Abstract
Purpose
The aim of this study is to evaluate the impact of scanning multiple mice simultaneously on image quantitation, relative to single mouse scans on both a micro-positron emission tomography/computed tomography (microPET/CT) scanner (which utilizes CT-based attenuation correction to the PET reconstruction) and a dedicated microPET scanner using an inexpensive mouse holder “hotel.”
Methods
We developed a simple mouse holder made from common laboratory items that allows scanning multiple mice simultaneously. It is also compatible with different imaging modalities to allow multiple mice and multi-modality imaging. For this study, we used a radiotracer (64Cu-GB170) with a relatively long half-life (12.7 h), selected to allow scanning at times after tracer uptake reaches steady state. This also reduces the effect of decay between sequential imaging studies, although the standard decay corrections were performed. The imaging was also performed using a common tracer, 2-deoxy-2-[18 F]fluoro-d-glucose (FDG), although the faster decay and faster pharmacokinetics of FDG may introduce greater biological variations due to differences in injection-to-scan timing. We first scanned cylindrical mouse phantoms (50 ml tubes) both in a groups of four at a time (multiple mice mode) and then individually (single mouse mode), using microPET/CT and microPET scanners to validate the process. Then, we imaged a first set of four mice with subcutaneous tumors (C2C12Ras) in both single- and multiple-mice imaging modes. Later, a second set of four normal mice were injected with FDG and scanned 1 h post-injection. Immediately after completion of the scans, ex vivo biodistribution studies were performed on all animals to provide a “gold-standard” to compare quantitative values obtained from PET. A semi-automatic threshold-based region of interest tool was used to minimize operator variability during image analysis.
Results
Phantom studies showed less than 4.5 % relative error difference between the single-and multiple-mice imaging modes of PET imaging with CT-based attenuation correction and 18.4 % without CT-based attenuation correction. In vivo animal studies (n=4) showed <5 % (for 64Cu, p>0.686) and <15 % (for FDG, p>0.4 except for brain image data p=0.029) relative mean difference with respect to percent injected dose per gram (%ID/gram) between the single- and multiple-mice microPET imaging mode when CT-based attenuation correction is performed. Without CT-based attenuation correction, we observed relative mean differences of about 11 % for 64Cu and 15 % for FDG.
Conclusion
Our results confirmed the potential use of a microPET/CT scanner for multiple mice simultaneous imaging without significant sacrifice in quantitative accuracy as well as in image quality. Thus, the use of the mouse “hotel” is an aid to increasing instrument throughput on small animal scanners with minimal loss of quantitative accuracy.
Keywords: Image quantitation, MicroPET, Attenuation correction, High throughput, Imaging, Biodistribution
Introduction
In vivo molecular imaging using mouse models is an important tool in preclinical research, being utilized in the development of novel imaging agents, drug treatments, and disease models [1–8]. Recently, there has been significant progress in the technology of mouse imaging including the development of new instruments, advances in the development of more specific imaging probes, and improvements in imaging techniques and/or methodology [9–11]. It is now possible to perform imaging across a wide array of applications ranging from the generation of high-quality 3D anatomical images to the imaging of gene expression in vivo [12, 13]. A major limitation to taking full advantage of state-of-the-art in vivo mouse imaging systems is the cost associated with each imaging study due to the significant capital investment in a shared instrument and ongoing operational costs [14, 15]. This is especially true for micropositron emission tomography (microPET) and small-animal magnetic resonance imaging (MRI), which are amongst the most expensive imaging instruments in many small-animal-imaging labs. In addition, these instruments are typically used to scan single animals sequentially with the intention of maintaining high image quality and quantitative accuracy. However, many mouse-imaging studies require multi-modality imaging and a large number of animals and serial scans to generate sufficient statistical power. Imaging a large number of animals sequentially on such instruments increases the cost of imaging and also limits instrument availability for other investigators. In addition, it generates relatively large volumes of data for storage and processing. The significantly high imaging costs and long data processing times in some cases limit the full application of small-animal imaging on biomedical research. To overcome some of these problems, mouse holders that allow scanning multiple mice simultaneously have been developed both for positron emission tomography (PET) and MRI [16–18]. There are, however, some major concerns and limitations that may need to be addressed when imaging multiple mice simultaneously. First, there should be a reliable method of delivering anesthesia to each mouse controlling the environment during each imaging session. For some applications, it is also important to have easy access for placing devices for physiological and temperature monitoring. In addition, each modality may impose unique requirements. For example, the bore size and type of MRI scanner determine the capability of scanning multiple mice, which may require custom-built multiple mice holders specific to a given scanner [18]. For PET, a major concern over multiple mice scanning is the effect it may have on image quality and quantitative accuracy. This is due to the increased signal attenuation and increased total injected dose and their effect on scatter, deadtime, and depth-of-interaction effects. Some of these effects have been discussed using phantom and animal studies [19–23]. Specifically, Aide et al. [21] using a custom-built four-mice holder bed replacing the single mouse bed provided by the vendor showed that the Inveon microPET scanner is capable of imaging four mice simultaneously without significant degradation in image quality or accuracy when routine attenuation correction mode is used during acquisition.
In this study, we developed a very inexpensive, simple modular mouse holder “hotel” made from disposable items found within a typical small-animal-imaging laboratory. It can be used to image multiple mice without replacing the scanner bed or other utilities such as anesthesia supply. As much as possible, the holder is designed to allow pre-scan preparation and animal placement procedures comparable to individual scanning without a significant increase in setup time. With a bore diameter of 12 cm of the Inveon and R4 microPET scanners, up to seven mouse holders can easily be assembled using only the existing single mouse anesthesia supply channel. This means that the bore diameter of the specific scanner limits the number of animal that can be scanned simultaneously with the proposed mouse holder design. However, other limitations should also be taken in to consideration if high image quality and better quantitation accuracy is desired. As reported in Siepel et al., Disselhorst et al., Aide et al., and Sheruma et al. [19–22], variations of spatial resolution and sensitivity can have significant impact on the image quality and quantitative accuracy of animals scanned off-center, relative to those scanned near the center of field of view of the scanner. Photon attenuation and scatter also produce significant differences in quantitation accuracy between animals placed at center of field of view and off-center [22]. All of these factors may cause notable variability and/or bias in quantitation analysis between animals scanned simultaneously in a group. Thus, care should be taken in selecting optimum number of animals and their position in the scanner to maintain uniformity in image quality and quantitation accuracy. One way to achieve this is to select suitable even number of animals (e.g., for Inveon two, four, or six) and place each animal at equidistance distance from the center of field of view. To ease animal handling and placement, further reduction of animals may also be necessary. For Inveon, selecting four animals for simultaneous scanning allows easy handling and placement of animals while increases the throughput of the scanner by fourfold.
Other than increasing the throughput of microPET scanners, the proposed mouse holder design is also developed to be compatible for simultaneous imaging of multiple mice with other modalities such as MRI, micro-computed tomography (microCT), and microSPECT allowing multiple mice and multi-modality imaging. Currently, this simple multiple mouse holder has been adopted for routine molecular imaging using sequential microPET and microCT scans within our small-animal-imaging facility and recently with a dedicated Inveon microPET/CT scanner. This has significantly reduced the scan time while also allowing experiments to be designed with statistically significant larger cohort numbers of animals, as well as decreasing storage requirements and the time for post-acquisition data processing and analysis. This study compares the quantitative impact of scanning four animals relative to single animals on either a multi-modality microPET/CT scanner (which utilizes CT-based attenuation correction to the PET reconstruction) or a dedicated microPET scanner, in which microCT scans, if performed, are on a different instrument and used simply for anatomical reference.
Materials and Methods
Construction of a Multiple Mice Holder “Hotel”
The need for a simple modular mouse holder to allow simultaneous multiple scanning, and movement of subjects between scanners resulted in the development of a cheap, easy to make disposable holder (see Fig. 1). Materials were chosen that are compatible with most imaging modalities including microCT, microPET, microSPECT, and MRI with minimal effect on image quality. In addition, the ability to stack individual holders permits placement of single, three, four, five, and seven mice in a scanner with isoflurane anesthesia. Table 1 includes a list of some of the materials used to construct the mouse holder “hotel.” The assembly of two or more mouse holders for multiple mice scan is very easy and can be done quickly before the imaging study. For example, to assemble a holder for three mice, it takes 30 min or less, and the holder is reusable.
Fig. 1.
Construction of single mouse holder and assembly into three- or four-animal holders. Sixty-milliliter plastic syringe (a) is cut open (b) to allow placement of animal. Rubber plunger is cut (d) to allow insertion to the end of a 10 ml syringe (e) to use as nose cone. (f) Shows completed single holder and (g) shows modular assembly into three- or four-mice holder using nylon screws. (h) Shows four-mice holder ready for placement of animals.
Table 1.
List of materials used to reconstruct the multiple-mice holder “hotel”
|
Phantom Scan
Cylindrical mouse phantoms (50 ml polypropylene centrifuge tubes) filled with known dose of 18F solution were scanned both individually and simultaneously in a group of four on a multi-modality Siemens Inveon microPET-CT scanner (which utilizes CT-based attenuation correction to the PET reconstruction) and on a dedicated Siemens R4 microPET scanner that does not utilize CT-based attenuation correction. The goal of this study is to assess the impact of multiple mice imaging on microPET quantitative accuracy with and without CT-based attenuation correction. The phantoms were also used to compute calibration factors of each scanner, which was used to correct the quantitation accuracy of mice image data collected using either modality.
Animal Preparation
All animal studies were carried out in compliance with Federal and local institutional rules for the conduct of animal experimentation and approved by the Stanford Institutional Animal Care and Use Committee (approval number A3213-01). All experiments strictly followed the panel’s specific guidelines regarding the care, treatment, and euthanasia of animals used in the study. Female athymic nude mice (nu/nu) were obtained from Charles River Laboratories (Boston, MA) at 7–8 weeks old and kept under sterile conditions. The experimental-group nude mice were inoculated subcutaneously in the right shoulder with 1 × 106 cultured C2C12/Ras cells. When the tumors reached 0.5–0.8 cm in diameter, measured using calipers, the tumor-bearing mice were imaged and then subjected to ex vivo biodistribution studies.
MicroPET Imaging
A total of eight mice were used for this imaging study. The first four mice with tumors were injected with a 64Cu-labeled radiotracer (64Cu-GB170) [24] with doses (51.1 µCi for mouse 1, 52.5 µCi for mouse 2, 49.2 µCi for mouse 3, and 55.4 µCi for mouse 4) via the tail vein according to the protocol specified for the imaging research project that require a series of acquisitions every 24-h intervals. For this study, imaging was performed 19 h after injection in between the first 24-h interval acquisition. The interval of 19 h was chosen based on the availability of the imaging instrument. 64Cu has the advantage of minimizing decay between imaging studies due to a relatively long half-life (12.7 h). It allows scanning at a later time point when the tracer distribution and clearance stabilizes, which may significantly minimize variations due to tracer movement between scans. We first imaged simultaneously in a four-mouse holder (Fig. 2) using Inveon microPET/CT to acquire a CT-based attenuation corrected microPET data, which is a default setting for routine microPET/CT scans. We then immediately repeated the scan using a dedicated microPET scanner (R4) without CT-based attenuation correction. Following this, we also imaged single animals sequentially using Inveon microPET/CT and then microPET (R4) scanners. For comparison and validation, we repeated the experiment on a different cohort of mice using FDG, the most common tracer in PET imaging but with much shorter half-life (1.8 h) than 64Cu (12.7 h) labeled radiotracer. The second set of four mice (without tumor) were injected with FDG (~100 µCi per animal) and imaged in groups of four and then singly 1 h post-injection to minimize inter-scan biological variations. We acquired ten scans of 5 min each static PET for each tracer (64Cu and FDG) in list mode data and reconstructed using a two-dimensional ordered subsets expectation maximum algorithm for both Inveon and R4. Before image analysis, all reconstructed images were decay-corrected and calibrated to convert the default pixel intensity unit (counts per pixel per second) into counts per milliliter (ml) per second by scanning a cylindrical phantom with known activity, assuming a tissue density of 1 g/ml. An image region of interest (ROI)-derived %ID/g of tissue was then determined by dividing counts per milliliter per second by the injected dose (ID).
Fig. 2.
Preparation of four-mice simultaneous scan using the simple reconstructed multiple mice holder “hotel.” Hotel is shown loaded on Siemens microPET R4 bed ready to position into detector ring.
Image Analysis
For image analysis, we used the Inveon Research Workplace (IRW 3.0) software (Siemens Medical Solutions USA, Inc., Knoxville, TN). Both PET image data obtained using single- and multiple-mice imaging modes were registered to the corresponding CT anatomical image. Both manually drawn and semi-automatic threshold-based ROIs were drawn on CT for the tumor and other major organs (brain, heart, liver, and kidneys). The mean ROI value was extracted from each organ for image-based biodistribution comparison. To correct for partial volume effect, we scanned four different sizes of hollow spheres with inner diameter (3.95, 3.95, 6.23, and 7.86 mm) filled with the same concentration (15 µCi/ml) of activity and each mounted in a 5 cm diameter cylindrical phantom filled with water at equal distance from the scanner center (12.7 mm). Using the recovery coefficient method [24], we corrected the microPET value of small organs such as kidneys, which initially showed notable underestimation compared with the gamma counter values as described in the following section.
Ex Vivo Biodistribution Study
Immediately after microPET imaging studies were completed (<5 min), the mice were killed, and the tumor and other major organs (including brain, heart, liver, and kidneys) were removed and individually weighted for ex vivo biodistribution using the Packard Cobra Gamma Counter (GMI inc, Ramsey, Minnesota, USA). The radioactivity counts obtained from the Gamma Counter for each sample were expressed as a percentage of the injected radioactive dose per gram of tissue (%ID/g) and used as a “gold-standard” to compare image quantitation accuracy with quantitative values extracted using imaging.
Statistical Analysis
Statistical analysis was performed using Wilcoxon rank sum test assuming independent samples from identical continuous distributions with equal median utilizing Matlab software. P<0.05 was designated as significantly different.
Results
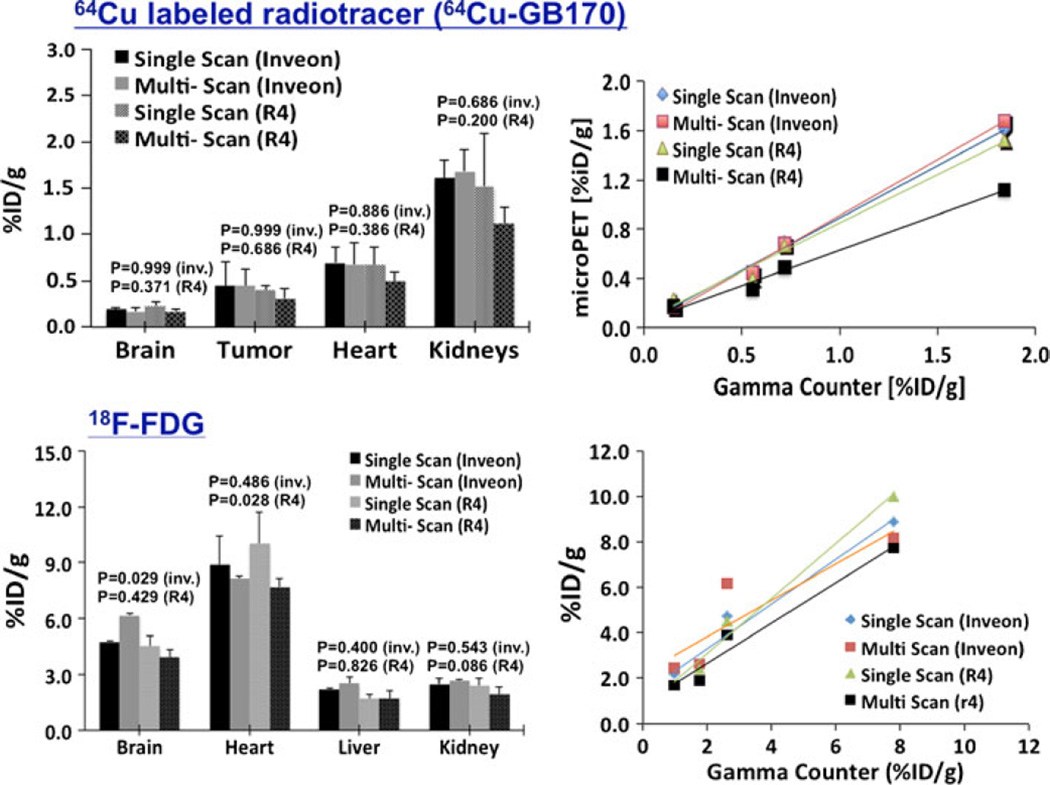
Fig. 3 shows the result of analysis of the cylindrical phantom images acquired using both single-scan and multiple-scan techniques on both microPET/CT (Inveon) and microPET (R4) scanners. The same ROIs were drawn on each one of the singly and multiply (groups of four) scanned cylindrical phantom images. From the ROIs drawn, mean ROI values were extracted and used to compute calibration factors (CF), given as ratio of dose concentration (dose/volume) of the cylindrical phantom to the mean ROI obtained from the images. The result indicates that less than 4.5 % relative difference of CF is obtained for Inveon with CT-based attenuation correction, while over 18.4 % is observed for R4 with no CT-based attenuation correction.
Fig. 3.
Comparison of computed calibration for microPET/CT (Inveon) and microPET (R4) scanners using both the single- and multiple-mice-imaging mode.
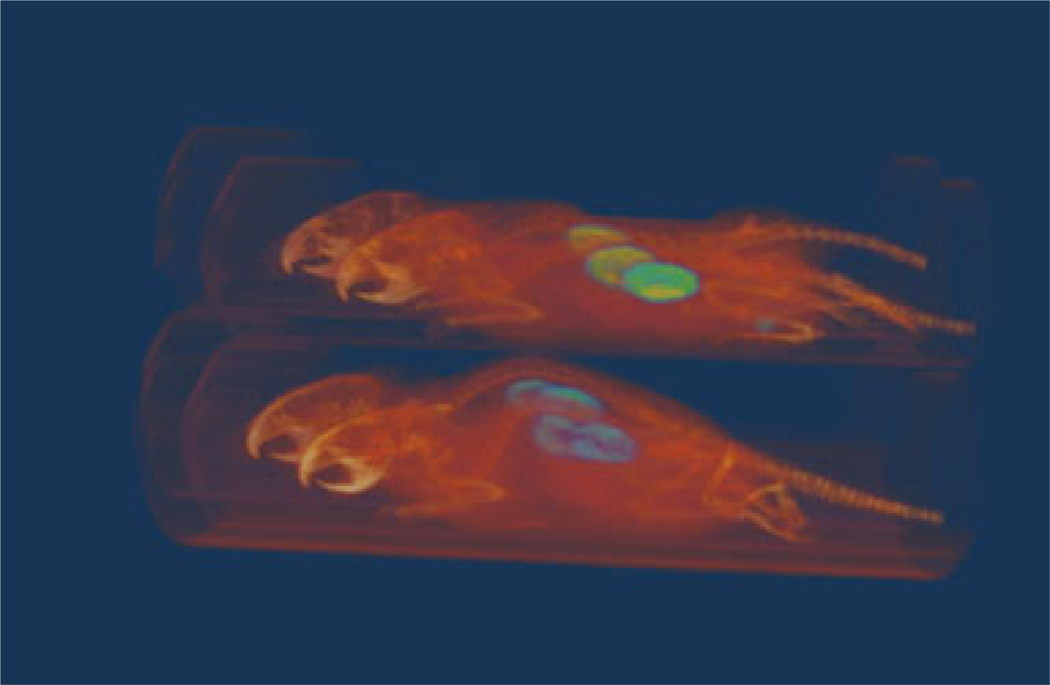
Fig. 4 shows a 3D rendering of fused microPET/CT of a four mice image that was scanned simultaneously using microPET/CT, demonstrating the image quality of multiple-mice mode images. Qualitatively, the images are similar in image quality as single-mouse mode images where all major organs and tumor are equally well visible and accessible. Quantitatively, all images, except FDG brain and heart image data, showed small difference (below acceptable statistically significant) between single- and multiple-mice mode scans when CT-based attenuation correction is used. When using 64Cu-labeled radiotracer, we observed a mean difference less than 5 % with P>0.686 (Fig. 5, top-left). For R4 (with no CT-based attenuation correction), images acquired with multiple scan-mode showed a slight drop (<11% mean difference, P>0.200) of measured mean ROI compared with single-scan mode. Due to a very low statistical power (n=4) used in the study, however, the computed P may not be so conclusive. Other than the mean difference, we furthermore observed a slightly better than average inter-animal variability (coefficient of variation<4 %) when multiple-mice scan mode is used compared with scanning animals individually. With the exception of image acquired with the multiple-mice scan mode without CT-based attenuation correction, all 64Cu-labeled images also showed similar linear increase (average slope of 0.848, R2>0.991) of tracer uptake when plotted with respect to ex vivo biodistribution study (Fig. 5, top-right) after a partial volume correction was performed using a recovery coefficient method [25].
Fig. 4.
Sample of fused 3D view of microPET/CT image for four mice simultaneously scanned.
Fig. 5.
Left: quantitation comparison between single scan and multiple mice scan mode of microPET/CT (Inveon) and dedicated microPET (R4) using four mice (n=4) imaged first in a group of four and then singly with a custom-made animal holder “hotel”; right: linear increase of uptake in selected organ of mouse versus the ex vivo gamma counter measurements assumed as “gold standard.” The solid lines show the respective linear fit of the extracted mean ROI values from the images. The computed P confirms the small difference (below acceptable statistically significant) between single- and multiple-mice mode scans though P may not be so conclusive due to a very low statistical power (n=4) used in the study.
For FDG, the measured mean ROI difference between single- and multiple-mice mode scans was less that 15 % for both modalities with P>0.4 except for brain image data with P=0.029 (Fig. 5, bottom-left). The FDG study provided slightly greater differences compared with 64Cu-labeled. The short half-life of FDG and faster radiotracer kinetics between back-to-back FDG scans may account some of the differences observed in this part of the study. However, after partial volume correction, FDG image data were also linearly correlated with the gamma counter data (average slope, 0.969; R2>0.924).
Discussion
Our study showed the potential use of the Inveon microPET/CT scanner for multiple mice simultaneous imaging without a significant degradation in image quality, which also agrees to a similar study performed by Aide et al. [22]. In addition, the Inveon aided by CT-based attenuation correction provided adequate quantitation accuracy compared with single-mouse scan. For applications that require increasingly high throughput, we also showed that the dedicated microPET systems without CT-based attenuation can be used to scan multiple animals with slightly reduced quantitation accuracy (<15 %), especially when the correct calibration factor suitable for the number of animals to be scanned is used. The multiple-mice scanning technique has a major benefit of saving investigators time and money. A routine 5-min static PET scan in our small-animal-imaging facility requires approximately 15 min of scan time in Inveon scanner, including the CT scan for attenuation correction. At current rates, this will cost an investigator about $28.00 per scan. The use of simple mice “hotel” developed in our lab to scan at least four mice provides a fourfold cost-reduction on the usage of the instrument without including the gain in the cost of the trace, which is needed in much lower dose to perform the planned scans due to reduced the reduce total scan time. If dedicated microPET is used, more than a twofold increase in the number of animals to be scanned is possible at the same scanning cost and time demonstrating the real benefit of high-throughput microPET. The increase in the number of animals in the study without increasing imaging cost and time will increase the size of the cohort and the statistical power, which is important in improving the quality of research per study.
One potential issue with multiple mice scanning is the animal handling, preparation, and anesthesia supply generally given using isoflurane inhalation. We have successfully imaged short static (5–10 min) images of up to seven mice sharing the same isoflurane supply channel using three- and four-way branch Luer tubing assembly connections to split the single isoflurane gas chamber into seven. Ideally, however, the next generation of customized mouse “hotels” including heated cover with temperature and respiratory pads will ensure more uniform animal scanning with relatively longer time for dynamic studies.
Multiple-Mice, Multi-modality Imaging Application
The 60-ml syringe was selected as the individual mouse holder unit due to its ability to comfortably hold a typical adult mouse (up to 30 g), disposability if required, and ease of cutting to allow assembly into a multiple animal hotel. Additionally, the material used in the syringe has minimal impact on any of the modalities in which the holder might be used such as PET, CT, SPECT, and MRI. Removal of a section of the top of the syringe allows easy placement of mice into each holder, and removal of the lower tip (Luer connector) allows insertion of a cut-off 10 ml syringe barrel as an isoflurane anesthesia nose cone. Removal of the edges from the other end of the syringe barrel permits efficient stacking to allow three (2+1), four (2+2), and seven (2+3+2) multiple animal holders to be assembled using nylon screws allowing fast assembly and disassembly for efficient animal loading in the scanner (Fig. 1). Single holders are often used for small field of view scanners such as 4 cm axial×6 cm transaxial CT scanner, while three- and four-mice holders are routinely used on the GE CT120 microCT scanner, Gamma Medica Flex SPECT/CT, Siemens microPET R4, and Siemens Inveon microPET/CT scanners at our imaging facility.
Conclusion
When CT-based attenuation correction is performed, scanning single or multiple mice provides equivalent quantification of uptake of small-animal PET images and that use of the mouse “hotel” is a valid aid to increasing instrument throughput on small animal scanners. Routine use of the three- and four-animal holders has allowed a significant increase in the throughput of animal studies performed at the Stanford Small Animal Imaging Facility, as well as permitting improved utilization of radiotracers, which are often the most expensive and time-consuming reagents to prepare. This will be especially valuable for studies involving short half-life radionuclide probes such as 11C (20.4 min) and 13N (10.0 min) when single animal scanning may result is unacceptable probe decay between sequential scans.
Acknowledgments
The authors acknowledge the use of imaging instruments and image analysis support in the Stanford Imaging Facility. This work was supported by grants NCI P50 CA114747 and NIH U54 CA119367 and by the Stanford Cancer Center.
Footnotes
Conflict of Interest. No other potential conflict of interest relevant to this article exists.
References
- 1.Henkelman M. Systems biology through mouse imaging centers: experience and new directions. Ann Rev Biomed Eng. 2010;12:143–166. doi: 10.1146/annurev-bioeng-070909-105343. [DOI] [PubMed] [Google Scholar]
- 2.Agdeppa ED, Spilker ME. A review of imaging agent development. The AAPS Journal. 2009;11:286–299. doi: 10.1208/s12248-009-9104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hargreaves RJ. The role of molecular imaging in drug discovery and development. Clin Pharmacol Ther. 2008;83:349–353. doi: 10.1038/sj.clpt.6100467. [DOI] [PubMed] [Google Scholar]
- 4.Ahn BC. Applications of molecular imaging in drug discovery and development process. Curr Pharm Biotechnol. 2011;12:459–468. doi: 10.2174/138920111795163904. [DOI] [PubMed] [Google Scholar]
- 5.Medarova Z, Pham W, Kim Y, Dai G, Moore A. In vivo imaging of tumor response to therapy using a dual-modality imaging strategy. Int J Cancer. 2006;118:2796–2802. doi: 10.1002/ijc.21672. [DOI] [PubMed] [Google Scholar]
- 6.Michalski MH, Chen X. Molecular imaging in cancer treatment. Eur J Nucl Med Mol Imaging. 2011;38:358–377. doi: 10.1007/s00259-010-1569-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pike LS, Tannous BA, Deliolanis NC, et al. Imaging gene delivery in a mouse model of congenital neuronal ceroid lipofuscinosis. Gene Ther. 2011;18:1173–1178. doi: 10.1038/gt.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yong J, Rasooly J, Dang H, et al. Multimodality imaging of B-cells in mouse models of type I and II diabetes. Diabetes. 2011;60:1383–1392. doi: 10.2337/db10-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyons S. Advances in imaging mouse tumour models in vivo. Journal of Pathology. 2005;205:194–205. doi: 10.1002/path.1697. [DOI] [PubMed] [Google Scholar]
- 10.Golestani R, Wu C, Tio RA, et al. Small-animal SPECT and SPECT/CT: application in cardiovascular research. Eur J Nucl Med Mol Imaging. 2010;37:1766–1777. doi: 10.1007/s00259-009-1321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roncali E, Cherry SR. Application of silicon photomultipliers to positron emission tomography. Annals of Biomedical Engineering. 2011;39:1358–1377. doi: 10.1007/s10439-011-0266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton CS, Ma Y, Smith SD, Benveniste H. High resolution 3D in vivo mouse brain imaging at 9.4 T bruker MRI system-Bioengineering Conference; 2007 NEBC '07 IEEE 33rd Annual Northeast; 2007. pp. 45–46. [Google Scholar]
- 13.Hong H, Yang Y, Cai W. Imaging gene expression in live cells and tissues. Cold Spring Harbor Protoc. 2011:354–365. doi: 10.1101/pdb.top103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kluanberg BA, Davis JA. Considerations for laboratory animal imaging center design and setup. ILAR J. 2008;49:4–16. doi: 10.1093/ilar.49.1.4. [DOI] [PubMed] [Google Scholar]
- 15.Pauux AL, Ong LC, Teh I, et al. Comparison of imaging techniques to monitor tumor growth and cancer progression in living animals. Int J Mol Imaging. 2011 doi: 10.1155/2011/321538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen TE, Yoder KK, Normandin MD, et al. A rat head holder for simultaneous scanning of two rats in small animal PET scanners: design, construction, feasibility testing and kinetic validation. J Neurosci Methods. 2009;176:24–33. doi: 10.1016/j.jneumeth.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bock NA, Konyer NB, Henkelman RM. Multiple-mouse MRI. Magnetic Resonance Med. 2003;49:158–167. doi: 10.1002/mrm.10326. [DOI] [PubMed] [Google Scholar]
- 18.Dazai J, Spring S, Cahill LS, Henkelman RM. Multiple-mouse neuroanatomical magnetic resonance imaging. J Vis Exp. 2011:e2497. doi: 10.3791/2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siepel FJ, Van Lier MGJTB, Chen M, et al. Scanning multiple mice in a small-animal PET scanner: influence on image quality. Nuclear Instruments and Methods in Physics Research A. 2010;621:605–610. [Google Scholar]
- 20.Disselhorst JA, Boerman OC, Oyen WJG, Slump CH, Visser EP. Spatial resolution of the Inveon small-animal PET scanner for the entire field of view. Nuclear Instruments and Methods in Physics Research A. 2010;615:245–248. [Google Scholar]
- 21.Aide N, Cd D, Ml B, Meryet-Figuiere M, Poulain L. High-throughput small animal PET imaging in cancer research: evaluation of the capability of the Inveon scanner to image four mice simultaneously. Nuclear Medicine Communications. 2010;31:851–858. doi: 10.1097/MNM.0b013e32833dc61d. [DOI] [PubMed] [Google Scholar]
- 22.Sheruma N, Peter LK, Wencke L, Steven RM. Maximizing the useful field of view of the microPET: feasibility of imaging large animals; IEEE Nuclear Science Symposium Conference Record; 2006. pp. 1853–1856. [Google Scholar]
- 23.Aide N, Visser P, Lheureux S, Heutte N, Szanda I, Hicks R. The motivations and methodology for high-throughput PET imaging of small animals in cancer research. Eur J Nucl Med Imaging. 2012;30:1497–1509. doi: 10.1007/s00259-012-2177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren G, Blum G, Verdoes M, et al. Non-invasive imaging of cysteine cathepsin activity in solid tumors using a 64Cu-labeled activity-based probe. PLoS ONE. 2011;6:e28029. doi: 10.1371/journal.pone.0028029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srinivas M, Dhurairaj T, Basu S, Bural G, Surti S, Alavi A. A recovery coefficient method for partial volume correction of PET images. Ann Nucl Med. 2009;23:341–348. doi: 10.1007/s12149-009-0241-9. [DOI] [PubMed] [Google Scholar]