Abstract
In osteoclastogenesis, the intercellular adhesion molecule (ICAM)-1 provides a high-affinity adhesion between the osteoblast and the osteoclast precursor, thereby facilitating the interaction between receptor activator nuclear factor κB ligand (RANKL) and its receptor RANK. However, the role of soluble ICAM (sICAM) in that process remains obscure. Therefore, the purpose of this study was to determine whether sICAM and ICAM-1 play an active role in the formation and maturation of osteoclasts. Monocytes isolated from healthy donors and cultured alone or with human osteoblast were stimulated with macrophage colony-stimulating factor, sRANKL, ICAM-1 monoclonal antibody (mAb), leucocyte function antigen (LFA)-1 mAb, and/or sICAM to produce mature osteoclasts. Release of TRAP 5b and resorption area were analyzed as markers of osteoclast formation and function, respectively. The effect of ICAM-1 and sICAM stimulation on apoptosis, cathepsin K, αvβ3, collagen-1, and on RANKL/osteoprotegerin (OPG)/RANK expression was evaluated. sICAM did not modify the release of TRAP 5b from osteoclast precursors in both mono and co-culture, but induced a significant increase in resorption area in both culture systems, as well as a positive effect on cathepsin K and αvβ3 protein expression. Cross-linking ICAM-1 on osteoblast resulted in increased RANKL mRNA and caspase-3 protein expression, decreased collagen-1 mRNA expression, and decreased osteoblast survival. Stimulation of preosteoclast with sICAM produced a significant increase in preosteoclast survival and a decrease in caspase-3 expression. These results indicate that ICAM-1 and sICAM have a dual effect on bone homeostasis, increasing osteoclast activity while lowering osteoblast anabolic activity.
Keywords: osteoclast, ICAM-1, sICAM, bone resorption, adhesion molecules
Introduction
Skeletal homeostasis is maintained at the cellular level by the actions of osteoblasts and osteoclasts, respectively forming and removing bone. This dynamic process is continuously adjusting to the physiological needs of the body. When an imbalance between bone formation and resorption occurs, it generally leads to skeletal disease. Most of these diseases are the consequence of an increase in osteoclast activity, resulting in net bone loss [1]. Osteoclasts are multinucleated giant cells from the monocyte/macrophage lineage. Numerous mediators and cellular events are needed to allow the differentiation of monocytes into mature osteoclast. The paramount importance of receptor activator of nuclear factor-κB ligand (RANKL) and its receptor (RANK) is well described in this process [2,3]. In vivo, interactions between osteoblast/stromal cells and osteoclast precursors appear essential for osteoclastogenesis. The importance of the intercellular adhesion molecule (ICAM)-1 in osteoclast formation is already described. It is believed that the high-affinity cellular adhesion supported by ICAM-1 facilitates the interaction of RANKL with its receptor RANK, leading to increased bone resorption [4–6].
In skeletal diseases, ICAM-1 expression is increased in the synovium of rheumatoid arthritis (RA) and osteoarthritis (OA) patients and in patients suffering from aseptic loosening of orthopedic implants [7]. We have also demonstrated a specific ICAM-1 expression on osteoblasts recovered from osteoporotic and OA patients [8]. Although these reports indicate the presence of ICAM-1 in osteoclast-mediated diseases, there is no particular role identified so far for ICAM-1 in bone metabolism other than the stabilization of cellular interaction.
ICAM-1 also exists in a soluble isoform (sICAM), but no specific physiological function is identified so far in bone. In other settings, sICAM acts as a competitive inhibitor of ICAM-1/leucocyte function antigen (LFA, β2-integrin)-1 interaction [9,10], while others have reported sICAM ability to induce intracellular signaling and produce inflammatory mediators such as tumor necrosis factor-α (TNF-α), interferon-γ, interleukin-6 (IL-6), and macrophage inflammatory protein 1 and 2 (MIP-1α, MIP-2) [11–14]. Interestingly, TNF-α, IL-6, and MIP-1α have all been described as modulators of osteoclast activity [15–18]. However, it is unknown whether sICAM could affect osteoclast maturation and formation secondary to its action on osteoclast differentiation factors (ODF).
Finally, it was demonstrated that both ICAM-1 and sICAM could affect cell viability in bone. The ICAM-1+ osteoblast phenotype, which is capable of supporting osteoclast differentiation as opposed to the ICAM-1− phenotype, appears to decrease osteoblast survival while the presence of sICAM tends to protect the osteoblast against cell death [19,20].
Therefore, the objective of this study is to clarify the actions of ICAM-1 and sICAM in osteoclast formation and function. We asked the following questions: (1) Can sICAM affect osteoclast differentiation and function through a modification of the RANKL/RANK/ osteoprotegerin (OPG) profile and/or cell viability? (2) Can the engagement of ICAM-1 affect the RANKL/RANK/OPG axis and/or cell viability? Our hypothesis is that both ICAM-1 and sICAM play an active role in the formation and function of osteoclast through an effect on the RANKL/RANK/OPG axis and/or cell survival. By incubating sICAM and anti-ICAM-1 monoclonal antibody with osteoclast precursors and osteoblasts, we hope to simulate the ICAM-1/LFA-1 interaction, which should allow us to study the specific role(s) of this axis in osteoclast metabolism.
Materials and methods
Isolation and culture of human monocytes
Monocytes were isolated from the peripheral blood of three healthy male subjects (age range, 31–38 years) on several occasions. Blood was then diluted 1 : 1 in α-minimal essential medium (MEM; Sigma, St. Louis, MO, USA) and 3 volumes of blood mixture were layered onto 1 volume of Ficoll-Paque (Amersham Biosciences, Uppsala, Sweden). After centrifugation for 20 min at 693 g, the interface layer was resuspended in MEM and centrifuged for 10 min at 510 g. Cells were then incubated 20 min at 4°C with MACS CD14 Microbeads (Miltenyi Biotec, Auburn, CA, USA) and passed through a MACS magnetic cell separator. The sorted CD14+ fraction was washed, resuspended in MEM, and counted in a hemocytometer following lysis of red blood cell with a 5% (v/v) acetic acid solution. Then, 2 × 106 peripheral blood mononuclear cells (PBMC) in MEM were added to 12-well culture plates, or 1 × 106 PBMCs were added to a 16-well BD BioCoat Osteologic Bone Cell Culture System plate (BD Biosciences, Mississauga, ON, Canada) for 2 h at 37°C in 5% CO2; cultures were then rinsed with the medium to remove nonadherent cells. Cells were cultured in MEM, 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, and 100 g/ml streptomycin (Wisent, St. Bruno, QC, Canada; MEM/FBS) at 37°C in 5% CO2. Medium was changed every 3 days and supplemented or not with appropriate mediators as per specific experimental conditions tested.
Co-cultures of osteoblasts and monocytes
Human normal osteoblasts were purchased from Promocell (Heidelberg, Germany) and cultured in 25 cm2 plastic cell culture flasks (Corning, NY, USA) with BGJb media containing 10% FBS until confluency. Once confluent, osteoblasts were stimulated overnight with 10 ng/ml TNF-α (to increase ICAM-1 expression), then removed from the flask and added to preincubated PBMCs (M-CSF, 50 ng/ml for 72 h) on a 12-well plate or on a 16-well BD BioCoat Osteologic Bone Cell Culture System plate in a ratio of 1:10. Cells were washed with culture media after 8 h and then incubated with or without M-CSF, sICAM, anti-ICAM-1 monoclonal antibody (mAb), anti-LFA-1 mAb, or sRANKL. Medium was replaced twice a week, and supernatants were kept for further testing.
Assessment of osteoclast formation
After 20 days of incubation with various mediators, osteoclast formation was assessed by the presence of osteoclast-derived TRAP 5b in the supernatant of the BD BioCoat Osteologic Bone Cell Culture System plate. TRAP-5b was measured in samples from each condition using an enzyme-linked immunoassay (ELISA) kit purchased from Suomen Bioanalytiikka Oy (Turku, Finland) and corresponds to the supernatant of days 17 to 20 of our experiments. Assay sensitivity is 0.06 U/l, and experiments were performed according to the manufacturer’s specifications. Absorbance was measured with a microELISA Vmax photometer (Molecular Devices, Menlo Park, CA, USA).
Assessment of osteoclast function
Functional evidence of osteoclast formation was evaluated by measuring the resorption area on the BD BioCoat Osteologic Bone Cell Culture System plate after 20 days of culture. Cells were first washed from wells using 1 N NH4OH for three periods of 5 min and then rinsed in phosphate-buffered saline (PBS). Resorption area was determined using a Microst Automated Image Analyzer (Millenium Biologix, Mississauga, ON, Canada). Specimens from each experimental condition were read twice and the results expressed as percentage of resorption of the substrate.
RANKL, RANK, OPG, and collagen-1 expression from preosteoclasts and osteoblasts
Monocytes cultured in 12-well plates were stimulated with M-CSF (50 ng/ml) and sRANKL (30 ng/ml) for 10 days before sICAM stimulation (16 h at 37°C in 5% CO2). Osteoblasts on 12-well plates were incubated overnight with 10 ng/ml TNF-α, washed three times with PBS, and stimulated for 16 h at 37°C in 5% CO2 with or without anti-ICAM-1 monoclonal antibody (mAb) (Dako, Mississauga, ON, Canada) (100 ng/ml, 500 ng/ml, or 1 μg/ml).
RANKL, RANK, and OPG mRNA expression (Table 1) was next verified with reverse transcriptase-polymerase chain reaction (RT-PCR). Total RNA was extracted from preosteoclasts or osteoblasts using Trizol reagent (Invitrogen Life Technologies, Burlington, ON, Canada) according to the manufacturer’s specifications. After precipitation, pellets were washed in 70% ethanol, resuspended in RNase-free water, the amount of RNA determined using SyberGreen, and then stored at –80°C. RT-PCR cDNA synthesis was carried out using 1 μg total RNA and a SuperScript one-step RT-PCR kit manufactured by Invitrogen Life Technologies. The oligonucleotide primers were specific, and sequences are reported in Table 1. The PCR products were analyzed and verified by electrophoresis on 1.2% agarose gels in a Tris-borate-ethylenedi-aminetetraacetic acid (EDTA) buffer. Semiquantitative measurements of the reaction products were made by taking density readings using a digital imaging system (G-image 2000; Canberra Packard Canada, Mississauga, ON, Canada). Results were calculated as relative units to GAPDH (n = 5 from each condition). Quantitative PCR for collagen 1 was performed using 25-μl reaction products with SYBR Green master mix (Qiagen). After an activation phase (10 min at 95°C), amplification was performed for 40 cycles at 95°C for 15 s and 60°C for 60 s. Incorporation of the dye into PCR products was monitored using a Mx3000P spectrofluorometric thermal cycler (Stratagene). The threshold was set above the nontemplate control background and within the linear phase of target gene amplification to calculate the cycle number at which the transcript was detected (n = 5).
Table 1.
Reverse transcriptase-polymerase chain reaction (RT-PCR) primers
| Molecules | Primers |
|---|---|
| GAPDH | 5′-CCACCCATGGCAAATCCATGGCA-3′(s) |
| 5′-TCTAGACGGCAGGTCAGGTCCAQCC-3′(as) | |
| RANKL | 5′-GCCAGTGGGAGATGTTAG-3′(s) |
| 5′-TTAGCTGCAAGTTTTCCC-3′(as) | |
| RANK | 5′-TTAAGCCAGTGCTTCACGGG-3′(s) |
| 5′-ACGTAGACCACGATGATGTCGC-3′(as) | |
| OPG | 5′-GCTAACCTCACCTTCGAG-3′(s) |
| 5′-TGATTGGACCTGGTTACC-3′(as) | |
| Collagen-1 | 5′-GGACACAATGGATTGCAAGG-3′(s) |
| 5′-TAACCACTGCTCCACTCTGG -3′ (as) |
Cell viability
PBMC were plated onto 12-well plates in MEM/FBS and incubated for 10 days with M-CSF (50 ng/ml) and sRANKL (30 ng/ml) to form preosteoclasts (5% CO2 at 37°C). Media was changed every 3 days and supplied with fresh mediators. At 10 days, preosteoclasts were rested overnight in mediator-free media before the addition of increasing concentrations of sICAM for 24 h. Normal human osteoblast were plated onto 12-well plates and cultured overnight in BGJb +10% FBS +1% PS (5% CO2 at 37°C) before the addition of increasing concentrations of anti-ICAM-1 mAb for 24 h. At the end of the incubation period, an MTT assay was performed on preosteoclasts and osteoblasts. Briefly, preosteoclast and osteoblast cultures were washed with PBS, and 500 μl culture medium containing 50 μl 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide (MTT) reagent was added for 1 h before the absorbance was measured at 440 nm (5% CO2 at 37°C). Each condition was repeated five times (n = 5).
Western blot
Caspase-3, cathepsin K, αvβ3 integrin, and β-actin protein expression were determined from either osteoclasts or normal osteoblasts (antibodies from Calbiochem, San Diego, CA, USA). Briefly, 20 μg protein extract was subjected to 12% sodium dodecyl (SDS)-polyacrylamide gel electrophoresis under reducing conditions and transferred onto nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). The membrane was next rinsed in TTBS (Tris 20 nM, NaCl 137 mM, 0.1% Tween 20, pH 7.4), saturated overnight at 4°C under agitation in TTBS containing 5% (w/v) skimmed milk before the addition of the first antibody and incubation at room temperature for 1 h. The membrane was then washed several times with TTBS and incubated again 1 h with the second antibody (antirabbit IgG-HRP; New England Biolabs, Ipswich, MA, USA). Detection was carried out using LumiGLO Chemiluminesent Substrat (Cell Signaling Technology, Beverly, MA, USA) membranes exposed to Kodak X-Omat film (Eastman Kodak, Rochester, NY, USA) and then subjected to a digital imaging systems (G-image 2000) for protein measurement.
Statistical analysis
The results are expressed as mean ± SEM. Assays were performed in triplicate unless otherwise stated. The data were analyzed statistically by the two-tailed Mann–Whitney U test. P values < 0.05 were considered significant.
Results
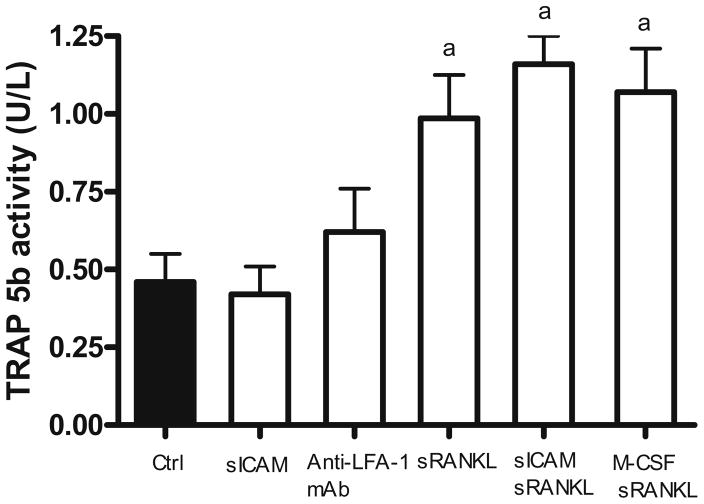
First, we wanted to verify whether sICAM could induce the differentiation of human monocytes into osteoclasts (Fig. 1). In this setting, tartrate-resistant acid phosphatase (TRAP)-5b activity measured from supernatant of human monocytes cultures reflects the number of osteoclasts formed. Compared to control (no mediator), significant changes in TRAP-5b activity were observed when monocytes were incubated with sRANKL alone (P < 0.005) or in combination with M-CSF (P < 0.001), which serves as a positive control. However, no change in TRAP-5b activity was demonstrated following sICAM or anti-LFA-1 mAb stimulation alone. The combination of sICAM and sRANKL induced the highest TRAP-5b activity, but this failed to reach significance when compared to sRANKL stimulation alone. These results indicate that sICAM or LFA-1 engagement does not appear to influence differentiation of monocytes into osteoclasts in vitro.
Fig. 1.
Differentiation of human monocytes into osteoclasts. Peripheral blood mononuclear cells (PBMC) were cultured for 20 days onto BD BioCoat Osteologic Bone Cell Culture System plate (n = 8). Osteoclast formation was assed by an enzyme-linked immunosorbent assay (ELISA) specific for osteoclast-derived TRAP 5b released in the supernatant (days 17 to 20). Results are expressed as mean ± SEM. a P ≤ 0.05 group vs. control. M-CSF, 50 ng/ml; sRANKL, 30 ng/ml; sICAM, 1 μg/ml; anti-LFA-1 mAb, 1 μg/ml
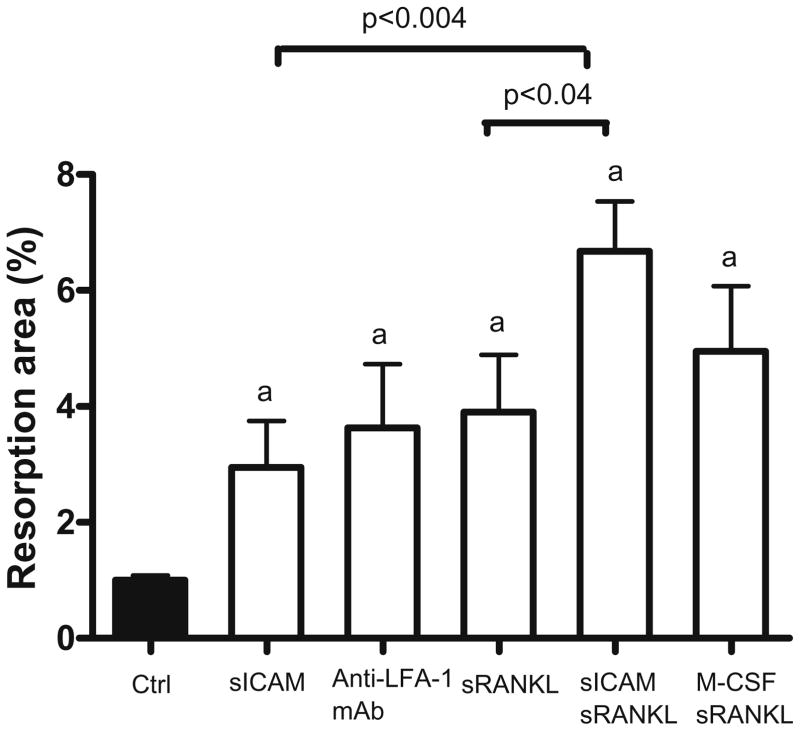
We then sought to determine if sICAM could influence the resorption capacity of osteoclasts formed from human monocytes in vitro (Fig. 2). Interestingly, compared to control, there was a 3-fold increase in resorption area when cells were stimulated with sICAM alone (P < 0.02), while incubation with sRANKL alone resulted in a 4-fold increase in resorption area (P < 0.009). Cross-linking LFA-1 receptors with anti-LFA-1 mAb resulted in a 3.3-fold increase in resorption area (P < 0.01), which is comparable to sICAM action. The combined effect of M-CSF and sRANKL resulted in a 5-fold increase in resorption surface (P < 0.002). The most important increase in osteoclast resorption area was observed when sICAM and sRANKL were combined together (6.5-fold increase; P < 0.0001), which resulted in statistically significant variations compared to individual effects of these effectors (sICAM + sRANKL vs. sICAM, P < 0.0004; sICAM + sRANKL vs. sRANKL, P < 0.04). Taken together, these results indicate that sICAM can positively modulate osteoclast function.
Fig. 2.
Resorption area following monocytes stimulation with various mediators. PBMC were cultured for 20 days onto BD BioCoat Osteologic Bone Cell Culture System plate (n = 8). Osteoclast function was assed by computer analysis of the resorption area. Results are expressed as mean ± SEM. a P ≤ 0.05 group vs. control. M-CSF, 50 ng/ml; sRANKL, 30 ng/ml; sICAM, 1 μg/ml; anti-LFA-1 mAb, 1 μg/ml
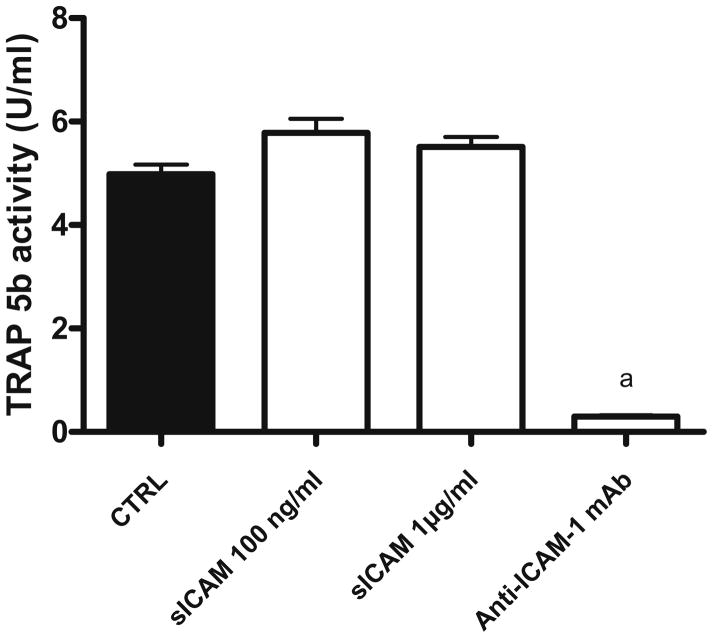
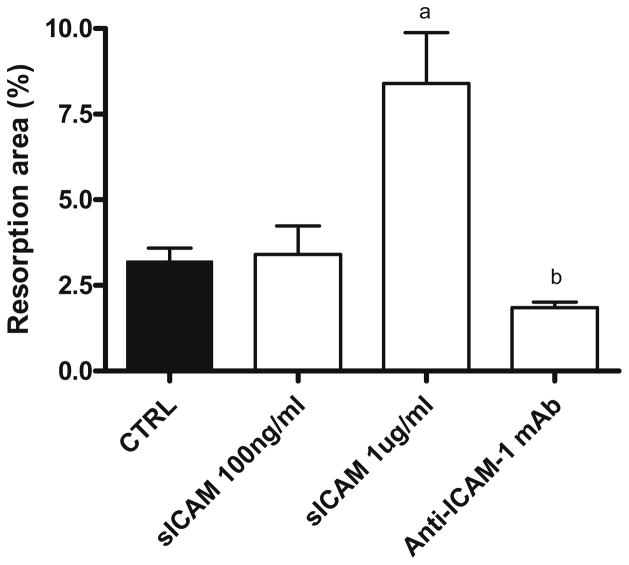
To verify to which extent sICAM could actually affect osteoclast maturation when compared to membrane-bound ICAM-1, we co-cultured osteoblasts with preosteoclasts in different conditions and looked at osteoclast formation (Fig. 3). When compared to control, the addition of anti-ICAM-1 mAb almost completely inhibited osteoclast formation, while sICAM did not significantly modify TRAP-5b activity. However, osteoclast function (Fig. 4), as measured by resorption area in wells, was significantly increased in the presence of sICAM (2.6-fold) whereas anti-ICAM-1 mAb significantly inhibited it. Comparing monocultures and co-cultures (Figs. 2, 4), it is interesting to note that sICAM induces the same increase in the resorption area in both culture systems (close to 2.5 fold), confirming its importance. Further, in co-culture, both TRAP-5b activity and the resorption area following the addition of anti-ICAM-1 mAb are similar to values observed in monoculture.
Fig. 3.
TRAP-5b activity following coculture of human preosteoclast and osteoblast with various mediators. Osteoblast and PBMC were plated onto BD BioCoat Osteologic Bone Cell Culture System plate (n = 8) for 20 days. Osteoclast formation was assed by an ELISA specific for osteoclast-derived TRAP 5b released in the supernatant (days 17 to 20). Results are expressed as mean ± SEM. a P ≤ 0.05 group vs. control. Anti-ICAM-1 mAb, 2 μg/ml
Fig. 4.
Resorption area following coculture of human preosteoclast and osteoblast with various mediators. Osteoblasts and PBMC were plated onto BD BioCoat Osteologic Bone Cell Culture System plate (n = 8) for 20 days. Osteoclast function was assed by computer analysis of the resorption area. Results are expressed as mean ± SEM. a P ≤ 0.01 group vs. control; b P ≤ 0.0001 group vs. control. Anti-ICAM-1 mAb, 2 μg/ml
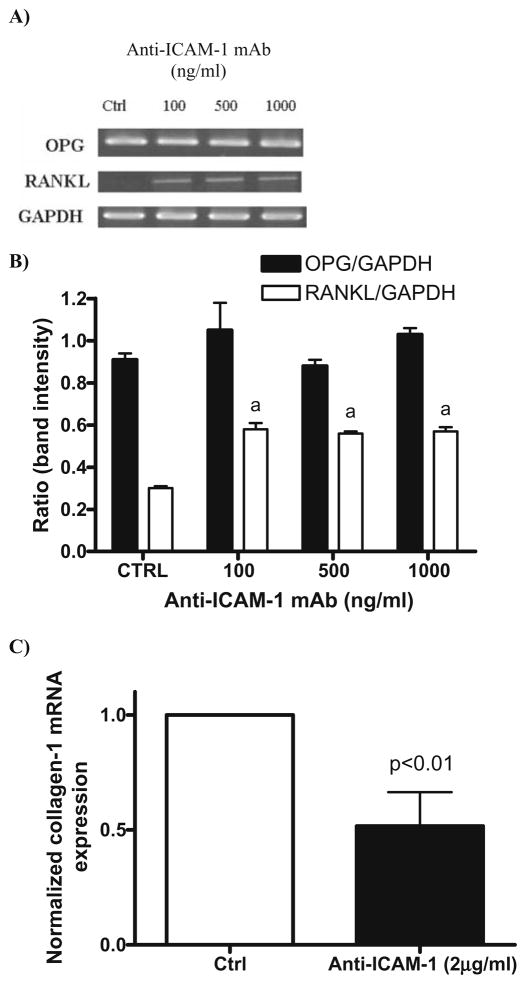
We next evaluated if the effect of both ICAM-1 and sICAM on osteoclastogenesis could be explained in part by their respective actions on the OPG/RANK/RANKL axis (Fig. 5). sICAM did not affect the expression of RANK (in preosteoclasts), or RANKL or OPG (in osteoblasts) (data not shown). However, RANKL expression by osteoblasts was significantly increased when membrane ICAM-1 was cross-linked with specific mAb (2-fold, P ≤ 0.01). Further, this increase in RANKL expression is accompanied by a 50% decrease in collagen type 1 mRNA expression. These results show that ICAM-1 does play a direct role in osteoclast formation and function in addition to its role as a stabilizer of osteoblasts and preosteoclasts. Moreover, it appears that the ICAM-1 phenotype on osteoblast facilitates bone resorption over bone deposition. However, the action of sICAM in this setting does not appear to be secondary to an effect on the OPG/RANK/RANKL axis.
Fig. 5.
RANKL, OPG, and collagen-1 expression by human osteoblasts following ICAM-1 engagement. Normal human osteoblasts were cultured overnight with increasing concentrations of anti-ICAM-1 mAb. A RANKL, OPG, and GAPDH expression were analyzed with semiquantitative RT-PCR. B Results expressed as the ratio of RANKL/ GAPDH or OPG/GAPDH band intensity. C Normalized collagen-1 expression assessed by qRT-PCR. Results represent the mean and the SEM of 5 experiments (n = 5). a P ≤ 0.01 group vs. control
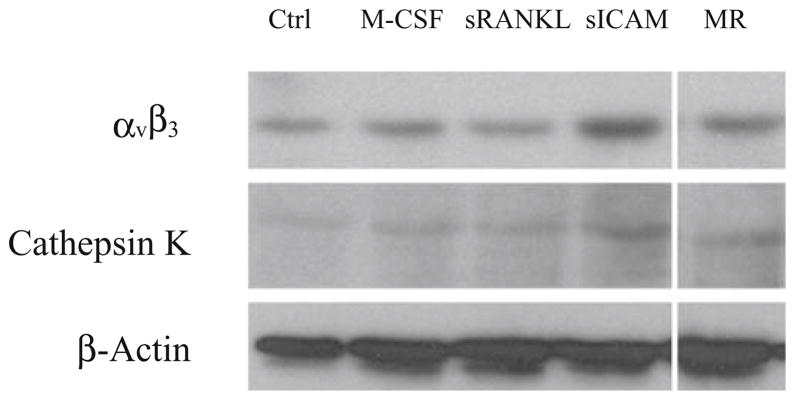
Because sICAM does not affect osteoclast differentiation but increases the resorption area, and given that it does not alter the OPG/RANK/RANKL axis, we looked at its action on proteins involved in osteoclast function (Fig. 6). Interestingly, sICAM induced the highest cathepsin K and αvβ3 protein expression amongst the mediators tested. This observation explains, at least in part, the action of sICAM on osteoclast function.
Fig. 6.
Action of sICAM on cathepsin K and αvβ3 protein expression. PBMC were cultured for 20 days in the presence of M-CSF (50 ng/ml), sRANKL (30 ng/ml), and/or sICAM (1 μg/ml). Protein expression was analyzed by Western blot (n = 3). MR, M-CSF + sRANKL
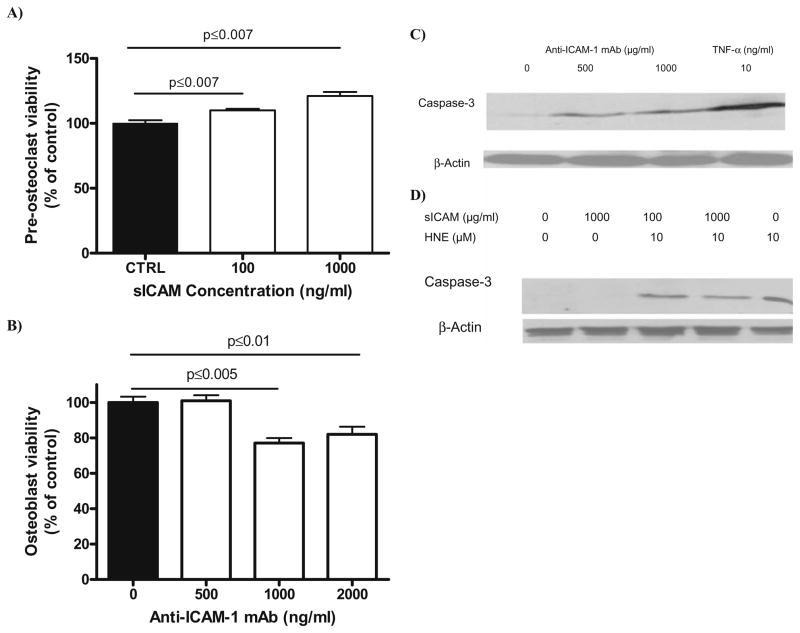
Finally, we tested the effect of sICAM and ICAM-1 on preosteoclast and osteoblast survival, as well as caspase-3 protein expression (Fig. 7). sICAM induced a significant increase in preosteoclast survival compared to control (20% increase) as opposed to anti-ICAM-1 mAb, which induced a 20% decrease in osteoblast viability. When we looked at caspase-3 protein expression, a similar effect was observed where preosteoclast caspase-3 expression appeared to be lowered when cells were pretreated with sICAM, while engagement of ICAM-1 on osteoblast stimulated the expression of caspase-3. Together, these observations indicate the ability of both mediators to modify cell survival, possibly through an action on the apoptotic cascade.
Fig. 7.
Survival analysis and caspase-3 protein expression in preosteo-clast and normal human osteoblasts following incubation with sICAM or Anti-ICAM-1 mAb. Preosteoclasts incubated overnight with increasing concentration of sICAM (A) and normal human osteoblasts incubated overnight with increasing concentration of anti-ICAM-1 mAb (B) were submitted to an MTT assay and cell viability recorded. Results are expressed as mean ± SEM (n = 5). Caspase-3 protein expression assessed by western blot in osteoblasts (C) and preosteoclasts (D). Osteoblasts were incubated overnight with anti-ICAM-1 mAb and caspase-3 protein expression was recorded (n = 3). Preosteoclasts were pretreated overnight with sICAM and then incubated in the presence of HNE for 2 h before caspase-3 protein expression determination (n = 3). HNE, 4-hydroxynonenal
Discussion
Cell–cell interactions between the osteoblasts/stromal cells and osteoclast precursors are considered a prerequisite for osteoclast maturation and function [5,6,21–23]. Some of these interactions are mediated through adhesion molecules such as ICAM-1, whose membrane expression in osteoblast was previously described [8,19]. Because its presence on osteoblasts is increased following exposure to mediators involved in osteoclast maturation and because both osteoclast maturation and function can be blocked by antibody directed against it, ICAM-1 is considered an important mediator in osteoclastogenesis [4–6]. However, apart from providing a high-affinity adhesion between the osteoblast and the osteoclast precursor, no other action has been described so far for ICAM-1 in the setting of bone metabolism [4]. Further, we are not aware of any reports on the potential role of sICAM in osteoclast development. In this study, we report for the first time an increase in osteoclast function and survival mediated through sICAM. Moreover, we also report that ICAM-1 engagement on osteoblasts leads to increased RANKL mRNA expression, decreased collagen type-1 mRNA expression, and decreased osteoblast survival.
The finding of an isolated increase in osteoclast function following exposure to sICAM without a concomitant increase in osteoclast number is intriguing. sICAM has been shown to bind to LFA-1 with the resultant activation of NF-κB [13]. It does appear that the effect of sICAM in this study is mediated through LFA-1, given the similar responses observed in Figs. 1 and 2 for sICAM and LFA-1. However, because it affects survival and function at the same time, other pathways may be involved in sICAM signal transduction. As shown in this study, sICAM does not affect osteoclast differentiation, but increases resorption, survival, and cathepsin K and αvβ3 protein expression, two proteins essential for proper osteoclast bone resorption. Therefore, it appears that sICAM increases the amount of bone that each individual osteoclast can remodel. Interestingly, bone-resorbing activity of osteoclasts formed in co-cultures of cells from CD11a −/− mice is reduced, confirming the importance of LFA-1 in osteoclast function [24]. If indeed sICAM intracellular signaling is mediated through LFA-1, as suggested by this study and others [13], our results complement those of Tani-Ishii et al. in that LFA-1 appears to determine osteoclast function [24]. Further, the same group did report a 20%–30% decrease in the number of osteoclasts formed in co-culture of cells from CD11a −/− mice and suggested that this observation was secondary to the loss of the high-affinity adhesion mediated by ICAM-1/LFA-1, leading to fewer RANKL/RANK interactions [24]. Because we did not demonstrate sICAM ability to stimulate osteoclast differentiation, our results and those from the previously cited study suggest that there is no differentiation signal mediated through LFA-1. We still need, however, to determine which pathways are involved in that process. Moreover, although others have previously described sICAM ability to confer cellular protection against apoptosis [20], it remains difficult to appreciate the exact contribution of increased survival on bone resorption in this study (20% increase in cell survival and a threefold increase in resorption area). Therefore, the functional significance of sICAM ability to increase preosteoclast survival remains to be demonstrated.
On the other hand, it has been previously described that ICAM-1+ osteoblasts could support osteoclastogenesis but did not proliferate well, being stopped in the G0/G1 phase of the cell cycle [19]. It has also been shown that co-cultures of osteoblasts and osteoclast precursors result in a decrease in alkaline phosphatase activity and, presumably, a decrease in bone matrix deposition [25]. Further, Tanaka et al. stated that ICAM-1+ osteoblasts might be apoptotic because of increased Fas and c-Myc expression combined with reduced bcl-2 expression [19]. Finally, osteoblast adhesion to myeloma cells mediated through ICAM-1 appeared to promote the transduction of apoptogenic signals [20]. In this study, ICAM-1 engagement on osteoblasts led to a decrease in collagen-1 mRNA expression, an increase of RANKL mRNA expression, and an increase in caspase-3 protein expression. Taken together with a decrease in osteoblast survival, these observations imply that osteoblast/preosteoclast adhesion through ICAM-1 would result in a dual effect on bone homeostasis. We therefore propose the following actions for ICAM-1 in osteoclast biology. First, engagement of the ICAM-1/LFA-1 axis would lead to an increase in RANKL expression and provide a high-affinity link between osteoblasts and osteoclast precursors, facilitating RANKL/RANK interaction and therefore osteoclastogenesis. At the same time, the arrest of osteoblast proliferation combined with the generation of apoptotic signals and the alteration in collagen metabolism would lead to a reduction of bone matrix deposition, favoring an overall imbalance between bone resorption and bone formation. Moreover, the presence of sICAM (and possibly the engagement of ICAM-1 with LFA-1 on osteoclast precursors) would lead to an increase in osteoclast function, at least in part through increased survival and through a positive effect on proteins important for bone resorption.
One question raised by this study is whether the decrease in osteoclast formation observed in co-culture when anti-ICAM-1 mAbs are added is secondary to apoptosis and loss of osteoblast or to inhibition of cell interactions. A previous study has shown that low concentrations of anti-ICAM-1 mAb do reduce osteoclast formation [4]. When similar low concentrations were used in this study, we failed to demonstrate an effect on caspase-3 (not shown). However, the exact contribution of osteoblast apoptosis when high concentrations of mAb are used remains to be demonstrated in osteoclast biology.
Limitations of this study include the limited number of osteoclast differentiation factors tested following sICAM stimulation and the timing at which the experiments were conducted, given that we screened for mRNA production at the fusion stage of osteoclast development. It is possible that sICAM effect on osteoclast function is not transmitted through LFA-1 but is secondary to the generation of untested mediators or on mediators tested but secreted later in the maturation/activation process. Further, by studying both differentiation and function at the same time, it becomes difficult to assess the effect of sICAM on bone resorption only, because of the simultaneous presence of PBMC, preosteoclasts, resting osteoclasts, and active osteoclasts in culture. Fuller et al. recently described an experiment where mature, active osteoclasts were sedimented on bone slices, allowing the study of activation only [26]. We are now planning such experiments to clarify the pathways responsible for sICAM action, mainly looking at its effect on intracellular signaling. We also want to confirm that the sICAM signal is indeed transmitted through LFA-1.
In this study, we showed for the first time that sICAM can increase bone resorption, at least in part because of increased preosteoclast survival and through its action on cathepsin K and αvβ3. Further, ICAM-1/LFA-1 engagement appears to directly stimulate bone resorption while altering bone deposition. In light of these results, it appears that the ICAM-1/LFA-1 axis in an important determinant of osteoclast physiology.
Acknowledgments
The authors would like to thank the Fond de Recherche en Santé du Québec and the MENTOR program for the funding and support in preparing this manuscript.
Contributor Information
Julio C. Fernandes, Orthopaedic Research Laboratory, Department of Orthopaedics, Centre hospitalier Sacré-Coeur, 5400 Boul., Gouin Ouest, Montréal, Québec, Canada H4J 1C5. Osteoarthritis Research Unit, Department of Orthopaedics, Centre hospitalier de l’Université de Montréal-Hôpital Notre-Dame, Montréal, Québec, Canada
Qin Shi, Orthopaedic Research Laboratory, Department of Orthopaedics, Centre hospitalier Sacré-Coeur, 5400 Boul., Gouin Ouest, Montréal, Québec, Canada H4J 1C5.
Mohamed Benderdour, Orthopaedic Research Laboratory, Department of Orthopaedics, Centre hospitalier Sacré-Coeur, 5400 Boul., Gouin Ouest, Montréal, Québec, Canada H4J 1C5.
Daniel Lajeunesse, Osteoarthritis Research Unit, Department of Orthopaedics, Centre hospitalier de l’Université de Montréal-Hôpital Notre-Dame, Montréal, Québec, Canada.
Patrick Lavigne, Orthopaedic Research Laboratory, Department of Orthopaedics, Centre hospitalier Sacré-Coeur, 5400 Boul., Gouin Ouest, Montréal, Québec, Canada H4J 1C5. Department of Orthopaedics, Centre hospitalier Maisonneuve-Rosemont, Montréal, Québec, Canada.
References
- 1.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 2.Hofbauer LC, Heufelder AE. Osteoprotegerin and its cognate ligand: a new paradigm of osteoclastogenesis. Eur J Endocrinol. 1998;139:152–154. doi: 10.1530/eje.0.1390152. [DOI] [PubMed] [Google Scholar]
- 3.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okada Y, Morimoto I, Ura K, Watanabe K, Eto S, Kumegawa M, Raisz L, Pilbeam C, Tanaka Y. Cell-to-cell adhesion via intercellular adhesion molecule-1 and leukocyte function-associated antigen-1 pathway is involved in 1alpha,25(OH)2D3, PTH and IL-1alpha-induced osteoclast differentiation and bone resorption. Endocr J. 2002;49:483–495. doi: 10.1507/endocrj.49.483. [DOI] [PubMed] [Google Scholar]
- 5.Kurachi T, Morita I, Murota S. Involvement of adhesion molecules LFA-1 and ICAM-1 in osteoclast development. Biochim Biophys Acta. 1993;1178:259–266. doi: 10.1016/0167-4889(93)90202-z. [DOI] [PubMed] [Google Scholar]
- 6.Harada H, Kukita T, Kukita A, Iwamoto Y, Iijima T. Involvement of lymphocyte function-associated antigen-1 and intercellular adhesion molecule-1 in osteoclastogenesis: a possible role in direct interaction between osteoclast precursors. Endocrinology. 1998;139:3967–3975. doi: 10.1210/endo.139.9.6171. [DOI] [PubMed] [Google Scholar]
- 7.Lavigne P, Benderdour M, Shi Q, Lajeunesse D, Fernandes JC. Involvement of ICAM-1 in bone metabolism: a potential target in the treatment of bone diseases? Expert Opin Biol Ther. 2005;5:313–320. doi: 10.1517/14712598.5.3.313. [DOI] [PubMed] [Google Scholar]
- 8.Lavigne P, Benderdour M, Lajeunesse D, Shi Q, Fernandes JC. Expression of ICAM-1 by osteoblasts in healthy individuals and in patients suffering from osteoarthritis and osteoporosis. Bone (NY) 2004;35:463–470. doi: 10.1016/j.bone.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Rieckmann P, Michel U, Albrecht M, Bruck W, Wockel L, Felgenhauer K. Soluble forms of intercellular adhesion molecule-1 (ICAM-1) block lymphocyte attachment to cerebral endothelial cells. J Neuroimmunol. 1995;60:9–15. doi: 10.1016/0165-5728(95)00047-6. [DOI] [PubMed] [Google Scholar]
- 10.Kusterer K, Bojunga J, Enghofer M, Heidenthal E, Usadel KH, Kolb H, Martin S. Soluble ICAM-1 reduces leukocyte adhesion to vascular endothelium in ischemia-reperfusion injury in mice. Am J Physiol. 1998;275:G377–G380. doi: 10.1152/ajpgi.1998.275.2.G377. [DOI] [PubMed] [Google Scholar]
- 11.Lukacs NW, Chensue SW, Strieter RM, Warmington K, Kunkel SL. Inflammatory granuloma formation is mediated by TNF-alpha-inducible intercellular adhesion molecule-1. J Immunol. 1994;152:5883–5889. [PubMed] [Google Scholar]
- 12.McCabe SM, Riddle L, Nakamura GR, Prashad H, Mehta A, Berman PW, Jardieu P. sICAM-1 enhances cytokine production stimulated by alloantigen. Cell Immunol. 1993;150:364–375. doi: 10.1006/cimm.1993.1204. [DOI] [PubMed] [Google Scholar]
- 13.Schmal H, Czermak BJ, Lentsch AB, Bless NM, Beck-Schimmer B, Friedl HP, Ward PA. Soluble ICAM-1 activates lung macrophages and enhances lung injury. J Immunol. 1998;161:3685–3693. [PubMed] [Google Scholar]
- 14.Van Seventer GA, Shimizu Y, Horgan KJ, Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990;144:4579–4586. [PubMed] [Google Scholar]
- 15.Konig A, Muhlbauer RC, Fleisch H. Tumor necrosis factor alpha and interleukin-1 stimulate bone resorption in vivo as measured by urinary [3H]tetracycline excretion from prelabeled mice. J Bone Miner Res. 1988;3:621–627. doi: 10.1002/jbmr.5650030607. [DOI] [PubMed] [Google Scholar]
- 16.Kotake S, Sato K, Kim KJ, Takahashi N, Udagawa N, Nakamura I, Yamaguchi A, Kishimoto T, Suda T, Kashiwazaki S. Inter-leukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclast-like cell formation. J Bone Miner Res. 1996;11:88–95. doi: 10.1002/jbmr.5650110113. [DOI] [PubMed] [Google Scholar]
- 17.Kukita T, Nomiyama H, Ohmoto Y, Kukita A, Shuto T, Hotokebuchi T, Sugioka Y, Miura R, Iijima T. Macrophage inflammatory protein-1 alpha (LD78) expressed in human bone marrow: its role in regulation of hematopoiesis and osteoclast recruitment. Lab Invest. 1997;76:399–406. [PubMed] [Google Scholar]
- 18.Thomson BM, Mundy GR, Chambers TJ. Tumor necrosis factors alpha and beta induce osteoblastic cells to stimulate osteoclastic bone resorption. J Immunol. 1987;138:775–779. [PubMed] [Google Scholar]
- 19.Tanaka Y, Maruo A, Fujii K, Nomi M, Nakamura T, Eto S, Minami Y. Intercellular adhesion molecule 1 discriminates functionally different populations of human osteoblasts: characteristic involvement of cell cycle regulators. J Bone Miner Res. 2000;15:1912–1923. doi: 10.1359/jbmr.2000.15.10.1912. [DOI] [PubMed] [Google Scholar]
- 20.Silvestris F, Cafforio P, Calvani N, Dammacco F. Impaired osteoblastogenesis in myeloma bone disease: role of upregulated apoptosis by cytokines and malignant plasma cells. Br J Haematol. 2004;126:475–486. doi: 10.1111/j.1365-2141.2004.05084.x. [DOI] [PubMed] [Google Scholar]
- 21.Jimi E, Nakamura I, Amano H, Taguchi Y, Tsurukai T, Tamura M, Takahashi N, Suda T. Osteoclast function is activated by osteoblastic cells through a mechanism involving cell-to-cell contact. Endocrinology. 1996;137:2187–2190. doi: 10.1210/endo.137.5.8612568. [DOI] [PubMed] [Google Scholar]
- 22.Udagawa N, Takahashi N, Akatsu T, Sasaki T, Yamaguchi A, Kodama H, Martin TJ, Suda T. The bone marrow-derived stromal cell lines MC3T3-G2/PA6 and ST2 support osteoclast-like cell differentiation in cocultures with mouse spleen cells. Endocrinology. 1989;125:1805–1813. doi: 10.1210/endo-125-4-1805. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka Y, Morimoto I, Nakano Y, Okada Y, Hirota S, Nomura S, Nakamura T, Eto S. Osteoblasts are regulated by the cellular adhesion through ICAM-1 and VCAM-1. J Bone Miner Res. 1995;10:1462–1469. doi: 10.1002/jbmr.5650101006. [DOI] [PubMed] [Google Scholar]
- 24.Tani-Ishii N, Penningeer JM, Matsumo G, Teranaka T, Umemoto T. The role of LFA-1 in osteoclast development induced by co-cultures of mouse bone marrow cells and MC3T3-G2/PA6 cells. J Periodont Res. 2002;37:184–191. doi: 10.1034/j.1600-0765.2002.00610.x. [DOI] [PubMed] [Google Scholar]
- 25.Orlandini SZ, Formigli L, Benvenuti S, Lasagni L, Franchi A, Masi L, Bernabei PA, Santini V, Brandi ML. Functional and structural interactions between osteoblastic and preosteoclastic cells in vitro. Cell Tissue Res. 1995;281:33–42. doi: 10.1007/BF00307956. [DOI] [PubMed] [Google Scholar]
- 26.Fuller K, Kirstein B, Chambers TJ. Murine osteoclast formation and function: differential regulation by humoral agents. Endocrinology. 2006;147:1979–1985. doi: 10.1210/en.2005-1340. [DOI] [PubMed] [Google Scholar]