Abstract
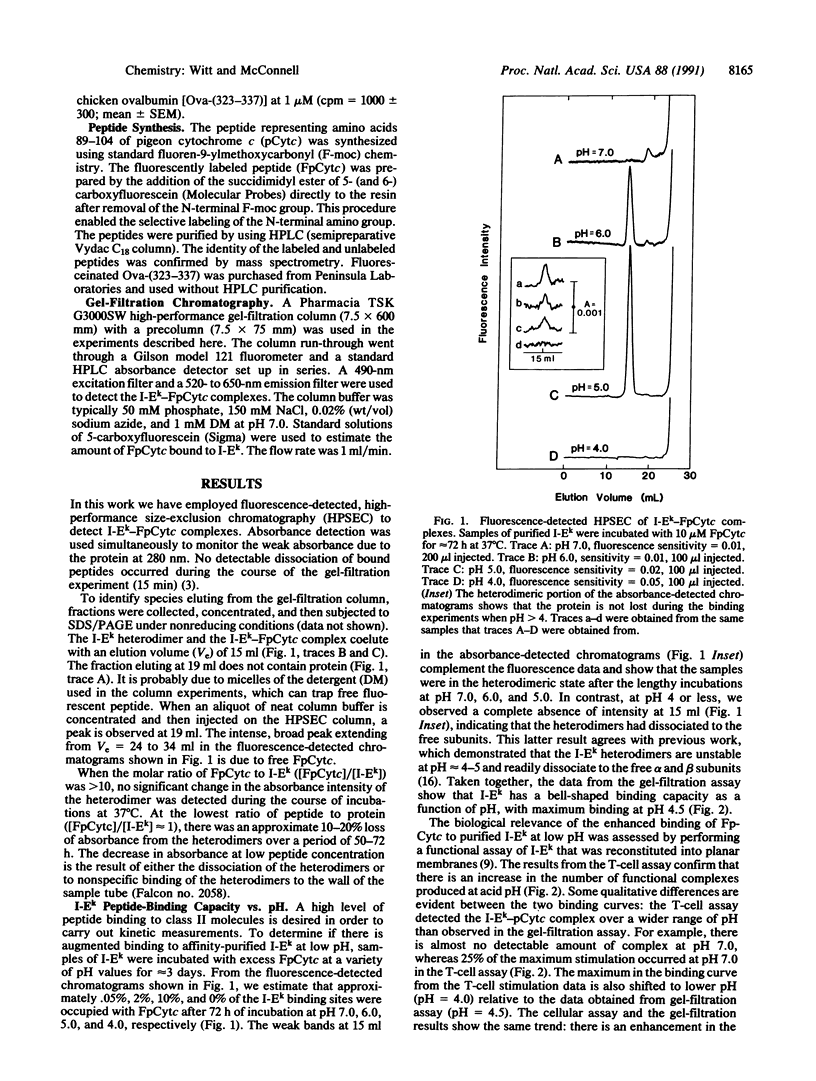
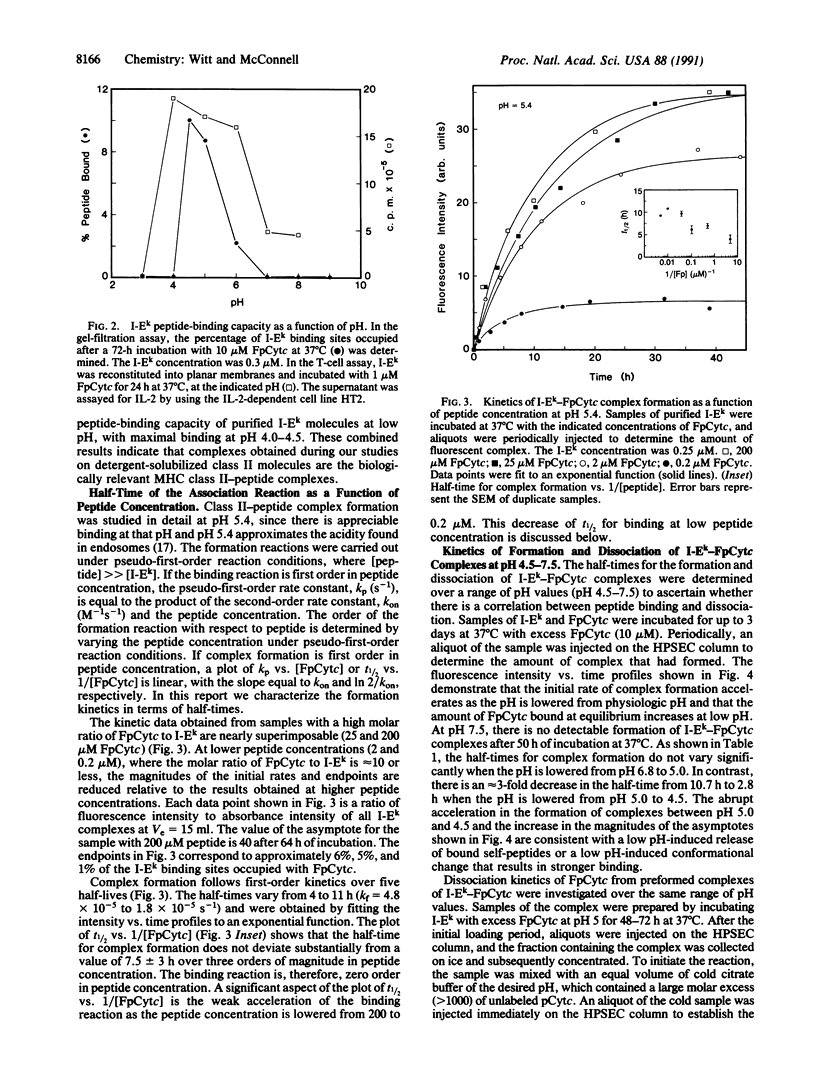
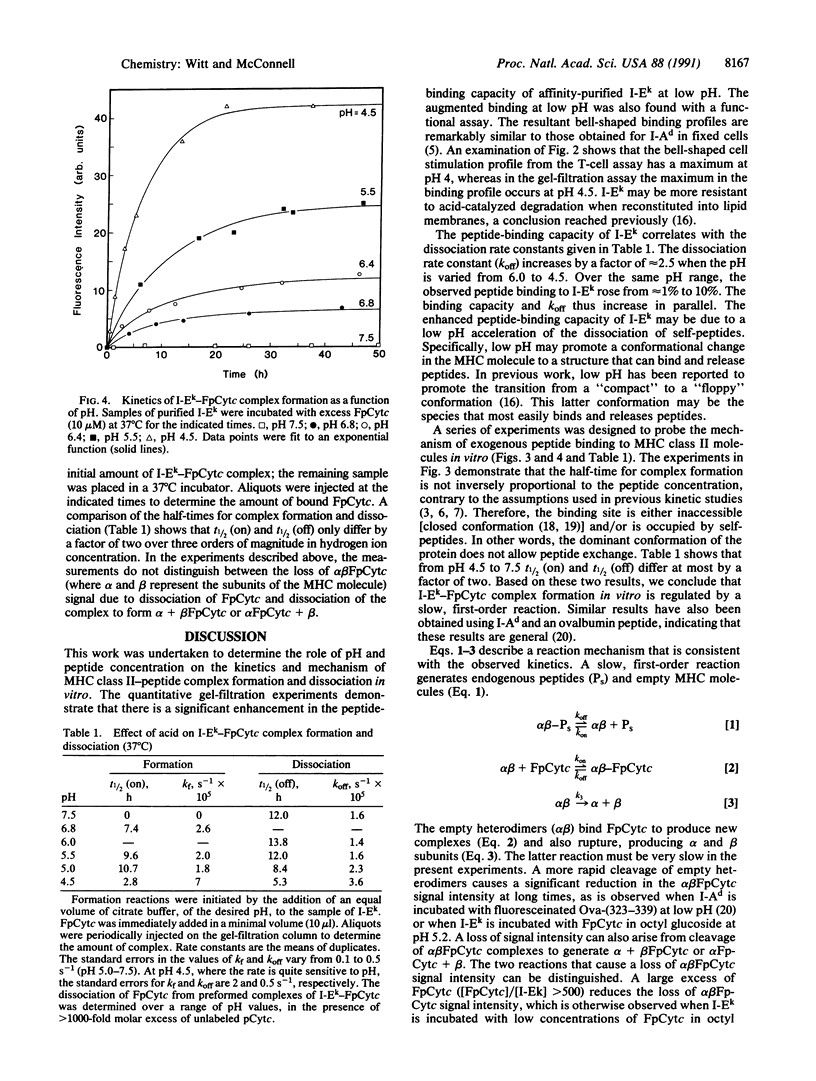
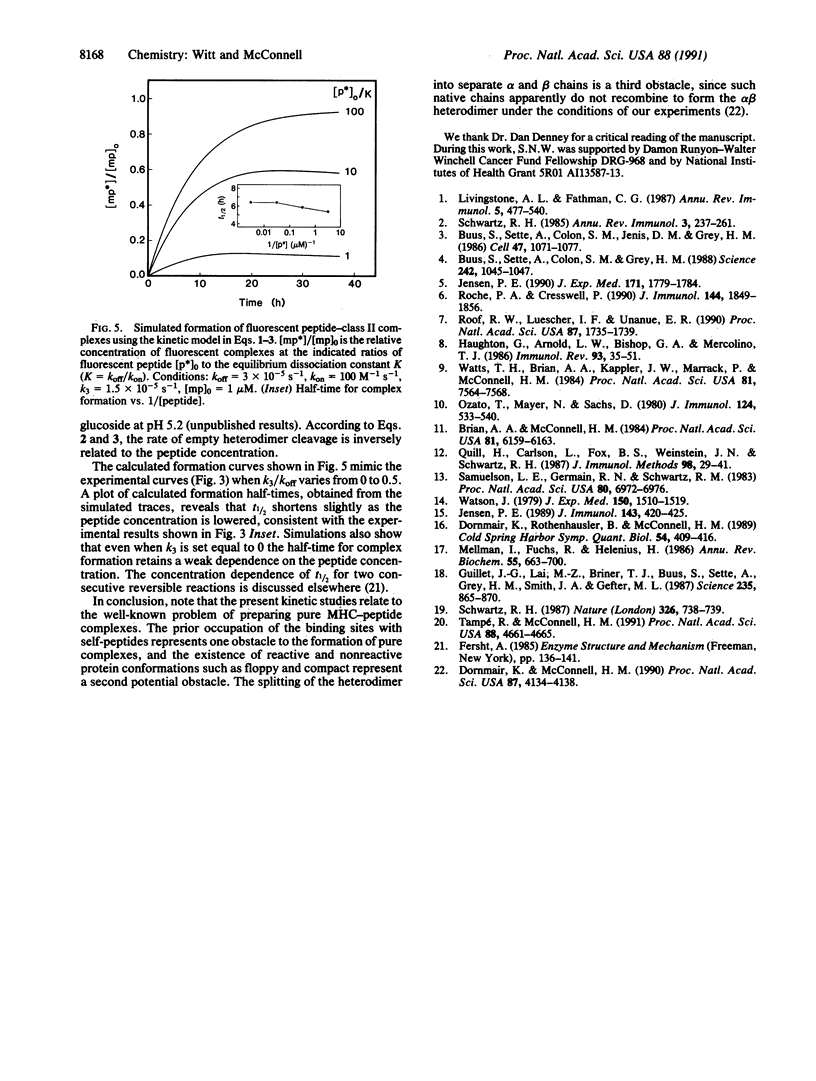
Major histocompatibility complex class II molecules have been reported to bind antigenic peptides very slowly in vitro. To investigate the molecular events that govern the slow binding reaction, we have determined the dependence of complex formation and dissociation on peptide concentration. The complex between the purified major histocompatibility complex class II protein I-Ek and a fluoresceinated peptide representing amino acids 89-104 of pigeon cytochrome c (FpCytc) was studied. Two important results emerge from this study. (i) At pH 5.4, the half-time for I-Ek-FpCytc complex formation is equal to approximately 7 hr for peptide concentrations that vary over a range of three orders of magnitude. There is in fact a small but significant decrease in the half-time for complex formation at low peptide concentrations. The small decrease in half-time is related to the release of endogenous peptides. (ii) At large ratios of peptide to protein [( FpCytc]/[I-Ek] greater than 40), the half-times for I-Ek-FpCytc complex formation and dissociation are equal to one another to within a factor of two between pH 7.5 and 4.5. The percent results demonstrate that a slow, first-order reaction precedes complex formation between I-Ek and FpCytc. This first-order reaction may involve a protein conformational change in addition to the release of endogenous peptides.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brian A. A., McConnell H. M. Allogeneic stimulation of cytotoxic T cells by supported planar membranes. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6159–6163. doi: 10.1073/pnas.81.19.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Grey H. M. Autologous peptides constitutively occupy the antigen binding site on Ia. Science. 1988 Nov 18;242(4881):1045–1047. doi: 10.1126/science.3194755. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Jenis D. M., Grey H. M. Isolation and characterization of antigen-Ia complexes involved in T cell recognition. Cell. 1986 Dec 26;47(6):1071–1077. doi: 10.1016/0092-8674(86)90822-6. [DOI] [PubMed] [Google Scholar]
- Dornmair K., McConnell H. M. Refolding and reassembly of separate alpha and beta chains of class II molecules of the major histocompatibility complex leads to increased peptide-binding capacity. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4134–4138. doi: 10.1073/pnas.87.11.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornmair K., Rothenhäusler B., McConnell H. M. Structural intermediates in the reactions of antigenic peptides with MHC molecules. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):409–416. doi: 10.1101/sqb.1989.054.01.050. [DOI] [PubMed] [Google Scholar]
- Guillet J. G., Lai M. Z., Briner T. J., Buus S., Sette A., Grey H. M., Smith J. A., Gefter M. L. Immunological self, nonself discrimination. Science. 1987 Feb 20;235(4791):865–870. doi: 10.1126/science.2433769. [DOI] [PubMed] [Google Scholar]
- Haughton G., Arnold L. W., Bishop G. A., Mercolino T. J. The CH series of murine B cell lymphomas: neoplastic analogues of Ly-1+ normal B cells. Immunol Rev. 1986 Oct;93:35–51. doi: 10.1111/j.1600-065x.1986.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Jensen P. E. Regulation of antigen presentation by acidic pH. J Exp Med. 1990 May 1;171(5):1779–1784. doi: 10.1084/jem.171.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. E. Stable association of processed antigen with antigen-presenting cell membranes. J Immunol. 1989 Jul 15;143(2):420–425. [PubMed] [Google Scholar]
- Livingstone A. M., Fathman C. G. The structure of T-cell epitopes. Annu Rev Immunol. 1987;5:477–501. doi: 10.1146/annurev.iy.05.040187.002401. [DOI] [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Quill H., Carlson L., Fox B. S., Weinstein J. N., Schwartz R. H. Optimization of antigen presentation to T cell hybridomas by purified Ia molecules in planar membranes. Ia molecule polymorphism determines the antigenic fine specificity of the response to cytochrome c peptides. J Immunol Methods. 1987 Apr 2;98(1):29–41. doi: 10.1016/0022-1759(87)90432-7. [DOI] [PubMed] [Google Scholar]
- Roche P. A., Cresswell P. High-affinity binding of an influenza hemagglutinin-derived peptide to purified HLA-DR. J Immunol. 1990 Mar 1;144(5):1849–1856. [PubMed] [Google Scholar]
- Roof R. W., Luescher I. F., Unanue E. R. Phospholipids enhance the binding of peptides to class II major histocompatibility molecules. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1735–1739. doi: 10.1073/pnas.87.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samelson L. E., Germain R. N., Schwartz R. H. Monoclonal antibodies against the antigen receptor on a cloned T-cell hybrid. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6972–6976. doi: 10.1073/pnas.80.22.6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. Antigen presentation: fugue in T-lymphocyte recognition. Nature. 1987 Apr 23;326(6115):738–739. doi: 10.1038/326738a0. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Tampé R., McConnell H. M. Kinetics of antigenic peptide binding to the class II major histocompatibility molecule I-Ad. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4661–4665. doi: 10.1073/pnas.88.11.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979 Dec 1;150(6):1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts T. H., Brian A. A., Kappler J. W., Marrack P., McConnell H. M. Antigen presentation by supported planar membranes containing affinity-purified I-Ad. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7564–7568. doi: 10.1073/pnas.81.23.7564. [DOI] [PMC free article] [PubMed] [Google Scholar]



