Abstract
Invertebrate iridescent virus 6 (IIV6) was evaluated for mode of transmission and ability to cause infection in the root weevil, Diaprepes abbreviatus (L.). This is the first evidence of IIV6 infection in D. abbreviatus, which caused both patent and sub-lethal covert infections in both larvae and adults. Adults and larvae were successfully infected with IIV6 by puncture, injection and per os. Transmission of IIV6 was demonstrated between infected and healthy individuals regardless of gender. Virus was detected in egg masses produced by virus-infected females suggesting IIV6 is transmitted transovarially. Virus particles were observed in the cytoplasm of weevil cells, and were shown to infect fat bodies, muscle, and nerve tissues, as visualized using transmission electron microscopy. Patent infections resulted in death of individuals within 3 to 4 days post infection. Individuals with covert infections tested positive for virus infection on day 7 by polymerase chain reaction analysis. Sequencing of PCR amplicons confirmed virus infection. Discovery of new pathogens against root weevils may provide new management tools for development of control strategies based on induced epizootics. This is the first report of a virus infecting D. abbreviatus.
Keywords: Beetle, biological insecticide, Chilo iridescent virus, Citrus, entomopathogen, IIV6, ingestion, injection, insect virus, transovarial, sexually transmitted, mating, weevil
Introduction
The Diaprepes root weevil Diaprepes abbreviatus (L.) is now regarded as a principal threat to the sustainability of the citrus industry in Florida. Since its introduction into Florida in the early 1960's (Woodruff, 1964), the weevil has spread throughout the citrus producing regions of peninsular Florida. Adult females oviposit on leaves. Upon hatching, neonate larvae fall to the ground and burrow into the soil where they feed on progressively larger roots as they grow. Tree decline occurs over time as primary roots are damaged and infected by root rot pathogens (Rogers et al., 1996). Tree death results when larval feeding girdles the structural root, or root crown. Feeding damage by adults on leaves is considered secondary. Few effective and environmentally appropriate control options are available to growers for such subterranean pests. We undertook a search for pathogens of D. abbreviatus for use in generating new management strategies for the control of D. abbreviatus. Viral pathogens that infect but do not kill their host cause ‘covert’ infections. Advances in molecular biology have provided new uses for these types of viral pathogens. Viral pathogens that cause covert infections could be used as delivery systems for ‘designed control’ to express gene products in pests. This area of research has been suggested as the focus in the development of highly specific biological control agents (Bergoin and Tijssen, 1998; Burand, 1998; Williams, 1998). The invertebrate iridescent virus 6 (also known as Chilo iridescent virus) (Iridoviridae: Iridovirus), has an icosahedral symmetry with a particle diameter of 120–130 nm, containing a single copy linear dsDNA genome varying in size from 140 to 210 kbp. Historically, IIV6 was isolated from the rice stem borer, Chilo suppressalis Walker (Fukaya and Nasu, 1966). Since iridoviruses have been reported to infect Coleopteran species (Ohba, 1975), including the scarab, Sericesthis pruinosa (Day and Mercer, 1964) and boll weevils, Anthonomus grandis (McLaughlin, Scott, and Bell, 1972), we decided to see if an invertebrate iridescent virus would infect D. abbreviatus. Herein we report on the first known viral infection in D. abbreviatus.
Materials and Methods
Source of Diaprepes root weevil.
Insects were obtained from a laboratory colony maintained at the U.S. Horticultural Laboratory of the USDA-ARS at Ft. Pierce, FL as described by Lapointe and Shapiro (1999). Larvae were reared on artificial diet (product no. F1675, Bio-Serv, Inc., www.bio-serv.com/insect/home.html) and allowed to pupate and emerge as adults in individual plastic diet cups (PC100 1 oz. cups and lids, Jet Plastica, www.jetplastics.com/). Adults were placed in screened cages (30 × 30 × 30 cm) in a temperature-controlled growth chamber (26° C, 16:8 L:D) and provided with water and citrus foliage.
Virus source.
Isolates of IIV-6 were obtained from Dr. J. Kalmakoff, University of Otago, Dunedin, New Zealand, and Joel Funk, USDA-ARS, Western Cotton Research Laboratory, 4135 E. Broadway Rd., Phoenix, AZ 85040. The virus was maintained through serial passage into third instar Trichoplusia ni (Hübner), harvested 6 days post injection, and purified using differential centrifugation (Marina et al., 1999). Purified virus was resuspended in 0.1 M Tris buffer, pH 7.02 and used or stored at − 40 °C.
Virus Inoculations.
The nomenclature used throughout is after Williams (1996). Adults or larvae were exposed to iridescent virus-6 (IIV6) by puncture, microinjection, or per os. Control weevils were treated in the same fashion, but were inoculated with sterile water. A preliminary test was conducted using insect pins dipped in purified IIV6 to inoculate D. abbreviatus larvae. Inoculated larvae were then tested by polymerase chain reaction (PCR) analysis for presence of virus. Punctures were done with no. 1 insect pins dipped in sterile water for controls, or in purified IIV6 for virus-exposed groups. Microinjections were done with a glass 25 µl syringe and a 30 1/2 gauge needle. Insects were injected on the right lateral side of the abdomen, approximatly one-fourth the distance of the body length from the anus, with ∼4 µl of either sterile water or purified virus in water (∼1.4 µg protein/µl, readings were done at a 1:10 dilution, GeneQuant, Pharmacia Biotech, RNA/DNA calculator, www.pharmacia.com/). Weevils were then tested by PCR at least 15 d post treatment. Weevils inoculated per os were fed either a 20 % (w:v) sucrose solution or purified virus in 20 % sucrose solution (1:10 dilution) over a 24 h period. Feeding was accomplished by providing adult D. abbreviatus access to a single young citrus leaf, placed on top of a drop (1 ml) of a purified virus sucrose solution. In this manner the larvae would ingest both the liquid and eat the leaf which would have virus on its surface. Inoculum not stated as ‘purified virus’ means inoculum that was made from the homogenates of three IIV6-infected D. abbreviatus larvae, used at least 30 days post infection and were virus positive as determined by PCR. These infected weevils were homogenized in 0.5 ml of PBS 1X, pH 7.2 with a plastic pestle and acid-washed micro glass beads in a 1.5 ml centrifuge tube. The homogenate was then centrifuged for 1 min at 14,000 rpm. The supernatant was transferred to a clean 1.5 ml vial and the pellet was processed for DNA extraction (AquaPure Genomic DNA Isolation Kit, BioRad, www.bio-rad.com). The supernatant was centrifuged again for 2 min at 14,000 rpm and transferred to a clean 1.5 ml tube. The supernatant volume was increased by the addition of 3 ml of 0.1 M Tris buffer, pH 7.02. The supernatant was then sterilized using a 0.45 µm membrane syringe filter. Larvae were inoculated by injection with ∼4 µl of the sterile filtrate (∼1.8 µg protein/µl, reading done at 1:10 dilution, GeneQuant, Pharmacia Biotech, RNA/DNA calculator) and later tested by PCR at least 15 days post injection. Controls were injected with ∼4 µl of syringe-sterilzed 0.1 M Tris buffer, pH 7.02.
Vertical transmission.
Paired wax paper sheets 2 cm X 20 cm, were provided to adults (Wolcott, 1933) in cages to collect eggs. Wax paper sheets were collected and replaced daily. Egg masses (100–150 eggs per mass) were collected and tested for the presence of virus starting 15 days post treatment. Treatments included females exposed to virus per os, females exposed by virus injection, females paired with virus-infected males that had either been fed purified virus, or injected with virus inoculum. Eggs from all treatments were collected daily starting 15 days post pairing and stored at −20 °C until processed for PCR analysis.
Horizontal transmission.
A total of 40 adults were evaluated (21 females and 19 males). Twenty-one females were caged together and provided fresh, young, citrus leaves and water. Ten females were injected with virus inoculum and caged with 11 healthy target females for 60 days. Target females were assayed by PCR 30 to 40 days post-treatment. Ten males were injected with virus inoculum and caged with 9 healthy target males for 60 days. Target males were assayed by PCR 30 to 40 days post-treatment. Cages that had previously held virus-infected adult D. abbreviatus for 30 days were used to test the possibility of transmission from contaminated surfaces. The insects, food, and water were removed from the cages. Five pairs (10 adults) of healthy target individuals were placed into the ‘contaminated’ cages, with fresh food and water sources. Weevils were analyzed 30 to 40 days later by PCR. Frass from adult D. abbreviatus was rehydrated with 100 µl of PBS buffer, pH 7.2, in an attempt to detect IIV6 in the excreta of D. abbreviatus. A total of 12 different samples of frass from adults, 20 days post virus exposure, were analyzed by PCR.
Electron Microscopy.
Adult and larval D. abbreviatus inoculated per os, by puncture, or injection with purified IIV6, were killed in an atmosphere of chloroform. The insects were then placed in fixing buffer (3 % v/v glutaraldehyde/0.1 M potassium phosphate buffer, pH 7.2), at room temperature for 2 days. Each insect was then cut into 4 pieces and placed in fresh fixing buffer for 3 days. The samples were washed 3 times, 20 min each, in rinsing buffer (0.1 M potassium phosphate buffer, pH 7.2), and post-fixed overnight in rinsing buffer plus 2 % (v/v) osmium tetroxide at room temperature. The samples were then washed 5 times in rinsing buffer, dehydrated in acetone and embedded in Spurr's resin (Spurr 1969). For internal orientation, 1 µm sections were made and stained with methylene blue/ azure A and 0.05 % basic fuchsin (Schneider 1981). Thin sections were made using an LKB Huxley ultramicrotome (LKB-Produkter AB, Bromma, Sweden), mounted on uncoated 200 mesh copper grids, and stained with 1% aqueous uranyl acetate (Stempak and Ward, 1964) and lead citrate [1 pellet of NaOH (0.1 to 0.2 g) into 50 ml of autoclaved water in a sealable autoclave tube, add 0.25 g lead citrate, shaken until dissolved] (Bozzola and Russell, 1992; Fahmy, 1967). The sections were viewed and photographed with a Philips 201 transmission electron microscope, TEM, (Philips Scientific & Analytical Equipment).
Molecular analyses
PCR Analysis and DNA Sequencing
Consensus primers were designed for PCR/sequencing based on a conserved region within the capsid protein gene from three insect iridoviruses: IIV1, IIV6, IIV22, (GeneBank accession no. M33542; M99395; M32799 respectively) (Webby and Kalmakoff, 1998). Amplification by PCR was conducted with consensus primers, P1FOR (5′ ACY TCW GGK TTY ATC GAT ATC GCC ACT 3′) and P2REV (5′ TTR ATW GCA TGA GAG AAR CGA ATA TC 3′), corresponding to IIV6 major capsid protein nucleotide positions 679–705 and 1548–1573 respectively (synthesized by Invitrogen, Carlsbad, CA, USA). The PCR mix was 1µl of DNA, 2 µl of primers (50 µM each), 3 µl of 25 mM MgCl2, 45µl of Platinum® PCR Supermix (Invitrogen, www.invitrogen.com). Cycles were run in an automated Peltier Thermal Cycler, (PTC 200) (MJ Research, www.mjr.com). The amplification protocol: Denature at 95 °C for 10 min, at 94 °C for 2 min, at 41 °C for 2 min, at 72 °C for 5 min. Then 30 cycles at: denaturing 94 °C for 1 min, annealing at 41 °C for 1 min, elongation at 72 °C for 3 min, with a final cycle at 94 °C for 3 min, at 41 °C for 1 min, at 72 °C for 5 min, hold at 4 °C. A 20 µl sample of each reaction mixture was fractionated by electrophoresis in a 1 % agarose gel in TAE 1X buffer and the fragments stained with ethidium bromide. The gel-purified 893 bp DNA fragment was sequenced from 3 virus positive larvae, 3 adults and 2 egg masses, with an ABI Prism 310 genetic analyzer (PE Applied Biosystems, www.appliedbiosystems.com) using the Dye Deoxyterminator-Tag cycle sequencing technique, as per instructions (PE Applied Biosystems).
Results and Discussion
D. abbreviatus were successfully infected with purified IIV6 by all three methods of inoculation, puncture, microinjection, and per os (Fig. 1, 2). Although we did not set up the experiments to validate which method was more efficient at causing virus infection, it is of interest to note that none of the methods caused 100% infection. Such a result suggests there may be some inherent resistance to IIV6 infection within the D. abbreviatus population. Iridoviruses are DNA viruses that are easily detectable using PCR. The amplicon sequence identity to IIV6, for virus-positive eggs, larvae, and adult D. abbreviatus, was 100 % when sequenced in both directions. The low annealing temperature of 41 °C produced a smaller, weak, nonspecific band (Fig. 2). This band was sequenced from 3 different samples that had no identity to IIV6. Analysis by PCR and TEM of tissues showed IIV6 within cells of adults and larval D. abbreviatus. Virus was observed in fat body, muscle, nerve and tracheal cells from D. abbreviatus that had been injected (Fig. 1). The majority of IIV6- infected weevils developed covert infections.
Figure 1.
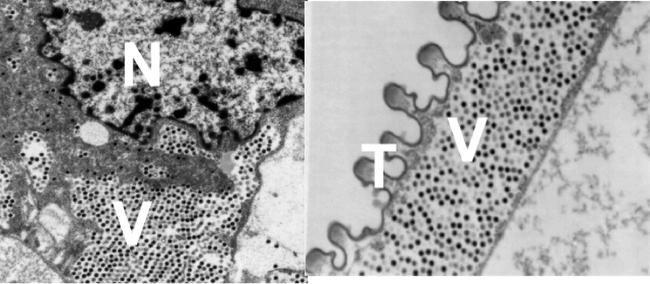
Transmission electron micrographs showing iridescent virus-6 inside Diaprepes root weevil cells. a) Virions were observed in the cytoplasm near cell nuclei (N). b) Tracheal epithelial cells (T) were heavily infected, with virions (V). Magnification ∼15,000X. Virions ∼120 nm in diameter.
Figure 2.
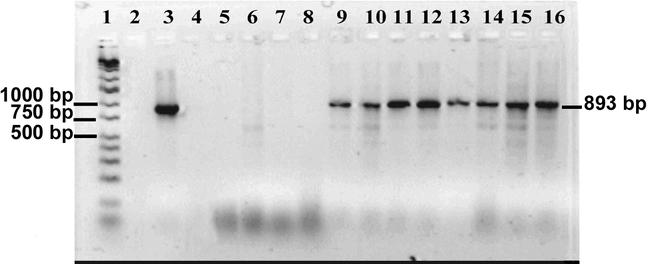
Gel of amplified virus-specific DNA from Diaprepes root weevil. Lanes: 1. Ladder wide-range DNA marker, (16 fragments, 50–10,000 bp), 2. Blank, 3. Positive control purified iridescent virus-6 (IIV6) (amplified DNA fragment ∼893 bp). 4. Blank. 5. Water control. 6. Adult weevil control injected with 4 µl of water. 7. Larvae weevil control injected with 4 µl of water. 8. Weevil egg control. 9. Adult weevil inoculated by puncturing with an insect pin dipped in purified virus. 10. Larvae inoculated by puncturing. 11. Adult weevil injected with 4 µl of homogenate made from an individual with a patent infection. 12. Larvae injected with 4 µl of homogenate virus inoculum. 13. Adult inoculated per os with purified IIV6 in 20 % sucrose (1 µl virus:10 µl sucrose solution). 14. Larvae inoculated per os. 15. Egg mass of 10–20 eggs from adult female inoculated by injection. 16. Egg mass of 10–20 eggs from adult female inoculated per os.
Of the 16 larvae inoculated by puncture, 13 larvae (81.2 %) tested negative for virus, while 3 (18.8 %) tested positive for virus at 20 days post inoculation. The ability to recover inoculum after infecting larvae through puncturing led us to test whether infection rates could be increased by microinjecting the inoculum. When inoculation was via microinjection in larvae, we used virus inoculum prepared from 3 infected D. abbreviatus larvae. As a result of injection approximately 10% of the larvae died within 1–2 days due to damage caused by the injection procedure (10 died out of 90 injected controls, 8 died out of 90 virus injected). Of the surviving virus-injected larvae, 9.8% (8 of 82) displayed patent infections, and 22% (18 of 82) had covert infections. Of the remaining larvae, 68%, (56 of 82) tested negative for virus infection. The low incidence of virus detection for virus-injected individuals was surprising, so this method was repeated. Over several months, batches of larvae were injected. Of the 269 larvae injected with 4 µl of virus inoculum, ranging from 8 to 10 weeks old, 149 (55.4 %) tested negative for virus, while 120 (44.6 %) tested positive by PCR analysis for virus when sampled 20 days post treatment. Administration of virus per os resulted in similar rates of infected males 31% and females 26% (Fig. 2).
Vertical transmission.
Virus positive eggs were detected by PCR 15 days after pairing healthy target females with virus-exposed males (Fig 2). When healthy females were paired with males fed a virus-sucrose solution, 32 egg masses from 8 different pairings resulted in 14 egg masses (43.8 %) that were negative for virus, while 18 masses (56.2 %) tested positive for virus. Of 21 egg masses tested from 10 pairings of males injected with virus inoculum with healthy target females 19 egg masses (90.4 %) were negative for virus, while 2 egg masses (9.5 %) tested positive for virus. Females paired with males that ingested virus produced more virus positive egg masses compared with females paired with males injected with virus. However, since the amount of ingested virus versus injected virus was not quantified it was not valid to make a direct comparison between these two methods of virus infection. However, it is evident that virus is being passed from infected males to healthy females and then to eggs. Evidence for virus replication in D. abbreviatus is the visual detection of virus in various tissues by TEM, the ability to transmit the virus through serial passages by microinjection from infected to healthy individuals, and the production of a large virus pellet from 3 infected larvae which could not be generated from the inoculum (12 µl). However, the lower infection rates from injected individuals may also be due to the abnormal pathway of infection in which males were injected with virus as oppose to ingesting virus. Thus virus movement through the male insect could be taking longer prior to becoming a contagion, thereby delaying the virus infection of females, and subsequently the egg masses. A preliminary examination of egg masses from females that were directly fed virus in a sucrose solution, resulted in 63.6 % virus positive (7 of 11). Horizontal transmission of virus was demonstrated from virus-exposed adults to healthy target D. abbreviatus, whether grouped by gender or paired for mating. When grouped by gender the virus-injected females that were caged with healthy target females produced an increase of infected females by 45.5 % (5 of 11) while virus-injected males resulted in an 88.9 % increase of infection in targeted males (8 of 9). Observations show that males have an aggressive mating behavior and will mount other males, but females have been reported to mount other females (Harari et al., 1999). The close aggregative behavior and attempted mating brings individuals into close proximity which may allow for a higher incidence of virus transmission through either direct contact (males often grasp with their mouthparts during mounting behavior) or as an aerosol (transmission through spiracles). Virus transmission by virus-infected males to healthy target females resulted in 30% (3 of 10) females testing virus negative, while 70% (7 of 10) were positive for virus.
Healthy target D. abbreviatus did become infected after being in contact with virus contaminated surfaces. The results showed that overall 40% were infected (4 of 10). Although the sample size was small, females were 20% infected (1 of 5), while males were 60% infected (3 of 5). The higher infection of males under these conditions may be due to the male tendencies to search for females, and to physically contact more individuals, especially with the mandibles, thus increasing the likelihood of virus ingestion. Also, a more active individual will have a higher respiratory rate.
Evidence presented here shows that IIV6 can infect D. abbreviatus, that IIV6 can be passed vertically (transovarially), horizontally (between individuals), and even through contaminated surfaces (in the absence of infected individuals). The virus infection rates of ∼30% on average, appears to be typical for iridovirus infection in insects (Williams, 1998). Other typical characteristics of iridovirus infection observed were the location of virus particles that were found at assembly sites within areas of the cytoplasm adjacent to the cell nucleus (Fig. 1). Examination of infected individuals showed virus in tracheal epithelial cells and in muscle and nerve tissues (Fig. 1). Analysis of extracted DNA using PCR amplification detected viral DNA in virus-exposed adults and larvae (Fig. 2). Amplicon sequence analysis had 98–100% with an average of 99.3% identity to the IIV6 major capsid protein gene. Expression of pathogenicity was highly variable with the majority of D. abbreviatus infections being covert (Fig. 3). In all cases, infection by IIV6 was verified by PCR, and successful passage of virus from inoculum made from virus infected individuals to healthy weevils. In the cases of typical patent infections, larvae darkened and became blue-black at death. We have not noted obvious iridescence probably because paracrystalline viral arrays do not form, or do not form in sufficient quantity to produce ‘Bragg’ reflections (Klug et al., 1959, Williams, 1996). Although some of these viruses have been reported to produce iridescence, recent reports suggest that iridescence is a minor characteristic of these viruses (Williams, 1996).
Figure 3.

Larvae of the Diaprepes root weevil, 24 h post inoculation with homogenate from a iridescent virus-6 (IIV6) infected larvae, showing the range of pathogenicity. 1. Control larvae injected with water. 2. Larvae darkening, blackish color usually dies 3 – 4 d post inoculation. 3. Larvae yellowish color, usually dies 5 – 7 d post inoculation. 4. Larvae normal coloration, covertly infected, no visible symptoms.
Modes of transmission and persistence of iridovirus in nature are still being discovered (Williams, 1998). In this study, virus transmission between caged D. abbreviatus may occur through contamination of surfaces, contact during mating, or through the spiracles as an aerosol. Sexual transmission from males to females may be expected based on the observations by Marina et al. (1999) which reported sexual transmission of iridovirus in mosquitoes. Transmission of virus during sex may be accomplished during the transfer and absorption of proteins by females during mating (Harari et al., 1999; Wolfner, 1997). Results show that infected females produced infected eggs, showing IIV6 can be vertically transmitted to the eggs. Vertical transmission has also been reported for iridoviruses in mosquitoes (Woodward and Chapman, 1968) and insect tissues found to be infected included ovaries, as well as the fat body, tracheal epithelium, imaginal discs, epidermis, hemocytes, esophagus, nerve, and muscles (Hall and Anthony, 1971). Visualization of massively infected cells surrounding the trachea suggest that one mode of virus infection may be as an aerosol (Fig. 1). Further studies on transmission modes and their efficiency under lab and field conditions are presently being planned.
The viral pathogen, IIV6, has the potential to be developed into an efficient biological control agent, although not in the classical sense of a severely pathogenic virus (Hall, 1985). Covert viruses will need to be bioengineered before they produce the desired effects needed for use in pest management programs. Furthermore, the viral interactions with the target host and the behavior of the target pest also play a role in selecting potential viral agents. The ability of IIV6 to be transovarially transmitted shows it could be used to target the subterranean larvae of D. abbreviatus, which is the crucial stage causing losses in citrus. The gregarious nature of the D. abbreviatus adults makes transmission per os, or as an aerosol, highly feasible since many individuals come into repeated contact with infected individuals. The potential ability of IIV6 to become established in the D. abbreviatus population appears likely and could produce long-term effects. Even at low infection rates, these rates could accumulate over time with repeated releases of infected adult males (Marschall and Ioane, 1982, Purrini, 1989, Zelazny et al., 1992), especially in a species like D. abbreviatus where females will mate repeatedly and males have aggressive tendencies. One example of this has been reported in a wild population of crane flies, Tipulidae (Ricou, 1975). In Ricou's study the infection levels were reported to have developed over the course of a year or two to 90%, with a reported decrease of the crane fly population density in the following years (Ricou, 1975).
As more iridoviruses are studied, we may gain a more accurate picture of the percentages of insects in a population that are covertly infected. Iridoviruses may be found to be more infectious than previously thought (Williams, 1998). Conceivably IIV6 would not be lost from an insect population as easily as more lethal viral diseases that rapidly kill their hosts before others can become infected. Other entomopathogenic viruses may also have potential for use as biological control agents and/or as molecular tools. The study of these viruses may increase our understanding of the immune response of insects to virus infection. They may also allow the development of new management strategies through the engineering of insect viruses to deliver sterilizing or lethal gene products (Williams, 1998).
Acknowledgments
We gratefully acknowledge Dr. J. Kalmakoff, University of Otago, Dunedin, New Zealand for providing invertebrate iridescent virus 6 (IIV6) from infected T. ni larvae. We also wish to acknowledge Anna S. Hill for technical support.
Footnotes
The mention or use of products within does not imply nor guarantee endorsement by the USDA to the exclusion of other similar products that may also be suitable.
References
- Bergoin M, Tijssen P. 1998 Invertebrate iridescent viruses. In: Miller LK and Ball LA, editors. The Insect Viruses. 6:141–169.Plenum Publishing Corporation. New York, NY. USA. [Google Scholar]
- Burand JP. 1998 Invertebrate iridescent viruses. In: Miller LK and Ball LA, editors. The Insect Viruses. 69–90.Plenum Publishing Corporation. New York, NY. USA. [Google Scholar]
- Bozzola JJ, Russell LD. 1992 Specimen Staining and Contrast Methods for Transmission Electron Microscopy. In: Bozzola JJ, Russell LD, editors. Electron Microscopy. Principles and Techniques for Biologists. 5:117–118.Jones and Bartlett Publishers, Boston. USA. [Google Scholar]
- Day MF, Dudzinski ML. The effect of temperature on the development of Sericesthis iridescent virus. Australian Journal of Biological Science. 1966;19:481–493. [Google Scholar]
- Day MF, Mercer EH. Properties of an iridescent virus from the beetle, Sericesthis pruinosa. Australian Journal of Biological Science. 1964;17:892–902. [Google Scholar]
- Fahmy A. 1967 An extemporaneous lead citrate stain for electron microscopy. Proceedings of the 25th Annual EMSA Meeting, pp. 148–149. [Google Scholar]
- Fukaya M, Nasu S. A Chilo iridescent virus (CIV) from the rice stem borer, Chilo suppressalis Walker (Lepidoptera: Pyralidae) Applied Entomology and Zoology. 1966;1:69–72. [Google Scholar]
- Hall DW. 1985 Pathobiology of invertebrate icosahedral cytoplasmic deoxyriboviruses (Iridoviridae). In: Maramorosch K, editor. Viral Insecticides for Biological Control. Academic Press, Inc. London. [Google Scholar]
- Hall DW, Anthony DW. Pathology of a mosquito iridescent virus (MIV) infecting Aedes taeniorhyncus. Journal of Invertebrate Pathology. 1971;18:61–69. doi: 10.1016/0022-2011(91)90009-f. [DOI] [PubMed] [Google Scholar]
- Harari AR, Handler AM, Landolt PJ. Size-assortive mating, male choice and female choice in the curculionid beetle Diaprepes abbreviatus. Animal Behaviour. 1999;58:1191–1200. doi: 10.1006/anbe.1999.1257. [DOI] [PubMed] [Google Scholar]
- Klug A, Franklin RE, Humphreys-Owen SPF. The crystal structure of Tipula iridescent virus as determined by Bragg reflection of visible light. Biochemica et Biophysica Acta. 1959;32:203–219. doi: 10.1016/0006-3002(59)90570-0. [DOI] [PubMed] [Google Scholar]
- Lapointe SL, Shapiro JP. Effect of soil moisture on development of Diaprepes abbreviatus (Coleoptera: Curculionidae) Florida Entomologist. 1999;82:291–299. [Google Scholar]
- Marina CF, Arrendondo-Jimenez JI, Castillo A, Williams T. Sublethal effects of iridovirus disease in a mosquito. Oecologia. 1999;119:383–388. doi: 10.1007/s004420050799. [DOI] [PubMed] [Google Scholar]
- Marschall JK, Ioane I. The effect of re-release of Oryctes rhinoceros baculovirus in the biological control of rhinoceros beetle in western Samoa. Journal of Invertebrate Pathology. 1982;39:267. [Google Scholar]
- McLaughlin RE, Scott HA, Bell MR. Infection of the boll weevil by Chilo iridescent virus. Journal of Invertebrate Pathology. 1972;19:285–290. [Google Scholar]
- Ohba M. Studies on the multiplication of Chilo iridescent virus. 3. Multiplication of CIV in the silkworm, Bombyx mori L. and field insects. Scientific Bulletin of Faculty of Agriculture, Kyushu University. 1975;30:71–81. [Google Scholar]
- Purrini K. Baculovirus oryctes release into Oryctes monoceros population in Tanzania, with special reference to the interaction of virus isolates used in our laboratory infection experiments. Journal of Invertebrate Pathology. 1989;53:285. [Google Scholar]
- Ricou G. Production de Tipula paludosa Meig. en prarie en function de l'humiditié du sol. Revue d'Ecologie et de Biologie du Sol. 1975;12:69–89. [Google Scholar]
- Rogers S, Graham JH, McCoy CW. Insect-plant pathogen interactions: preliminary studies of Diaprepes root weevil injuries and Phytophthora infections. Proceedings of the Florida State Horticultural Society. 1996;109:57–62. [Google Scholar]
- Schneider H. 1981 Plant anatomy and general botany. In Clark G. editor. Staining procedures for biological stain commission. 4th edition. Pp. 315–373.Williams and Wilkins, Baltimore, MD. USA. [Google Scholar]
- Spurr AR. A low-viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructure Research. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stempak JG, Ward RT. An improved staining method for electron microscopy. Journal of Cell Biology. 1964;22:697–701. doi: 10.1083/jcb.22.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webby R, Kalmakoff J. Sequence comparison of the major capsid protein gene from 18 diverse iridoviruses. Archives of Virology. 1998;143:1949–1966. doi: 10.1007/s007050050432. [DOI] [PubMed] [Google Scholar]
- Williams T. The Iridoviruses. Advances in Virus Research. 1996;46:345–413. doi: 10.1016/s0065-3527(08)60076-7. [DOI] [PubMed] [Google Scholar]
- Williams T. 1998 Invertebrate iridescent viruses. In Miller LK. and Ball LA, editors. The Insect Viruses. 31–68.Plenum Publishing Corporation. New York, NY. USA. [DOI] [PubMed] [Google Scholar]
- Wolcott GN. Otiorhynchids oviposit between paper. Journal Economic Entomology. 1933;26:1172–1173. [Google Scholar]
- Wolfner MF. Tokens of love: Functions and regulation of Drosophila male accessory gland products. Insect Biochemistry and Molecular Biology. 1997;7:179–192. doi: 10.1016/s0965-1748(96)00084-7. [DOI] [PubMed] [Google Scholar]
- Woodruff RE. 1964 A Puerto Rican weevil new to the United States (Coleoptera:Curculionidae). Florida Department of Agriculture, Division of Plant Industry Entomology Circular. No. 30. 2. pp. [Google Scholar]
- Woodward DB, Chapman HC. Laboratory studies with the mosquito iridescent virus (MIV) Journal of Invertebrate Pathology. 1968;11:296. doi: 10.1016/0022-2011(68)90162-6. [DOI] [PubMed] [Google Scholar]
- Zelazny B, Lolong A, Pattang B. Oryctes rhinoceros (Coleoptera: Scarabaeidae) populations suppressed by a baculovirus. Journal of Invertebrate Pathology. 1992;59:61. [Google Scholar]


