Abstract
After ingestion of infected blood by a mosquito, malarial parasites are fertilized in the mosquito midgut and develop into motile ookinetes. These ookinetes invade epithelial cells by rupturing the cell membrane and migrate through the cytoplasm toward the basal lamina, on which they develop to oocysts. Here we report that a microneme protein with a membrane-attack complex and perforin (MACPF)-related domain, which we name membrane-attack ookinete protein (MAOP), is produced in the ookinete stage and plays an essential role in midgut invasion by the ookinete. Ookinetes with the MAOP gene disrupted completely lost infectivity to the midgut. After ingestion of blood infected with the disrupted parasite, the midgut epithelium remained intact, making a clear contrast with the damaged midgut epithelium invaded by wild-type ookinetes. Electron microscopic analysis showed that the disruptant ookinetes migrate to the gut epithelium and attach to the cell surface at the apical tip, but are unable to enter the cytoplasm by rupturing the cell membrane. These results indicate that the MAOP molecule acts on the plasma membrane of the host-cell-like mammalian MACPF family proteins that create pores in the membrane of target cells. Another previously identified MACPF-related molecule is produced in the liver-infective sporozoite and has a crucial role in traversing the liver sinusoidal cell boundary. The present finding, thus, suggests that conserved mechanisms for membrane rupture involving MACPF-related proteins are used in different host invasive stages of the malarial parasite, playing a key role in breaching biological barriers of host organs.
Keywords: malaria, ookinete, host cell invasion, membrane rupture, membrane-attack complex and perforin-related proteins
Malaria is still the most important parasitic disease in the world, responsible for 300 million to 500 million cases of illness and 1.5 million to 2.7 million deaths annually. Because of the spread of chloroquine-resistant malaria parasites and insecticide-resistant mosquitoes, malaria is still a major public health problem.
Malarial parasites are transmitted to humans by anopheline mosquitoes. After ingestion of infected blood by a mosquito, malarial parasites are fertilized in the mosquito midgut and develop into motile zygotes, called ookinetes. Ookinetes invade the midgut epithelial cell from the luminal surface by rupturing the cell membrane and move through the cytoplasm to the side of the basal lamina, sometimes penetrating additional epithelial cells laterally (1, 2). On the basal lamina, ookinetes transform to oocysts and finally develop to sporozoites, the mosquito salivary gland-invasive stage.
During this midgut invasion, many ookinetes are killed by the insect's defense system, and the number of malarial parasites is greatly reduced (2-4). The midgut epithelium is, therefore, one of the most important biological barriers against malarial infection. To breach this barrier and migrate to the site of further development, ookinetes have the ability to traverse the host cell.
In the vertebrate host, liver infective sporozoites must cross the liver sinusoidal cell wall, the boundary between hepatocytes and the blood circulation (5, 6). Crossing this layer is necessary for sporozoites to infect the hepatocyte and develop to the erythrocyte-infective form. Sporozoites have cell traversal ability to breach this barrier. In a previous paper, we reported that a sporozoite protein with a membrane-attack complex and perforin (MACPF) domain, named SPECT2, is essential for this ability (6). SPECT2 is specifically produced in the liver-infective sporozoite and localized to micronemes. spect2-disrupted parasites cannot traverse the sinusoidal cells and remain in the sinusoid, resulting in great reduction of the liver infectivity. The MACPF domain of SPECT2 is highly conserved with the mammalian MACPF-family proteins, especially in the region around an amphipathic helix-loop-helix motif that has been suggested to be important for integration into the cell membrane (7). Because mammalian MACPF family proteins act by forming pores in the target cell membrane, the presence of a MACPF domain in SPECT2 strongly suggests that SPECT2 participates in host cell invasion by rupturing the cell membrane, although the direct evidence has not been obtained in this stage.
Malarial parasites have additional MACPF-related genes in the genome (8), but their expressed stages and functions remain unclear. We speculated that some of these genes may be used by ookinetes, when ookinetes traverse the mosquito midgut epithelial cells. To test this idea and to elucidate roles of MACPF-related genes in the malarial lifecycle, we investigated the expression of MACPF-related genes in the ookinete stage. Here we report that membrane-attack ookinete protein (MAOP), a microneme protein paralogous to a sporozoite MACPF-related protein, is produced in the ookinete stage and plays an essential role in midgut invasion by the ookinete. We show that maop- disrupted ookinetes cannot invade the midgut epithelium by rupturing the epithelial cell membrane. This finding suggests that conserved mechanisms for membrane rupture are used in different host invasive stages and play a key role in breaching biological barriers of host organs.
Materials and Methods
Parasite Preparations. Female 6- to 10-week-old BALB/c mice infected with the Plasmodium berghei ANKA strain were prepared by peritoneal injection of infected blood that was stored at -70°C. Infected mice were used within one blood passage for mosquito biting. Ookinete culture was carried out as described (9). Ookinetes were purified from the culture by erythrocyte lysis in 0.83% NH4Cl and used for further analysis. For the purification of sporozoites, infected mosquitoes were anesthetized in CO2 20-24 days after an infective blood meal. The salivary glands and midgut were dissected out, washed in saline, and separately collected in 70 μl of medium 199 (GIBCO/BRL) on ice. Collected tissues were gently ground in the medium to release sporozoites. After removal of tissue fragments by centrifugation at 18 × g for 3 min, and sporozoites were collected from the supernatant by centrifugation at 5,000 × g for 3 min. For purification of merozoites, infected rat blood was cultured for 16 h in RPMI medium 1640 (GIBCO/BRL) containing 20% FCS, under 10% O2/5% CO2. Mature schizonts were purified from the cultured blood by density gradient using Nycoprep 1.077A (Axis-Shield, Huntingdon, U.K.).
Construction of Ookinete EST Database. To search for malarial genes involved in mosquito midgut infection, we established an EST database of P. berghei ookinetes, composed of 11,814 ESTs. Cultured mature ookinetes were purified by density gradient using Nycoprep 1.077A, and poly(A) (+) RNA was extracted from these parasites by using a microprep mRNA purification kit (Amersham Pharmacia). A cDNA library was made from the mRNA as described (5). Sequence analysis using the blast program showed that this database includes ESTs of known ookinete stage-specific genes: 33 circumsporozoite protein and thrombospondin-related anonymous protein-related protein (CTRP); 18 von Willebrand factor A domain-related protein; 91 chitinase; and 216 secreted ookinete adhesive protein (9-12).
Genomic Southern Blot Hybridization. Genomic DNA of P. berghei parasites (3 μg) was digested with a restriction enzyme, DraI, ScaI, EcoI, HindIII, XhoI SmaI, or SnaI, separated on 0.8% agarose gel and then transferred to a nylon membrane (Hybond-N, Amersham Pharmacia). DNA fragments were amplified by PCR using genomic DNA as template with the following primers: 5′-GCGCTCGAATGAATCCCTTTTATATTTTCAC-3′ and 5′-AAGGTACCTATCTCATTTCGCACTTATGATCC-3′. They were labeled with alkaline phosphatase by using CDP-Star kit (Amersham Pharmacia). Hybridization and signal detection procedures were carried out according to the manufacturer's instructions.
Antibody Preparation and Western Blot Analysis. Production of recombinant MAOP in Escherichia coli was performed by essentially the same procedure described (5, 9). A DNA fragment encoding MAOP (amino acid residues 106-238) was amplified by PCR using genomic DNA as a template with a primer pair, 5′-GGCGAATTCGTAGACAGAATGCAAAACATACGT-3′ and 5′-GGACTCGAGTCCAACACCTAAATATTCTGTTCC-3′, and subcloned into the expression plasmid, pGEX6P-1 (Amersham Pharmacia) by using the unique restriction site, EcoRI/XhoI. Recombinant MAOP was produced as a soluble GST fusion protein and affinity-purified on glutathione Sepharose (Amersham Pharmacia). Recombinant MAOP was separated from GST according to the manufacturer's instructions and used for immunization of rabbits. Specific antibodies were affinity purified by using a N-hydroxysuccinimide-activated column (Amersham Pharmacia) coupled with recombinant MAOP. Western blot analysis was performed as described (9).
Immunocytochemistry. Immunofluorescence microscopy was performed as described (9). Briefly, purified parasites on glass slides were fixed in acetone for 2 min and rinsed in PBS. The slides were then blocked in PBS containing 1% BSA and incubated for 60 min with purified anti-MAOP antibodies diluted in the same buffer (20 μg/ml final concentration). After being rinsed five times in PBS, the slides were incubated for 60 min with FITC-conjugated anti-rabbit IgGs (Zymed) diluted 1:40 in the same buffer, and again rinsed five times in PBS. For nuclear staining, 4′,6-diamidino-2-phenylindole (0.02 μg/ml final concentration) was added to the secondary antibody solution. Samples were mounted in PermaFluor (Thermo Shandon, Marseille, France), and micrographs were obtained with an Olympus BX60 fluorescence microscope with a C4742-95 digital color camera (Hamamatsu Photonics, Hamamatsu City, Japan). The images were processed by using aquacosmos (Hamamatsu Photonics) and photoshop (Adobe Systems, Mountain View, CA).
Targeted Disruption of the MAOP Gene. For construction of the targeting vector, two fragments of the MAOP gene were amplified by PCR using genomic DNA as template with the primer pairs 5′-TCAGAGCTCGATTGTCATTATGGCTATTTTTCC-3′ and 5′-TCAGGATCCTGCACCTAAATTGTCAAGTTTGTG-3′, and 5′-GCGCTCGAATGAATCCCTTTTATATTTTCAC-3′ and 5′-AAGGTACCTATCTCATTTCGCACTTATGATCC-3′. These fragments were ligated to either side of the selectable marker gene in plasmid vector pBluescript (Strategene). The gene-targeting experiment was performed as described (13).
Evaluation of Ookinete Infectivity to Mosquitoes. After checking the number of exflagellated parasites in their blood (>30 per 105 erythrocytes), infected mice were subjected to biting by Anopheles stephensi mosquitoes for 20 min. Fully engorged mosquitoes were selected and maintained at 20°C. These mosquitoes were dissected 14 days after feeding, and oocysts in the midgut were carefully counted under a microscope.
Light and Electron Microscopy Analyses. Mosquitoes (5-7 days after emergence) were fed on mice infected with wild type or disruptants. Midguts of fully engorged mosquitoes were carefully dissected 21 h after an infective blood meal, fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) containing 4% sucrose and postfixed in 1% osmium tetroxide in 0.1 M phosphate buffer (pH 7.4). The fixed samples were dehydrated in a graded series of ethanol and embedded in epon resin.
For light microscopy, semithin sections (300 nm) were prepared from these samples (30 midguts) and stained with toluidine blue. Sections were examined under a light microscope, and degenerated epithelial cells, which are characterized by heterogenous staining and protrusion from the epithelium, were counted. The total number of degenerated cells in 6,000 epithelial cells was compared between wild type and disruptants. For negative control, mosquitoes were fed on uninfected mice, and the midguts were examined in the same way.
For transmission electron microscopy, 50-100 serial sections (70-90 nm thick) were prepared from the posterior portion of the midgut and stained with uranyl acetate and lead citrate. For both wild type and disruptants, a total of ≈2,100 ultrathin sections were prepared from 30 mosquitoes. These sections were carefully examined with a HITACHI H-800 transmission electron microscope, and locations of ookinetes in the midgut were recorded.
Results
Identification of cDNA Encoding MAOP from P. berghei Ookinete EST Database. To examine whether MACPF-related proteins are produced by the ookinete, the ookinete EST database was screened by tblastn using the amino acid sequence of SPECT2 as a query (6). By this screening, one EST with high homologies to SPECT2 was identified in the database. The nucleic acid sequence of this gene, which was obtained from the P. berghei genome database, contained a single exon encoding a 815-aa secretory-like protein with a MACPF domain. The aligned region was located in the MACPF domain-coding region of both genes. The MACPF domain of this molecule was located in the middle of the sequence and shares 53.5% and 52.0% sequence similarity with human complement component C9 (P02748) and human perforin (M31951), respectively. These similarities seem to be of significance, because human C9 and perforin share only ≈51.5% sequence similarity in the domain. A second structural analysis revealed that the MACPF domain contains two amphiphilic α-helices separated by a short turn, which is suggested as a membrane-spanning motif (7). This protein has no other amino acid sequence motifs suggesting its function. Genomic Southern blot analysis showed that this gene occurs in a single-copy in the P. berghei genome (data not shown).
blast homology searches of the Malaria Genome Project sequence databases (14, 15) identified complete or partial sequences of orthologous genes throughout several Plasmodium species, including the rodent malarial parasite, Plasmodium yoelii (P. yoelii perforin-like protein 3, ref. 8), and the human malarial parasites, Plasmodium falciparum (P. falciparum perforin-like protein 3, ref. 8) and Plasmodium vivax. The amino acid sequence identities of this molecule with orthologues in P. falciparum and P. vivax are 67.7% and 64.5%, respectively, and significantly higher in the MACPF domain (84.1% and 82.2%, respectively), suggesting the functional importance of this domain.
MAOP Is Produced Specifically in the Ookinete Stage. To examine whether this gene is expressed specifically in the ookinete stage, the expression profile was investigated through the malarial life cycle. Immunofluorescence analysis showed that MAOP production was restricted to the ookinete stage (Fig. 1A). Signals were mainly localized in the apical end of the ookinete and showed patchy distribution, suggesting localization in micronemes. Western blot analysis (Fig. 1B) showed that MAOP is produced by mature ookinetes as an 85-kDa protein.
Fig. 1.
MAOP is specifically expressed in the ookinete stage. (A) Indirect immunofluorescent microscopy of all four invasive forms of the malarial parasite. Parasites were stained with primary antibodies against MAOP followed by FITC-conjugated secondary antibodies. Corresponding phase contrast (Phase) or 4′,6-diamidino-2-phenylindole (DAPI)-stained images are shown under each panel. (Scale bars, 5 μm.) (B) Western blot analysis of MAOP production. Infected blood was subjected to ookinete culture in vitro. MAOP production was compared before (0 h) and after (21 h) cultivation. maop-disrupted ookinetes [maop(-)1] were used as a negative control (KO; see also Fig. 2). MAOP was detected as a single band of 85 kDa (arrowhead) only in wild-type ookinetes.
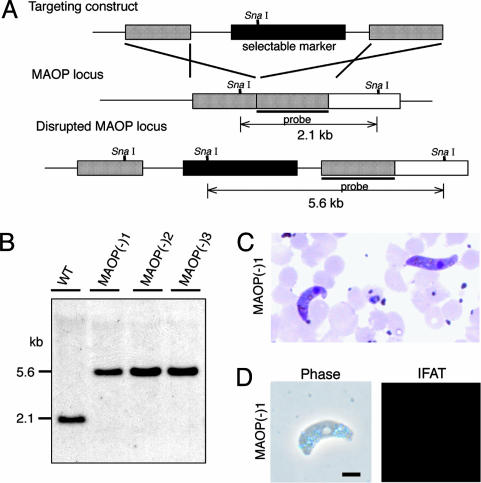
Parasite Infectivity to Mosquitoes Was Lost by Disruption of the MAOP Gene. To investigate the function of this gene, maop-disrupted parasites were prepared. The targeting construct used is composed of a selectable marker that confers pyrimethamine (antimalarial drug) resistance to parasites and maop gene sequences ligated at both ends (Fig. 2A). Merozoites of P. berghei were transfected with this construct by electroporation, and parasites with the maop locus disrupted by homologous recombination were selected by pyrimethamine. Three disruptant clones were obtained from three independent recombination events. Disruption of maop loci was confirmed by genomic Southern blot analysis (Fig. 2B), Western blot analysis (Fig. 1B), and immunocytochemistry (Fig. 2D).
Fig. 2.
Targeted disruption of the MAOP gene. (A) Schematic representation of targeted disruption of the MAOP gene. The targeting vector (Top) containing a selectable marker gene is integrated into the MAOP gene locus (Middle) by double crossover. This recombination event resulted in the disruption of the MAOP gene and confers pyrimethamine resistance to disruptants (Bottom). (B) Genomic Southern hybridization of wild type (WT) and maop(-) populations. Genomic DNA isolated from the respective parasite populations was digested with SnaI and hybridized with the probe indicated by a solid bar in A. By integration of the targeting construct, the size of detected fragments was increased from 2.1 to 5.6 kbp. The result is shown for three independently prepared populations, maop(-)1, maop(-)2, and maop(-)3. (C) Giemsa-stained ookinetes of maop-disruptants that were collected from the mosquito midgut 16 h after blood meal. (D) Immunofluorescence microscopy of maop(-)1 parasite. Ookinetes were collected from the culture and stained with primary antibody against MAOP followed by FITC-conjugated secondary antibodies. maop(-)1 ookinetes were not stained with anti-MAOP antibodies. The corresponding phase contrast (phase) is shown at Left. The same results were obtained in other two disruptant populations. (Scale bar, 5 μm.)
All parasite populations showed normal exflagellation numbers (>30 exflagellations per 105 erythrocytes) and developed into mature ookinetes in vitro. They also developed to mature ookinetes in the midgut of A. stephensi mosquitoes (Fig. 2C). They did not show any morphological differences from wild-type parasites under microscopic observation. This finding showed that the disruption did not affect parasite development to ookinetes.
To assess the ability of these parasite populations to infect the insect vector, mosquitoes were dissected 14 days after an infective blood meal, and oocysts in the midgut were counted (Table 1). In the wild-type populations, all 30 mosquitoes dissected were infected, and a total of 10,856 oocysts were found in the midguts. In contrast, no oocysts were found in total of 90 mosquitoes fed on the mice infected with disruptant populations. Furthermore, no sporozoites were collected from mosquitoes fed on mice infected with disruptants. These results demonstrated that ookinetes completely lost their infectivity to the mosquito host by maop disruption.
Table 1. Midgut infection of maop-disrupted parasites in A. stephensi mosquitoes.
| Parasite population | Infected, % | No. of oocysts per 30 mosquitoes* | No. of sporozoites per 30 mosquitoes† |
|---|---|---|---|
| Wild type | 100 | 10,856 | 5,270,000 |
| maop(−)1 | 0 | 0 | 0 |
| maop(−)2 | 0 | 0 | 0 |
| maop(−)3 | 0 | 0 | 0 |
On day 14 after feeding, oocysts formed in the midgut were counted under the microscope.
On day 20, sporozoites in the midgut were collected and counted.
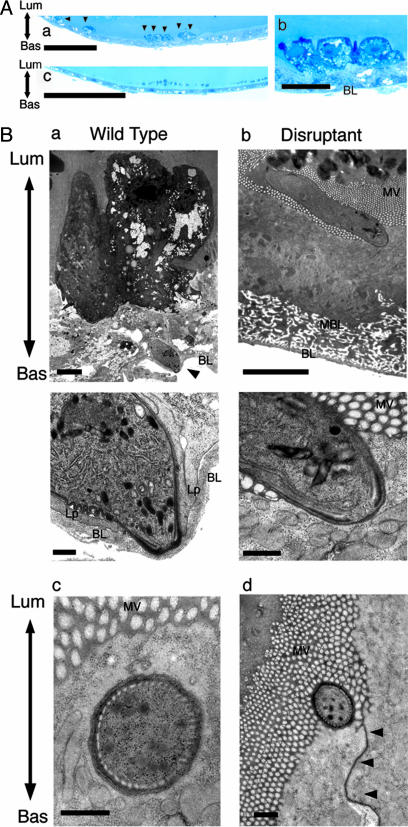
MAOP Disruptants Cannot Invade Midgut Epithelial Cells. Loss of midgut infectivity by maop disruption suggested that disruptants could not invade the midgut epithelium. To test this possibility, mosquito midguts were examined by light microscopy 21 h after engorgement, when wild-type ookinetes have already arrived at the basal lamina and begun transforming to oocysts. In mosquitoes infected with wild-type parasites, degenerated cells were easily identified in the midgut epithelium by toluidine blue staining (Fig. 3A). Degenerated cells were heterologously stained and protruded from the epithelium, suggesting damage caused by ookinete invasion. Six thousand epithelial cells from 30 mosquitoes were examined, and a total of 148 degenerated cells were detected. In contrast, no degenerated cells were found in the same number of epithelial cells from 30 mosquitoes fed on mice infected with maop disruptants (Fig. 3B). Their midgut epithelium remained intact after the blood meal and was indistinguishable from those of control mosquitoes fed on uninfected mice (data not shown). These results indicated that MAOP gene-disrupted ookinetes lost their ability to invade the epithelial cells.
Fig. 3.
maop-disrupted ookinetes cannot invade the midgut epithelium. (A) Representative light microscopic views. Mosquitoes were fed on infected mice, and the midguts were dissected after 21 h. Semithin sections were prepared from the midgut and stained with toluidine blue (see also Table 2). (a) Mosquito midgut epithelium invaded by wild-type ookinetes. Arrows indicate invaded epithelial cells, which are deeply stained with toluidine blue. Seven cells are seen in this view. (Scale bar, 20 μm.) (b) A higher-magnification view of the same section. Three epithelial cells that are severely damaged by ookinete invasion protrude from the epithelium (see also Ba). (Scale bar, 5 μm.) (c) Midgut epithelium after ingestion of the blood infected with disruptants. The apical surface of the epithelium is flat and cells are uniformly stained. The appearance of the epithelium is the same as after ingestion of noninfected blood (data not shown), indicating that the midgut epithelium is not damaged. (Scale bar, 20 μm.) (B) Representative transmission electron microscopic views. Mosquitoes were fed on mice infected with wild type (a) or disruptants (b) and dissected after 21 h. Ultra-thin sections were prepared from the midgut (see also Table 2). (a) (Upper) A wild-type ookinete (arrowhead) that has arrived at the basal lamina (BL). The initially invaded cell (at the upper side) is severely damaged and protrudes from the epithelium. It loses microvilli and is stained with high electron density. Lum, the luminal side of the epithelium; Bas, the basal side of the epithelium. (Scale bar, 3 μm.) (Lower) A higher-magnification view of the same section. An ookinete has already exited from the host cell and attaches to the basal lamina at the apical end. Extending lamellipodia (Lp) of the neighboring epithelial cells are observed at both sides of the ookinete, suggesting that epithelium-repairing procedure has already begun (4). (Scale bar, 300 nm.) (b) (Upper) An ookinete of the disrupted parasite that attaches to the apical surface of the midgut epithelium. The surface of the attached cell is invaginated toward the inside, but the cell is not impaired. It has the same appearance as neighboring cells, including dense microvilli (MV), normal electron staining of the cytoplasm, and conserved complex structure of the basal membrane labyrinth (BML). (Scale bar, 3 μm.) (Lower) A high-magnification view of the same section. The apical surface of the ookinete and the cell membrane of the epithelial cell closely adhere to each other, but the cell membrane is intact and can be followed along the attaching surface. This finding suggests that disruptants lack ability to rupture the cell membrane, which is essential for host cell traversal. (Scale bar, 300 nm.) (c) A cross section of the apical tip of the maop-disrupted ookinete, which may adhere to the apical surface of the epithelial cell and push the cell membrane into the cytoplasm. Whereas the cell membrane is invaginated by this pressing, the epithelial cell remains intact. (Scale bar, 300 nm.) (d) A cross section of the apical tip of the maop-disrupted ookinete attached to the apical surface of the epithelial cell. The attaching site is adjacent to the cell junction that is indicated by arrowheads. (Scale bar, 300 nm.)
Disruptant Ookinete Cannot Rupture the Cell Membrane of the Midgut Epithelial Cell. Electron microscopic analysis was performed to investigate which step of midgut invasion the MAOP gene is involved in. Thirty fully engorged mosquitoes were dissected 21 h after engorgement, and 50-100 serial ultrathin sections were prepared from each. In total, ≈2,100 ultrathin sections were prepared for analyses of disruptants and of the wild-type, respectively. These sections were carefully examined along the gut epithelium, that is, from the luminal layer posessing microvilli to the basal lamina of the midgut epithelial cell. Ookinetes were categorized into four groups according to the locations where they were found: lying in microvilli, attaching to the luminal surface of the epithelium, in the epithelial cell cytoplasm, and arriving at the basal lamina. The results are summarized in Table 2. In total, similar numbers of ookinetes were found in disruptants and the wild type, suggesting that disruptants can normally migrate to the gut epithelium. However, ookinete distribution in the epithelium was clearly different between disruptants and wild type. In the wild type, almost all ookinetes had invaded the midgut epithelium, and over half of them were attached to the basal lamina (Fig. 3Ba). The epithelial cells initially invaded by ookinetes were heavily damaged. They were characterized by high electron density staining, protrusion from the epithelium, absent or scant microvilli, and degeneration of cytoplasmic organelles such as mitochondria.
Table 2. Comparison of location in the midgut epithelium between wild-type and disruptant ookinetes.
| No. of ookinetes/total (%)
|
||
|---|---|---|
| Location | maop(−) | Wild type |
| Among microvilli | 36/80 (45) | 1/81 (1) |
| Adhering to the membrane* | 44/80 (55) | 0/81 (0) |
| Cytoplasm† | 0/80 (0) | 11/81 (14) |
| Basal lamina‡ | 0/80 (0) | 69/81 (85) |
Twenty-one hours after a blood meal, ookinete location in the midgut epithelium was analyzed by transmission electron microscopy.
Attaching to the epithelial cell membrane at the apical tip.
Found in the cytoplasm but not arrived at the basal lamina.
Attaching to the basal lamina.
In contrast, disruptants were found neither in the cytoplasm of the midgut epithelial cell nor beneath the basal lamina. All of the disruptants were located outside the epithelium, and over the half of them adhered to the membrane of the epithelial cell, attaching their apical tip to epithelial cell surface. Surprisingly, the epithelial cell surface where the ookinete attached was invaginated toward the cytoplasm, but no apparent breakage was observed in the extended cell membrane (Fig. 3Bb). The attached epithelial cells were kept intact, as shown by staining of normal electron density, dense microvilli, retention of the complex structure of the basal membrane labyrinth, and intact cytoplasmic organelles. Thus, they were ultrastructurally undistinguishable from the neighboring epithelial cells, in clear contrast with epithelial cells damaged by penetration of the wild type. Among these adhering ookinetes, eight were obtained as cross-sections observed just under the apical epithelial cell membrane (Fig. 3Bc). They were judged to be topologically outside the cell, because these ookinetes showed host cell membrane around their own plasma membrane, and because the attached cells were intact as described above. Taken together, these results show that disruptant ookinetes can migrate to the gut epithelium and attach to the cell surface at the apical tip, but cannot enter the cytoplasm because of loss of the ability to disrupt the cell membrane.
Discussion
Recently, we reported that a MACPF-related protein is essential for cell passage activity of the sporozoite, playing a critical role in its traversal of the liver sinusoidal Kupffer cells (6). We proposed that this molecule participates in rupture of the cell membrane, from the observation that sporozoites disrupted in this gene completely lost cell wounding activity. Based on this inference and on a finding that a related protein, MAOP, is expressed in the ookinete stage, we examine here the possibility that MAOP participates in ookinete invasion into the mosquito midgut epithelium, especially in rupture of the cell membrane.
The gene-disruption experiment showed that ookinetes with the MAOP gene inactivated could not invade the midgut epithelium. Detailed analysis by electron microscopy revealed that they attached to the epithelial cell surface, but could not proceed into the cytoplasm, confirming that this molecule is essential for rupturing the epithelial membrane before midgut invasion.
This finding indicates that conserved mechanisms for membrane rupture are used by the ookinete and the sporozoite for host cell passage. Both stages play central roles in malaria transmission, breaking through the cellular barrier of the new host and migrating to the site where they can develop into the next invasive forms. MACPF proteins may support this invasion by disrupting the host cell membrane.
Primary structures of these malarial MACPF-related proteins show high homology with mammalian MACPF family proteins in the MACPF domain (16, 17). A helix-turn-helix motif, which has been reported to be important for membrane integration, is also conserved in them (7), and their similarities to mammalian MACPF family proteins are highest exactly in this region. Therefore, it is most likely that they may be integrated into the host cell membrane and break it by pore formation activity. It is also possible that MAOP creates routes for other micronemal molecules to enter the cytoplasm of the host cell, enabling them to disrupt the cytoskeleton supporting the membrane. Elucidation of the whole mechanism of membrane rupture is indispensable for understanding malarial transmission at the molecular level and the creation of novel transmission-blocking strategies on this basis.
Ultrastructural study of disruptant ookinetes also showed that membrane rupture is initiated by parasite attachment to the host membrane at the apical tip. The attachment seems tight and irreversible, because most disruptants remained attached to the cell surface, even when wild-type ookinetes had already arrived at the basal lamina. This apical attachment of the ookinetes is similar to that of the merozoite just about to enter the erythrocyte. Before cell entry, the merozoite makes apical junction with the erythrocyte membrane, which involves specific interactions between erythrocyte surface receptors and parasite attachment proteins (18). Therefore, it is possible that the ookinete attachment involves adhesive proteins interacting with receptors on the epithelial cell surface to initiate its commitment to midgut invasion. Although such adhesive molecules have not yet been identified, they might provide important clues for understanding the mechanisms of ookinete epithelial cell entry and be possible targets for blocking malarial transmission.
It was reported that ookinetes of the avian malarial parasite, P. gallinaceum, invade the midgut epithelial cell through the apical lateral cell membrane when they infect the mosquito Aedes aegypti (1). In our preliminary electron microscopic analysis, at least 40% (18 of 44) of disruptant ookinetes attached to the cell membrane adjacent to the cell junction (Fig. 3Bd). Therefore, it is possible that ookinete invasion occurs at the crevices between epithelial cells in Anopheline mosquitoes. However, electron microscopic analysis could not define the invasion site for all ookinetes. Other approaches are also required to demonstrate the possibility. maop-disrupted ookinetes would help further investigation as a useful tool.
Application of reverse genetics to this stage has revealed some other genes involved in midgut invasion by the ookinete, but their disruption did not result in complete loss of infectivity, except for circumsporozoite protein and thrombospondin-related anonymous protein related protein (CTRP) that is essential for parasite motility itself (13, 19, 20). For example, the peritrophic matrix is one of the most important barriers against ookinete invasion, but the effect of disruption of the gene for chitinase, an enzyme necessary for digesting the matrix, on the ookinete infectivity was limited (11). Although the role of the epithelial cell membrane has not been especially emphasized, our study revealed that it functions as an important barrier against ookinete invasion. The cell surface with the disruptant ookinete attached was bent deeply into the cytoplasm, but the plasma membrane was not impaired. This observation shows that the cell membrane is solid enough to endure the mechanical pressure of the parasite. Supposedly, the luminal side of the epithelial cell membrane is strongly supported by underlying cytoskeleton, because it must endure the tension of the midgut dilatation by blood feeding and also support the ellaborate structure of microvilli. MAOP may be necessary to weaken the membrane and prepare for parasite entry.
So far, the function of MACPF family proteins has been demonstrated in the mammalian host defense system (7, 21). This system kills invading microorganisms or cells infected with them by pore-formation activity of MACPF proteins. Our results first revealed that MACPF family proteins are used conversely by the parasite invading the host cell. This finding, together with identification of related genes in the genomes of some pathogenic microorganisms by recent genome research (8), indicates the possibility that similar molecular mechanisms for host cell invasion may be used by these pathogens. They include Apicomplexan parasites such as Toxoplasma gondii and Eimeria tenella. In fact, some Apicomplexan parasites exhibit host cell invasion with membrane rupture. For instance, Eimeria papillata shows host cell invasion motility similar to the Plasmodium ookinete in invading intestinal epithelial cells (22, 23). Furthermore, they create a tight junction between the apical end and the luminal surface of the epithelial cell and then enter the cytoplasm by rupturing the cell membrane. Therefore, elucidation of the action of MACPF-related molecules in malarial host invasion might lead to an understanding of the pathogenesis of these microorganisms at the molecular level.
In summary, we have shown that rupture of the midgut cell membrane is a critical step in malarial infection of the mosquito and that a MACPF-related protein plays an essential role in this step. Our findings suggest that the molecular basis of host cell invasion may be conserved between the ookinete and the sporozoite. Investigations of these stages may complement each other, and future transmission blocking strategies might be developed on this common molecular basis.
Acknowledgments
We thank Gerard R. Wyatt for reading the manuscript. This study was supported by Grants-in-Aid for Scientific Research on Priority Areas 16017243, 14207011, and 16659110 (to Y.C.) and 16390124 and 16659111 (to M.Y.) from the Ministry of Education, Science, Culture, and Sports of Japan, a grant from the Research for the Future Program from the Japan Society for the Promotion of Science (to Y.C.), and a grant from the Japan Science and Technology Agency (to Y.C.).
Author contributions: K.K., T.I., Y.C., and M.Y. designed research; K.K., T.I., T.M., and M.Y. performed research; M.Y. analyzed data; and K.K. and M.Y. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: MACPF, membrane-attack complex and perforin; MAOP, membrane-attack ookinete protein.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY756182).
References
- 1.Zieler, H. & Dvorak, J. A. (2000) Proc. Natl. Acad. Sci. USA 97, 11516-11521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han, Y. S., Thompson, J., Kafatos, F. C. & Barillas-Mury, C. (2000) EMBO J. 19, 6030-6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blandin, S., Shiao, S. H., Moita, L. F., Janse, C. J., Waters, A. P., Kafatos, F. C. & Levashina, E. A. (2004) Cell 116, 661-670. [DOI] [PubMed] [Google Scholar]
- 4.Vlachou, D., Zimmermann, T., Cantera, R., Janse, C. J., Waters, A. P. & Kafatos, F. C. (2004) Cell Microbiol. 6, 671-685. [DOI] [PubMed] [Google Scholar]
- 5.Ishino, T., Yano, K., Chinzei, Y. & Yuda, M. (2004) PLoS Biol. 2, 77-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishino, T., Chinzei, Y. & Yuda, M. (2004) Cell Microbiol., in press. [DOI] [PubMed]
- 7.Peitsch, M. C., Amiguet, P., Guy, R., Brunner, J., Maizel, J. V., Jr., & Tschopp, J. (1990) Mol. Immunol. 27, 589-602. [DOI] [PubMed] [Google Scholar]
- 8.Kaiser, K., Camargo, N., Coppens, I., Morrisey, J. M., Vaidya, A. B. & Kappe, S. H. (2004) Mol. Biochem. Parasitol. 133, 15-26. [DOI] [PubMed] [Google Scholar]
- 9.Yuda, M., Sawai, T. & Chinzei, Y. (1999) J. Exp. Med. 189, 1947-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuda, M., Yano, K., Tsuboi, T., Torii, M. & Chinzei, Y. (2001) Mol. Biochem. Parasitol. 116, 65-72. [DOI] [PubMed] [Google Scholar]
- 11.Dessens, J. T., Mendoza, J., Claudianos, C., Vinetz, J. M., Khater, E., Hassard, S., Ranawaka, G. R. & Sinden, R. E. (2001) Infect. Immun. 69, 4041-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dessens, J. T., Siden-Kiamos, I., Mendoza, J., Mahairaki, V., Khater, E., Vlachou, D., Xu, X. J., Kafatos, F. C., Louis, C., Dimopoulos, G. & Sinden, R. E. (2003) Mol. Microbiol. 49, 319-329. [DOI] [PubMed] [Google Scholar]
- 13.Yuda, M., Sakaida, H. & Chinzei, Y. (1999) J. Exp. Med. 190, 1711-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlton, J. M., Angiuoli, S. V., Suh, B. B., Kooij, T. W., Pertea, M., Silva, J. C., Ermolaeva, M. D., Allen, J. E., Selengut, J. D., Koo, H. L., et al. (2002) Nature 419, 512-519. [DOI] [PubMed] [Google Scholar]
- 15.Gardner, M. J., Hall, N., Fung, E., White, O., Berriman, M., Hyman, R. W., Carlton, J. M., Pain, A., Nelson, K. E., Bowman, S., et al. (2002) Nature 419, 498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lichtenheld, M. G., Olsen, K. J., Lu, P., Lowrey, D. M., Hameed, A., Hengartner, H. & Podack, E. R. (1988) Nature 335, 448-451. [DOI] [PubMed] [Google Scholar]
- 17.Peitsch, M. C. & Tschopp, J. (1991) Curr. Opin. Cell Biol. 3, 710-716. [DOI] [PubMed] [Google Scholar]
- 18.Miller, L. H., Aikawa, M., Johnson, J. G. & Shiroishi, T. (1979) J. Exp. Med. 149, 172-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dessens, J. T., Beetsma, A. L., Dimopoulos, G., Wengelnik, K., Crisanti, A., Kafatos, F. C. & Sinden, R. E. (1999) EMBO J. 18, 6221-6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Templeton, T. J., Kaslow, D. C. & Fidock, D. A. (2000) Mol. Microbiol. 36, 1-9. [DOI] [PubMed] [Google Scholar]
- 21.Morgan, B. P. (1999) Crit. Rev. Immunol. 19, 173-198. [PubMed] [Google Scholar]
- 22.Chobotar, B., Danforth, H. D. & Entzeroth, R. (1993) Parasitol. Res. 79, 15-23. [DOI] [PubMed] [Google Scholar]
- 23.Danforth, H. D., Entzeroth, R. & Chobotar, B. (1992) Parasitol. Res. 78, 570-573. [DOI] [PubMed] [Google Scholar]