Abstract
Ant communities in longleaf pine habitats are poorly known and hence the naturally occurring ant assemblages of a large portion of southeastern North America are not well understood. This study examined the diverse ant community found in the longleaf pine flatwoods of north Florida and tested how ant diversity changes along a herbaceous ground cover gradient. Restoring the ground cover to its original floral composition is an important focus of longleaf pine conservation and hence it is important to understand how native faunal communities vary with ground cover variation. Using 4 sampling methods, we characterized the ant community and analyzed its within-habitat variation among 12 study sites. We found the highest plot species richness (55 species) and within-habitat species richness (72 species) ever recorded for North American ants. The ants formed three distinct communities. The low-diversity arboreal and subterranean assemblages varied little across forest stands while the diversity of the species-rich ground foraging ant community was negatively correlated with percent herbaceous cover. The imported fire ant, Solenopsis invicta (monogyne form), was unexpectedly found to be abundant in high herbaceous cover sites. Floral restoration of the pine flatwoods, which is increasing the proportion of herbaceous cover, is likely to cause an increase in the abundance of the imported fire ant.
| Abbreviation: | |
|---|---|
| ANF | Apalachicola National Forest |
Keywords: Formicidae, Pinus palustris, Solenopsis invicta, conservation biology, ant community
Introduction
Community level studies of ants in longleaf pine (Pinus palustris) habitats have investigated subsets of the overall ant community (Tschinkel 1988; Prather 1997; Tschinkel and Hess 1999) or produced checklists of species found in a particular area (Van Pelt 1956; Deyrup and Trager 1986; Campbell 1996). Little work has been done to examine the entire ant community across numerous sites in any specific longleaf pine habitat. We therefore know little about the ant communities that, until recently, occurred naturally throughout a large part of the southeastern United States.
Learning more about longleaf pine ant communities is important not only to further our understanding of North American ants but also to guide current longleaf pine conservation efforts. Before Europeans arrived in North America longleaf pine covered over 30 million hectares and dominated the Atlantic and Gulf coastal plains. Today longleaf pine is found in less than 3% of its historical range (Frost, 1993) and what does remain forms some of North America's most threatened habitats (Noss 1989; Simberloff 1993; Collins 2001). While the floral diversity found in some longleaf pine habitats is among the highest known for any temperate zone plant communities (Peet and Allard 1993), studies of faunal communities in these habitats are rare (reviews in Hermann 1993; Stout and Marion 1993). Animal research has focused on a few threatened or economically important species such as Picoides borealis (red cockaded woodpecker, Walters 1991), Gopherus polyphemus (gopher tortoise, Aresco and Guyer 1999) and Colinus virginianus (bobwhite quail, Rosene 1969). Effective preservation of remnant longleaf pine forests demands more research to catalog resident species, to understand species associations, and to identify organisms that need special attention because of their rarity or endemism. Carefully planned community studies can efficiently fill some of these needs and add to our general knowledge of these habitats.
Studying the ant community can also help us understand an important component of longleaf pine ecosystems. Social insects often constitute more than half of all the insect biomass in many terrestrial habitats (Wilson 1990) and ants in particular are one of the most well represented groups (Hölldobler and Wilson 1990; Tobin 1995; Longino and Colwell 1997). A large body of literature documenting the various ecological roles ants fill in terrestrial ecosystems (Beattie 1985; Wilson 1990; Gotwald 1995) highlights the important direct and indirect effects ants can have on many organisms. Within longleaf pine habitats ants are likely to interact with many resident species and affect populations of a diverse collection of flora and fauna.
One important role filled by ants in a longleaf pine ecosystem has already been found. Hess and James (1998) discovered that the arboreal ant Crematogaster ashmeadi is the dominant food item in the diet of the adult endangered Red Cockaded Woodpeckers of the Apalachicola National Forest (ANF). This finding has stimulated research investigating C. ashmeadi's autecology (Hahn and Tschinkel 1997; Baldacci and Tschinkel 1999; Tschinkel 2002) and the arboreal ant community of the ANF's pine forests (Prather 1997; Hess and James 1998; Tschinkel and Hess 1999). The more diverse ant community found beneath the trees has not been studied.
Our research examined the complete ant community found in the ANF's pine flatwoods. We sampled ants, using four methods, in twelve sites. The aims of our work were to establish the species composition of the longleaf pine flatwoods ant community, to determine its dominant species, and to assess how ant diversity changes along a herbaceous ground cover gradient. We specifically focused on examining the ant community across this gradient because this habitat characteristic is an important focus of contemporary longleaf pine conservation.
Materials and Methods
Study Area/Study sites
The ANF lies in the Big Bend region of Florida and contains over 50,000 ha of longleaf pine forest. Pine flatwoods (Abrahamson and Hartnett 1992), our focal study habitat, develop on slightly undulating, sandy plains with a high water table, and are common here (Fig 1). Under natural conditions, these stands once contained widely spaced old growth longleaf pine trees (Platt et al. 1988) and a grass-dominated ground cover. Contemporary longleaf pine forests, in contrast, are almost exclusively second growth stands, are more densely stocked, and contain populations of younger, smaller trees (Noel et al. 1998). Anthropogenic changes to the natural fire regime have also decreased the dominance of herbaceous plant cover in many ANF longleaf pine stands (James et al. 1997, U.S. Department of Agriculture 1999a).
Figure 1.

Pine flatwoods of the Apalachicola National Forest. (A & B). Stands with a high percentage of herbaceous vegetation. (C & D). Stands with a woody plant dominated ground cover.
Current ANF management plans call for restoring and maintaining longleaf pine stands in a state that “falls within the natural variation of this habitat type” (U.S. Department of Agriculture 1999b). To this end, the Forest Service has decreased logging to increase the average tree age and size. Restoring the ground cover, and maintaining it in a more natural state, is being attempted through the use of prescribed fire. The natural fire regime once suppressed the growth and establishment of understory woody plants, stimulated herbaceous plant growth and reproduction, and did little harm to mature longleaf pine trees. The Forest Service is mimicking this process by trying to burn the ground vegetation, during the growing season, every three years (Ferguson 1998, U.S. Department of Agriculture 1999a). It is not known how this management will affect resident animal communities.
The twelve study sites we sampled from were chosen from a larger pool of 24 pine flatwood stands. Prior sampling, for an unrelated study (James et al. 1997), allowed us to classify these stands into low, medium, or high grassy cover and we randomly selected four sites from each of these classes. Seven of our study sites, and all four sites with high herbaceous cover, were located in the Apalachicola Ranger District. Of the two management districts in the ANF, the Apalachicola Ranger District historically experienced more cattle grazing and prescribed fires than the Wakulla Ranger District. Apalachicola Ranger District pine flatwoods also contain, in general, a higher percentage of wiregrass (Aristida stricta) in their ground cover. None of our high herbaceous study sites were located in the Wakulla Ranger District because we had no prior surveyed stands from this area in this class. Distances between our study sites ranged from 4 to 55 km.
Ant sampling
Pitfall trapping, ground baiting, subterranean baiting, and tree baiting were used to sample the ant community. These methods were used together in order to comprehensively sample ants above, below, and at the ground surface. At each site a 70 m × 80 m plot was established and subdivided into fifty-six 10m × 10m squares (Fig. 2). The corners of these squares were marked with flagging, forming a grid with 72 evenly spaced sample points (hereafter referred to as bait stations). These plots were sampled in June and July of 1998.
Figure 2.

Sampling grid design used to sample the ant community within each plot.
Pitfall trapping.
Pitfall traps were positioned in the center of each of the 56 squares within a sample plot and left for six days. The traps were made from plastic Solo© (www.solocup.com) drink cups (0.27 L volume, 8.8 cm diameter at cup lip), buried so that the lip of the cup was placed flush with the ground surface, and partially filled with an ethylene glycol and water mixture. A 22.8 cm styrofoam plate was suspended over each trap to prevent rain from falling directly into the cups. This protocol was developed from prior test sampling (Lubertazzi, unpublished data) where collection curves for a variety of pitfall trap sizes, number of traps, and trapping duration were compared to establish a design that would capture most of the ant diversity within a site. Ants captured in pitfall traps were sorted from the cups in the lab, identified to species, and their abundance recorded.
Ground baiting.
Ground foraging ants were sampled by placing baited tubes at each bait station within a plot. Baits consisted of 1 cm × 9 cm glass tubes containing a small portion of Spam©, a protein rich food source readily consumed by most omnivorous ants. Bait tubes were placed within 5 cm of a bait station with the lip of the tube touching the ground, and collected after ∼ 30 minutes. Ant species that were both readily identifiable and easily quantified were immediately recorded as occupying such baits, others were collected and brought to the lab to be counted and identified.
Maximum ant foraging diversity in the ANF's pine flatwoods was found from 23° C - 29.5° C but even at these temperatures, foraging activity may be curtailed by a recent soaking rain (Lubertazzi, unpublished data). Collecting conditions were therefore standardized by sampling each plot in the mid morning when the temperature was ∼ 28° C and only on sunny days when no heavy rain had fallen during the previous 12 hours.
Tree baiting.
Every tree > 2 m tall within a plot was baited using one cm diameter filter paper disks dipped in a blended cat food/water/oil mixture (2:1:0.25). These baits were placed on a tree, at each of the four cardinal directions, 2 m from the ground. After approximately 30 minutes the abundance of each ant species at each bait was recorded. This protocol was slightly modified from that of Tschinkel and Hess (1999).
Tree baiting was carried out in the late morning and early afternoon. The temperature at this time of day does not appear to influence arboreal ant activity (Tschinkel and Lubertazzi, unpublished data) but ground-nesting species that may forage on the trees could be less active during this warmest part of the day.
Subterranean baiting.
Subterranean ant species were sampled at each bait station using buried baits. This sampling was initiated at least one day after the ground baiting at each plot; buried baits were left for two days. Two-ml polypropylene cryogenic vials, with their sides perforated using a heated wire, were used as bait tubes (MacKay and Vinson 1989). These vials were filled halfway with Spam© and buried to a depth of 10 cm. A wire attached to each vial, and extending to the soil surface, was used to pull the bait tubes from the ground. Each vial was quickly placed in its own container once it was pulled from the ground, preventing ants from escaping. Ants were sorted, counted, and identified to species in the lab.
Environmental variables
Aristida stricta (wiregrass), Serenoa repens (saw palmetto), Ilex coriacea and I. glabra (gallberry), Quercus minima, and Q. pumila (runner oak) are common ground cover plants in ANF longleaf pine stands. Within any site one, or at most a few, of these species generally constitute most of the ground cover biomass. We sampled the ground vegetation within each plot by placing a 1-m2 quadrat at each bait station and visually estimating the percent cover of these common plants. The percent cover of other plant species, classed as other-herbaceous or other-woody vegetation, the percent exposed ground, and a ground litter score (sparse, light, medium or heavy) was also noted for each sample quadrat. To quantify the forest stand the diameter at breast height of all longleaf pines within each plot was measured and recorded.
Analysis
Ant species richness was compared among sites by using the total number of species observed within a site and by rarefying the pitfall trap data. Rarefaction can provide estimates, through interpolation, of the expected number of species found in a random sub-sample from a larger pool of samples (Simberloff 1979). Site species richness, as determined from pitfall data, could thereby be compared on an equivalent basis among sites without the problems associated with unequal sample sizes. We choose to not use baiting data in this analysis because the clumped occurrences of ants recruited to baits may bias rarefaction estimates (Simberloff 1979; Gotelli and Graves 1996; Collins 1998). The evenness of the ant community was compared among sites through the use of log abundance-rank plots (May 1975) and by using the rarefaction curves generated from the pitfall trap data.
The subterranean baiting, tree baiting, and pitfall trap data sets were examined with correspondence analysis to determine how each site's ant community was similar, or different, from other sites. Correspondence analysis is an exploratory data analysis method that can reveal such patterns through ordinating site-by-species contingency table data (Greenacre and Verba 1984; Zorrilla et al. 1986; James and McCulloch 1990). Each site is reduced to a plotted point that is positioned in an ordination graph according to its species composition and each species' abundance. Differences between sites are shown through the relative distance and direction between each site's ordinated position. In our analysis, points positioned close to one another represent sites having similar ant communities.
Ground baiting samples were used to identify the dominant ground-foraging species. In order to better categorize these data according to what we know about the biology of ant foraging behavior, and the ability of particular ant species to numerically and behaviorally dominate other ants, the number of workers of the particular species found at each bait sample were converted to an abundance score (1, 1 ant; 2, 2–5 ants; 3, 6–20 ants, 4, 21–50 ants; 5, 51–100 ants; and 6, >100 ants). A species with consistently low abundance scores (1–2) at baits is likely to forage singly and/or not recruit well to food finds. Intermediate scores (3–4) are indicative of species that can recruit to food but are not overwhelmingly numerically or behaviorally dominant. Species with consistently high scores (5–6) are potentially important competitors that could deny other ants access to resources such as food and nesting sites. Our categories make it easier to not only differentiate species along this continuum of increasing dominance but also make it simpler to differentiate species within these groupings – especially for those species that are dominant. The more a species dominates food finds, the greater the range and variability in the number of workers that may be found at a bait and the less useful raw worker numbers are for deriving useful comparative statistics.
We calculated the average abundance score for each species within a site by taking the sum of a species' abundance scores for that site divided by the number of baits it occupied. An ant species found with 3, 10, 149, and 242 workers (101 mean workers per bait and an average abundance score of 4) is easily shown to be less dominant than a species with 101 workers at each of four baits (average abundance score of 6). The proportional site abundance score, calculated as a species' average site abundance score multiplied by the proportion of all ant-occupied baits a species was found at within that site, was used to compare the dominance of species within a site. Greater relative dominance is indicated by a higher score and species that increasingly dominate a site (evidenced by increasingly disparate average abundance scores and by occupying more of the baits at a site) show a relatively larger difference between their score and scores of other species.
Results
Ant diversity
A total of 72 ant species (Appendix A), representing 25 genera and 5 subfamilies, were found among the nearly 110,000 ants sampled. Total site species richness, found by combining the species data for all the sampling methods for each site, ranged from 31 to 55 species (Fig. 3). The pitfall traps yielded 25 – 46 species per site (Fig. 3, Appendix B) with non-pitfall trap samples adding an additional 3 – 9 species per site. Species richness estimates of the pitfall trap data, rarefied to 1250 individuals (the lowest total ant abundance in pitfalls in any site was 1263 individual ants), gives a site species richness range of 23–39 species (Fig. 3). The rarefied pitfall trap species richness, total pitfall trap species richness, and total site species richness all show close agreement in how they order the sites from low to high species richness.
Figure 3.
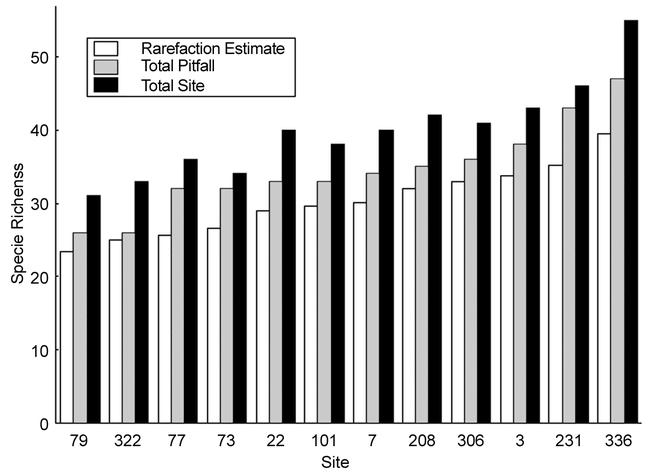
Species richness, by site, using three different richness values: rarefaction estimate of each site's pitfall trap samples standardized at 1250 individuals, uncorrected species richness for the pitfall trap data, and total site species richness combining all sampling methods. The sites are ordered along the abscissa from low to high species richness using the rarefaction estimate of species richness.
The ground foraging ant community sampled by the pitfall traps is most even in sites 231 and 336. The initial steep rise of their rarefaction curves shows these sites accumulate species at a faster rate, compared to all other sites, as additional ants are sampled (Fig. 4). This contrasts with the shallow rarefaction curves of sites 79, 322 and 73, plots that exhibited the lowest evenness. Log abundance-rank plots (Fig 5) showed similar results. The ant assemblages found in the subterranean and arboreal zones (see results below) are not even. Each of these areas is numerically dominated by a single ant species.
Figure 4.
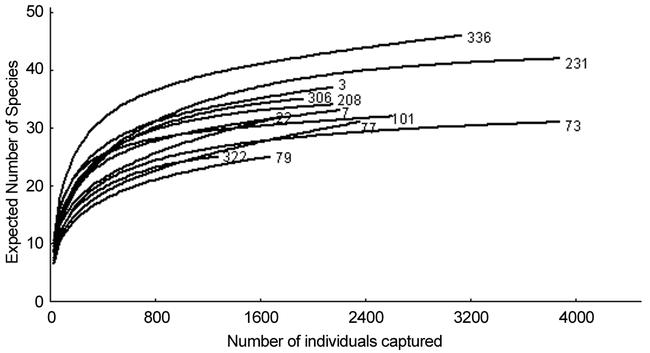
Rarefaction curves, by site, for ants captured in pitfall traps.
Figure 5.
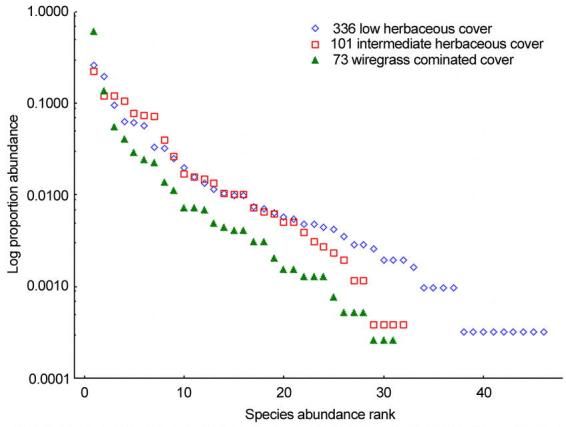
Log abundance-rank plot of pitfall trap data from three sites. Evenness increases in going from high herbaceous (site 73), intermediate herbaceous (101) to low herbaceous (336) ground cover.
Eight of the 72 ant species (11%) we found are not native to Florida (Deyrup, Davis, and Cover 2000). Solenopsis invicta (monogyne form), Cardiocondyla wroughtonii and Cyphomyrmex rimosus were found in many plots, Brachymyrmex musculus and Pheidole moerens were locally abundant in a few plots and Paratrechina vividula, Pyramica margaritae and Pyramica membranifera were very rarely found.
Environmental variation
Vegetation sampling.
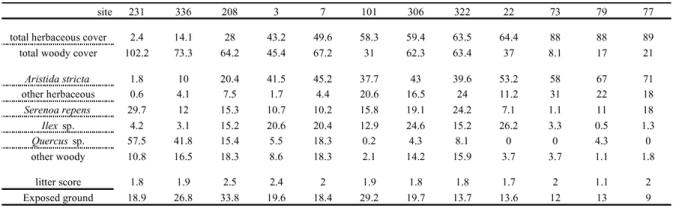
The sites varied greatly in the proportions of their dominant ground cover plants (Table 1). A. stricta was abundant in areas containing a high percentage of herbaceous cover and Quercus species were common in areas dominated by woody plants. Reducing the vegetation data to herbaceous and non-herbaceous classes (Table 1) shows that our study plots sampled the full range of the herbaceous ground cover gradient. Sites varied from 2.4% to 89% herbaceous cover.
Table 1.
Ground cover attributes. All numbers are percent cover except the litter scores. The estimate of each plant class coverage was not discounted if there was other vegetation above, below, or intertwined within plants as they were quantified. Therefore summing all the measured classes within a site may give a total coverage > 100%. Litter score is the average litter score of the 56 vegetation samples in a plot. Exposed ground is the average percent of ground not covered by ground cover vegetation. Sites are ordered by their percent herbaceous cover (the sum of Aristida stricta and other herbaceous plants).
Other stand attributes.
The number of trees found within each plot and the mean diameter at breast height (DBH) of the trees within these stands varied among sites (Table 2c). Site 79 had the fewest trees (53) and the largest average DBH (35.6 cm). The greatest number of trees was found in site 101 (282) and the lowest mean DBH was recorded in site 322 (19.1 cm). While all of these stands are considered mature forest, variation is found among sites in their tree demographics.
Table 2.
Summary of ant sampling data by site and method. Sites are arranged from left to right in order of increasing herbaceous cover.

Average ground-litter ranks (sparse = 1, light = 2, medium = 3, and heavy = 4) were also variable (Table 1). Nine sites had an average litter rank score between 1.6 and 2.0. Site 79 had the lowest litter score at 1.1 while the highest litter scores were 2.5 for site 208 and 2.4 for site 3. Percent exposed ground varied from 9% in site 77 to 33% in site 208 (Table 1).
Ant sampling
Pitfall traps.
Excluding gynes, 29,065 individual ants from 70 species were captured in 664 pitfall traps (Table 2a, Appendix B). Thirteen traps were lost during sampling. Ordination of the pitfall trap data (Fig. 6) shows five sites (3, 7, 101, 208, and 322) grouped in a loose cluster slightly above and to the left of the centroid (the 0, 0 coordinate). Five other sites were arranged progressively further to the right of this cluster by their increasingly higher dimension 1 values, and a few sites were found toward the lower left corner of the ordination. The patterning of sites along the second dimension forms an arc, indicating correspondence analysis is not deriving a second dimension solution that is orthogonal to the variation captured in the first dimension (ter Brakk 1996). This problem does not influence the quality of the first dimension result and the interpretation of these data will focus on the positioning of sites along this single dimension.
Figure 6.

Correspondence analysis ordination of sites for the pitfall trap data. Symbols indicate the percentage of herbaceous cover found at each site. The ant community shows consistent change, in moving from left to right along the first dimension, along the herbaceous ground cover gradient.
Coding sites by their percentage herbaceous ground cover (Fig. 6) shows ant community change, moving from left to right along the first dimension, parallels an increase in the percentage of herbaceous ground cover found within a site. Dimension 1 site scores show a strong positive correlation with percent herbaceous cover (Spearman r = 0.95, p<0.000). Other environmental variables, such as the amount of litter (Table 1), the number of longleaf pine trees in a plot (Table 2), and the mean diameter at breast height, show no association with the ground cover/ground-foraging ant community correlation. The amount of exposed ground (Table 1) did tend to decrease with increasing amounts of herbaceous cover but this trend was not significant.
Ant diversity also changes along with the ground vegetation. Species richness was negatively correlated with percent herbaceous ground cover (Spearman r, using rarefaction species estimates from the pitfall trap data = −0.88, p<0.0001, Fig. 7). Evenness decreased with decreases in species richness (Fig. 4 and 5) and is therefore also negatively correlated with increasing amounts of herbaceous ground cover.
Figure 7.
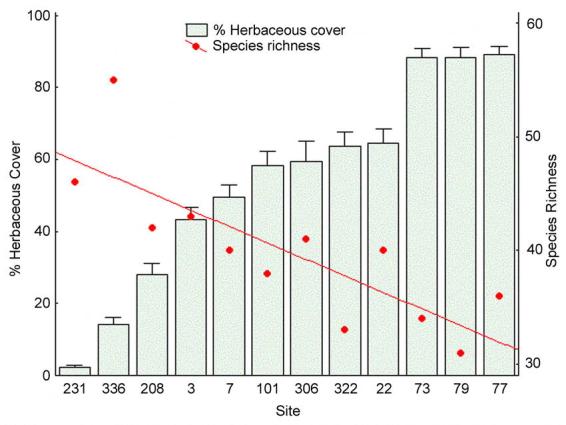
Percent herbaceous cover and rarefaction estimate of species richness by site. Herbaceous cover error bars are ± 1SE. The line shown is a best fit line of the species richness data and illustrates the negative correlation of species number and percent herbaceous ground cover.
One way to determine which ant species are important in positioning sites along the ordination axis is to examine how each species' site abundance is correlated with its dimension scores. Aphaenogaster flemingi, C. rimosus, Hypoponera opaciceps, and S. invicta are positively correlated with increasing dimension 1 scores and therefore show an increase in abundance with an increasing amount of herbaceous ground cover. Camponotus floridanus abdominalis, Crematogaster cerasi, Formica archboldi, Leptothorax pergandei, Paratrechina parvula, Pheidole dentata, Pheidole morrisi, Pogonomyrmex badius, Solenopsis carolinensis, and Trachymyrmex septentrionalis are all negatively correlated with dimension 1 values. These latter species tend to decrease as the amount of herbaceous vegetation increases.
Ground Baiting.
Thirty ant species were found among the approximately 34,000 individual ants recorded foraging on 587 baits. The per-site percentage of ant-occupied baits ranged from slightly less than half to close to 90% (Table 2b, Appendix C).
S. invicta, P. dentata, and P. morrisi were the only species that were both abundant at baits and present at more than a few baits within a site (Table 3). S. invicta had a high proportional abundance in 5 sites, obtained the highest proportional abundance scores of any species, and overwhelmingly dominated baits within sites 73 and 79. P. morrisi had a high proportional abundance in site 231 and P. dentata obtained a moderately high proportional abundance in 4 sites. The latter species also shared a high proportional abundance score with S. invicta in site 306 and with P. morrisi in site 231.
Table 3.
Abundant ground-foraging ant species. Species shown are those found at many baits or with a high average abundance score within a site. Sites are ordered from low to high herbaceous cover. The top two values in a cell show the total number of baits a species occupied within a site (out of a possible 56) and the average abundance score at those baits. The bottom value in a cell is the proportional abundance score for a species within a site. The derivations of these values are explained in the methods section. The proportional abundances were all multiplied by 100 to eliminate decimal places. Looking at the scores across a row shows how a species' occurrence at baits changes as the amount of herbaceous cover increases. Examining each column reveals the relative dominance of each species within a site.

A number of species with low proportional abundance scores (Table 3) were found at many baits (Odontomachus brunneus) or were abundant at the few baits on which they were found (C. cerasi, Crematogaster lineolata, Monomorium viride, P. moerens, P. parvula, and S. carolinensis).
Tree Baiting.
Close to 1,800 pines were sampled and approximately 39,000 ants from 13 species were found (Table 2c, Appendix D). Eight of these 13 species were rarely sampled on the trees and were more abundant in samples from the ground surface. The true arboreal dwelling species were not commonly encountered using sampling methods other than tree baits. Ants living in the longleaf pines are segregated from those living at and below the ground surface.
The arboreal community is a low diversity assemblage dominated by C. ashmeadi. This species was overwhelmingly more abundant than any other arboreal ant (Table 2c, Appendix D), occupying close to half of all the trees baited and making up 80% of the total number of ants sampled on longleaf pines. Other species consistently found on longleaf pine trees include Camponotus nearticus, Leptothorax smithi, Solenopsis picta, and Forelius pruinosus.
Correspondence analysis reveals the arboreal community is consistent among most sites. The ordination shows one main cluster of sites (Fig. 8) and two sites (231 and 73) with seemingly divergent arboreal ant communities. M. viride was found on five trees scattered within plot 231 and three of these trees yielded only a single M. viride forager. Ignoring this aberrant species occurrence, the arboreal ant community in this site is similar to most others. Site 73 had a high abundance of S. invicta and S. picta and was the only site containing B. musculus, B. obscurior and P. moerens. Three of these five species are exotics. This arboreal ant assemblage was clearly different from that found in other plots.
Figure 8.
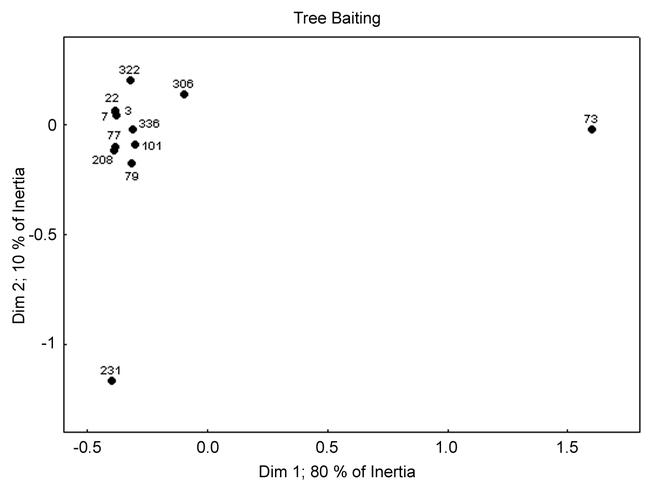
Correspondence analysis ordination of sites for the tree baiting data.
Subterranean Baiting.
Approximately 7,700 individual ants were collected from subterranean baits (Table 2d, Appendix E). Twenty ant species were found among the 485 ant-occupied bait tubes. Ants occupied 56 % of the 864 baits deployed. The remaining tubes either still contained Spam and were presumably not found by foraging ants, or the tubes no longer contained any food. As with the arboreal ant species, many of the subterranean species were not commonly encountered using other sampling methods. The subterranean ant community is segregated from other portions of the pine flatwoods ant community. S. carolinensis was one exception in being found on a number of ground baits.
The subterranean ant community is primarily composed of Solenopsis species from the subgenus Diplorhoptrum. S. carolinensis was numerically dominant and S. carolinensis, S. abdita, S. tonsa, and S. tennesseensis were commonly found in many sites. Two other Diplorhoptrum, S. nickersoni and S. pergandi, were also present but were less commonly encountered. The only other species frequently found foraging on underground baits was Brachymyrmex depilis.
Correspondence analysis ordination of these data (Fig. 9) places sites 231, 79, and 73 away from a cluster of similar sites. Samples from site 73 yielded only one S. carolinensis and this was the only site containing P. moerens. Site 79 also had relatively few S. carolinensis but also contained an unusually high number of S. invicta. While site 231 contains an abundance of S. carolinensis, its ordination position is affected by the highest single site abundance of S. nickersoni and S. pergandi.
Figure 9.
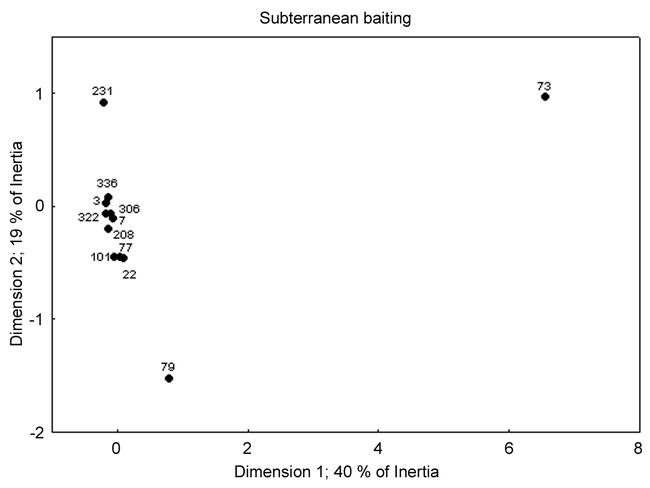
Correspondence analysis ordination of sites for the subterranean baiting data.
Discussion
The ground-foraging ant community
In moving from a predominately shrubby ground cover to one dominated by wiregrass, the ground foraging ant community in pine flatwoods becomes less diverse and its species composition changes. Ant community change has been correlated with vegetation change in other studies (Greenslade 1975; Majer 1985; Andersen 1997; Morrison 1998; Bestelmeyer and Wiens 1996) and species turnover tends to increase with increasing habitat divergence. Examining the pine flatwoods' ant community across such a breadth of percent herbaceous cover as we did, the changes we found are not unusual, nor entirely unexpected. Both competitive interactions and environmental conditions within the ant community change across the ground cover gradient and these factors may be important in causing some of the changes we observed.
Competition is known to be important in structuring ant communities (Vepsäläinen and Pisarski 1982; Hölldobler and Wilson 1990). Ants that are abundant, that can monopolize food finds, and that prevent other ant colonies from nesting in their vicinity can affect the abundance and distribution of ants found within their resident communities. In high woody and mixed herbaceous-woody ground cover sites, P. dentata showed the greatest numerical dominance at ground baits (Table 3, Appendix C). P. dentata is known to quickly and aggressively respond to non-nestmate conspecifics and other ant species that it commonly encounters (Carlin and Johnston 1984; Wilson 1975, 1976; Jones and Phillips 1987), illustrating that it is an important behaviorally dominant species. P. morrisi, a species closely related to P. dentata, also dominated numerous baits in a few high woody cover sites (Table 3, Appendix C) but this ant does not appear to be as strong a competitor as P. dentata (Feener 1987). Neither of these Pheidole species were found at a majority of the ground baits within any one site and therefore, presuming their appearance at baits is indicative of their nest density and the competitive reach of these two species, their ability to eliminate other co-competitors from the areas they occur in is limited.
In areas with an abundance of wiregrass, S. invicta is the dominant ground-foraging ant species (Table 3, Appendix C). This ant defends an absolute territory (Tschinkel et al. 1995), the polygyne form is known to displace other ant species (Porter and Savignano 1990), and it has negative effects on native animals in its introduced range (Tschinkel 1993a; Vinson 1997; Mueller et al. 1999; Allen et al. 2001; Wojcik et al. 2001). This species also has a mature colony size (Tschinkel 1993b) that is an order of magnitude larger (hundreds of thousands versus a maximum of four thousand workers) than P. dentata (Calabi and Traniello 1989) and P. morrisi (Johnson 1988). In two sites (73 and 79) the fire ant was found at over 75% of the ground bait samples. S. invicta is clearly the strongest ant competitor found on the ground surface in the flatwoods and it is likely to have a negative influence on some co-occurring ant species.
Several environmental conditions, including soil moisture, depth to water table and temperature at the ground surface, are likely to vary in parallel with the ground vegetation in pine flatwoods, both as cause and/or as effect. Even seemingly minor changes in these conditions can be important to individual ants and these differences may explain some of the community change we observed (Andersen 1995). Small insects are disproportionately subject to desiccation and therefore are highly sensitive to ambient moisture and temperature differences. Simple ground-shading and plant transpiration changes found across the range of ground cover sampled could create a corresponding gradient of temperature/moisture microclimate conditions at the ground surface. We found that areas dominated by woody plant cover, for example, tend to have more exposed ground. Both sunlight and air movement increases at the ground surface in such areas, creating hotter and dryer conditions on clear, sunny days. Sites dominated by wiregrass, on the other hand, have a thick layer of intertwined grass blades covering most of the ground surface. Such vegetation can protect ants foraging on the ground surface from the more extreme conditions found just above the grass layer. Ant foraging is known to occur within species-typical temperature limits (Whitford and Ettershank 1975; Bernstein 1979; Porter and Tschinkel, 1987) and hence microclimate variation could limit the occurrence of certain species to a portion of the ground cover gradient.
Colony level life history traits are also influenced by, and can depend upon, moisture and temperature conditions. Some ground-nesting ants may be limited to sites with sufficient depth to the water table, making large areas of the flatwoods, with its shallow water table, unusable to them. This may be why such deep-nested species such as P. badius, Prenolepis imparis and S. geminata are essentially absent from the flatwoods. Most ants also require an adequate range of temperature for rearing their brood. It has been shown, for example, that brood development in S. invicta occurs only between 22 and 35° C (Porter 1988). Experimental study of environmental variables across the ground cover gradient, and examining how these factors influence particular ant species' distributions, should prove fruitful in explaining some of the difference we observed in the ground-foraging community.
Arboreal and subterranean ant communities
The arboreal and subterranean ants form two distinctive communities that overlap little with the ground-foraging community or each other, nor are they influenced by changes in ground cover, pine stand characteristics or the ground foraging ant community. Some ground foraging species do venture into these other two zones but their presence is rare and sporadic.
Living in trees or underground requires special adaptations not present in “ordinary,” more generalized ground-foraging ants, and probably limit the number of species that live exclusively above and below the ground. Arboreal ants, for example, are on average twice as resistant to desiccation as ground-foraging (even desert) species (Hood and Tschinkel 1990), perhaps because trees do not offer refuge from persistently dry conditions, as soil does. Greater air movement, higher temperature, and increased exposure on trees may exacerbate water loss. Underground, the threat of desiccation is lower but foraging for food can be energetically expensive. The friable, sandy soils we found in most of our plots means constructing and maintaining an anastomosing, ever changing series of tunnels is a constant challenge. The ants foraging on underground baits were small bodied and relatively short-legged, and thus able to move freely within small interstitial soil spaces and soil passages (Kaspari and Weiser 1999) rather than having to build and maintain large passageways. Larger-bodied ants must construct tunnels to forage underground and are unlikely to find enough food to offset the costs incurred by this constant digging. Subterranean army ants of the genus Nievamyrmex do appear to be an exception to this rule. We rarely encountered these ants in our samples but 3 species are resident in the ANF.
The arboreal ant community we found is essentially the same as reported by Tschinkel and Hess (1999). They surveyed a number of pine habitats within the ANF, found little change in community composition among larger longleaf pine trees, and reported C. ashmeadi as the dominant species. The arboreal ant assemblage in mature longleaf pine stands appears to be remarkably consistent within and across north Florida pine habitats.
The subterranean foraging ant assemblage is qualitatively similar, in being primarily composed of ants from the tribe Diplorhoptrum, to that found in a Gainesville longleaf pine woodland and an adjacent open field (Thompson 1980). S. carolinensis was also numerically dominant in both of these sites. Unfortunately Thompson reported limited quantitative data and her study only included a single woodland and open field site. Nevertheless, this limited evidence suggests the flatwoods subterranean ant community may be consistent across a wide geographic area.
Hölldobler and Wilson's (1990) dominance impoverishment rule states that low diversity ant communities are often numerically and behaviorally dominated by a single species. The subterranean and arboreal communities we found conform to this rule. Both consist of a small number of species and each is dominated behaviorally and numerically by a single species, the arboreal community by C. ashmeadi (Table 2c) and the subterranean community by S. carolinensis (Table 2d). The more diverse ground foraging ant community showed greater variation in the relative abundance of behaviorally and numerically dominant species both among and between sites, at least for the limited baiting we conducted.
Site 73 had the highest within-site abundance above and below the ground of Solenopsis invicta, and was the only site that differed significantly in its arboreal and subterranean ant composition. This flatwoods stand was abiotically aberrant in having a hardened clay layer at least 10 cm thick at the ground surface. The topsoil horizon was hard enough to cause considerable difficulty in digging holes for the subterranean baits. This site was also the only plot within an active cattle-grazing allotment although cows were not observed during our sampling. It is unclear what the interrelationships are among the surface hardpan, cattle grazing, the high abundance of S. invicta and the low ant diversity found within this site.
Overall ant diversity
The diversity of the pine flatwoods ant community is remarkable for a Nearctic ant community. The high plot species richness (55 species) and high habitat species richness (72 species) we observed are the highest ever reported for a North American ant community. At a regional scale, this species richness is not as surprising. Florida contains more ant species (218) than many other U.S. states (Mark Deyrup 2003). Faunal surveys from a number of Florida locations have revealed high beta species richness (Van Pelt 1958; Deyrup and Trager 1986; Deyrup et al. 1988; Ferster and Prusak 1994), and new species are still being found (Deyrup 1991; Deyrup and Davis 1998; Deyrup and Lubertazzi 2001).
Florida's high species richness can be partially explained by two factors, biogeography and the presence of many introduced species (Deyrup and Trager 1986, Deyrup 2003). From a biogeographical perspective, elements of ant faunas from the tropical West Indies, temperate North America, and even genera associated with southwestern North American deserts can be found within the state (Deyrup and Trager 1986). Florida also harbors many introduced organisms (Simberloff 1997) including more than 50 exotic ant species (Deyrup, Davis, and Cover 2000). Thorough sampling of other Florida habitats may reveal high ant species richness similar to that of the flatwoods.
The total species richness we found within this habitat may be greater than what was once found in pristine longleaf pine flatwoods. Eight exotic species are present and a number of other species were only found in sites with a ground cover dominated by woody plants. If long term prescribed burning does eventually restore a wiregrass-dominated ground cover to all of our study sites, it is unlikely the within-habitat species richness would remain as high as we observed.
Implications for longleaf pine conservation
Longleaf pine conservation management appears unlikely to produce any change in the arboreal or subterranean ant assemblages. Increasing the age, size, or number of trees may increase the volume of arboreal habitat but these trees will still be inhabited by arboreal ants. Similarly, the subterranean environment does not contain enough differences across our sites that suggest non-subterranean ant specialists are likely to invade this zone.
The most interesting ant community change we observed was associated with the groundcover vegetation; ant diversity at the ground surface decreased as the percentage of herbaceous ground cover increases. As the continued use of prescribed fire increases the proportion of herbaceous ground cover within the pine flatwoods, presumably ant diversity will decrease in parallel. This will not necessarily be a negative influence of conservation management on ant diversity. It may be that wiregrass dominated vegetation naturally supports fewer ant species than are found in sites with woody plant cover. It is also unlikely that the long-term application of prescribed fires will shift every flatwoods stand to a wiregrass-dominated ground flora. Other factors, such as the depth of the water table, are likely to be just as important in determining the nature of the ground vegetation, and the ants found there, as fire. Provided ANF management plans continue to value sound conservation management practices it is likely this forest will continue to provide longleaf pine stands with a range of ground covers.
More worrisome is the fact that S. invicta was found to be abundant in sites that contained an abundance of wiregrass. It seems evident that the herbaceous ground cover in some areas, under the current prescribed fire regime, will be increasingly dominated by wiregrass. It is possible the imported fire ant is displacing native ants from these areas and that its negative effects will increase in these stands. Manipulative experiments excluding this invasive ant are clearly needed to examine these questions.
S. invicta may represent an unquantified negative cost of floral restoration efforts in these forests. With its no-stone-unturned foraging strategy this ant is likely to have direct negative effects on seed dispersal (Zettler et al. 2001; Christian 2001) and many resident invertebrate species (Forys et al. 2001a; Forys et al. 2001b). The diverse collection of native invertebrates (Hanula and Franzreb 1998; Hermann et al. 1998; Landau and Prowell 1999) are also likely to fill specialized ecological roles that help maintain the high floral diversity of longleaf pine habitats. If the fire ant reduces native invertebrate populations this may indirectly harm the flora. Pollination and seed dispersal, for example, are important species interactions that could be affected by abundant fire ant populations.
It would be useful to better understand what factors are allowing the fire ant to thrive in high herbaceous longleaf pine sites. This ant has not previously been associated with natural forest habitats (Tschinkel 1988). S. invicta may be responding to favorable resource levels, favorable conditions, or relaxed predation pressure from Diplorhoptrum (Buren 1983), or other, ant species in high herbaceous sites. Further study is needed to understand how prevalent this ant is in other sites and to track its abundance through time in areas where prescribed fires are increasing the percentage of herbaceous ground cover.
Acknowledgments
Keith Mason, Eric Walters, Fran James, Thomas Miller, Alice Winn, Don Levitan, Virge Kask, Debbie Cassill, Scott Powell, John Bouma, Jenee Redman, Kevin Haight, Chuck Hess and Andy Davis all provided invaluable aid and/or advice with various aspects of this work. Without Mark Deyrup's myrmecological expertise and kind help, identifying all the ants collected during this project would not have been possible. The managers of the Apalachicola National Forest were also gracious in helping us carry out this research within their forest. This work was financially supported by a Florida State University College of Arts and Sciences Teaching Fellowship and a Florida State University Fellowship to David Lubertazzi and by National Science Foundation Grant IBN 9317853 to Walter R. Tschinkel.
Appendix A.
Ant species found at each site using all sampling methods.
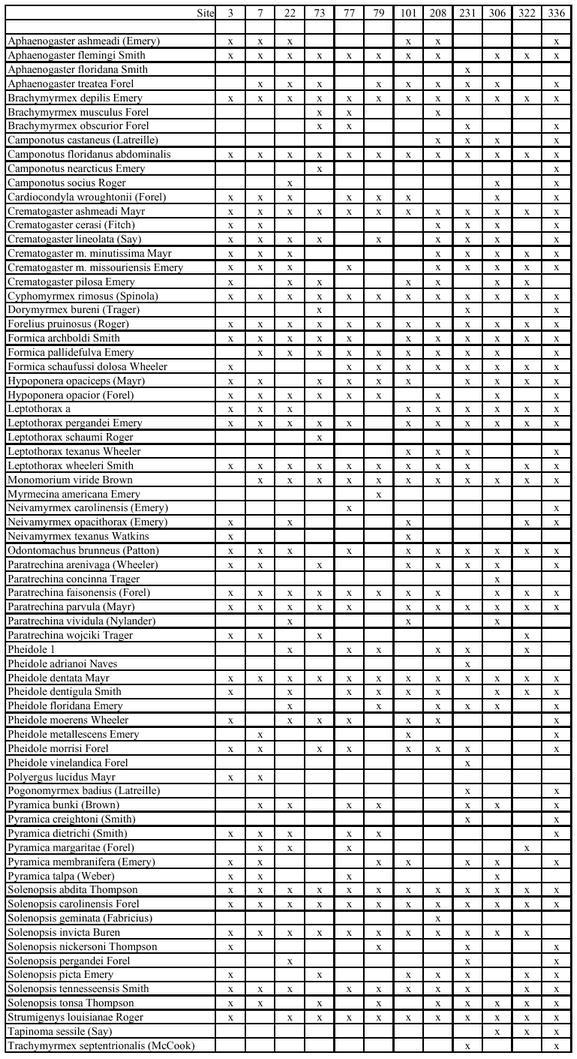
Appendix B.
Pitfall trap species-by-site contingency table.
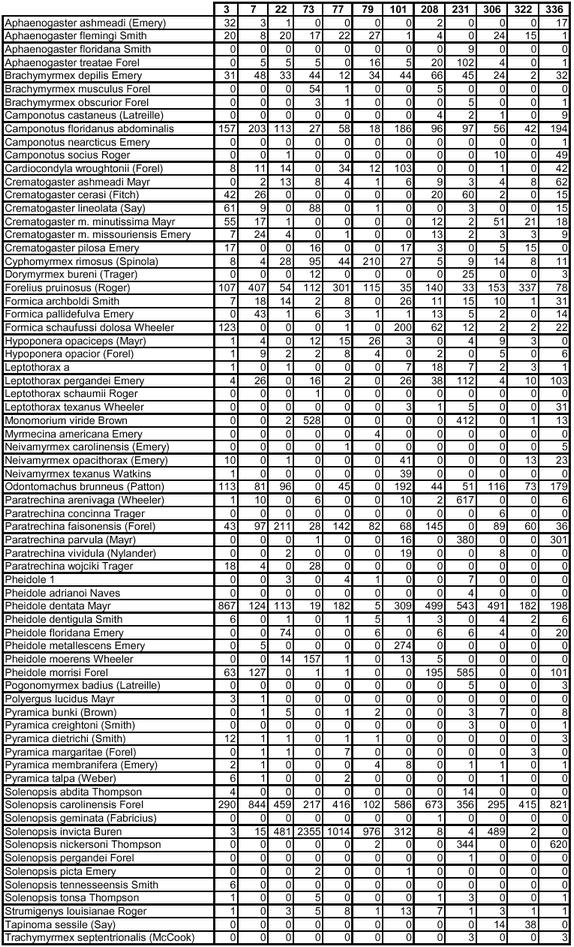
Appendix C.
Ground bait species-by-site contingency table.
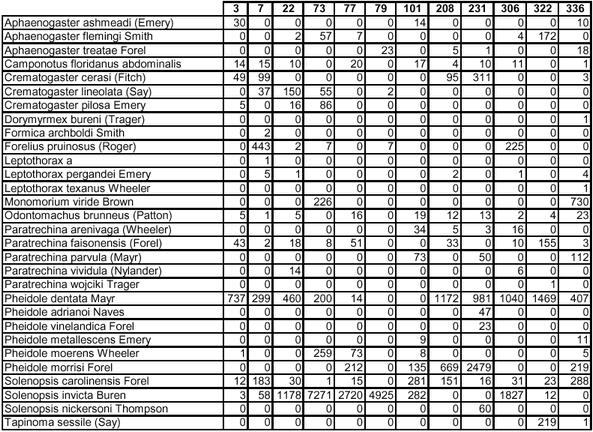
Appendix D.
Tree bait species-by-site contingency table.

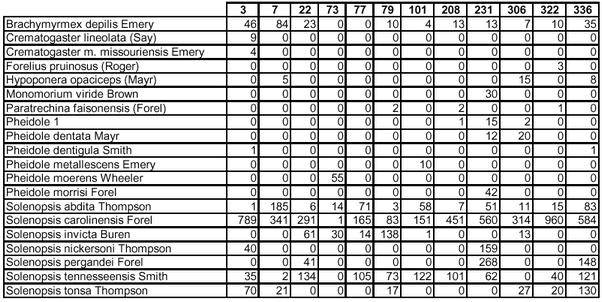
Appendix E.
Subterranean bait species-by-site contingency table.
References
- Abrahamson WG, Hartnett DC. 1992 Pine flatwoods and dry prairies. Pages. 103–149.In: Myers RL, Ewel JJ, editors. Ecosystems of Florida. Orlando: University of Central Florida Press. [Google Scholar]
- Allen CR, Forys EA, Rice KG, Wojcik DP. Effects of fire ants (Hymenoptera: Formicidae) on hatching turtles and prevalence of fire ants on sea turtle nesting beaches in Florida. Florida Entomologist. 2001;84:250–253. [Google Scholar]
- Andersen AN. A classification of Australian ant communities, based on functional groups which parallel plant life-forms in relation to stress and disturbance. Journal of Biogeography. 1995;22:15–29. [Google Scholar]
- Andersen AN. Functional groups and patterns of organization in North American ant communities: a comparison with Australia. Journal of Biogeography. 1997;24:433–460. [Google Scholar]
- Aresco MJ, Guyer C. Burrow abandonment by gopher tortoises in slash pine plantations of the Conecuh National Forest. Journal of Wildlife Management. 1999;63:26–35. [Google Scholar]
- Baldacci J, Tschinkel WR. An experimental study of colony-founding in pine saplings by queens of the arboreal ant, Crematogaster ashmeadi. Insectes Sociaux. 1999;46:41–44. [Google Scholar]
- Beattie AJ. 1985 The evolutionary ecology of ant-plant mutualisms. Cambridge University Press. [Google Scholar]
- Bernstein RA. Schedules of foraging activity in species of ants. Journal of Animal Ecology. 1979b;48:921–942. [Google Scholar]
- Bestelmeyer BT, Wiens JA. The effects of land use on the structure of ground-foraging ant communities in the Argentine Chaco. Ecological Monographs. 1996;6:1225–1240. [Google Scholar]
- Buren WF. Artificial faunal replacement for imported fire ant control. Florida Entomologist. 1983;66:93–100. [Google Scholar]
- Calabi P, Traniello JAF. Social organization in the ant Pheidole dentata - physical and temporal caste ratios lack ecological correlates. Behavioral Ecology and Sociobiology. 1989;24:69–78. [Google Scholar]
- Campbell SM. 1996 Two scavenger ant (Hymenoptera: Formicidae) assemblages of xeric, upland longleaf pine (Pinus palustris) stands in the sandhill region of North Carolina. Masters thesis. North Carolina State University, Raleigh. [Google Scholar]
- Carlin NF, Johnston AB. Learned enemy specification in the defense recruitment system of an ant. Naturwissenschaften. 1984;71:156–157. [Google Scholar]
- Christian CE. Consequences of a biological invasion reveal the importance of mutualism for plant communities. Nature. 2001;413:635–639. doi: 10.1038/35098093. [DOI] [PubMed] [Google Scholar]
- Collins MD. 1998 The effect of spatial autocorrelation on rarefaction. Masters thesis. Florida State University, Tallahassee. [Google Scholar]
- Collins B, White PS, Imm DW. Introduction to ecology and management of rare plants of the southeast. Natural Areas Journal. 2001;21:4–11. [Google Scholar]
- Deyrup MA. Technomyrmex albipes, a new exotic ant in Florida (Hymenoptera: Formicidae) Florida Entomologist. 1991;74:147–148. [Google Scholar]
- Deyrup MA. An updated list of Florida ants (Hymenoptera : Formicidae) Florida Entomologist. 2003;86:43–48. [Google Scholar]
- Deyrup MA, Trager J. Ants of the Archbold Biological Station, Highlands County, Florida (Hymnenoptera:Formicidae) Florida Entomologist. 1986;69:206–229. [Google Scholar]
- Deyrup MA, Carlin N, Trager JC, Umphrey G. A review of the ants of the Florida Keys. Florida Entomologist. 1988;71:163–176. [Google Scholar]
- Deyrup MA, Johnson C, Wheeler GC, Wheeler J. A preliminary list of the ants of Florida. Florida Entomologist. 1989;71:91–101. [Google Scholar]
- Deyrup MA, Davis L. A new species of Aphaenogaster (Hymenoptera: Formicidae) from upland habitats in Florida. Entomological News. 1998;109:88–94. [Google Scholar]
- Deyrup MA, Davis L, Cover S. Exotic ants in Florida. Transactions of the American Entomological Society. 2000;126:293–326. [Google Scholar]
- Deyrup MA, Lubertazzi D. A new species of ant (Hymenoptera: Formicidae) from north Florida. Entomological News. 2001;112:15–21. [Google Scholar]
- Feener DH Jr. Response of Pheidole morrisi to two species of enemy ants, and a general model of defense behavior in Pheidole (Hymenoptera: Formicidae) Journal of the Kansas Entomological Society. 1987;60:569–575. [Google Scholar]
- Ferguson JP. 1998 Prescribed fire on the Apalachicola Ranger District: the shift from dormant season to growing season and effects on wildfire suppression. Pages. 120–126.In: Pruden TL, Brennan LA, editors. Fire in ecosystem management: shifting the paradigm from suppression to prescription. Proceedings of the 20th Tall Timbers Fire Ecology Conference. Tall Timbers Research Station, Tallahassee, Florida, USA. [Google Scholar]
- Ferster B, Prusak Z. A preliminary checklist of the ants (Hymenoptera: Formicidae) of Everglades national park. Florida Entomologist. 1994;77:508–512. [Google Scholar]
- Forys EA, Quistorff A, Allen CR. Potential fire ant (Hymenoptera: Formicidae) impact on the endangered Schaus Swallowtail (Lepidoptera: Papilionidae) Florida Entomologist. 2001a;84:254–258. [Google Scholar]
- Forys EA, Quistorff AC.R, Allen CR, Wojcik DP. The likely cause of extinction of the tree snail Orthalicus reses reses (Say) Journal of Molluscan Studies. 2001b;67:369–376. [Google Scholar]
- Frost CR. 1993 Four centuries of changing landscape pattern in the longleaf pine ecosystem. In: Hermann SM, editor. The longleaf pine ecosystem: ecology, restoration, and management. pp. 17–43.Proceedings of the 18th Tall Timbers Fire Ecology Conference. Tall Timbers Research Station, Tallahassee, Florida, USA. [Google Scholar]
- Gotelli NJ, Graves GR. 1996 Null Models in Ecology. Smithsonian Institution Press, Washington. [Google Scholar]
- Gotwald WH Jr. 1995 Army ants: the biology of social predation. Cornell University Press, Ithaca, New York. [Google Scholar]
- Greenacre MJ, Verba ES. Graphical display and interpretation of antelope census data in African wildlife areas, using correspondence analysis. Ecology. 1984;65:984–997. [Google Scholar]
- Greenslade PJM. 1975 Survey of the ant fauna of Kunoth Paddock, Hamilton Downs Station, NT. CSIRO Technical Memorandum 2/1975, CSIRO Division of Soils, Canberra. [Google Scholar]
- Hahn DA, Tschinkel W. Settlement and distribution of colony-founding queens of the arboreal ant, Crematogaster ashmeadi, in a longleaf pine forest. Insectes Sociaux. 1997;44:323–336. [Google Scholar]
- Hanula JL, Franzreb K. Source distribution and abundance of macroarthropods on the bark of longleaf pine: Potential prey of the red-cockaded woodpecker. Forest Ecology and Management. 1998;102:89–102. [Google Scholar]
- Hermann SM. 1993 The longleaf pine ecosystem: ecology, restoration, and management. Proceedings of the 18th Tall Timbers Fire Ecology Conference. Tall Timbers Research Station, Tallahassee, Florida, USA. [Google Scholar]
- Hermann SM, Hook TV, Flowers RW, Brennan LA, Glitzenstein JS, Streng DR, Walker JL, Myers RL. Fire and biodiversity: studies of vegetation and arthropods. Transactions of the North American Wildlife and Natural Resources Conference. 1998;63:384–401. [Google Scholar]
- Hess CA, James FC. Diet of the red-cockaded woodpecker in the Apalachicola National Forest. Journal of Wildlife Management. 1998;62:509–517. [Google Scholar]
- Hölldobler B, Wilson EO. 1990 The Ants. The Belknap Press of Harvard University of Massachusetts, Cambridge, MA. [Google Scholar]
- Hood WG, Tschinkel WR. Desiccation resistance in arboreal and terrestrial ants. Physiological Entomology. 1990;15:23–35. [Google Scholar]
- James FC, Hess CA, Kufrin D. Species-centered environmental analysis: indirect effects of fire history on red-cockaded woodpeckers. Ecological Applications. 1997;7:118–129. [Google Scholar]
- James FC, McCulloch CE. Multivariate analysis in ecology and systematics: panacea or Pandora's box? Annual Review of Ecology and Systematics. 1990;21:129–166. [Google Scholar]
- Johnson C. 1988 Colony structure and behavioral observations in Pheidole morrisi (Hymenoptera: Formicidae). Pages. 371–383.In: Trager JC, editor. Advances in myrmecology. E.J. Brill, New York. [Google Scholar]
- Jones SR, Phillips SA Jr. Aggressive and defensive propensities of Solenopsis invicta (Hymenoptera: Formicidae) and three indigenous ant species in Texas. The Texas Journal of Science. 1987;39:107–115. [Google Scholar]
- Kaspari M, Weiser MD. The size-grain hypothesis and interspecific scaling in ants. Functional Ecology. 1999;13:530–538. [Google Scholar]
- Landau D, Prowell D. 1999 A partial checklist of moths from longleaf pine savannas in Louisiana (Insecta: Lepidoptera). Transactions of the American Entomological Society. Philadelphia. 125:127–138. [Google Scholar]
- Longino JT, Colwell RK. Biodiversity assessment using a structured inventory: capturing the ant fauna of a tropical rain forest. Ecological Applications. 1997;7:1263–1277. [Google Scholar]
- MacKay WP, Vinson SB. A versatile bait trap for sampling ant populations. Notes from the Underground. 1989;3:14. [Google Scholar]
- Majer JD. Recolonization by ants of rehabilitated mineral sand mines on North Stradbroke Island, Queensland, with particular reference to seed removal. Australian Journal of Ecology. 1985;10:31–48. [Google Scholar]
- May RM. 1975 Patterns of species abundance and diversity. In: Cody ML, Diamond JM, editors. Ecology and Evolution of Communities. pp. 81–120.The Belknap Press of Harvard University Press, Cambridge, Mass. [Google Scholar]
- Morrison LW. The spatiotemporal dynamics of insular ant metapopulations. Ecology. 1998;79:1135–1146. [Google Scholar]
- Mueller JM, Dabbert CB, Demarais S, Forbes AR. Northern bobwhite chick mortality caused by red imported fire ants. Journal of Wildlife Management. 1999;63:1291–1298. [Google Scholar]
- Noel JM, Platt WJ, Moser EB. Structural characteristics of old- and second-growth stands of longleaf pine (Pinus palustris) in the gulf coastal region of the U. S.A. Conservation Biology. 1998;12:533–548. [Google Scholar]
- Noss RF. Longleaf pine and wiregrass: keystone components of an endangered ecosystem. Natural Areas Journal. 1989;9:211–213. [Google Scholar]
- Peet RK, Allard DJ. 1993 Longleaf pine vegetation of the southern Atlantic and eastern Gulf Coast regions: a preliminary classification. In: Hermann SM, editor. The longleaf pine ecosystem: ecology, restoration, and management. pp. 45–67.Proceedings of the 18th Tall Timbers Fire Ecology Conference. Tall Timbers Research Station, Tallahassee, Florida, USA. [Google Scholar]
- Platt WJ, Evans GW, Rathburn SL. The population dynamics of a long-lived conifer (Pinus Palustris) The American Naturalist. 1988;131:491–525. [Google Scholar]
- Porter SD. Impact of temperature on colony growth and developmental rates of the ant, Solenopsis invicta. Journal of Insect Physiology. 1988;34:1127–1133. [Google Scholar]
- Porter SD, Tschinkel WR. Foraging in Solenopsis invicta (Hymenoptera: Formicidae): effects of weather and season. Environmental Entomology. 1987;16:802–808. [Google Scholar]
- Porter SD, Savignano DA. Invasion of polygyne fire ants decimates native ants and disrupts arthropod community. Ecology. 1990;71:2095–2106. [Google Scholar]
- Prather WC. 1997 Community structure of the arboreal ants in the longleaf pines of the Apalachicola National Forest. Masters thesis. Florida State University, Tallahassee. [Google Scholar]
- Rosene W. 1969 The bobwhite quail: its life and management. Rutgers University Press, New Brunswick, NJ. [Google Scholar]
- Simberloff D. 1979 Rarefaction as a distribution-free method of expressing and estimating diversity. In: Grassle JF, Patil GP, Smith W, Taillie C, editors. Ecological Diversity in Theory and Practice. pp. 159–176.International Publishing House, Fairland. [Google Scholar]
- Simberloff D. 1993 Species-area and fragmentation effects on old-growth forests: prospects for longleaf pine communities. In:. Hermann SM, editor. The longleaf pine ecosystem: ecology, restoration, and management. pp. 1–13.Proceedings of the 18th Tall Timbers Fire Ecology Conference. Tall Timbers Research Station, Tallahassee, Florida, USA. [Google Scholar]
- Simberloff D. 1997 The biology of invasions. In: Simberloff D, Schmitz DC, Brown TC, editors. Strangers in Paradise. pp. 3–17.Island Press, Washington, D.C. [Google Scholar]
- Stout IJ, Marion WR. 1993 Pine flatwoods and xeric pine forests of the southern (lower) coastal plain. In: Martin WH, Boyce SG, Echternacht AC, editors. Biodiversity of the southeastern United States. pp. 373–446.John Wiley and Sons, Inc, New York. [Google Scholar]
- ter Brakk CJF. 1996 Ordination. In: Jongman RGH, ter Braak CJF,. van Tongeren OFR, editors. Data Analysis in Community and Landscape Ecology. pp. 91–173.Cambridge University Press, New York. [Google Scholar]
- Thompson CR. 1980 Solenopsis (Diplorhoptrum) (Hymenoptera: Formicidae) of Florida. Ph.D. dissertation University of Florida. [Google Scholar]
- Tobin JE. 1995 Ecology and diversity of tropical forest canopy ants. In: Lowman MD, Nadkarni NM, editors. Forest Canopies. pp. 129–147.Academic Press, New York. [Google Scholar]
- Tschinkel WR. Distribution of the fire ants Solenopsis invicta and S. geminata (Hymenoptera: Formicidae) in northern Florida in relation to habitat and disturbance. Annals of the Entomological Society of America. 1988;81:76–81. [Google Scholar]
- Tschinkel WR. 1993a The fire ant (Solenopsis invicta): still unvanquished. In: McKnight BN, editor. Biological pollution: The control and impact of invasive exotic species. pp. 121–136.Indiana Academy of Science, Indianapolis. [Google Scholar]
- Tschinkel WR. Sociometry and sociogenesis of colonies of the fire ant Solenopsis invicta during one annual cycle. Ecological Monographs. 1993b;63:425–457. [Google Scholar]
- Tschinkel WR. 2002 The natural history of the arboreal ant, Crematogaster ashmeadi. 15 pp. Journal of Insect Science. 2:12. Available online: insectscience.org/2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschinkel WR, Adams ES, Macom T. Territory area and colony size in the fire ant Solenopsis invicta. Journal of Animal Ecology. 1995;64:473–480. [Google Scholar]
- Tschinkel WR, Hess CA. The arboreal ant community of a pine forest in northern Florida. Annals of the Entomological Society of America. 1999;92:63–70. [Google Scholar]
- U.S. Department of Agriculture. 1999a Final environmental impact statement for the revised land and resource management plan for national forests in Florida. USDA Management Bulletin R8-MB-83B, Forest Service Southern Region. [Google Scholar]
- U.S. Department of Agriculture. 1999b Revised land and resource management plan for national forests in Florida. USDA Management Bulletin R8-MB-83A, Forest Service Southern Region. [Google Scholar]
- Van Pelt AF Jr. The ecology of the ants of the Welaka Reserve, Florida (Hymenoptera:Formicidae) The American Midland Naturalist. 1956;56:358–387. [Google Scholar]
- Van Pelt AF Jr. The ecology of the ants of the Welaka reserve, Florida (Hymenoptera:Formicidae). Part II. Annotated list. The American Midland Naturalist. 1958;59:1–57. [Google Scholar]
- Vepsäläinen K, Pisarski B. Assembly of island ant communities. Annales Zoologici Fennici. 1982;19:327–335. [Google Scholar]
- Vinson SB. Invasion of the red imported fire ant (Hymenoptera: Formicidae): spread, biology, and impact. American Entomologist. 1997;43:23–39. [Google Scholar]
- Walters JR. Application of ecological principles to the management of endangered species: the case of the red-cockaded woodpecker. Annual Review of Ecology and Systematics. 1991;22:505–523. [Google Scholar]
- Whitford WG, Ettershank G. Factors affecting foraging activity in Chihuahuan desert harvester ants. Environmental Entomology. 1975;4:689–696. [Google Scholar]
- Wilson EO. Enemy specification in the alarm-recruitment system of an ant. Science. 1975;190:798–800. doi: 10.1126/science.1198097. [DOI] [PubMed] [Google Scholar]
- Wilson EO. The organization of colony defense in the ant Pheidole dentata Mayr (Hymenoptera: Formicidae) Behavioral Ecology and Sociobiology. 1976;1:63–81. [Google Scholar]
- Wilson EO. 1990 Success and dominance in ecosystems: the case of Social Insects. Ecology Institute, Oldendorf/Luhe. [Google Scholar]
- Wojcik DP, Allen CR, Brenner RJ, Forys EA, Jouvenaz DP, Lutz RS. Red imported fire ants: impact on biodiversity. American Entomologist. 2001;47:16–23. [Google Scholar]
- Zettler JA, Spira TP, Allen CR. Ant-seed mutualisms: Can red imported fire ants sour the relationship? Biological Conservation. 2001;101:249–253. [Google Scholar]
- Zorrilla JM, Serrano JM, Casado MA, Acosta FJ, Pineda FD. Structural characteristics of an ant community during succession. Oikos. 1986;47:346–354. [Google Scholar]


