Abstract
The response of male moths from two pheromone races of the European corn borer, Ostrinia nubilalis, was measured in a flight tunnel assay to different ratios of structurally different compounds that comprise the sex pheromone of the Asian corn borer, Ostrinia furnacalis. For both O. nubilalis races, between 1 and 5% of the males completed upwind flights to two different blends of the O. furnacalis pheromone components (the 2:1 Z/E12-14:OAc female-produced blend, and a 97:3 Z/E mix), confirming that rare males exist in the O. nubilalis populations that can detect and respond to mixtures of the O. furnacalis pheromone components. Rare males that responded to the O. furnacalis blends also responded to their own O. nubilalis blends (97:3 or 1:99 Z/E11-14:OAc), indicating that rare O. nubilalis males are not preferentially sensitive to mixtures of the O. furnacalis compounds, but rather that they have a broad range of response specificity, which includes recognition of a wide range of conspecific female-produced ratios, and also recognition of heterospecific mixtures. The results support the hypothesis that saltational shifts in pheromone blend composition (Roelofs et al., 2002) can lead to the evolution of a new species-specific communication system, in part because the broad response specificity of some males includes the ability to respond in an agonistic manner to novel mixtures of compounds.
| Abbreviation: | |
|---|---|
| E race | using a 1:99 Z/E11-14:Oac blend |
| Z race | using a 97:3 Z/E11-14:OAc blend |
| Z/E-11 | the European corn borer, O. nubilalis, blends (Z/E11-14:OAc). |
| Z/E-12 | the Asian corn borer, O. furnacalis, blend (Z/E12-14:OAc). |
| bivoltine | one generation per year |
| univoltine | two generations per year |
Keywords: European corn borer, Ostrinia nubilalis, Asian corn borer, Ostrinia furnacalis, flight tunnel, sex pheromone, pheromone evolution
Introduction
Recently, Roelofs et al., (2002) reported that females of the European corn borer, Ostrinia nubilalis, and the Asian corn borer, Ostrinia furnacalis, had the genes for key desaturase enzymes that are involved in producing both species' pheromone blends, but that in each species the gene for the related species is not expressed. Female O. nubilalis use a Δ11-desaturase, which is common to many moths, and acts on a chain-shortened 14-carbon fatty acyl chain to produce a mixture of (Z)- and (E)-11-tetradecenoic acids. The acids are reduced and acetylated to give the pheromone components, (Z)- and (E)-11-tetradecenyl acetates.
In most O. nubilalis populations in Europe and North America, the major component is the Z isomer; trapping data show that males respond maximally to the 97:3 blend of Z/E11-14:OAc produced by females (designated the ‘Z race’). However, males from some populations in Europe and the United States respond to a different female-produced blend, 1:99 Z/E11-14:OAc (designated the ‘E race’) (Klun et al., 1973; Kochansky et al., 1975; Klun, 1975; Anglade et al., 1984). In New York state, three different races of O. nubilalis have been described, each differing in pheromone blend and voltinism (number of generations per year; Roelofs et al., 1985): 1) a bivoltine Z race (two generations, using a 97:3 Z/E11-14:OAc blend); 2) a univoltine Z race (single generation, using a 97:3 Z/E11-14:OAc blend); and 3) a bivoltine E race (1:99 Z/E11-14:OAc).
In contrast, O. furnacalis uses a Δ14-desaturase, which acts on a 16-carbon fatty acid, and after chain shortening, produces a pheromone blend comprised of (Z)- and (E)-12-tetradecenyl acetate (in a 2:1 Z/E mix; Zhao et al., 1990, 1995). The study by Roelofs et al., 2002 suggested that the gene controlling production of the O. furnacalis Δ14-desaturase had been present as a pseudogene in the Ostrinia genome for millions of years, but it was not expressed in ancestral Ostrinia females until recently. Additionally, they showed that the gene controlling the O. nubilalis Δ11-desaturase is present, but not expressed, in O. furnacalis females.
The desaturase studies raise the possibility that a new pheromone communication system can arise as a result of changes in regulatory pathways controlling gene expression. However, for the new pheromone to be effective in mate location it is necessary for some males to be able to detect and respond to the new blend. Roelofs et al., (2002) reported on a preliminary study showing that a few rare males of one of the O. nubilalis races did, in fact, respond to both species' pheromone blends. Here we report on an expanded flight-tunnel study, with two of the pheromone races of O. nubilalis that occur in New York state, showing that rare males exist in both pheromone races that respond to mixtures of the O. furnacalis pheromone components.
Methods and materials
Insects
Colonies of the univoltine Z and bivoltine E races of O. nubilalis from New York state were maintained in separate walk-in environmental chambers. Mating and larval rearing conditions were as described in Roelofs et al., (1987) at a constant 25° C, 16:8 L:D photoperiod. Pupae were sexed and the male pupae placed on a layer of vermiculite in plastic and screen emergence cages inside a walk-in environmental chamber (25° C; 16:8 L:D photoperiod). Cages of adults were separated daily so that individuals of known age could be used for flight tunnel tests.
Chemicals
The chemicals were obtained from the Pherobank (www.pherobank.nl), and mixtures were prepared in hexane and applied to red rubber septa (Thomas Scientific, www.thomassci.com/index.jsp) at a dose of 30 µg/septum (30 µg in 300 µl solvent; Glover et al., 1987; Linn et al., 1997).
Flight tunnel
Adults were tested in the sustained-flight tunnel during their second night as adults, under standard conditions for O. nubilalis (Glover et al., 1987; Linn et al., 1997): 19° C, 60% relative humidity, 0.50 m/sec air flow, and illumination of 11 lux of red light at the tunnel floor, during the 2nd – 6th hour of scotophase. Adults were taken to the room housing the flight tunnel 1 hour prior to the start of the 8-hour scotophase, placed individually in screen release cages that were then placed on a wooden rack next to the flight tunnel, so they could acclimate to the flight tunnel room environment. Adult moths were tested individually, and scored for the following behaviors: 1) taking flight from the release cage (150 cm from source); 2) initiation of zig-zag flight directed upwind a minimum 10 cm distance toward the odor source (140 cm); 3) upwind flight to within 40 cm of the source; and 4) upwind flight making contact with the source and attempting copulation.
Two experiments were conducted, with individual males tested to more than one treatment during each test period. After recapture, males were removed from the flight tunnel and allowed a minimum period of 30 minutes prior to retesting with a different blend. Preliminary tests with the Z race males showed that 4 tests could be made in one 4 hour test period with no significant change in the proportion of upwind flights (total N = 50, proportion of upwind flights for 4 tests: 90, 92, 88, 92%; see also Cosséet al., 1995).
Nomenclature
To aid in the reading of the text in the sections below, the following abbreviated nomenclature for the sex pheromone blends is adopted:
Z/E-11 refers to the European corn borer, O. nubilalis, blends (Z/E11-14:OAc).
Z/E-12 refers to the Asian corn borer, O. furnacalis, blend (Z/E12-14:OAc).
Experiment 1
Males of O. nubilalis Z and E races were tested to a series of treatments in the following order.
For the Z race:
First, a 30 µg dose of the 2:1 Z/E-12 blend.
Second, a 30 µg dose of the 97:3 Z/E-12 blend.
Third, a 30 µg dose of the Z race 97:3 Z/E-11 blend.
For the E race:
First, a 30 µg dose of the 2:1 Z/E-12 blend.
Second, a 30 µg dose of the E race 1:99 Z/E-11 blend.
Experiment 2
Males of the Z race were divided into 2 groups, and tested to treatments in the following order:
Group 1:
First, a 30 µg dose of the 2:1 Z/E-12 blend.
Second, if a male responded to 1 it was then tested to a 30 µg dose of the 2:1 Z/E-11 blend.
Group 2.:
First, to a 30 µg dose of the 2:1 Z/E-11 blend.
Second, if a male responded to 1 it was then tested to a 30 µg dose of the 2:1 Z/E-12 blend.
All males from group 1 and 2 that responded to either or both of the 2:1 Z/E blends were then tested to the Z race 97:3 Z/E-11 blend.
Statistical analysis
The number of moths in each treatment that exhibited each behavior in the flight tunnel sequence was combined for all testing periods and converted to a percent value for graphical display. Pairwise comparisons were made between treatments for each behavior, using χ2 analysis, according to the JMP statistical analysis program for Macintosh (P < 0.05).
Results
Experiment 1
A total of 343 Z race males were tested to two Z/E-12 mixtures and the Z race 97:3 Z/E-11 blend (Fig. 1). Males first were tested to the 2:1 Z/E-12 blend, with 25% taking flight, 11% initiating upwind flight, 6% flying to the 40 cm mark, and 4% making source contact. These males were then tested to the 97:3 Z/E-12 blend, and whereas values were lower for each behavior compared with the 2:1 Z/E blend, there were no significant differences (18, 4, 3, and 2% respectively). However, response values for both Z/E-12 blends were significantly lower than the proportion responding to the third treatment, the Z race 97:3 Z/E-11 blend (98, 92, 92, and 91% touching the source, respectively).
Figure 1.
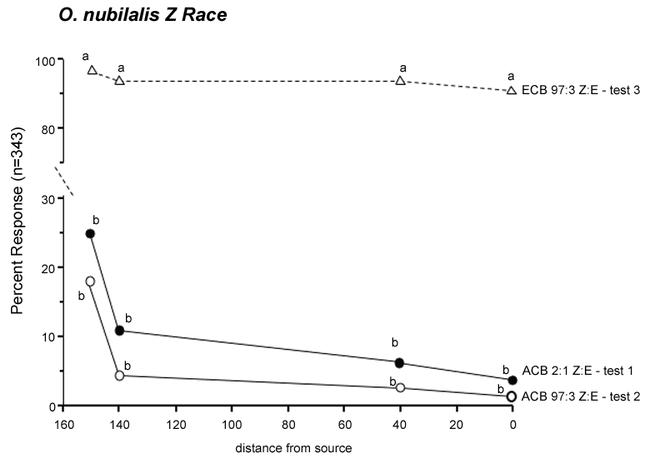
Percent response of 343 O. nubilalis Z race males tested to treatments in the following order: O. furnacalis 2:1 Z/E blend (ACB, test 1), O. furnacalis 97:3 Z/E blend (ACB, test 2), and then to the 97:3 Z/E O. nubilalis blend (ECB, test 3). Values are for 4 behaviors: taking flight, 150 cm; initiation of upwind flight, 140 cm; upwind flight to the 40 cm mark, and touching the source, 0 cm. For each behavior/distance from source, letters that differ between blends are significantly different (χ2; P < 0.05).
Figure 2 shows that of the 52 instances in which a Z race male initiated upwind flight to either of the Z/E-12 blends, 60% were only to the 2:1 Z/E-12 blend, 22% were only to the 97:3 Z/E-12 blend, and 18% flew upwind to both mixtures. All of the males that flew upwind to either or both of the Z/E-12 blends also responded to the Z race 97:3 Z/E-11 blend.
Figure 2.
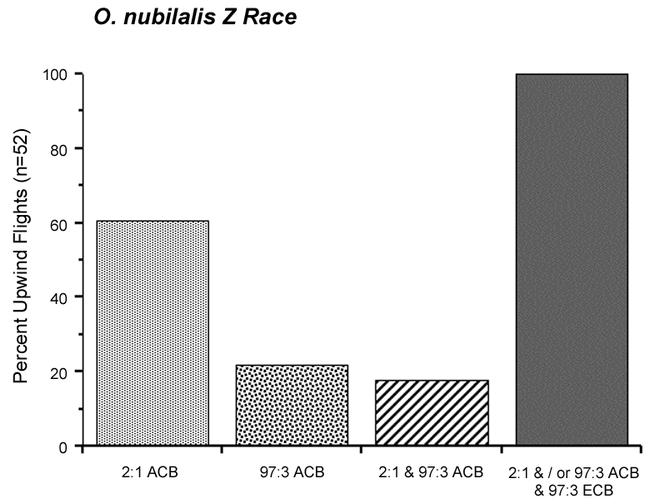
Percent upwind flights of 52 O. nubilalis Z race males that responded to the 2:1 and/or 97:3 Z/E O. furnacalis blends (ACB), and then the 97:3 Z/E O. nubilalis blend (ECB). Data from Figure 1.
A total of 732 E race males were tested to 2 mixtures (Fig. 3). Males were tested first to the 2:1 Z/E-12 blend, with 32% taking flight, 13% initiating upwind flight, 4% flying to the 40 cm mark, and 3% touching the source. These values were significantly lower than those with the second treatment, the E race 1:99 Z/E-11 blend (90, 73, 70, 70%, respectively).
Figure 3.
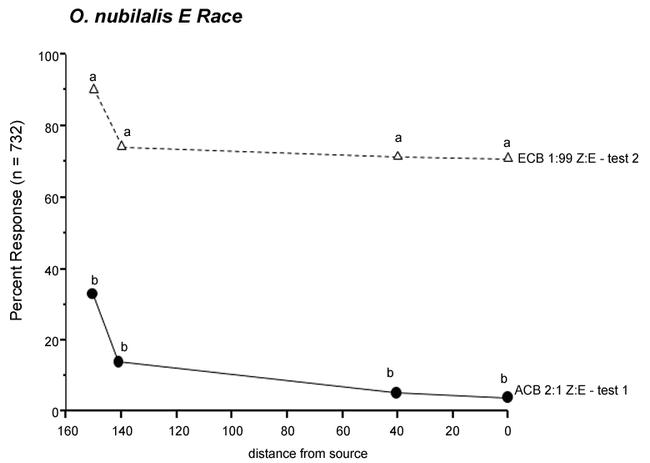
Percent response of 732 O. nubilalis E race males to 2 blends, in the following order: O. furnacalis 2:1 Z/E blend (ACB, test 1), and then to the 1:99 Z/E O. nubilalis blend (ECB, test 2). Values are for 4 behaviors: taking flight, 150 cm; initiation of upwind flight, 140 cm; upwind flight to the 40 cm mark, and touching the source, 0 cm. For each behavior/distance from source, letters that differ between blends are significantly different (χ2; P < 0.05).
Experiment 2
One hundred fifty-four Z race males were tested to the 2:1 Z/E-11 blend, with 78% taking flight, 54% initiating upwind flight, 17% flying to the 40 cm mark, and 15% reaching the source (Fig. 4). One hundred fifty-seven Z race males were tested to the 2:1 Z/E-12 blend, and the responses for all behaviors were significantly lower than observed with the 2:1 Z/E-11 blend (16, 9, 4, and 3% respectively).
Figure 4.
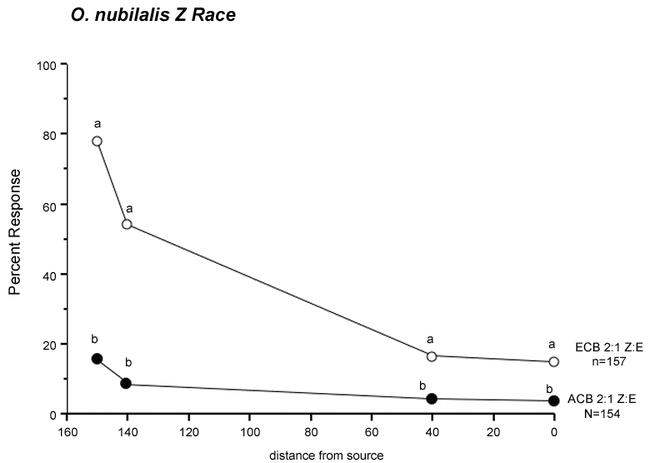
Percent response of O. nubilalis Z race males to the O. furnacalis 2:1 Z/E blend (ACB, n = 154), or to the 2:1 Z/E O. nubilalis blend (ECB, n = 157). Values are for 4 behaviors: taking flight, 150 cm; initiation of upwind flight, 140 cm; upwind flight to the 40 cm mark, and touching the source, 0 cm. For each behavior/distance from source, letters that differ between blends are significantly different (χ2; P < 0.05).
Figure 5 shows further that of the 14 Z race males that initiated upwind flight to the 2:1 Z/E-12 blend, 40% also subsequently flew to the 2:1 Z/E-11 blends. In contrast, of the 86 Z race males that initiated upwind flight to the 2:1 Z/E-11 blend, only 11% initiated upwind flight to the 2:1 Z/E-12 blend. However, all of the 100 males that responded to either the 2:1 Z/E-11 or Z/E-12 blends made successful flights to the Z race 97:3 Z/E-11 blend.
Figure 5.
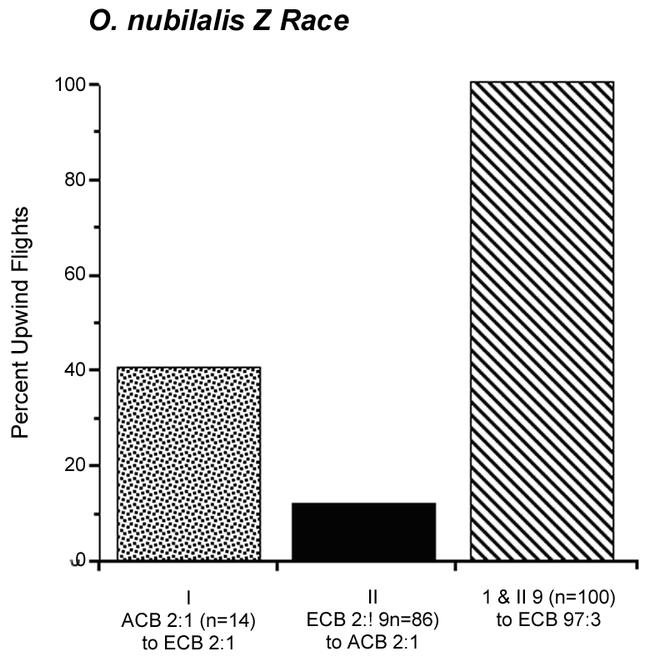
I, II: Percent upwind flights of O. nubilalis Z race males in Figure 4 responding to the 2:1 Z/E O. furnacalis (I; n = 14, ACB) or O. nubilalis (II; n = 86, ECB) blend and then to the untested 2:1 Z/E blend. III, percent upwind flights of males in I and II that then responded to the 97:3 Z/E O. nubilalis blend (ECB).
Discussion
Rare males in O. nubilalis
Our results extend those of Roelofs et al., (2002) by showing that rare males exist in both the Z and E races of O. nubilalis that can exhibit upwind anemotactic flight to mixtures of the pheromone components of the Asian corn borer, O. furnacalis. With the O. furnacalis female-produced blend (2:1 Z/E12-14:OAc) 4% of Z race males, and 3% of E race males, flew upwind to the source. These values compare well with 4% (5/119) reported by Roelofs et al., (2002) for Z race males, using a different protocol in which males were first tested to the Z race 97:3 Z/E-11 blend and then to the 2:1 Z/E-12 blend.
What is remarkable is not only did males respond to a mixture of structurally different isomers, but to a ratio (2:1 Z/E-12) significantly different from the normal 97:3 and 1:99 Z/E-11 blends of the two races. Previous flight tunnel studies with O. nubilalis, designed to determine the response specificity of males in both races, showed that a very low proportion (5 – 15%) flew upwind to a 30 µg dose of the 2:1 Z/E-11 blend (Glover et al., 1987; Linn et al., 1997). This ratio thus represents an extreme limit for both races.
The current results show that a similar low proportion of Z race males flew upwind to the same 2:1 Z/E-11 mix, and that some, but not all, of these males also responded to the 2:1 proportion of structurally different isomers that comprise the O. furnacalis pheromone (Z/E12-14:OAc). Interestingly, some rare Z race males also flew to a 97:3 Z/E-12 blend, and further, some of these males flew upwind to the 2:1 and 97:3 Z/E-11 blends as well. In fact, all of the males that exhibited an oriented flight to one or both of the O. furnacalis mixtures also subsequently flew upwind to the O. nubilalis blend of their race. The combined results suggest that the rare males are not preferentially sensitive to mixtures of the O. furnacalis compounds, but rather that these males have: 1) broadly tuned sensory cells that may respond to a range of compounds (Baker, 2002); and 2) a broad range of response specificity that includes variability for ratios of species-specific pheromone components, and variability for mixtures of structurally different compounds from related species.
A major question for future study is how olfactory information for the O. furnacalis components is processed by the antenna of O. nubilalis. Are there olfactory sensilla with receptor neurons specific for the Δ12 acetates? Are they different from ones for the Δ11's, and do all males in the population have the sensory capability to detect these compounds? Equally intriguing is the question of the relationship between the evolution of the new desaturases and new olfactory receptors for the products of those enzymes. One possibility is that a diverse olfactory receptor multigene family has coevolved with the female desaturase multigene family, allowing for the rapid evolution of male response to novel female pheromone blends (Roelofs et al., 2002). A few electrophysiological studies have shown that males of some moth species possess very broadly tuned sensory neurons, enabling them to respond to a variety of pheromone components, which may even represent those of different species; one of the best studied cases involves the yponomeutid moths (Löfstedt et al., 1990, 1991; see Baker 2002). Another wide-spread phenomena involves antagonism of upwind flight by detection of hetero-specific pheromone components between closely related species, such as, in the case of O. nubilalis, with the compound Z9-14:OAc (Glover et al., 1989).
Sex pheromone blends and response specificity
Sex pheromones in almost all moth species are mixtures of chemicals, and signal (or blend) specificity has been regarded as a dominant feature of pheromone communication systems (Roelofs, 1978; Baker, 1989; Linn and Roelofs, 1989; 1995). Because closely related moth species share common biosynthetic pathways, they often utilize common chemicals, but in species-specific blends and isomeric ratios. The evolution of specific blends has provided a high degree of specificity to each communication channel.
Variability is an important feature of pheromone communication systems, both with respect to the range of female-produced ratios of compounds in a population, and the breadth of individual male responsiveness (Baker, 1989; Löfstedt, 1990, 1993; Linn and Roelofs, 1989, 1995; Haynes, 1997; Phelan, 1992, 1997). However, studies documenting variability have generally focused on the defined set of female-produced components, and did not systematically include blends of closely related species, looking for phylogenetic relationships such as the one seen here for the two Ostrinia species. Structure-activity studies have shown that responses can occur to analogues of pheromone components that differ slightly in chain length and functional group (see Schwarz et al., 1989 for one such study with O. nubilalis). Low levels of response to pheromones of related species also have been documented in many field trapping experiments, but it has generally been thought that these behavioral responses would result in ‘mating mistakes’, and that males with such broad response specificities have a lower fitness than males who respond to a small range of mixtures centered on the average female-produced blend.
Our results support the idea that males in many species may be able to respond in an agonistic manner to a number of phylogenetically related pheromone blends, or even to novel blends that may arise as a result of changes in regulatory pathways controlling gene expression. The asymmetric tracking model (Phelan, 1992, 1997), in fact, predicts that selection will act on males to produce variation in response specificity, allowing for the opportunity to respond to a new female-produced mix in the event that such an occurrence should arise. As documented by at least two studies, this situation could arise through mutation in an existing pheromone pathway (such as with the cabbage looper moth, Trichoplusia ni; Haynes, 1997), or with a shift to a new pheromone blend (O. nubilalis/O. furnacalis; Roelofs et al., 2002; see also discussions in Löfstedt, 1990; Baker 2002; and Phelan, 1992, 1997). If successful mating occurs, subsequent shifts in sensitivity, or in behavioral (agonist/antagonist) properties of sensory inputs, would allow males to track variation in female blend composition as well as quality (Phelan, 1992, 1997; Butlin and Trickett, 1997; Haynes, 1997), allowing for the possibility of a new, isolated, communication channel to develop. Future behavioral, as well as electrophysiological, studies hopefully will include pheromone blends and pheromone components of related species, in order to further test these ideas.
Acknowledgments
We thank Kathy Poole, Callie Musto, Karrie Catropia, and Katie Straight for maintaining the univoltine and bivoltine O. nubilalis colonies, and Satoshi Nojima for preparing the solutions for flight tunnel tests.
References
- Anglade PJ, Stockel J. IWGO Cooperators. 1984 Intraspecific sex-pheromone variability in the European corn borer, Ostrinia nubilalis Hbn. (Lepidoptera, Pyralidae). Agronomie. 4:183–187. [Google Scholar]
- Baker TC. Sex pheromone communication in the Lepidoptera: New research progress. Experientia. 1989;45:248–262. [Google Scholar]
- Baker TC. Mechanisms for saltational shifts in pheromone communication systems. Proceedings of the National Academy of Sciences, USA. 2002;99:13368–13370. doi: 10.1073/pnas.222539799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butlin RK, Trickett AJ. 1997 Can population genetic simulations help interpret pheromone evolution? In: RT Cardé, AK Minks, editors. Insect Pheromone Research: New Directions. pp. 548–562.Chapman and Hall. [Google Scholar]
- Cossé AA, Campbell MG, Glover TJ, Linn CE Jr, Todd JL, Baker TC, Roelofs WL. Pheromone behavioral responses in unusual male European corn borer hybrid progeny not correlated to electrophysiological phenotypes of their pheromone-specific antennal neurons. Experientia. 1995;51:809–816. [Google Scholar]
- Glover TJ, Tang X-H, Roelofs WL. Sex pheromone blend discrimination by male moths from E and Z strains of European corn borer. Journal of Chemical Ecology. 1987;13:143–151. doi: 10.1007/BF01020358. [DOI] [PubMed] [Google Scholar]
- Glover TJ, Perez N, Roelofs WL. Comparative analysis of sex pheromone-response antagonists in three races of European corn borer. Journal of Chemical Ecology. 1989;15:863–873. doi: 10.1007/BF01015182. [DOI] [PubMed] [Google Scholar]
- Haynes K. 1997 Genetics of pheromone communication in the cabbage looper moth, Trichoplusia ni. In: RT Cardé, AK Minks, editors. Insect Pheromone Research: New Directions. pp. 525–534. [Google Scholar]
- Klun JA. Insect sex pheromones: Intraspecific pheromonal variability of Ostrinia nubilalis in North America and Europe. Environmental Entomology. 1975;4:891–894. [Google Scholar]
- Klun JA, Chapman OL, Mattes KC, Wojtkowski PW, Beroza M, Sonnet PE. Insect sex pheromones: Minor amount of opposite geometrical isomer critical to attraction. Science. 1973;181:661–663. doi: 10.1126/science.181.4100.661. [DOI] [PubMed] [Google Scholar]
- Kochansky J, Cardé RT, Liebherr J, Roelofs WL. Sex pheromones of the European corn borer in New York. Journal of Chemical Ecology. 1975;1:225–231. [Google Scholar]
- Linn CE Jr, Roelofs WL. Response specificity of male moths to different blends and dosages of sex pheromone. Chemical Senses. 1989;14:421–437. doi: 10.1007/BF01012203. [DOI] [PubMed] [Google Scholar]
- Linn CE Jr, Roelofs WL. 1995 Pheromone communication in the moths and its role in the speciation process. In: Lambert DH, Spencer H, editors. Speciation and the recognition concept: Theory and application. Pages. 263–300.Johns Hopkins University Press, Baltimore. [Google Scholar]
- Linn CE Jr, Young MS, Gendle M, Glover TJ, Roelofs WL. Sex pheromone blend discrimination in two races and hybrids of the European corn borer moth, Ostrinia nubilalis. 1997. Physiological Entomology. 1997;22:212–223. [Google Scholar]
- Löfstedt C. Population variation and genetic control of pheromone communication systems in moths. Entomologia Experimentalis et Applicata. 1990;54:199–218. [Google Scholar]
- Löfstedt C. Moth pheromone genetics and evolution. Philosophical Transactions of the Royal Society of London B. 1993;340:167–177. [Google Scholar]
- Löfstedt C, Hansson BS, Dijkerman HJ, Herrebout WM. Behavioral and electrophysiological activity of unsaturated analogues of the pheromone tetradecyl acetate in the small ermine moth Yponomeuta rorellus. Physiological Entomology. 1990;15:47–54. [Google Scholar]
- Löfstedt C, Herrebout WM, Menken J. Sex pheromones and their potential role in the evolution of reproductive isolation in small ermine moths (Yponomeutidae) Chemoecology. 1991;2:20–28. [Google Scholar]
- Phelan PL. 1992 Evolution of sex pheromones and the role of asymmetric tracking. In, Insect Chemical Ecology: An Evolutionary Approach, eds. B. D. Roitberg, M. B. Isman. Chapman and Hall, pp. 265–314. [Google Scholar]
- Phelan PL. 1997 Evolution of mate-signaling in moths: phylogenetic considerations and predictions from the asymmetric tracking hypothesis. In: Choe JC, Crespi BJ, editors. The Evolution of Mating Systems in Insects and Arachnids. pp. 240–256.Cambridge university Press. [Google Scholar]
- Roelofs WL. Threshold hypothesis for pheromone perception. Journal of Chemical Ecology. 1978;4:685–699. [Google Scholar]
- Roelofs WL, Du J-W, Tang X-H, Robbins PS, Eckenrode CJ. Three European corn borer populations in New York based on sex pheromones and voltinism. Journal of Chemical Ecology. 1985;11:829–836. doi: 10.1007/BF01012071. [DOI] [PubMed] [Google Scholar]
- Roelofs WL, Glover T, Tang X-H, Sreng I, Robbins P, Eckenrode C, Löfstedt C, Hansson BS, Bengtsson BO. Sex pheromone production and perception in European corn borer moths is determined by both autosomal and sex-linked genes. Proceedings of the National Academy of Sciences, USA. 1987;84:7585–7589. doi: 10.1073/pnas.84.21.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs WL, Liu W, Hao G, Jiao H, Rooney AP, Linn CE Jr. Evolution of moth sex pheromones via ancestral genes. Proceedings of the National Academy of Sciences, USA. 2002;99:13621–13626. doi: 10.1073/pnas.152445399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz M, Klun JA, Fritz GL, Uebel EC, Raina AK. European corn borer sex pheromone: Structure-activity relationships. Journal of Chemical Ecology. 1989;15:601–617. doi: 10.1007/BF01014704. [DOI] [PubMed] [Google Scholar]
- Zhao C-H, Löfstedt C, Wang W. Sex pheromone biosynthesis in the Asian corn borer, Ostrinia furnacalis (II). Biosynthesis of (E) – and (Z) 12-tetradecenyl acetate involves delta-14 desaturation. Archives of Insect Biochemistry and Physiology. 1990;15:57–65. [Google Scholar]
- Zhao C-H, Fang L, Bengtsson M, Löfstedt C. Substrate specificity of acetyltransferase and reductase enzyme systems used in pheromone biosynthesis by Asian corn borer, Ostrinia furnacalis. Journal of Chemical Ecology. 1995;21:1795–1810. doi: 10.1007/BF02035148. [DOI] [PubMed] [Google Scholar]


