Abstract
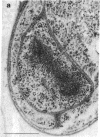
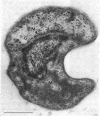
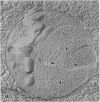
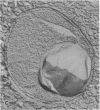
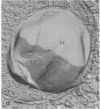
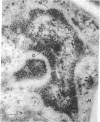
The freshwater budding eubacterium Gemmata obscuriglobus possesses a DNA-containing nuclear region that is bounded by two nuclear membranes. The membrane-bounded nature of the nucleoid in this bacterium was shown by thin sectioning of chemically fixed cells, thin sectioning of freeze-substituted cells, and freeze-fracture/freeze-etch. The fibrillar nucleoid was surrounded by electron-dense granules that were in turn enveloped by two nuclear membranes separated by an electron-transparent space. Immunogold labeling of thin sections of conventionally fixed cells with anti-double-stranded DNA antibody demonstrated double-stranded DNA associated with fibrillar material within the membrane boundary. The occurrence of a membrane-bounded nucleoid in a eubacterial prokaryote is a significant exception to the evidence supporting the prokaryote/eukaryote dichotomous classification of cell structure.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AFZELIUS B. A. The ultrastructure of the nuclear membrane of the sea urchin oocyte as studied with the electron microscope. Exp Cell Res. 1955 Feb;8(1):147–158. doi: 10.1016/0014-4827(55)90051-3. [DOI] [PubMed] [Google Scholar]
- Bomar D., Giovannoni S., Stackebrandt E. A unique type of eubacterial 5S rRNA in members of the order Planctomycetales. J Mol Evol. 1988;27(2):121–125. doi: 10.1007/BF02138371. [DOI] [PubMed] [Google Scholar]
- Bourgeois C. A., Hubert J. Spatial relationship between the nucleolus and the nuclear envelope: structural aspects and functional significance. Int Rev Cytol. 1988;111:1–52. doi: 10.1016/s0074-7696(08)61730-1. [DOI] [PubMed] [Google Scholar]
- Branton D., Bullivant S., Gilula N. B., Karnovsky M. J., Moor H., Mühlethaler K., Northcote D. H., Packer L., Satir B., Satir P. Freeze-etching nomenclature. Science. 1975 Oct 3;190(4209):54–56. doi: 10.1126/science.1166299. [DOI] [PubMed] [Google Scholar]
- Brock T. D. The bacterial nucleus: a history. Microbiol Rev. 1988 Dec;52(4):397–411. doi: 10.1128/mr.52.4.397-411.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crèvecoeur M., Deltour R., Bronchart R. Quantitative freeze-fracture study of plasmalemma and nuclear envelope of Zea mays root cells during early germination. J Ultrastruct Res. 1982 Jul;80(1):1–11. doi: 10.1016/s0022-5320(82)80027-0. [DOI] [PubMed] [Google Scholar]
- Depierre J. W., Dallner G. Structural aspects of the membrane of the endoplasmic reticulum. Biochim Biophys Acta. 1975 Dec 29;415(4):411–472. doi: 10.1016/0304-4157(75)90006-4. [DOI] [PubMed] [Google Scholar]
- Dorward D. W., Garon C. F. DNA Is Packaged within Membrane-Derived Vesicles of Gram-Negative but Not Gram-Positive Bacteria. Appl Environ Microbiol. 1990 Jun;56(6):1960–1962. doi: 10.1128/aem.56.6.1960-1962.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Scheer U., Krohne G., Jarasch E. D. The nuclear envelope and the architecture of the nuclear periphery. J Cell Biol. 1981 Dec;91(3 Pt 2):39s–50s. doi: 10.1083/jcb.91.3.39s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzmann P. D., Skerman V. B. Gemmata obscuriglobus, a new genus and species of the budding bacteria. Antonie Van Leeuwenhoek. 1984;50(3):261–268. doi: 10.1007/BF02342136. [DOI] [PubMed] [Google Scholar]
- Graham L. L., Beveridge T. J. Evaluation of freeze-substitution and conventional embedding protocols for routine electron microscopic processing of eubacteria. J Bacteriol. 1990 Apr;172(4):2141–2149. doi: 10.1128/jb.172.4.2141-2149.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson W. G., Kusano T., Yamaki H., Balakrishnan R., King M., Murchie J., Schaechter M. Binding of the origin of replication of Escherichia coli to the outer membrane. Cell. 1982 Oct;30(3):915–923. doi: 10.1016/0092-8674(82)90296-3. [DOI] [PubMed] [Google Scholar]
- Hobot J. A., Villiger W., Escaig J., Maeder M., Ryter A., Kellenberger E. Shape and fine structure of nucleoids observed on sections of ultrarapidly frozen and cryosubstituted bacteria. J Bacteriol. 1985 Jun;162(3):960–971. doi: 10.1128/jb.162.3.960-971.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R. A. Fine structure of division in ciliate protozoa. I. Micronuclear mitosis in Blepharisma. J Cell Biol. 1967 Aug;34(2):463–481. doi: 10.1083/jcb.34.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesack W., Stackebrandt E. Evidence for unlinked rrn operons in the Planctomycete Pirellula marina. J Bacteriol. 1989 Sep;171(9):5025–5030. doi: 10.1128/jb.171.9.5025-5030.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T. P. Ultrastructural changes in the spermatid nuclear envelope of Periplaneta americana during spermiogenesis. Tissue Cell. 1973;5(2):323–331. doi: 10.1016/s0040-8166(73)80026-6. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Reed R. The role of small nuclear ribonucleoprotein particles in pre-mRNA splicing. Nature. 1987 Feb 19;325(6106):673–678. doi: 10.1038/325673a0. [DOI] [PubMed] [Google Scholar]
- Nanninga N. Preservation of the ultrastructure of Bacillus subtilis by chemical fixation as verified by freeze-etching. J Cell Biol. 1969 Sep;42(3):733–744. doi: 10.1083/jcb.42.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanninga N., Woldringh C. L. The interpretation of chemically fixed and freeze-fractured bacterial nucleoplasm. Acta Histochem Suppl. 1981;23:39–53. [PubMed] [Google Scholar]
- Newport J. W., Forbes D. J. The nucleus: structure, function, and dynamics. Annu Rev Biochem. 1987;56:535–565. doi: 10.1146/annurev.bi.56.070187.002535. [DOI] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Ryter A. Contribution of new cryomethods to a better knowledge of bacterial anatomy. Ann Inst Pasteur Microbiol. 1988 Jan-Feb;139(1):33–44. doi: 10.1016/0769-2609(88)90095-6. [DOI] [PubMed] [Google Scholar]
- STANIER R. Y., VAN NIEL C. B. The concept of a bacterium. Arch Mikrobiol. 1962;42:17–35. doi: 10.1007/BF00425185. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Gunderson J. H., Elwood H. J., Alonso R. A., Peattie D. A. Phylogenetic meaning of the kingdom concept: an unusual ribosomal RNA from Giardia lamblia. Science. 1989 Jan 6;243(4887):75–77. doi: 10.1126/science.2911720. [DOI] [PubMed] [Google Scholar]
- Stackebrandt E., Ludwig W., Schubert W., Klink F., Schlesner H., Roggentin T., Hirsch P. Molecular genetic evidence for early evolutionary origin of budding peptidoglycan-less eubacteria. Nature. 1984 Feb 23;307(5953):735–737. doi: 10.1038/307735a0. [DOI] [PubMed] [Google Scholar]
- Szollosi D. Development of Pleistophora sp. (Microsporidian) in eggs of the polychaete Armandia brevis. J Invertebr Pathol. 1971 Jul;18(1):1–15. doi: 10.1016/0022-2011(91)90002-8. [DOI] [PubMed] [Google Scholar]
- Vossbrinck C. R., Maddox J. V., Friedman S., Debrunner-Vossbrinck B. A., Woese C. R. Ribosomal RNA sequence suggests microsporidia are extremely ancient eukaryotes. 1987 Mar 26-Apr 1Nature. 326(6111):411–414. doi: 10.1038/326411a0. [DOI] [PubMed] [Google Scholar]
- Watson S. W., Graham L. B., Remsen C. C., Valois F. W. A lobular, ammonia-oxidizing bacterium, Nitrosolobus multiformis nov.gen.nov.sp. Arch Mikrobiol. 1971;76(3):183–203. doi: 10.1007/BF00409115. [DOI] [PubMed] [Google Scholar]
- Weisburg W. G., Hatch T. P., Woese C. R. Eubacterial origin of chlamydiae. J Bacteriol. 1986 Aug;167(2):570–574. doi: 10.1128/jb.167.2.570-574.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston S., Sueoka N. DNA-membrane association is necessary for initiation of chromosomal and plasmid replication in Bacillus subtilis. Proc Natl Acad Sci U S A. 1980 May;77(5):2834–2838. doi: 10.1073/pnas.77.5.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Fox G. E. The concept of cellular evolution. J Mol Evol. 1977 Sep 20;10(1):1–6. doi: 10.1007/BF01796132. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldringh C. L., Nanninga N. Organization of the nucleoplasm in Escherichia coli visualized by phase-contrast light microscopy, freeze fracturing, and thin sectioning. J Bacteriol. 1976 Sep;127(3):1455–1464. doi: 10.1128/jb.127.3.1455-1464.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]