E, Taniguchi E, and Eto M. 2002a Three-dimensional pharmacophore hypotheses of octopamine receptor responsible for the inhibition of sex-pheromone production in Plodia interpunctella. Internet Electron J Mol Des. 1:37–51.http://www.biochempress.com, ftp://biochempress.com/iejmd_2002_1_0037.pdf. [Google Scholar]
- Hirashima A, Eiraku T, Watanabe Y, Kuwano E, Taniguchi E, Eto M. Identification of novel inhibitors of calling and in vitro [14C]acetate incorporation by pheromone glands of Plodia interpunctella. Pest Manag Sci. 2001;57:713–720. doi: 10.1002/ps.345. [DOI] [PubMed] [Google Scholar]
- Hirashima A, Morimoto M, Kuwano E, Taniguchi E, Eto M. Three-dimensional common-feature hypotheses for octopamine agonist 2-(arylimino)imidazolidines. Bioorg Med Chem. 2002b;10:117–123. doi: 10.1016/s0968-0896(01)00247-4. [DOI] [PubMed] [Google Scholar]
- Hirashima A, Morimoto M, Ohta H, Kuwano E, Taniguchi E, and Eto M. 2002c Three-dimensional common-feature hypotheses for octopamine agonist 1-arylimidazolidine-2-thiones. Internet J Mol Sci. 3:56–68.http://www.mdpi.org/ijms/papers/i3020056.pdf. [Google Scholar]
- Hirashima A, Pan C, Katafuchi Y, Taniguchi E, Eto M. Synthesis and octopaminergic-agonist activity of 2-(arylimino)oxazolidines and 2-(substituted benzylamino)-2-oxazolines. J Pestic Sci. 1996;21:419–424. [Google Scholar]
- Hirashima A, Pan C, Kuwano E, Taniguchi E, Eto M. Three-dimensional pharmacophore hypotheses for the locust neuronal octopamine receptor (OAR3): 2. Agonists. Bioorg Med Chem. 1999c;7:1437–1443. doi: 10.1016/s0968-0896(99)00057-7. [DOI] [PubMed] [Google Scholar]
- Hirashima A, Rafaeli A, Gileadi C, Kuwano E. Three-dimensional quantitative structure-activity studies of octopaminergic agonists responsible for the inhibition of sex-pheromone production in Helicoverpa armigera. Bioorg Med Chem. 1999a;7:2621–2628. doi: 10.1016/s0968-0896(99)00189-3. [DOI] [PubMed] [Google Scholar]
- Hirashima A, Rafaeli A, Gileadi C, Kuwano E. Three-dimensional pharmacophore hypotheses of octopamine receptor responsible for the inhibition of sex-pheromone production in Helicoverpa armigera. J Mol Graphics Model. 1999b;17:43–50. doi: 10.1016/s1093-3263(99)00019-4. [DOI] [PubMed] [Google Scholar]
- Hirashima A, Shinkai K, Kuwano E, Taniguchi E, Eto M. Synthesis and octopaminergic-agonist activity of 3-(substituted phenyl)imidazolidine-2-thiones and related compounds. Biosci Biotech Biochem. 1998;62:1179–1184. doi: 10.1271/bbb.62.1179. [DOI] [PubMed] [Google Scholar]
- Hirashima A, Tarui H, Eto M. Synthesis and octopaminergic agonist activity of 2-(arylimino)thiazolidines, 2-(aralkylamino)-2-thiazolines and related compounds. Biosci Biochem Biotech. 1994;58:1206–1209. [Google Scholar]
- Jurenka RA. Signal transduction in the stimulation of sex pheromone biosynthesis in moths. Arch Insect Biochem Physiol. 1996;33:245–258. [Google Scholar]
- Jurenka RA, Jacquin E, Roelofs WL. Stimulation of pheromone biosynthesis in the moth Helicoverpa zea, Action of a brain hormone on pheromone glands involves Ca2+ and cAMP as second messengers. Proc Natl Acad Sci USA. 1991;88:8621–8625. doi: 10.1073/pnas.88.19.8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara Y, Kitamura C, Takahashi S, Hara H, Ishii S, Fukami H. Sex pheromone of the almond moth and Indian meal moth: cis-9, trans-12-tetradecadienyl acetate. Science. 1971;171:801–802. doi: 10.1126/science.171.3973.801. [DOI] [PubMed] [Google Scholar]
- Linn CE, Campbell MG, Poole KR, Wu WQ, Roelofs WL. Effects of photoperiod on the circadian timing of pheromone response in male Trichoplusia ni: Relationship to the modulatory action of octopamine. J Insect Physiol. 1996;42:881–891. [Google Scholar]
- Ma PWK, Roelofs W. Calcium involvement in the stimulation of sex pheromone production by PBAN in the European corn borer, Ostrinia nubilalis (Lepidoptera: Pyralidae) Insect Biochem Molec Biol. 1995;25:467–473. [Google Scholar]
- Matsumoto S, Ozawa R, Nagamine T, Kim G-H, Uchiumi K, Shono T, Mitsui T. Intracellular transduction in the regulation of pheromone biosynthesis of the silkworm, Bombyx mori: Suggested involvement of calmodulin and phosphoprotein phosphatase. Biosci Biotech Biochem. 1995;59:560–562. doi: 10.1271/bbb.59.560. [DOI] [PubMed] [Google Scholar]
- Nathanson JA. Characterization of octopamine-sensitive adenylate cyclase: Elucidation of a class of potent and selective octopamine-2 receptor agonists with toxic effects in insects. Proc Natn Acad Sci. 1985;82:599–603. doi: 10.1073/pnas.82.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson JA, Kaugars G. A probe for octopamine receptors: synthesis of 2-[(4-azido-2,6-diethylphenyl)imino]imidazolidine and its tritiated derivative, a potent reversible-irreversible activator of octopamine-sensitive adenylate cyclase. J Med Chem. 1989;32:1795–1799. doi: 10.1021/jm00128a022. [DOI] [PubMed] [Google Scholar]
- Orchard I. Octopamine in insects: Neurotransmitter, neurohormone and neuromodulator. Can J Zool. 1982;60:659–669. [Google Scholar]
- Pan C, Hirashima A, Kuwano E, Eto M. Three-dimensional pharmacophore hypotheses for the locust neuronal octopamine receptor (OAR3): 1. Antagonists. J Molec Model. 1997;3:455–463. doi: 10.1016/s0968-0896(99)00057-7. [DOI] [PubMed] [Google Scholar]
- Rafaeli A, Gileadi C. Modulation of the PBAN-induced pheromonotropic activity in Helicoverpa armigera. Insect Biochem Molec Biol. 1995a;25:827–834. [Google Scholar]
- Rafaeli A, Gileadi C. Factors affecting pheromone production in the stored product moth, Plodia interpunctella: a preliminary study. J Stored Prod Res. 1995b;31:243–247. [Google Scholar]
- Rafaeli A, Gileadi C. Down regulation of pheromone biosynthesis: Cellular mechanisms of pheromonostatic responses. Insect Biochem Molec Biol. 1996;26:797–807. [Google Scholar]
- Rafaeli A, Gileadi C. Neuroendocrine control of pheromone production in moths. Invertebrate Neuroscience. 1997;3:223–229. [Google Scholar]
- Rafaeli A, Gileadi C, Fan Y, Meixun C. Physiological mechanisms of pheromonostatic responses: effects of adrenergic agonists and antagonists on moth pheromone biosynthesis. J Insect Physiol. 1997;43:261–269. doi: 10.1016/s0022-1910(96)00088-1. [DOI] [PubMed] [Google Scholar]
- Rafaeli A, Gileadi C, Hirashima A. Identification of novel synthetic octopamine receptor agonists which inhibit moth sex pheromone production. Pestic Biochem Physiol. 1999;65:194–204. [Google Scholar]
- Rafaeli A, Soroker V, Kamensky B, Raina AK. Action of PBAN on in vitro pheromone glands of Heliothis armigera females. J Insect Physiol. 1990;36:641–646. [Google Scholar]
- Raina K. Neuroendocrine control of sex pheromone biosynthesis in Lepidoptera. Ann Rev Entomol. 1993;38:320–349. doi: 10.1146/annurev.en.38.010193.001553. [DOI] [PubMed] [Google Scholar]
- Smellie A, Kahn SD, Teig SL. Analysis of conformational coverage. 1. Validation and estimation of coverage. J Chem Inf Comp Sci. 1995a;35:285–294. [Google Scholar]
- Smellie A, Kahn SD, Teig SL. Analysis of conformational coverage. 2. Application of conformational models. J Chem Inf Comp Sci. 1995b;35:295–304. [Google Scholar]
- Smellie A, Teig SL, Towbin P. Poling-promoting conformational variation. J Comp Chem. 1995c;16:171–187. [Google Scholar]
- Soroker V, Rafaeli A. In vitro hormonal stimulation of acetate incorporation by Heliothis armigera pheromone glands. Insect Biochem. 1989;67:1–5. doi: 10.1016/0303-7207(89)90163-9. [DOI] [PubMed] [Google Scholar]
- Zhu J, Millar J, Loefstedt C. Hormonal regulation of sex pheromone biosynthesis in the turnip moth, Agrotis segetum. Archs Insect Biochem Physiol. 1995;30:41–59. [Google Scholar]
- Zhu J, Ryne C, Unelinus CR, Valeur PG, Lofstedt C. Reidentification of the female sex pheromone of the Indian meal moth Plodia interpunctella: evidence for a four-component pheromone blend. Entomol Experiment Appl. 1999;92:137–146. [Google Scholar]


