Abstract
Vascular development depends on the highly coordinated actions of a variety of angiogenic regulators, most of which apparently act downstream of vascular endothelial growth factor (VEGF). One potential such regulator is delta-like 4 ligand (Dll4), a recently identified partner for the Notch receptors. We generated mice in which the Dll4 gene was replaced with a reporter gene, and found that Dll4 expression is initially restricted to large arteries in the embryo, whereas in adult mice and tumor models, Dll4 is specifically expressed in smaller arteries and microvessels, with a striking break in expression just as capillaries merge into venules. Consistent with these arterial-specific expression patterns, heterozygous deletion of Dll4 resulted in prominent albeit variable defects in arterial development (reminiscent of those in Notch knockouts), including abnormal stenosis and atresia of the aorta, defective arterial branching from the aorta, and even arterial regression, with occasional extension of the defects to the venous circulation; also noted was gross enlargement of the pericardial sac and failure to remodel the yolk sac vasculature. These striking phenotypes resulting from heterozygous deletion of Dll4 indicate that vascular development may be as sensitive to subtle changes in Dll4 dosage as it is to subtle changes in VEGF dosage, because VEGF accounts for the only other example of haploid insufficiency, resulting in obvious vascular abnormalities. In summary, Dll4 appears to be a major trigger of Notch receptor activities previously implicated in arterial and vascular development, and it may represent a new opportunity for pro- and anti-angiogenic therapies.
The formation of a mature vasculature depends on the precisely coordinated and sequential actions of a variety of angiogenic growth factors, including members of the vascular endothelial growth factor (VEGF), angiopoietin, and ephrin families. As most elegantly demonstrated by genetic deletion studies in mice, VEGF is required at the earliest stages of vascular development in an exquisitely dose-dependent manner so that even haploid insufficiency results in early embryonic lethality (1, 2), whereas angiopoietins and ephrins are involved during later vascular remodeling (3–7). Angiogenic growth factors have been explored as therapeutic targets for the treatment of solid tumors, with the most successful approaches to date targeting VEGF (8, 9).
A genome-wide screen for genes reduced by VEGF blockade in tumors revealed striking regulation of delta-like 4 ligand (Dll4) (J.H., H.C.L., Y. Wei, and G.D.Y., unpublished results), a recently identified membrane-bound ligand for the Notch family of receptors (10–12). Both the Notch 1 and Notch 4 receptors display prominent arterial expression, whereas Notch 2 is highly expressed in the heart myocardium (13, 14). Consistent with their arterial expression patterns, combined deletion of both Notch 1 and Notch 4 results in severe albeit variable defects in arterial development (e.g., the aorta can be irregularly atretic along its length with regions entirely lacking a lumen), although defects sometimes extend into the venous circulation to the extent that the anterior cardinal vein can be completely absent (15, 16); in addition, uniform vessel networks initially form in the developing yolk sac in these embryo but fail to properly remodel into large vessels and small capillaries. Mice homozygous for a Notch 2 hypomorphic allele can survive to be born, although half die as embryos at embryonic day (E)16.5 due to severe heart defects including pericardial edema and an atrophic myocardial wall (25). In humans, missense mutations in Notch 3 have been implicated in a neurovascular disorder known as cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, in which neurological symptoms arise due to a slowly developing arteriopathy, involving regression of arterial vascular smooth muscle cells (13).
An especially important role for the Notches in arterial specification and maintenance is further supported by findings that Notches may directly regulate two related subfamilies of transcriptional repressors potentially involved in regulation of the arterial marker ephrinB2 [The Hairy/Enhancer of split (Hes) and Hes-related (HRT, also named CHF, Hey, HesR, and gridlock) transcriptional repressors] (13, 14, 17); combined deletion of Hey1 and Hey2 in mice leads to a phenotype very similar to that found for Notch 1/4 mutants (18).
The Notch receptors can use many different potential ligands, including members of the Delta and Jagged families (13, 14). Mice lacking Jagged 1 develop some of the vascular defects seen in the Notch mutants, implying that it might be a ligand for these Notch receptors during vascular development (13, 14). Moreover, mutations in Jagged 1 in humans are associated with Alagille syndrome, a developmental disorder that includes vascular defects. Previous studies have shown that Dll4 is expressed in arterial endothelial cells during development (10, 11, 16).
In this study, we describe mice in which the Dll4 gene is replaced by a reporter gene, and report that even heterozygous deficiency of Dll4 results in embryonic lethality with profound vascular defects remarkably reminiscent to those observed in mouse embryos lacking Notch receptors. Furthermore, use of the Dll4 reporter reveals sites of Dll4 expression, during vascular development, in the mature adult, as well as during tumor angiogenesis, with exquisite resolution. Our findings demonstrate that Dll4 is a requisite ligand for the Notches during vascular development and support further exploration of the Dll4/Notch signaling pathway as a potential target for both pro- and antiangiogenic therapies.
Materials and Methods
Targeting the Dll4 Gene in Mice. Recently described VelociGene technology (19) was used to generate a precise deletion and exchange of the Dll4 coding region, extending from the initiation to the termination codon (corresponding to an 8.1-kb region comprising all of the coding exons and intervening introns), with the β-galactosidase (β-gal) reporter gene as well as a neomycin-selection cassette. Briefly, a bacterial artificial chromosome (BAC) containing the 8.1-kb Dll4 coding region and 140 kb of flanking sequences (clone 475d4 from a 129/SvJ BAC library obtained from Incyte Genomics, Wilmington, DE) was modified to generate a BAC-based targeting vector, which was then linearized and used as a targeting vector to replace the Dll4 gene in F1H4 (C57BL/6:129 hybrid) mouse embryonic stem (ES) cells. Correctly targeted ES cells were identified by using the loss of native allele (LONA) assay as described (19). Two independent correctly targeted ES lines were used to generate chimeric male mice that were complete transmitters of ES-derived sperm. Chimeras were then bred to C57BL/6 and/or ICR females to generate F1 mice or embryos, which were genotyped by LONA assays (Fig. 1B) and β-gal histochemical assays. Mice derived from both ES lines behaved identically, and pooled data from both clones were used for statistics.
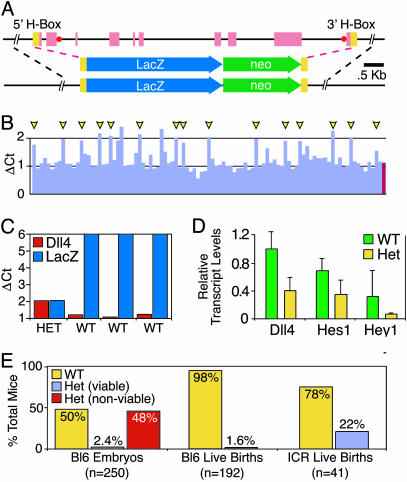
Fig. 1.
Generation and preliminary analysis of Dll4 gene-targeted mice. (A) Schematic representation of Dll4 gene targeting strategy achieved by using VelociGene (19) technology. (Top) A 8.1-kb Dll4 genomic region spanning exons encoding entire coding region (red boxes) along with flanking homology boxes (yellow boxes) used for bacterial homologous recombination approach to incorporate LacZ/Neo reporter cassette shown (Middle), yielding modified Dll4 genomic region (Bottom), in which the Dll4 coding region has been precisely replaced by the LacZ/Neo reporter. Location of short LONA probes also depicted (red circles). (B) Representative screening data from LONA screen (19) for correctly targeted ES cells; clones scoring positive in 16 of 16 independent LONA assays are indicated (yellow arrowheads). (C) Representative genotyping data from F1 animals by using the LONA assay (19) showing clear quantitative distinction between WT and Dll4Lz/+ Het mice. (D) Quantitative RT-PCR results of levels of Dll4, Hes1, and Hey1 transcripts in E9.5 yolk sac RNA from WT and Het embryos. (E) Percentage of embryos and live births that are WT versus Het for the modified Dll4Lz allele, with the heterozygous offspring further subdivided between those that appear viable (blue bars) versus nonviable (red bars) as discussed in the text; all offspring are generated from breedings between male chimeras that are complete transmitters of ES-derived sperm, to either C57BL/6 females (Left and Center) or ICR females (Right). Note that whereas ≈50% of embryos (Left, yellow bar) are heterozygous for the Dll4Lz allele, as would be expected, the vast majority of these appear nonviable, defining haploid insufficiency embryonic lethality.
Tumor Implantations. Lewis lung carcinoma cells (American Type Culture Collection) were s.c. implanted into the flank of Dll4 chimeric mice, harvested after 16 days, cut into 80-μm sections, and stained for CD31/platelet-endothelial cell adhesion molecule (PECAM) or β-gal as described (9, 20).
PECAM and Reporter Staining. Staining of whole-mounted embryos, as well as tissue sections from embryos and adults, were performed as described for CD31/PECAM-1 to define the vascular endothelium and for β-gal to visualize the Dll4 reporter gene product (7, 19).
Quantitative RT-PCR Analysis for Dll4, Hey1, and Hes1. The RT-PCR analysis was performed as described (21). The results are expressed as the ratio of the amount of the RNA of interest to the amount of control RNA (GAPDH) as described (22) on an Applied Biosystems 7900HT real-time PCR system by using specific primers and probes as follows: Dll4 primers: Dll4–1574F, GAGGTCCAAGCCGAACCTG and Dll4–1644R, ATCGCTGATGTGCAGTTCACA; Dll4 probe: Dll4–1594T, CGCTGCCGGCCTGGATTCAC; Hey1 primers: mHEY1–139F, CA AGCCCGGA AGA AGCG and mHey1–219R, TCGTCGCAATTCAGAAAGGC; Hey1 probe: mHey1–173T, AACGGCGCAGAGACCGCATCA; and mHes1 [oligonucleotide ID code Mm00468601_m1, Hes1 (Applied Biosystems, Assay on demand services)]. cDNAs were derived from six WT and three heterozygous (Het) embryos.
Results
Gene Targeting the Dll4 Locus Reveals Lethal Haploid Insufficiency. VelociGene technology (19) was used to create a targeting vector in which the entire Dll4 coding region was replaced with the LacZ reporter gene as well as a neomycin selection cassette (Fig. 1 A). Correct gene targeting in F1H4 (C57BL/6:129 hybrid) ES clones was scored with the described (19) LONA assay (Fig. 1B), revealing a conservatively estimated targeting frequency of ≈15%. Male chimeric mice (selected to be complete transmitters of ES-derived sperm) derived from two independent ES clones were initially bred to C57BL/6 females to generate F1 offspring. However, only three F1 mice heterozygous for the modified Dll4 allele (hereafter termed Dll4Lz/+ animals) were recovered from >300 offspring, leading to the suspicion that Dll4Lz/+ mice might be dying in utero. Embryos recovered from timed pregnancies revealed that viable F1 Dll4Lz/+ embryos could indeed be recovered at the expected frequency (≈50% of the embryos) at 8.5 days postcoitum (E8.5), and at this stage the heterozygous embryos were indistinguishable from their WT littermates (data not shown). However, Dll4Lz/+ embryos recovered at E9.5 and E10.5 exhibited severe morphological abnormalities as compared with their WT littermates, and could be scored with nearly 100% accuracy by their appearance before genotypic analysis. Obvious distinguishing characteristics of the E9.5 and E10.5 Dll4Lz/+ embryos included dramatic pericardial edema (Fig. 2B), lack of large conducting vessels within the yolk sac (Fig. 3B), and frequent necrosis in the posterior half of the embryo (see below); all Dll4Lz/+ embryos were still alive at E9.5 (i.e., their hearts were still beating), whereas the observed phenotypes became more severe and were associated with many dead embryos at E10.5.
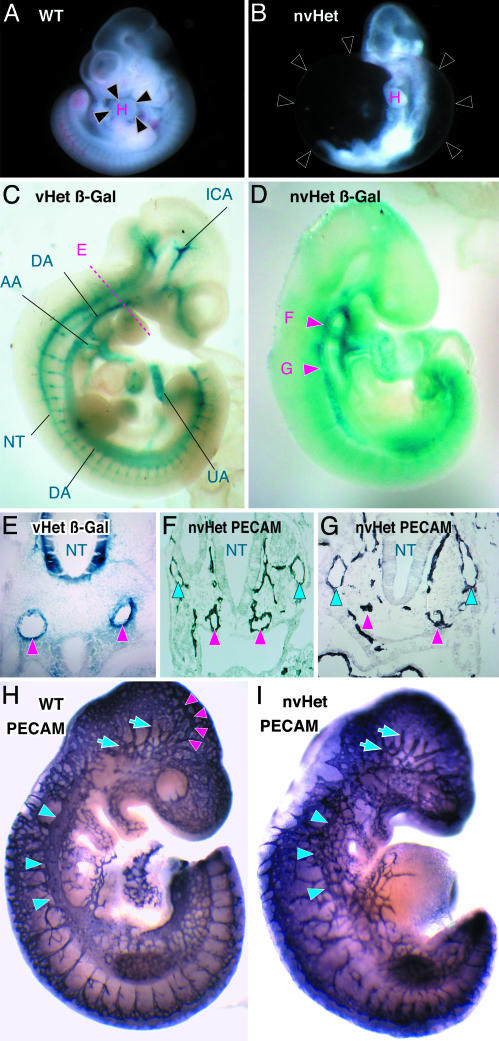
Fig. 2.
Characterization of Dll4 reporter expression patterns and vascular defects in Dll4Lz/+ embryos. (A and B) Freshly dissected E10.5 embryos representative of normal WT and nonviable Dll4Lz/+ heterozygous embryos (nvHet), with black arrowheads in B, indicating dramatic pericardial edema; H, heart. (C and D) Whole-mount β-gal staining reflecting Dll4 reporter gene expression at E10.5 in a viable Dll4Lz/+ heterozygous embryo (vHet) compared with an nvHet. In C, prominent reporter expression is notable in all of the major arterial structures [i.e., internal carotid artery (ICA), dorsal aorta (DA), aortic arch (AA), umbilical artery (UA), and intersomitic branches, unlabeled] as well as in the neural tube (NT), and the level of cross section depicted in E is also indicated, whereas in D arterial branches are less prominent or missing, and red arrowheads indicate the location of the dorsal aorta in nonconstricted region (F arrowhead) as well as constricted/atretic region (G arrowhead). (E) β-gal-stained cross section at the level depicted in C from a vHet embryo, showing that the Dll4 reporter is expressed exclusively in arterial endothelium (red arrowheads, indicating paired dorsal aortas) and neural tube but not in venous endothelium. (F) PECAM-1-stained cross section at the level depicted by the F arrowhead, in D, revealing relatively normal paired dorsal aortas (red arrowheads) and cardinal veins (blue arrowheads); perineural vessels around the neural tube are also seen. (G) PECAM-1-stained cross section at the level depicted by the G arrowhead, in D, revealing obvious defects in the dorsal aortas (red arrowheads) on the left, such that whereas arterial endothelial cells are in approximately correct position, they are not properly organized into a vessel structure, and no lumen can be seen; cardinal veins (blue arrowheads) appear relatively normal. (H and I) Whole-mount PECAM-1-stained WT versus nvHet embryos, comparing vasculature; in H, red arrowheads depict internal carotid artery, blue arrows in head region depict cephalic venous plexus, and blue arrowheads along back depict cardinal vein, all of which are missing or abnormal in nvHet in I, except for the cephalic venous plexus.
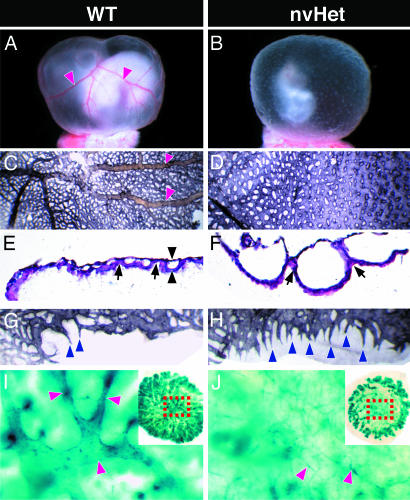
Fig. 3.
Abnormal yolk sac and placental vasculature in Dll4Lz/+ nvHet embryos compared with WT embryos. (A and B) Whole-mount views of freshly dissected embryos with intact yolk sacs showing lack of conducting arteries (red arrowheads in A) and orange peel-like appearance of yolk sacs in nvHet embryos. (C and D) Whole-mount flat views of PECAM-1-stained yolk sacs showing lack of conducting arteries (red arrowheads in C) as well as an almost sheet-like and fused primitive vascular network in nvHet embryos. (E and F) Hemotoxylin/eosin-stained paraffin cross sections of yolk sacs showing gross enlargement of the space between endoderm and mesoderm layers in nvHet embryos (black arrowheads), in part due to an almost complete lack of attachment sites (black arrows) between these layers, resulting in huge lacunae-like vessels in nvHet embryos as opposed to the normally sized and organized vessel cross sections seen in WT embryos. (G and H) High-power views of the periphery of the whole-mounted PECAM-1-stained yolk sacs from C and D. (I and J) Degenerative arterial changes in placentas derived from nonviable Dll4Lz/+ embryos (as compared with those derived from vHet Dll4Lz/+ embryos. (I and J Insets) Low-power whole-mount views of β-gal-stained placentas showing dramatic Dll4-reporter expression in umbilical arteries and their branches extending out to placental periphery, with corresponding high-power views highlighting apparent degenerative arterial changes in center of nvHet placentas that leave only atretic vascular remnants (red arrowheads).
Correlative analysis of genotypes with the above phenotypes from E9.5 and E10.5 litters revealed that the Dll4Lz/+ embryos fell into two classes, which we term “viable” and “nonviable” to reflect the probable fate of the embryos. Genotyping revealed that 50% of 250 characterized embryos at E9.5 and E10.5 were heterozygous Dll4Lz/+ embryos, as would be expected based on normal Mendelian inheritance, with 47.6% classified with the above nonviable phenotype and 2.4% appearing viable and indistinguishable from WT littermates (Fig. 1E Left). The observation that most Dll4Lz/+ E9.5 and E10.5 embryos had obvious vascular abnormalities that apparently precluded continued survival was consistent with the finding that only 1.6% of the 192 characterized pups (born to C57BL/6 females bred to male chimeric mice completely transmitting ES-derived sperm) surviving to birth were heterozygous for the Dll4Lz allele (Fig. 1E Center). However, Dll4Lz/+ pups that did survive to birth were indistinguishable from their WT littermates, and survived and remained indistinguishable into adulthood. Interestingly, when chimeric males were bred to females of the ICR outbred strain, a far greater but still dramatically deficient number (22%, as opposed to the expected 50%, of 41 characterized offspring) of heterozygous pups survived to birth (Fig. 1E Right). Together, these data demonstrate that haploid insufficiency of Dll4 results in embryonic lethality associated with obvious vascular abnormalities, albeit with incomplete penetrance that depends on background strain and thus depends on unlinked genetic modifiers.
To confirm that loss of a single Dll4 allele results in corresponding decreases in Dll4 mRNA, quantitative PCR (TaqMan) analysis was performed on cDNA derived from E9.5 embryos and yolk sacs. In addition to decreases in Dll4 transcript levels, decreases in downstream transcriptional targets (Hes1 and Hey1) of the Dll4/Notch signaling pathway were observed (Fig. 1D), suggesting that haploid insufficiency of Dll4 results in significantly diminished downstream signaling.
Dll4 Embryonic Expression Is Restricted to the Arterial Endothelium. Expression analysis of the β-gal reporter gene activity in both viable E9.5 Dll4Lz/+ embryos as well as chimeric embryos derived from Dll4Lz/+ ES cells revealed that Dll4 is normally remarkably restricted in expression to the endothelium of the developing arterial system (Fig. 2 C and E). These data are consistent with previous expression studies performed by using in situ hybridization (10, 11, 16). Dll4 reporter gene expression was strongest in the endothelium of the developing arches of the aorta, the dorsal aorta, the internal carotid artery, and the umbilical and vitelline arteries (Fig. 2C and data not shown). Whole-mount embryo analysis suggested that the Dll4 reporter was not detected in the venous side of the developing vasculature (Fig. 2C), and this finding was confirmed when cross sections stained for the Dll4 reporter (Fig. 2E, red arrowheads indicating paired dorsal aortas) were directly compared with sections stained with a pan-vessel-specific marker PECAM-1 (Fig. 2F, note blue arrowheads depicting cardinal veins not detected in E). Dll4 expression was also detected outside the vascular system in ventral regions of the neural tube (Fig. 2 C and E).
Defective Arteriogenesis and Remodeling in Dll4Lz/+ Embryos. The above Dll4 reporter expression patterns, together with the embryonic lethality associated with pericardial edema and gross morphologic defects in the yolk sac vasculature in Dll4Lz/+ embryos, demanded further exploration of the vascular abnormalities in these embryos. Staining of whole or sectioned E9.5 embryos for either the Dll4 reporter or for PECAM-1 revealed clearly abnormal vascular patterning in nonviable Dll4Lz/+ embryos, with somewhat variable degrees of severity. Nonviable Dll4Lz/+ embryos stained for the Dll4 reporter often revealed the loss of many of the well defined arterial branches as compared with viable Dll4Lz/+ embryos, with particularly consistent absence of the internal carotid artery (compare Fig. 2 C and D); similarly, PECAM-1 staining indicated that all Dll4Lz/+ embryos scored as nonviable had defective remodeling of the primitive vascular plexus of the head, which was characterized by the apparent lack of a defined arterial plexus as marked by the absence of the internal carotid artery, but a relatively normal venous plexus (Fig. 2 H and I). Furthermore, the aorta appeared variably stenosed or atretic along its length in the nonviable Dll4Lz/+ embryos (see arrowheads, Fig. 2D). Analysis of embryonic sections in the nonviable Dll4Lz/+ embryos confirmed the variable defects along the length of the aorta, with the paired and fused dorsal aortas appearing rather normal with patent lumens in some sections (e.g., see relatively normal paired aortas indicated by red arrowheads in Fig. 2F), whereas in other sections the aortas lacked lumens and appeared to be comprised only of nonorganized endothelial cell clusters in the correct position but lacking any structure (e.g., see red arrowheads in Fig. 2G). Consistent with abnormalities of the aortas and its arterial branches, smooth muscle coverage of these large arterial vessels was often lacking or markedly deficient in the Dll4Lz/+ embryos (data not shown). Perhaps related to the malformations of the dorsal aorta and its branches, nonviable Dll4Lz/+ embryos were often severely underdeveloped and necrotic posterior to the forelimb bud (Fig. 2B and data not shown).
In contrast to consistent abnormalities in the aortas and arterial branches in nonviable Dll4Lz/+ embryos, the cardinal veins often looked relatively normal throughout their length in many nonviable Dll4Lz/+ embryos (e.g., see blue arrowheads in Fig. 2 F and G). In some severely affected Dll4Lz/+ embryos, however, the anterior and posterior cardinal veins were also affected. In some cases, these veins were normally located but appeared variably stenotic or atretic along their length, whereas in the most severe cases, the primitive venous plexus had not properly coalesced into a single large vessel in the Dll4Lz/+ embryos (compare blue arrowheads delineating cardinal veins in Fig. 2H and I). Additional abnormalities were occasionally noted in the branching patterns of the intersomitic vessels (Fig. 2I).
Vascular Remodeling Defects in Dll4Lz/+ Yolk Sacs. In addition to the vascular defects within the embryo proper as defined above, Dll4Lz/+ embryos often lacked large blood-filled conducting vitelline arteries and collecting veins on the surface of their yolk sacs, as was usually obvious upon inspection of freshly dissected embryos (Fig. 3 A and B); the lack of large vessels was also associated with an orange peel-like texture to the surface of the Dll4Lz/+ yolk sacs. PECAM-1 staining of flattened yolk sacs revealed that the Dll4Lz/+ yolk sacs did indeed lack organization of endothelium into large vessels, and the endothelium instead formed an almost continuous surface punctuated with occasional holes (Fig. 3 C and D). Hemotoxylin/eosin-stained paraffin sections of E10.5 yolk sacs revealed that the abnormal yolk sac vascular surface was due to the formation of dramatically enlarged endothelial-lined lacunae between the endodermal and mesodermal layers of the yolk sac (Fig. 3 E and F), with a marked decrease in the invaginations representing the normal attachment sites between these two opposing layers. Interestingly, in the normally avascular periphery of the yolk sac where it connects to the placenta, vessels in the Dll4Lz/+ yolk sacs appeared to have frequent sprout-like projections, whereas the WT yolk sacs had only occasional sprouts (Fig. 3 G and H). Despite obvious yolk sac angiogenesis deficits, E8.5 embryos do form blood islands and later-staged embryos do have circulating red blood cells (data not shown), indicating that early hematopoiesis does occur in the Dll4Lz/+ yolk sacs.
Arterial Regression in Developing Dll4Lz/+ Placentas. As with other large arteries, Dll4 reporter gene analysis revealed strong Dll4 expression in the umbilical arteries, as well as their arterial branches in the center of the placenta, which then provide active sprouts to the placenta periphery (Fig. 3 I and Inset). In placentas from nonviable Dll4Lz/+ embryos, it is clear that an initial arterial network has formed and extended out to the placental periphery as evidenced by the presence of peripheral vascular sprouts; however, the major central placental arteries appear to undergo central degeneration and regression, leaving only atretic arterial remnants (Fig. 3 J and Inset).
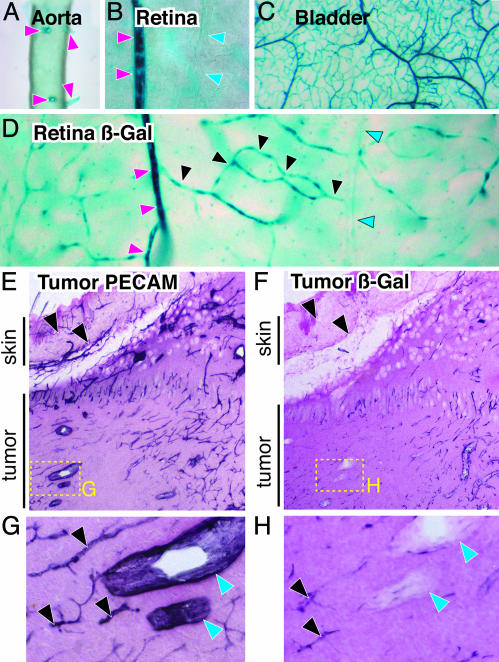
In Adult Mice, Dll4 Is Not Expressed in Major Arteries and Veins but Is Expressed in Smaller Arteries and Microvessels. Dll4 reporter analysis in adult mice reveals that Dll4 continues to be highly restricted to the arterial endothelium of the vascular system. In contrast to early embryos, however, where expression is highest in the largest vessels of the arterial system, Dll4 expression was barely detectable in the aorta and several of its largest branches (such as the carotid and femoral arteries) in the adult, although it was notably expressed in smaller arteries branching directly from the aorta, such as the intercostals (Fig. 4A). Whereas expression was highest in smaller arteries, there was no detectable expression in the corresponding veins paired with these arteries (Fig. 4B). However, expression remained high in the microvessels derived from small arteries (Fig. 4C). Visualization of a complete arterial venous circuit, as can conveniently be performed in planar views of the retina, reveals a striking and abrupt reduction in Dll4 expression just as microvessels join into collecting venules (Fig. 4D). Thus, high levels of Dll4 expression in the adult seem to be restricted to smaller arteries and to the capillary bed, but is minimal in the largest arteries as well as on the venous side of the circulation.
Fig. 4.
Dll4 reporter gene expression reveals prominent expression in smaller arteries and capillaries but not venous vessels in normal adult tissues, with apparent induction during tumor angiogenesis. (A) Whole-mount views of Dll4 reporter expression in aorta and its intercostal artery branches (red arrowheads) of a viable Dll4Lz/+ adult animal. (B) Side-by-side views (from retinal whole mounts) of a small artery (red arrowheads) next to a small vein (blue arrowheads). (C) Prominent Dll4 reporter gene expression in most tissues (urinary bladder wall is shown) seems to extend from small arteries into their downstream microvascular networks. (D) Dll4 expression in an individual vascular circuit (visualized within wholemounts of the adult retina): small arteries, red arrowheads; capillaries, black arrowheads; and post capillary venules, blue arrowheads. (E) Lower-power views of s.c. tumor sections stained with anti-PECAM-1, demonstrating robust staining in all large and small vessels, both in tumor and overlying skin (arrowheads). (F) In contrast, Dll4 reporter analysis reveals stronger expression in tumor vessels as compared with the vessels in the adjacent skin. (G and H) High-power views of boxed areas in E and F highlighting small tumor vessels (black arrowheads) versus large tumor veins (blue arrowheads).
Dll4 Is Induced in Restricted Portions of the Tumor Vasculature. Because we initially identified Dll4 in a genome-wide screen for VEGF-regulated genes in tumors (J.H., H.C.L., Y. Wei, and G.D.Y., unpublished results), we decided to exploit the Dll4 reporter to ascertain Dll4 expression patterns in tumors with higher resolution. In contrast to PECAM-1 expression, which is rather similarly expressed in tumor vessels as compared with nontumor vessels in adjacent skin, the Dll4 reporter is preferentially detected in tumor vasculature as opposed to normal tissue vasculature (compare relative staining in tumor versus skin vessels in Fig. 4 E and F), which is consistent with previous claims that Dll4 is induced in tumor vasculature (10, 11). As in normal adult tissues, however, Dll4 reporter gene expression in tumors was noted in microvessels and small arteries, but was relatively undetectable in small venules (Fig. 4 G and H). Thus, Dll4 is seemingly induced in tumor vessels as compared with normal tissue vessels, but retains its restriction in expression to small arteries and capillaries.
Discussion
Our analyses of Dll4Lz/+ mice add to a growing body of evidence that indicates that the Notch receptors and their ligands play critical roles during embryonic vascular development, and perhaps in vascular maintenance in the adult as well as during tumor angiogenesis (13, 14). The Notches are not as requisitely specific for the vasculature, as are angiogenic growth factors such as the VEGFs or angiopoietins, in that the Notches are also critically and directly involved in the development of nervous, hematopoietic, and other systems; e.g., our own data confirm that Dll4 is highly expressed in the developing neural tube. However, the Notches and their ligands presumably act in concerted and coordinated fashion with these more specific vascular growth factors; e.g., our evidence indicates that Dll4 expression in the vasculature may be directly regulated by VEGF, whereas previous evidence (13, 14) suggests that the Notches may in turn directly regulate the arterial marker ephrinB2 through the Hey2/Hes family of transcriptional factors, raising the possibility of a linear pathway from VEGF to arterialization via the Notches.
Our data are consistent with previous suggestions that the Notch receptors play a particularly important role in arterial specification and development. The phenotypes observed in our Dll4Lz/+ mice are remarkably reminiscent of those reported for mice homozygously deleted for both Notch 1 and Notch 4, suggesting that Dll4 is a major physiologic ligand for these receptors and initiates their activities during vascular development; interestingly, mice lacking Jagged 1 suggest that it is also playing a critical role during vascular development, and future studies will be required to distinguish the relative roles of these two requisite Notch ligands.
As with the vascular defects in mice null for both Notch 1 and Notch 4, the defects in Dll4Lz/+ mice were variable. The most consistent defects were seen in the developing aorta and its major arterial branches, where both Dll4 and its receptors appear to be preferentially expressed, although defects were also seen on the venous side in the most severely affected embryos; the Notch pathway may be directly required only on the arterial side, with the more sporadic venous defects occurring secondarily to severe arterial abnormalities, or the Notch pathway may also be directly acting on the venous side. Remarkably, rare embryos lacking one copy of Dll4 appeared viable and rather normal, as has been described for some of the Notch mutant mice. These findings raise the possibility that Dll4 and its Notch receptors are required only for a critical event during a developmental period, and that once embryos can somehow (and rarely) successfully transit this period in the reduction of Dll4 and Notch signaling, they can then proceed rather normally. Interestingly, the requirement for the Dll4/Notch pathway seems to depend on genetic background, because Dll4Lz/+ mice exhibit substantially higher survival rates when backbred one generation to the ICR outbred strain as compared with the C57BL/6 strain.
It is notable that the Dll4-associated phenotypes described above result from mere haploid insufficiency of this ligand. Such severe vascular defects associated with just a 2-fold reduction in a gene product have previously been noted only for VEGF, demonstrating a rather unusual and exquisite dosage dependence for Dll4. As seen in embryos with homozygous deletions of the VEGF gene, preliminary analyses of embryos bearing homozygous deletions of Dll4 reveal even more severe and consistent vascular abnormalities associated with complete lack of Dll4 (data not shown).
Our description of Dll4 expression patterns in normal adult tissues raises questions about a potential role for this ligand in maintaining normal tissue vasculature. In the adult, preferential expression of Dll4 in small arteries and capillaries once again points to specific roles on the arterial side of the circulation. The apparent regression of the arterial tree during placental development in Dll4Lz/+ mice raises questions about the potential need of Dll4 for arterial maintenance, and may be related to the paucity of smooth muscle coverage in the arteries of Dll4Lz/+ mice. In addition, the regression of these placental arterial vessels is reminiscent of, and may be mechanistically related to, the arterial regressions seen in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy patients. Conditional temporal ablation of Dll4 in adult settings seems likely to provide insight into its requirement in the adult as well as into its possible role in arterial maintenance, whereas conditional spatial ablation should provide insight into the requisite cell source of Dll4 for particular processes.
The localized expression of Dll4 within different parts of the tumor vasculature is consistent with recent findings, based initially on localized ephrinB2 expression, that tumor vessels are not homogenous or purely venous in character as previously thought, but instead capable of differentiating into and retaining both arterial and venous features (23, 24). Future studies will determine whether the Dll4/Notch pathway has any important functional roles during tumor angiogenesis, and whether targeting this pathway can have therapeutic benefits in cancer settings. The suggestion that this pathway is downstream of VEGF and requisite for normal vascular development supports the exciting possibility that it might prove to be an antiangiogenesis target alone or in concert with anti-VEGF approaches. Alternatively, promoting this pathway may prove useful in therapeutic attempts to improve collateral blood flow to ischemic tissues, which may largely depend on the growth and arterialization of preexisting microvessels (26).
Acknowledgments
We thank Drs. P. R. Vagelos and L. S. Schleifer and the rest of the Regeneron community for continued support, especially T. M. DeChiara, D. Frendewey, R. Chernomorsky, Y. Yu, W. Poueymirou, W. Auerbach, A. Elsasser, J. Yasenchack, and J. Rodriguez of the VelociGene group for generation of Dll4 targeting vector and mice; J. Bermudez, X. Ding, and J. Griffiths in the gene expression group for assistance with analysis of expression patterns; P. Boland in the tumor angiogenesis group for assistance with tumor implantation experiments; M. Simmons in the Vivarium for animal breeding; Y. Wei in the microarray group; and B. Ephraim, V. Lan, and S. Staton in the Imaging Technology group for assistance with graphics.
Author contributions: N.W.G., I.N., G.T., and G.D.Y. designed research; N.W.G., M.G.D., I.N., L.P., and V.H. performed research; D.M.V., A.J.M., N.C.A., H.C.L., and J.H. contributed new reagents/analytic tools; N.W.G., M.G.D., I.N., and G.T. analyzed data; and N.W.G., G.T., and G.D.Y. wrote the paper.
Abbreviations: Dll4, delta-like 4 ligand; VEGF, vascular endothelial growth factor; En, embryonic day n; HES, Hairy/Enhancer of split; β-gal, β-galactosidase; ES, embryonic stem; LONA, loss of native allele; PECAM, platelet-endothelial cell adhesion molecule; Het, heterozygous; vHet, viable Het; nvHet, nonviable Het.
References
- 1.Ferrara, N., Carver-Moore, K., Chen, H., Dowd, M., Lu, L., O'Shea, K. S., Powell-Braxton, L., Hillan, K. J. & Moore, M. W. (1996) Nature 380, 439–442. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet, P., Ferreira, V., Breier, G., Pollefeyt, S., Kieckens, L., Gertsenstein, M., Fahrig, M., Vandenhoeck, A., Harpal, K., Eberhardt, C., et al. (1996) Nature 380, 435–439. [DOI] [PubMed] [Google Scholar]
- 3.Suri, C., Jones, P. F., Patan, S., Bartunkova, S., Maisonpierre, P. C., Davis, S., Sato, T. N. & Yancopoulos, G. D. (1996) Cell 87, 1171–1180. [DOI] [PubMed] [Google Scholar]
- 4.Wang, H. U., Chen, Z. F. & Anderson, D. J. (1998) Cell 93, 741–753. [DOI] [PubMed] [Google Scholar]
- 5.Gerety, S. S., Wang, H. U., Chen, Z. F. & Anderson, D. J. (1999) Mol. Cell 4, 403–414. [DOI] [PubMed] [Google Scholar]
- 6.Gerety, S. S. & Anderson, D. J. (2002) Development (Cambridge, U.K.) 129, 1397–1410. [DOI] [PubMed] [Google Scholar]
- 7.Gale, N. W., Thurston, G., Hackett, S. F., Renard, R., Wang, Q., McClain, J., Martin, C., Witte, C., Witte, M. H., Jackson, D., et al. (2002) Dev. Cell 3, 411–423. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara, N. (2002) Semin. Oncol. 29, 10–14. [DOI] [PubMed] [Google Scholar]
- 9.Holash, J., Davis, S., Papadopoulos, N., Croll, S. D., Ho, L., Russell, M., Boland, P., Leidich, R., Hylton, D., Burova, E., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 11393–11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shutter, J. R., Scully, S., Fan, W., Richards, W. G., Kitajewski, J., Deblandre, G. A., Kintner, C. R. & Stark, K. L. (2000) Genes Dev. 14, 1313–1318. [PMC free article] [PubMed] [Google Scholar]
- 11.Mailhos, C., Modlich, U., Lewis, J., Harris, A., Bicknell, R. & Ish-Horowicz, D. (2001) Differentiation (Berlin) 69, 135–144. [DOI] [PubMed] [Google Scholar]
- 12.Liu, Z. J., Shirakawa, T., Li, Y., Soma, A., Oka, M., Dotto, G. P., Fairman, R. M., Velazquez, O. C. & Herlyn, M. (2003) Mol. Cell. Biol. 23, 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shawber, C. J. & Kitajewski, J. (2004) BioEssays 26, 225–234. [DOI] [PubMed] [Google Scholar]
- 14.Thurston, G. & Gale, N. W. (2004) Int. J. Hematol. 80, 7–20. [DOI] [PubMed] [Google Scholar]
- 15.Swiatek, P. J., Lindsell, C. E., del Amo, F. F., Weinmaster, G. & Gridley, T. (1994) Genes Dev. 8, 707–719. [DOI] [PubMed] [Google Scholar]
- 16.Krebs, L. T., Xue, Y., Norton, C. R., Shutter, J. R., Maguire, M., Sundberg, J. P., Gallahan, D., Closson, V., Kitajewski, J., Callahan, R., et al. (2000) Genes Dev. 14, 1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer, A. & Gessler, M. (2003) Trends Cardiovasc. Med. 13, 221–226. [DOI] [PubMed] [Google Scholar]
- 18.Fischer, A., Schumacher, N., Maier, M., Sendtner, M. & Gessler, M. (2004) Genes Dev. 18, 901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valenzuela, D. M., Murphy, A. J., Frendewey, D., Gale, N. W., Economides, A. N., Auerbach, W., Poueymirou, W. T., Adams, N. C., Rojas, J., Yasenchak, J., et al. (2003) Nat. Biotechnol. 21, 652–659. [DOI] [PubMed] [Google Scholar]
- 20.Holash, J., Maisonpierre, P. C., Compton, D., Boland, P., Alexander, C. R., Zagzag, D., Yancopoulos, G. D. & Wiegand, S. J. (1999) Science 284, 1994–1998. [DOI] [PubMed] [Google Scholar]
- 21.Livak, K. J. & Schmittgen, T. D. (2001) Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- 22.Daly, C., Wong, V., Burova, E., Wei, Y., Zabski, S., Griffiths, J., Lai, K. M., Lin, H. C., Ioffe, E., Yancopoulos, G. D. & Rudge, J. S. (2004) Genes Dev. 18, 1060–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gale, N. W., Baluk, P., Pan, L., Kwan, M., Holash, J., DeChiara, T. M., McDonald, D. M. & Yancopoulos, G. D. (2001) Dev. Biol. 230, 151–160. [DOI] [PubMed] [Google Scholar]
- 24.Shin, D., Garcia-Cardena, G., Hayashi, S., Gerety, S., Asahara, T., Stavrakis, G., Isner, J., Folkman, J., Gimbrone, M. A., Jr., & Anderson, D. J. (2001) Dev. Biol. 230, 139–150. [DOI] [PubMed] [Google Scholar]
- 25.McCright, B., Gao, X., Shen, L., Lozier, J., Lan, Y., Maguire, M., Herzlinger, D., Weinmaster, G., Jiang, R. & Gridley, T. (2001) Development (Cambridge, U.K.) 128, 491–502. [DOI] [PubMed] [Google Scholar]
- 26.Buschmann, I. & Schaper, W. (2000) J. Pathol. 190, 338–342. [DOI] [PubMed] [Google Scholar]
- 27.Duarte, A., Hirashima, M., Benedito, R., Trindade, A., Diniz, P., Bekman, E., Costa, L., Henrique, D. & Rossant, J. (2004) Genes Dev. 18, 2474–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krebs, L. T., Shutter, J. R., Tanigaki, K., Honjo, T., Stark, K. L. & Gridley, T. (2004) Genes Dev. 18, 2469–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]