Abstract
The mechanisms by which proteins are targeted to the membrane of eukaryotic flagella and cilia are largely uncharacterized. We have identified a new family of small myristoylated proteins (SMPs) that are present in Leishmania spp and related trypanosomatid parasites. One of these proteins, termed SMP-1, is targeted to the Leishmania flagellum. SMP-1 is myristoylated and palmitoylated in vivo, and mutation of Gly-2 and Cys-3 residues showed that both fatty acids are required for flagellar localization. SMP-1 is associated with detergent-resistant membranes based on its recovery in the buoyant fraction after Triton X-100 extraction and sucrose density centrifugation and coextraction with the major surface glycolipids in Triton X-114. However, the flagellar localization of SMP-1 was not affected when sterol biosynthesis and the properties of detergent-resistant membranes were perturbed with ketoconazole. Remarkably, treatment of Leishmania with ketoconazole and myriocin (an inhibitor of sphingolipid biosynthesis) also had no affect on SMP-1 localization, despite causing the massive distension of the flagellum membrane and the partial or complete loss of internal axoneme and paraflagellar rod structures, respectively. These data suggest that flagellar membrane targeting of SMP-1 is not dependent on axonemal structures and that alterations in flagellar membrane lipid composition disrupt axoneme extension.
INTRODUCTION
Eukaryotic flagella and cilia are complex surface organelles that play a key role in motility and sensory reception in many unicellular protists and metazoan organisms (Rosenbaum and Witman, 2002; Cole, 2003). The assembly and maintenance of the internal microtubule axoneme requires the continuous import of axoneme precursor proteins from the cytosol as well as the removal of proteins generated by turnover of axonemal structures. The import and export of these proteins appears to be largely mediated by intraflagellar transport (IFT) particles that move along the axonemal doublet microtubules just beneath the flagellar membrane (Kozminski et al., 1995; Rosenbaum and Witman, 2002; Cole, 2003). IFT particles moving from the base of the flagellum to the tip (anterograde transport) are associated with plus-end directed kinesin motor proteins, whereas IFT particles moving in the reverse direction associate with dynein motor proteins and recycle kinesin and discarded axoneme proteins back to the cytosol. Current models also suggest that IFT particles may play a role in targeting proteins to the flagella membrane, which often contains a distinct protein (and lipid) composition from the rest of the plasma membrane, with which it is contiguous (Rosenbaum and Witman, 2002). However, it has recently been shown that polycystin-2, a membrane protein in kidney cilia, is still targeted to the cilia membrane of mutant kidney cells partially deficient in IFT (Pazour et al., 1998). IFT-independent mechanisms (Kozminski et al., 1993) may thus contribute to the distinctive protein composition of the flagellar membrane.
Trypanosmatid parasites are protozoa that cause a number of important diseases in humans, including African sleeping sickness (Trypanosoma brucei), Chagas disease (T. cruzi), and the (muco)cutaneous and visceral leishmaniases (Leishmania spp). The flagellum of most trypanosomatid developmental stages emerges from a deep invagination in the plasma membrane, termed the flagellar pocket and contains the typical 9 + 2 array of microtubule doublets as well as a paracrystalline structure termed the paraflagellar rod (Gull, 1999). Although the trypanosomatid flagellum is clearly important for parasite migration and invasion of host tissues, it may also regulate processes such as secretion and endocytosis in the flagellar pocket and may be enriched in proteins involved in environmental or nutrient sensing (Gull, 1999; Bastin et al., 2000; Landfear and Ignatushchenko, 2001; McConville et al., 2002; Hill, 2003; Vaughan and Gull, 2003). Studies on trypanosomatid flagellar membrane proteins have provided new insights into the signals that target proteins to the flagellar membrane. Specifically, sorting of the L. enrietti glucose transporter isoform, ISO1, to detergent-soluble membranes in the flagellum, is mediated by a peptide sequence within the N-terminal cytoplasmic tail of this polytopic membrane protein (Piper et al., 1995; Snapp and Landfear, 1999). In contrast, dual acylation of the amino termini of T. cruzi flagellar calcium-binding protein, FCaFP, is required for flagellar localization (Godsel and Engman, 1999). Binding of FCaFP to the flagellar membrane is Ca2+-dependent, indicating the presence of a novel myristoyl/palmitoyl switch mechanism (Godsel and Engman, 1999).
In the present study, we describe the isolation, characterization, and flagellar targeting of a new diacylated membrane protein, termed small myristoylated protein-1 (SMP-1), in L. major. SMP-1 was initially identified in a Triton X-100–insoluble membrane fraction that contains plasma membrane components, such as the major glycosylphosphatidylinositol (GPI)-anchored proteins, free GPI glycolipids (GIPLs), and sterols and sphingolipids (Denny et al., 2001; Ralton et al., 2002). SMP-1 belongs to a new family of mono- or di-acylated SMPs that may exist in all trypanosomatids. We show that the insolubility of SMP-1 in cold Triton X-100 is due to association with membrane components rather than the flagellar axoneme and paraflagellar rod proteins. Remarkably, perturbation of these membranes with inhibitors of sterol and sphingolipid biosynthesis resulted in the appearance of swollen flagella that retain the SMP-1–positive limiting membrane, but lacked prominent axoneme and paraflagellar rod structures or their precursors. These findings strongly indicate that the targeting of SMP-1 to the flagellar membrane can occur independently of the axoneme or IFT and that the formation of the axoneme is critically dependent on membrane lipids.
MATERIALS AND METHODS
Cell Culture
L. major (LRC-L137, clone V121) promastigotes were cultivated at 27°C in Schneider's Drosophila medium containing 10% heat-inactivated fetal bovine serum (iFBS). L. mexicana (MNYC/BZ/62/M379) and L. donovani (L8) promastigotes were grown in RPMI or SDM79 medium, respectively, supplemented with 10% iFBS. For isolation of amastigote stages, BALB/c mice were subcutaneously infected with 5 × 106 stationary phase promastigotes, and lesions were harvested after 3–5 wk. Partial inhibition of sphingolipid and sterol biosynthesis was achieved by incubating L. major promastigotes in medium containing 1 μg/ml myriosin (Sigma, St. Louis, MO) or 2 μg/ml ketoconazole (Sigma), respectively (Ginger et al., 2001; Ralton et al., 2002).
Isolation of Detergent-resistant Membranes
Leishmania promastigotes were harvested by centrifugation (2000 × g, 25°C, 10 min), washed with phosphate-buffered saline (PBS), and resuspended in 500 μl ice-cold 1% Triton X-100 buffer (1% Triton X-100, 25 mM HEPES, pH 7.4, 1 mM EDTA, Complete protease inhibitor cocktail [20 μl, Roche, Indianapolis, IN]) at 0°C for 30 min. The extract was adjusted to 60% sucrose, placed at the bottom of an ultracentrifuge tube, overlaid with a discontinuous sucrose gradient, and centrifuged at 200,000 × g for 20–22 h at 4°C (Ralton et al., 2002). Proteins were solvent-precipitated from gradient fractions (Wessel and Flugge, 1984) and analyzed on 15% SDS-PAGE gels. Proteins were visualized with Coomassie Blue or silver staining or transferred to nitrocellulose membranes and detected by immunoblottting. Glycolipids were recovered by solvent partitioning an aliquot of each fraction (100 μl) with 200 μl 1-butanol. The organic phase was dried and glycolipids analyzed by high-performance TLC (HPTLC) on aluminum-backed silica gel HPTLC sheets (Merck, Rahway, NJ) developed in CHCl3/CH3OH/13 M NH3/1 M NH4OAc/H2O (180:140: 9:9:23 vol/vol; McConville and Blackwell, 1991). To assess the solubility of SMP-1 in Triton X-114, L. major promastigotes (2 × 107) were extracted with 200 μl 1% Triton X-114 as described in Ralton et al. (2002).
Isolation and Sequencing of SMP-1
Sucrose gradient fractions containing buoyant, Triton X-100–insoluble membranes were pooled, solvent-precipitated, and resolved on a large format 15% SDS-PAGE gel. The Coomassie Blue–stained bands were excised and reduced with 0.4% (vol/vol) β-mercaptoethanol in 0.2 M Tris, pH 8.4, containing 2 mM EDTA (60°C, 2 h) and then alkylated with 2% (wt/vol) iodoacetamide in 0.2 M Tris, pH 8.4, containing 2 mM EDTA (25°C, 1 h). The gel fragments were destained, washed with 0.1 M ammonium bicarbonate in 60% acetonitrile, 80–100% isopropanol and water, and then dried under vacuum. The gel was rehydrated, and the proteins were digested with 30 μl 20 ng/μl trypsin (Promega, Madison, WI; sequence grade) in 0.1 M ammonium bicarbonate containing 1 mM CaCl2 (37°C,16 h). Peptides were extracted with 100 μl 50% acetonitrile in 1% trifluoroacetic acid (TFA), dried under vacuum, and identified by liquid chromatography electrospray ionization MS/MS analysis. Peptide separations were achieved on a C18 Vydac column (2.2 mm × 5 cm, 300 μm), eluted in 0.5% acetic acid and a 5–60% acetonitrile gradient for >60 min. Peptides were detected with a LCQ mass spectrometer (ThermoFinnigan, Austin, TX). A cycle of one full-scan mass spectrum (300–2000 m/z using a 4.6-kV source voltage) followed by one data-dependant MS/MS spectrum with a collision energy of 35. Alternatively, proteins were solvent precipitated from sucrose gradient fractions and digested in 0.2% RapiGest (50 μl, Waters, Milford, MA) in 50 mM ammonium bicarbonate (25°C, 10 min). Proteins were reduced with 5 mM dithiothreitol (60°C, 30 min), alkylated with 15 mM iodoacetamide (25°C, 30 min), and digested with 1 μg trypsin (Promega, sequence grade) in 0.1 M ammonium bicarbonate containing 1 mM CaCl2 (37°C, 16 h). RapiGest was removed by acidifying the digest (10 μl, 0.5 M HCl) at 37°C for 30 min and centrifugation (16,000 × g,10 min), and the supernatant was analyzed by two-dimensional LC ESI MS/MS. The sample was loaded onto a column of fractogel EMD SO3 resin (1 cm × 2 mm, ID) at 70 μl/min with 0.5% acetic acid. Peptides were eluted onto a LC Packings C18, u-precolumn cartridge (5 × 0.3 mm, ID) with stepwise injections of 60 μl of 10, 25, 50, and 100 mM sodium acetate. After each stepwise injection peptides were eluted off the C18 precolumn with 5–60% acetonitrile gradient in 0.5% acetic acid and analyzed with an LCQ mass spectrometer as described above. MS/MS spectra were analyzed using SEQUEST to search L. major database November 2002, using a static modification of 57 Da for carboxamidomethylation of cysteines. Sequences encoding SMP-1–related proteins were identified by BLAST searching the kinetoplastid genomes (www.genedb.org). Protein sequences were aligned using ClustalX 1.83 (Thompson et al., 1997). Phylogenetic trees were inferred using PAUP*4b2 (Swofford, 1997).
Preparation of the L. major SMP-1 Antiserum
The synthetic peptide CPLSEEYRQHQAEKDK (225 nmol) consisting of the last C-terminal 16 amino acid residues of SMP-1 was coupled to diphtheria toxoid via a maleimidocaproyl N-hydroxysuccinimide linkage (Mimotopes, Victoria, Australia) mixed with 1.5 volumes of Freund's adjuvant (Sigma) and used to generate rabbit polyclonal antisera.
Metabolic Labeling
L. major promastigotes were harvested in mid-log phase and resuspended in Schneider's Drosophila medium containing 2 mg/ml defatted bovine serum albumin (BSA; 1.2 × 107 cells/ml) and either [9,10-3H]myristate or [9,10-3H]palmitate (1 and 5 μCi/ml, respectively), added as equimolar complexes with BSA (Ralton and McConville, 1998). After 24 h, metabolically labeled promastigotes were extracted in cold 1% Triton X-100 buffer (50 mM Tris, pH 7.4, 0.3 M NaCl, 5 mM EDTA, Complete protease inhibitor cocktail), at either 0 or 25°C for 30 min. Labeled SMP-1 was recovered by immunoprecipitation using the anti-SMP-1 rabbit antibody. L. major SMP-1 antiserum (10 μl) and preimmune serum (10 μl) were cross-linked to protein A agarose (20 μl in 500 μl PBS) with dimethyl pimelimidate (20 mM) in 0.2 M sodium borate, the reaction was quenched with 0.2 M ethanolamine, and antibody-coated beads were washed in PBS containing 10% BSA (4°C, 1 h). The Triton X-100 extract (200 μl) was precleared by incubation with beads coated with preimmune serum (4°C, 1 h), centrifuged to remove beads, and then incubated with anti-SMP-1–protein A agarose beads (4°C, 2 h). After centrifugation, the beads were washed with 50 mM Tris, pH 7.4, containing 0.1% Triton X-100, 0.3 M NaCl, and 5 mM EDTA, then boiled in reducing sample buffer for 5 min, and analyzed on 15% SDS-PAGE gel. 3H-myristolyated or -palmitoylated protein were detected by fluorography after treating the gel with Amplify (Amersham, Piscataway, NJ) and exposure to Biomax MR film (Eastman Kodak, Rochester, NY) at -70°C. Radiolabeled proteins in the Triton X-100–insoluble fraction, were recovered by boiling the pellet in sample buffer and analyzed on 15% SDS-PAGE gel, and the proteins were fixed in 25% isopropanol, 10% acetic acid. The gel was washed with water and incubated with 1 M hydroxylamine in 1 M Tris, pH 7 (16 h, 25°C; Olson et al., 1985). Radiolabeled myristolyated or palmitoylated proteins were detected by fluorography.
Immunoblotting
Nitrocellulose membranes with electrophoretically transferred proteins were blocked with 5% powdered skim milk in Tris-buffered saline containing 0.05% Tween 20. The blots were probed with the following antibodies (at 1:1000 dilution); mouse monoclonal anti-α-tubulin (Clone DM1A, Sigma), rabbit anti-SMP-1, rabbit anti-BiP (kindly provided by Dr J.D. Bangs), and rabbit anti-PSA2 (kindly provided by Dr E. Handman). Primary antibodies were diluted in 5% powdered skim milk. Secondary antibodies, anti-rabbit horseradish peroxidase–conjugated antibody were diluted 1:1000 in 1% powdered skim milk and detected using ECL Western detection system.
Immunofluorescence and Electron Microscopy
L. major promastigotes were fixed in 4% paraformaldehyde on ice for 15 min and then immobilized on poly-l-lysine–coated coverslips (4 × 106 cells/coverslip). In experiments shown in Figure 6, promastigotes were extracted with 1% Triton X-100 at either 0 or 25°C before immobilization on the coverslips. The cells were dehydrated with methanol (0°C, 5 min), washed with PBS, incubated with 50 mM ammonium chloride (0°C, 10 min) and blocked in 1% BSA in PBS (25°C, 30 min). The fixed cells were probed with SMP-1 antiserum (1:200 dilution), preimmune serum (1:200 dilution), antitubulin (1:500 dilution), anti-GIPL (L-5–28, 1:10 dilution; kindly provided by Dr. E. Handman), antiparaflagellar rod proteins (L8C4, 1:4 dilution, kindly provided by Prof. Keith Gull), or anti-HA (Roche, 1:80 dilution). Secondary antibodies, Alexa Fluor 488 goat anti-rabbit and Alexa Fluor 594 goat anti-mouse were used at 1:1000 dilution and Alexa Fluor 488 goat anti-rat at 1:200 dilution. All antibodies were diluted with 1% BSA in PBS. The coverslips were mounted in Mowiol 4–88 (Calbiochem, La Jolla, CA) containing Hoechst dye. Cells were viewed with a Zeiss Axioplan2 microscope (Thornwood, NY) equipped with a AxioCam Mrm digital camera. Electron microscopy was carried out as described (Mullin et al., 2001) except that the cells were stained with 1% aqueous uranyl acetate before being embedded in LR White (ProSciTech, Thuringowa, Queensland, Australia).
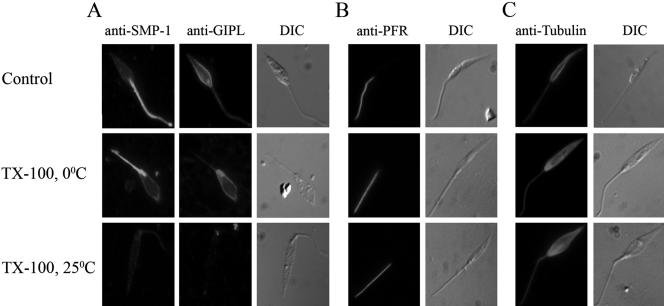
Figure 6.
SMP-1 and GIPL were extracted from the flagellum under the same conditions. L. major promastigotes were extracted in Triton X-100 at either 0 or 25°C. Immunofluorescence microscopy of detergent-extracted promastigotes probed with the anti-SMP-1 and anti-GIPL (L-5–28) antibodies or with the anti-PFR (L8C4) and anti-α-tubulin antibodies.
Cloning and Expression of Epitope-tagged Di-, Mono-, and Nonacylated SMP-1
For episomal expression in L. major, SMP-1 constructs were cloned into the vector pX containing the neomycin resistance gene and three copies of the influenza hemagglutinin peptide eptiope (HA) within the multiple cloning site (Mullin et al., 2001). SMP-1 was amplified from genomic DNA with primers 1 (TCCCCCGGGATGGGCGGCTCCAAC) and 2 (CGGGATCCCTTGTCCTTCTCCGC) containing unique BamHI and SmaI restriction sites (underlined) to allow in frame placement of the gene upstream of the triple HA epitope. SMP-1 mutants lacking N-terminal myristoylation or palmitoylation sites (due to mutation of Gly-2 to Ala and Cys-3 to Ser) were created by modification of the relevant nucleotides (in bold) using primers 3 (TCCCCCGGGATGGCCTGCGGTGCTTC) and 2 or primers 4 (TCCCCCGGGATGGGCAGCGGTGCTTC) and 2, respectively. These PCR products were then cloned into the triple HA modified pX-vector using the same restriction sites as mentioned above.
Constructs were transfected into L. major by electroporation and cultured in drug-free media for 24 h, and positive transfectants were selected by addition of 10 μg/ml neomycin. After adaptation, parasites were maintained in medium containing 100 μg/ml neomycin.
RESULTS
Identification of a New Family of SMPs in Trypanosomatids
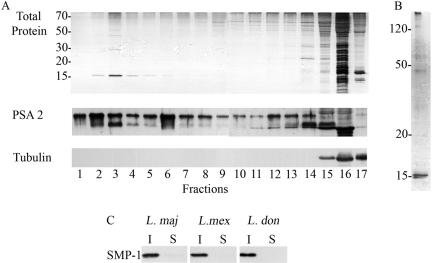
We have previously shown that the major glycolipids and GPI-anchored proteins of Leishmania promastigotes become incorporated into Triton X-100–resistant membranes (detergent-resistant membranes [DRMs]) upon reaching the plasma membrane (Ralton et al., 2002). To identify possible non–GPI-anchored proteins in the DRM fraction, L. major promastigotes were solubilized with cold 1% Triton X-100, and the lysate was subjected to flotation centrifugation in a sucrose density gradient. As shown previously, most cellular proteins remained at the bottom of the gradient, whereas the GPI-anchored protein, PSA2, was largely recovered in fractions at the top of the gradient (Figure 1A, fractions 1–6). The DRM fractions also contained a prominent 15-kDa protein (Figure 1A, top panel), which was resolved as a doublet on 15% SDS-PAGE gels (Figure 1B). Mass spectrometry of the tryptic peptides released from this doublet or tryptic digests of unfractionated DRMs identified two peptides, DNGLLFR and MDALPLSEEYR, derived from a putative 131 amino acid protein encoded by the L. major genome (Figure 2A). As the N-terminal sequence of this protein contained a strong myristoylation motif (MGXXXS/T; Towler et al., 1987), this protein was referred to as small myristoylated protein-1 (SMP-1). Western blot analysis of Triton X-100–soluble and –insoluble fractions of L. major, L. mexicana, and L. donovani promastigotes using a polyclonal antibody raised against the unique C-terminal peptide of SMP-1, indicated that this protein was expressed at comparable levels in these pathogenic species of Leishmania (Figure 1C).
Figure 1.
SMP-1 is a major protein in the buoyant Triton X-100–insoluble membranes. (A) L. major promastigotes were extracted in cold Triton X-100 and lysates subjected to flotation centrifugation in a sucrose density gradient. Protein in gradient fractions were analyzed by 15% SDS-PAGE and silver staining (top panel). PSA2 (a DRM component) and α-tubulin (cytoskeleton) were detected by Western blotting (bottom panels). Fractions 1–17 encompass a step gradient of 5–60% sucrose. (B) Large format 15% gel showing the 15-kDa protein doublet in the buoyant membrane fraction (Fractions 2–4 of A). (C) L. major, L. mexicana, and L. donovani promastigotes were extracted in cold Triton X-100 and insoluble (I) and soluble (S) fractions were analyzed by 15% SDS-PAGE. Western blotting with a polyclonal antiserum directed against the unique C-terminal peptide of SMP-1, revealed a single reactive protein at 15 kDa.
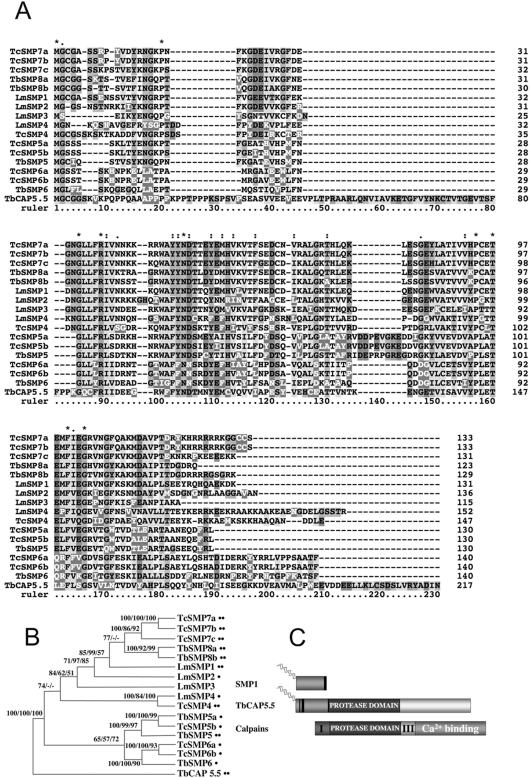
Figure 2.
Sequence comparison of trypanosomatid SMPs. (A) Alignment of the L. major, T. brucei, and T. cruzi SMP family members with the N-terminus of TbCAP5.5. Accession numbers for L. major and T. brucei proteins are as follows: LmSMP1 (AY642931), LmSMP2 (AY642932), LmSMP3 (CAC24686), LmSMP4 (AY642933),TbSMP5 (CAB95480), TbSMP6 (CAB95478), TbSMP8a (CAB95484), TbSMP8b (CAB95485), and TbCAP5.5 (AAG48626). Genedb numbers for the T. cruzi proteins are (prefix Tc00.104705350); TcSMP4 (6563.110), TcSMP5a (8999.260), TcSMP5b (6563.170), TcSMP6a (6563.180), TcSMP6b (8999.250), TcSMP7a (6563.70), TcSMP7b (9003.40), and TcSMP7c (9003.30). (B) Phylogenetic tree of the SMP family members. A neighbor joining distance tree rooted using the N-terminus of TbCAP5.5 is shown. Bootstrap support values for three types of analysis (neighbor joining/maximum parsimony/quartet puzzling) are shown at nodes. Bootstrap values <50% are shown as a dash. Monoacylated or diacylated proteins are indicated by single or double bullet points, respectively. (C) Schematic showing related protein domains in the SMPs, TbCAP5.5, and the calpain superfamily. Sequence identity is indicated by like-shaded cylinders. The diacylated N-terminal domain of CAP5.5 shares homology with the acylated SMPs. The central domain of TbCAP5.5 shares homology with the protease domain of the calpains but lacks other domains (domain I, III and the calcium-binding domains) typical of calpains.
BLAST searches of the L. major genome suggested that SMP-1 was a member of a new family of Leishmania proteins (Figure 2A). These proteins contain a relatively well-conserved N-terminal domain and a highly divergent C-terminal domain and may differ in the extent to which they are acylated (Figure 2A). Although SMP-1 is predicted to be both myristoylated and palmitoylated at positions Gly-2 and Cys-3, respectively, SMP-2 and SMP-4 only contain myristoylation sites (Figure 2A). In contrast, SMP-3 lacks both N-terminal myristoylation and palmitoylation sites (Figure 2A). BLAST searches of the genomes of T. brucei and T. cruzi indicated that SMP-like proteins are ubiquitously encoded in other trypanosomatidae and that they share some identity with the N-terminal domain of the recently identified T. brucei cytoskeletal protein, TbCAP5.5 (Hertz-Fowler et al., 2001). TbCAP5.5 is also N-terminally diacylated, but clearly differs from the SMPs in containing two distinct indels and a large calpain protease-like domain (shown schematically in Figure 2C). Phylogenetic analysis of the trypanosomatid SMP family revealed eight clusters. SMP-1, SMP-2, and SMP-3 proteins are unique to L major and lack any clear orthologues in T. brucei or T. cruzi. The SMP-4 cluster contains orthologues from T. cruzi and L. major united by a DD/S motif at position 22, the PY motif at 35, a Q at 166, and a basic C-terminus. The SMP-5 cluster has representatives in T. brucei and T. cruzi, and each member has a predominately acidic, eight amino acid indel in the middle plus a relatively short C-terminus ending in F/YRL. The SMP-6 cluster also has representatives in T. brucei and T. cruzi with clear signatures such as the threonine at 100, phenylalanine at 104, asparagine at 106, and a charged C-terminus ending in AA/TT/SF. The SMP-7 cluster is restricted to T. cruzi but perhaps weakly related to the SMP-8 cluster of T. brucei. Intriguingly, there is no clear trend for mono- or di-acylation in the SMP orthologue clusters with clear-cut orthologues such as the SMP-5s predicted to be monoacylated in T. cruzi but diacylated in T. brucei. Similarly, LmSMP-4 is predicted to be monoacylated, whereas TbSMP-4 may be diacylated. It is interesting that LmSMP-3, which lacks a clear acylation motif, is nested within a cluster of acylated proteins and is not an evolutionary outlier.
L. major SMP-1 Is Targeted to the Flagellum of Promastigote Stages
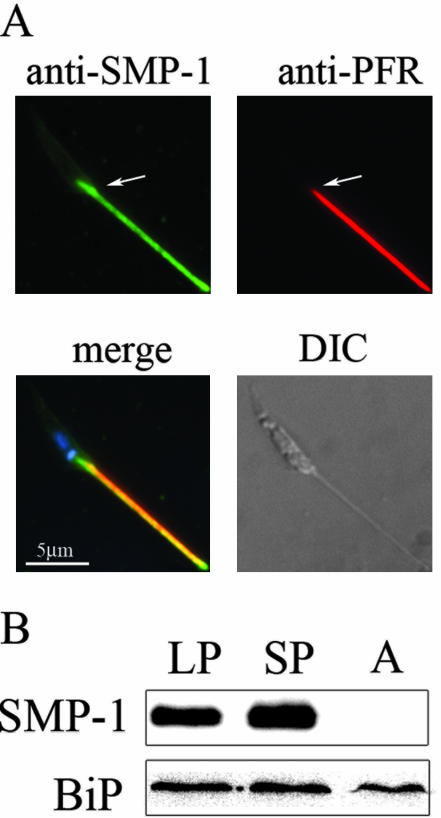
The polyclonal antibody raised against the unique C-terminal peptide of LmSMP-1 (abbreviated to SMP-1) was used to localize this protein to the flagellum of promastigote stages (Figure 3A). Staining extended from near the basal body, juxtaposed to the kinetoplast, to the distal tip of the flagellum (Figure 3A). This contrasts with the distribution of the paraflagellar rod proteins-1 and -2 (PFR1, and -2) that only associate with the flagellum after it emerges from the flagellar pocket (Figure 3A). SMP-1 staining was not observed around the flagellar pocket or to the rest of the cell body membrane (Figure 3A). Western blotting showed that SMP-1 was expressed in all promastigotes stages containing an elongated flagellum, but not the amastigote stage that contain a highly truncated flagellum (Figure 2C, lane 3). Finally, trypsin digestion of live or detergent-permeabilized promastigotes indicated that SMP-1 was primarily localized on the inner leaflet of the flagellar membrane (our unpublished results).
Figure 3.
Localization of SMP-1 to the flagellum. (A) L. major promastigotes were fixed and probed with the anti-SMP-1 polyclonal antibody (green signal) and the anti-PFR mAb, L8C4 (red signal) and visualized by indirect immunofluorescence microscopy. Merged image also shows Hoechst staining of the nucleus and kinetoplast (blue signal), whereas DIC refers to differential interference contrast image. Arrows indicate the point at which the flagellum emerges from the flagellar pocket. (B) L. major promastigotes at log phase (LP), stationary phase (SP), and lesion-derived amastigotes (A) were extracted in SDS sample buffer. Total protein was analyzed by 15% SDS-PAGE and Western blotting with anti-SMP-1 and anti-BiP antibodies.
Myristoylation and Palmitoylation of SMP-1 Is Required for Flagellar Targeting
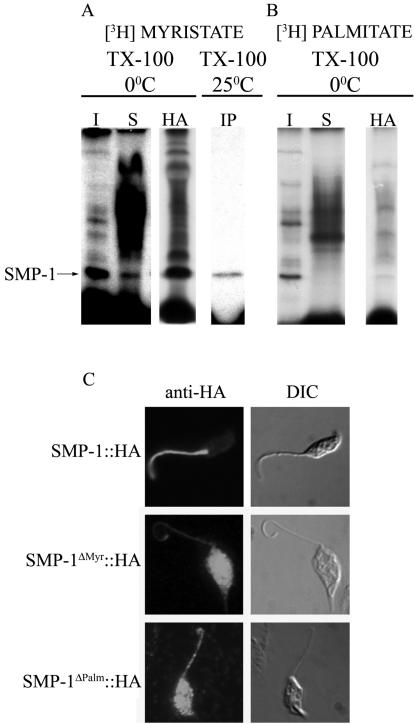
The acylation state of SMP-1 was investigated by metabolically labeling L. major promastigotes with [3H]myristate or [3H]palmitate and total cellular proteins analyzed by SDS-PAGE. A major [3H]myristate-labeled protein with the same molecular weight as SMP-1 was recovered in the Triton X-100–insoluble fraction (Figure 4A). This band could be solubilized in 1% Triton X-100 at 25°C and immunoprecipitated with the SMP-1 specific polyclonal antibody (Figure 4A). The labeled band was resistant to hydroxylamine treatment, indicating the presence of an amide linkage (Figure 4A). A protein with the same properties was also labeled with [3H]palmitate (Figure 4B). Unlike the [3H]myristate label, the [3H]palmitate label was removed after hydroxylamine treatment (Figure 4B), indicating the presence of a thiol ester linkage. Interestingly, the [3H]palmitate label was lost from SMP-1 when promastigotes were solubilized in Triton X-100 at 25°C (our unpublished results), suggesting that this modification is enzymatically removed.
Figure 4.
SMP-1 is both myristoylated and palmitoylated. (A) L. major promastigotes were metabolically labeled to steady state with [3H]myristic acid and extracted in 1% Triton X-100 at 4 or 25°C. Labeled proteins in the Triton X-100–insoluble (I) and –soluble (S) fractions as well as protein immunoprecipitated with the anti-SMP-1 antibody (IP) were analyzed by 15% SDS-PAGE and detected by fluorography. A lane containing cold Triton X-100–insoluble protein (duplicate of I) was treated with hydroxylamine (HA) before fluorography. (B) L. major promastigotes were metabolically labeled with [3H]palmitic acid and extracted in cold 1% Triton X-100. Protein in the insoluble (I) and soluble (S) fractions were analyzed by SDS-PAGE and detected by fluorography. A lane containing cold Triton X-100–insoluble protein (duplicate of I) was treated with hydroxylamine (HA) before fluorography. (C) Immunofluorescence microscopy of L. major promastigotes expressing HA-tagged forms of SMP-1. L. major promastigotes stably expressing equivalent levels of SMP-1::HA, SMP-1ΔMyr::HA (in which Gly2 is replaced with Ala) or SMP-1ΔPalm::HA (in which Cys3 is replaced with Ser) were fixed and probed with anti-HA antibody and Alexa Fluor 488 goat anti-rat antibody. DIC; differential interference contrast image. The cytoplasmic localization of SMP-1ΔMyr::HA and SMP-1ΔPalm::HA was confirmed by deconvolution microscopy of the fixed parasites (our unpublished results).
To confirm that Gly2 and Cys3 are indeed acylated, C-terminally HA-tagged chimeras of SMP-1 containing both residues or lacking one or the other (SMP-1::HA, SMP-1ΔMyr::HA and SMP-1ΔPalm::HA) were ectopically expressed in L. major promastigotes. All three chimeras were expressed at similar levels (∼10-fold lower than endogenous protein) and had the expected molecular weight of ∼19 kDa on SDS-PAGE (our unpublished results). As expected SMP-1::HA was targeted to the flagellum in the same way as the native protein (Figure 4C, top panel). In contrast, the two mutant SMP-1 proteins lacking the N-terminal Gly2 and Cys3 residues were targeted to the cytosol (Figure 4C, middle and bottom panels). These data strongly suggest that SMP-1 is myristoylated and reversibly palmitoylated and that both of these modifications are essential for flagellar targeting.
SMP-1 Is Not Associated with the Flagellum Cytoskeleton
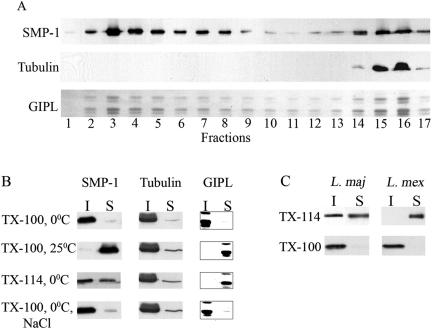
Diacylated proteins such as T. brucei CAP5.5 appear to be tightly associated with the subpellicular microtubules, as well as the cell body plasma membrane (Hertz-Fowler et al., 2001). To examine whether SMP-1 is similarly associated with an underlying cytoskeleton, notably the flagellar axoneme, L. major promastigotes were solubilized in cold 1% Triton X-100 and the lysates were floated in sucrose density gradients. Approximately 40–60% of SMP-1 as well as externally disposed surface glycolipids (GIPLs), which are not expected to interact with the cytoskeleton (Ralton et al., 2002), were recovered in the buoyant membrane fraction (Figure 5A). This fraction lacked tubulin, indicating that the DRM fraction does not contain a tightly bound microtubule component (Figure 5A). To further assess whether the SMP-1 and GIPL pools that remained at the bottom of the sucrose gradient were associated with the cytoskeleton, L. major promastigotes were extracted in 1% Triton X-100 at 25°C instead of 0°C. These conditions resulted in the total extraction of SMP-1 and GIPLs, whereas tubulin remained in the insoluble fraction (Figure 5B), indicating that all of the SMP-1 is associated with DRMs. We have previously shown that leishmanial DRM components, but not the microtubulin cytoskeleton, are more effectively extracted in cold 1% Triton X-114 than in 1% Triton X-100 (Ralton et al., 2002). As expected for a DRM component, SMP-1 was partially solubilized from L. major promastigotes in cold Triton X-114 (Figure 5B), without detectable solubilization of cytoskeletal components (Figure 5B). Interestingly, both SMP-1 (Figure 5C) and the major GIPLs (Ralton et al., 2002) were quantitatively solubilized from L. mexicana membranes in cold 1% Triton X-114 (Figure 5C), indicating species-specific differences in the lipid composition of leishmanial DRMs. Finally, unlike other trypanosomatid cytoskeleton-associated proteins (Vedrenne et al., 2002), the solubility of SMP-1 and GIPLs in cold 1% Triton X-100 was not increased by addition of high salt (Figure 5B). The absence of a strong association of SMP-1 with the flagellar cytoskeleton was further supported by immunofluorescence studies. Specifically, SMP-1 and GIPLs were poorly extracted with cold 1% Triton X-100, but were totally extracted when promastigotes were treated with 1% Triton X-100 at 25°C (Figure 6A). In contrast, the subpellicular/axoneme microtubules as well as the paraflagellar rod were not extracted under either conditions (Figure 6, B and C). Collectively, these data suggest that SMP-1 is largely associated with detergent-resistant membranes and that the flagellar localization of SMP-1 is not dependent on strong associations with the microtubule axoneme or the paraflagellar rod.
Figure 5.
SMP-1 is not associated with the flagellum cytoskeleton. (A) L. major promastigotes were extracted in cold 1% Triton X-100 and the lysate was subjected to flotation centrifugation in sucrose density gradient. Proteins were analyzed by SDS-PAGE and Western blotting with anti-SMP-1 and anti-α-tubulin antibodies. The major promastigote glycoinositolphospholipids (GIPL) were analyzed by HPTLC and detected with orcinol/H2SO4. Fractions 1–17 encompass a step gradient of 5–60% sucrose. (B) L. major promastigotes were extracted in 1% Triton X-100 at 0°C and 25°C, in 1% Triton X-114 (0°C) and with 1% Triton X-100 containing 1 M NaCl (0°C). The distribution of SMP-1, α-tubulin, and GIPL in the insoluble (I) and soluble (S) fractions was determined by Western blotting with SMP-1 and α-tubulin antibodies or by HPTLC and orcinol/H2SO4 staining. (C) L. major and L. mexicana promastigotes were extracted in either cold 1% Triton X-100 or cold 1% Triton X-114 and recovery of SMP-1 in the insoluble (I), and soluble (S) fractions was determined by SDS-PAGE and Western blotting (only reactive 15-kDa band shown).
Flagellar Localization of SMP-1 Is Not Affected by Changes in DRM Composition or Axoneme and Paraflagellar Rod Structure
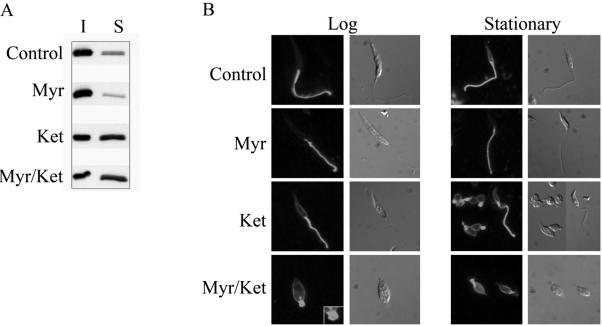
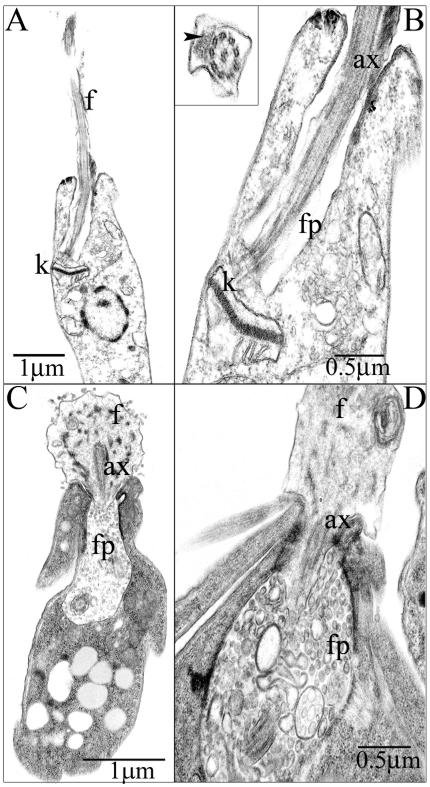
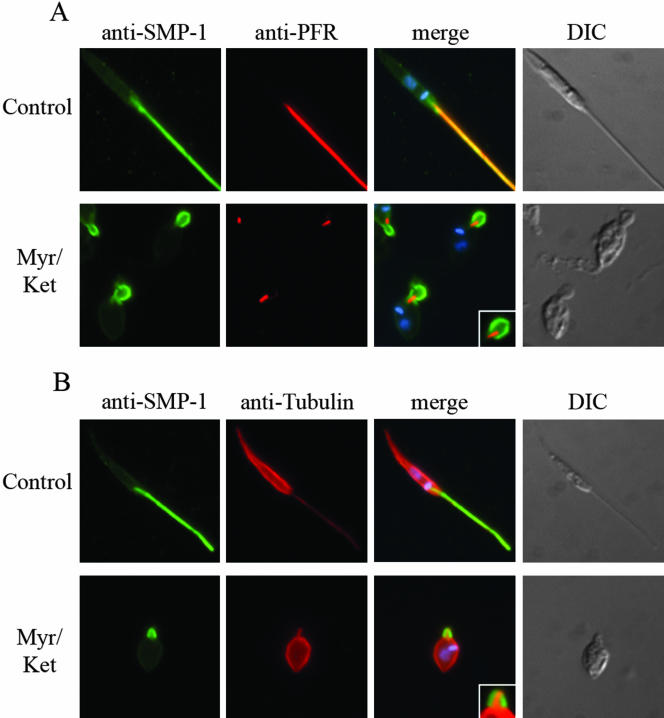
Leishmanial DRMs are enriched in the major cellular sterol and sphingolipids, ergosterol and inositolphosphoceramide, respectively (Denny et al., 2001; Ralton et al., 2002). To investigate whether the flagellar localization of SMP-1 is dependent on the lipid composition of the flagellar DRM, L. major promastigotes were treated with myriocin, an inhibitor of serine: palmitoyl-CoA transferase, the first committed enzyme in sphingolipid biosynthesis (Miyake et al., 1995) and ketoconazole, an inhibitor of sterol 14′-demethylase (Beach et al., 1988). At the concentrations used, these inhibitors cause a reduction of 70 and 50% in inositolphosphoceramide and sterol levels, respectively (Ginger et al., 2001; Ralton et al., 2002; our unpublished results). Myriocin-treatment alone had no effect on the growth rate of L. major promastigotes (our unpublished results), the solubility of SMP-1 and GIPLs in cold Triton X-100 (Figure 7A) or the flagellar localization of SMP-1 (Figure 7B). In contrast, ketoconazole-treatment significantly increased the solubility of both SMP-1 (Figure 7A) and the GIPLs (our unpublished results) in cold Triton X-100, demonstrating that changes in sterol composition alters the physical properties of DRMs. However, ketoconazole treatment had little effect on SMP-1 localization (Figure 7B), indicating that the targeting and/or retention of SMP-1 to the flagellar membrane is not dependent on a specific lipid composition. Interestingly, ketoconazole treatment caused the flagellum of some stationary phase promastigotes (∼ 40%) to retract, forming a rounded balloon-like structure at the anterior end of the promastigote (Figure 7B). This phenotype was induced in all (>90%) promastigotes when both ketoconazole and myriocin were added to either log or stationary phase cultures (Figure 7B). When ketoconazole/myriocin-treated parasites were fixed and examined by electron microscopy, dramatic alterations in both flagellar morphology and the structure of the flagellar pocket were observed (Figure 8). Specifically, the flagellum of combined drug-treated promastigotes became highly dilated at the point at which it emerged from the flagellar pocket. The 9 + 2 microtubule axoneme only partially extended into these swollen flagellum, and the lumen contained amorphous electrondense material and occasional membrane whorls (Figure 8, compare A,B with C,D). To investigate whether axoneme or paraflagellar rod precursors were still imported into the swollen flagellum, combined-drug–treated L. major promastigotes were fixed and probed with anti-α-tubulin and anti-PFR antibodies. As shown in Figure 9, the axonemes of drug-treated parasites were clearly truncated, whereas the flagellum lumen appeared to lack detectable pools of free tubulin. Moreover, levels of expression of the paraflagellar rod proteins were dramatically decreased in drug-treated parasites. In untreated promastigotes, the paraflagellar rod runs along the length of the axoneme from the point at which it emerges from the flagellar pocket to the distal tip (Figures 8B and 9A). In combined drug-treated promastigotes, the residual PFR signal was restricted to a single punctate structure at the base of the flagellum (Figure 9A). Finally, the flagellar pockets of drug-treated promastigotes were uniformally packed with membrane-bound vesicles 50–150 nm in diameter (Figure 8). Similar vesicles also appeared to be attached to the external surface of the dilated flagellum membrane. Collectively, these results suggest that partial inhibition of sterol and sphingolipid biosynthesis interferes with both the biogenesis of new flagellum in log phase parasites as well as the retraction of existing flagellum in stationary phase promastigotes. They also provide strong evidence that the flagellar targeting and/or retention of SMP-1 is not dependent on interactions with the axoneme.
Figure 7.
Inhibitors of sterol and sphingolipid biosynthesis perturb DRM formation and flagellar structure but not localization of SMP-1. (A) L. major promastigotes were grown in the absence or presence of 1 μg/ml myriocin (Myr), 2 μg/ml ketoconazole (Ket) or both drugs together (Myr/Ket) for 3 d and extracted in cold 1% Triton X-100. The distribution of SMP-1 in insoluble (I) and soluble (S) fraction was determined by Western blotting. (B) L. major promastigotes were grown in the presence of 1 μg/ml myriocin (Myr), 2 μg/ml ketoconazole (Ket), or both drugs together (Myr/Ket) for 1 (log) or 3 (stationary) days. Immunofluorescence microscopy of fixed promastigotes probed with the polyclonal anti-SMP-1 antibody.
Figure 8.
Ultrastructure of ketoconazole/myriocin-treated L. major promastigotes. L. major promastigotes were grown in the absence (A and B) or presence of myriocin and ketoconazole (1 and 2 μg/ml, respectively; C and D) for 3 d and fixed for electron microscopy. (B and D) Detail of the flagellar pocket and emergent flagellum. Inset in B shows detail of 9 + 2 microtubule axoneme and associated paraflagellar rod (arrowhead). f, flagellum; k, kinetoplast; ax, axoneme; fp, flagellar pocket.
Figure 9.
The swollen flagellar of drug-treated promastigotes lack axoneme or PFR precursors. L. major promastigotes were grown in the absence or presence of myriocin and ketoconazole (1 and 2 μg/ml, respectively), fixed, and probed with anti-SMP-1 (green signal) and anti-PFR (L8C4; red signal) antibodies (A) or anti-SMP-1 (green signal) and anti-α-tubulin antibodies (red signal; B). Inset in A and B (merge) shows detail of flagellar balloon structures showing absence of detectable pools of paraflagellar rod protein or α-tubulin in the lumen.
DISCUSSION
We have identified a novel family of SMPs in L. major that appear to be part of a larger family of proteins found in all trypanosomatids. Our data show that 1) SMP-1, the first of these proteins to be characterized in detail, is a major flagellar membrane protein in Leishmania, 2) that the dual myristoylation/palmitoylation signal in SMP-1 is required for localization of SMP-1 to detergent-resistant flagellar membranes, 3) that flagellar targeting and/or retention is not dependent on direct interactions with the axoneme or other cytoskeletal proteins, and 4) that disruption of sphingolipid and/or sterol biosynthesis has a profound affect on axoneme biogenesis or maintenance.
SMP-1 was initially identified as a major protein in the buoyant Triton X-100–insoluble membranes of Leishmania promastigotes. It is estimated from immunoprecipitation experiments that L. major promastigotes contain at least 105 copies of SMP-1 per cell. Assuming that the surface area of the flagellum is ∼0.5–1 μm2, that SMP-1 has a cross-sectional area of ∼15 nm2 and is exclusively located on the inner leaflet of the flagellar membrane, it is estimated that SMP-1 effectively coats the cytoplasmic face of the flagellar membrane. This high packing density may be facilitated by the use of the myristate/palmitate anchor, which would result in less perturbation of the flagellar membrane than a transmembrane polypeptide domain. Searches of the L. major genome revealed the presence of three other SMPs in this parasite (Figure 2). SMP-2 and -4 contain a strong myristoylation motif, but lack a clearly defined palmitoylation signal. These proteins also differ from SMP-1, and from each other, in containing distinct C-terminal sequences (Figure 2). The fourth member of the L. major SMP family, SMP-3, differed from the other members in lacking any identifiable acylation site or a comparable C-terminal domain. Although the functions of this protein family have yet to be elucidated, their diverse subcellular localizations (our unpublished results) and the presence in other trypanosomatids (T. brucei, T. cruzi; see Figure 2) suggests that they may be involved in multiple cellular processes. In this respect, it is of interest that the SMP “module” appears to act as a membrane anchor for the T. brucei cytoskeletal protein, TbCAP5.5 (Figure 2, A and B) and related proteins in the L. major genome (Hertz-Fowler et al., 2001). By analogy, the SMP family may be involved in targeting other proteins to surface or intracellular membranes. The potential importance of SMPs and other myristolyated proteins (Denny et al., 2000) in these parasites, is underlined by a recent study showing that the myristoyl-CoA: protein N-myristoyltranferase is essential for normal growth of L. major and T. brucei (Price et al., 2003).
Although the protein and lipid compositions of eukaryotic flagellar and cilia membranes differ from the abutting plasma membrane, little is known about flagellar membrane targeting signals or the mechanisms involved. Flagellar targeting of the L. enriettii glucose transporter, ISO1, is mediated by the N-terminal cytoplasmic domain of this polytopic membrane protein (Snapp and Landfear, 1999), whereas dual acylation appears to be both necessary and sufficient for flagellar localization of T. cruzi FCaBP (Godsel and Engman, 1999). Dual acylation was also found to be essential for flagellar targeting of SMP-1, as mutation of either the myristoylation or the palmitoylation sites prevented membrane association (our unpublished results) and resulted in a cytoplasmic localization. Dual acylation also appeared to be essential for membrane association as SMP-1ΔPalm::HA, which should still be cotranslationally myristoylated (Denny et al., 2000), was localized to the cytosol and soluble fractions. In contrast, the diacylated L. major protein, HASP-B, is targeted to Triton X-100–soluble domains of the plasma and endomembranes, rather than detergent insoluble-domains of the flagellum (Denny et al., 2000, 2001). Moreover, a GFP-chimera containing the monoacylated (myristoylated) N-terminal domain of HASPB was targeted to intracellular secretory/endosomal membranes (Denny et al., 2000). Collectively, these data suggest that other factors, such as protein conformation and/or additional peptide sorting signals, modulate the potential for myristoylation and palmitoylation to target HASPB and SMP proteins to different cellular localizations. Interestingly, the association of the diacylated T. cruzi protein FCaBP with flagellar membranes appears to be calcium-dependent (Godsel and Engman, 1999). In contrast, the association of SMP-1 with the flagellar membrane was not affected by calcium-chelating agents (our unpublished results), although it is possible that other factors (pH, phosphorylation, GTP-binding or oligomerization; Tang et al., 2004) could regulate flagellar localization.
A number of trypanosomatid proteins appear to be localized to the plasma membrane of the cell body through interactions with the underlying microtubule cytoskeleton (Piper et al., 1995; Hertz-Fowler et al., 2001; Vedrenne et al., 2002). However, several lines of evidence suggest that the flagellar localization of SMP-1 is not dependent on interactions with the axoneme microtubules or the paraflagellar rod. First, most of the SMP-1 floated with other DRM components in sucrose density gradients and was clearly separated from microtubules or tubulin. Although some SMP-1 remained at the bottom of the gradient, DRM components such as the GIPLs and PSA2 were similarly retained, presumably reflecting incomplete solubilization of the DRMs. Second, SMP-1 was solubilized to the same extent as the GIPLs, under a variety of detergent and high salt extraction conditions (1% Triton X-100, 25°C; 1% Triton X-114, 0°C) that left the cytoskeleton (subpellicular microtubules plus axoneme) essentially intact. Interestingly, these studies showed that the Triton X-114 solubility of SMP-1 and GIPL in L. major and L. mexicana differed markedly, indicating species-specific differences in the lipid composition of the DRMs. These results suggest that the Triton X-100 insolubility of SMP-1 is largely due to hydrophobic interactions, rather than interactions with the cytoskeleton (Ralton et al., 2002). Third, partial inhibition of sterol biosynthesis, which is expected to alter the properties of DRMs, increased the solubility of SMP-1 and GIPLs in cold Triton X-100. Finally, the subcellular distribution of SMP-1 was not significantly altered when promastigotes were treated with sterol and sphingolipid biosynthesis inhibitors, under conditions that caused the axoneme to completely dissociate from the flagellar membrane. Collectively, these data suggest that the flagellar localization of SMP-1 is not dependent on tight associations with the axoneme microtubules and/or the paraflagellar rod. Moreover they raise the possibility that SMP-1 is targeted to the flagellar membrane via an IFT-independent mechanism. Transport of polycistin-2 to the membrane kidney epithelia cilia also appears to occur via an IFT-independent mechanism (Pazour et al., 2002). A calcium-dependent process involving flagellar matrix phosphoproteins has been proposed to operate in regulating the directed flow of flagellar membrane proteins in Chlamydomonas reinhardtii (Bloodgood and Salomonsky, 1994) and related proteins could play a role in regulating SMP-1 flagellar localization in Leishmania.
Sterol biosynthetic inhibitors such as ketoconazole and fluconazole are used as anti-Leishmania drugs (Arana et al., 2001; Alrajhi et al., 2002; Davies et al., 2003). However, the precise mode of action of these drugs, in terms of cellular processes affected, remain unclear. In this study we show that sublethal concentrations of ketoconazole affect flagellum structure in stationary phase promastigotes. This affect was dramatically accentuated and observed in all growth phases when promastigotes were treated with both ketoconazole and myriocin, although the sphingolipid inhibitor had no detectable effect on flagellum morphology when used alone. Partial inhibition of sterol/sphingolipid biosynthesis induced the flagellar membrane to dilate, usually at the point at which the flagellum emerged from the flagellar pocket, and was associated with a dramatic shortening of the axoneme and the loss of the paraflagellar rod. The dilated flagellum contained electron-dense material that did not appear to comprise axoneme or paraflagellar rod precursors. This flagellum phenotype is distinct from those found Chlamydomonas mutants deficient in retrograde or anterograde IFT, which are characterized by swellings at the distal tip of the flagellum or production of very short flagellum, respectively (Kozminski et al., 1995; Pazour et al., 1998; Piperno et al., 1998; Marshall and Rosenbaum, 2001). This phenotype is also distinct from T. brucei PFR-A mutants, which accumulate PFR-C protein at the distal end of the flagellum (Bastin et al., 1999). We speculate that perturbation of sterol and sphingolipid biosynthesis affects either the import of axoneme precursors or axonemal stability. Axonemal instability could result from functional loss of plus-end capping proteins (Carvalho et al., 2003; Howard and Hyman, 2003) that are likely to exist at the distal tip of trypanomatid flagellum (Briggs et al., 2004). Axonemal truncation was associated with the loss of the paraflagellar rod and the degradation of PFR proteins. A similar loss of PFR proteins was observed in another L. mexicana mutant that exhibited a severely shortened flagellum (Wiese et al., 2003). Together these results suggest that perturbation of the lipid composition of the plasma membrane has a profound effect on axoneme extension. Whether stage-specific changes in lipid composition also modulate changes in flagellar length observed during promastigote-amastigote transitions is being investigated.
Ketoconazole/myriocin treatment also caused the accumulation of numerous vesicles within the flagellar pocket. These vesicles are the same size as the intraluminal vesicles of the multivesicular endosomes and lysosomal compartments of Leishmania promastigotes (Mullin et al., 2001). Inhibition of sterol/sphingolipid biosynthesis may induce or accelerate the fusion of endosomal/lysosomal compartments with the flagellar pocket membrane. Alternatively, endosomal compartments may constitutively fuse with the flagellar pocket and release these vesicles, which become trapped in drug-treated parasites because of the dilation of the flagellar membrane (see Figure 8). Interestingly, a very similar phenotype is observed in a L. major mutant lacking serine:palmitoylCoA transferase, the target of myriocin (Zhang et al., 2003; Denny et al., 2004). These observations support the notion that changes in bulk lipid composition rather than depletion of specific sterol or sphingolipid molecular species are responsible for this unusual flagellum phenotype and that sphingolipid biosynthesis inhibitors may increase the efficacy of sterol biosynthetic inhibitors as anti-leishmanial agents.
Acknowledgments
We thank Professor K. Gull (University of Oxford), Dr. E. Handman (The Walter and Eliza Hall Institute of Medical Research), and Dr. J. D. Bangs (University of Madison, Madison Medical School) for generously providing antibodies. This work was funded by an Australian National Health and Medical Research Council (NHMRC) program grant (215201) and Wellcome Trust Equipment grant (058965/Z/99/Z). M.J.M. is a NHMRC Principal Research Fellow and Howard Hughes International Fellow. G.I.M. is an ARC Professor Fellow and Howard Hughes International Fellow. Preliminary sequence data were produced by the Leishmania Sequencing Group at the Sanger and EULEISH Sequencing Centre and by The Institute for Genomic Research (supported by an award from the National Institute of Allergy and Infectious Diseases, National Institutes of Health).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–06–0457. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–06–0457.
References
- Alrajhi, A.A., Ibrahim, E.A., De Vol, E.B., Khairat, M., Faris, R.M., and Maguire, J.H. (2002). Flucozole for the treatment of cutaneous leishmaniasis caused by Leishmania major. N. Engl. J. Med. 346, 891-895. [DOI] [PubMed] [Google Scholar]
- Arana, B., Rizzo, N., and Diaz, A. (2001). Chemotherapy of cutaneous leishmaniasis: a review. Med. Microbiol. Immunol. 190, 93-95. [DOI] [PubMed] [Google Scholar]
- Bastin, P., Pullen, T.J., Moreira-Leite, F.F., and Gull, K. (2000). Inside and outside of the trypanosome flagellum: a multifunctional organelle. Microbes Infect. 2, 1865-1874. [DOI] [PubMed] [Google Scholar]
- Bastin, P., Pullen, T.J., Sherwin, T., and Gull, K. (1999). Protein transport and flagellum assembly dynamics revealed by analysis of the paralysed trypanosome mutant snl-1. J. Cell Sci. 112, 3769-3777. [DOI] [PubMed] [Google Scholar]
- Beach, D.H., Goad, L.J., and Holz, G.G. (1988). Effects of antimycotic azoles on growth and sterol biosynthesis of Leishmania promastigotes. Mol. Biochem. Parasitol. 31, 166-185. [DOI] [PubMed] [Google Scholar]
- Bloodgood, R.A., and Salomonsky, N.L. (1994). The transmembrane signaling pathway involved in directed movements of Chlamydomonas flagellar membrane glycoproteins involves the dephosphorylation of a 60-kD phosphoprotein that binds to the major flagellar membrane glycoprotein. J. Cell Biol. 127, 803-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs, L.J., McKean, P.G., Baines, A., Moreira-Leite, F.F., Davidge, J., Vaughan, S., and Gull, K. (2004). The flagellar connector of Trypanosoma brucei: an unusual mobile transmembrane junction. J. Cell Sci. 117, 1641-1651. [DOI] [PubMed] [Google Scholar]
- Carvalho, P., Tirauer, J.S., and Pellman, D. (2003). Surfing on microtubule ends. Trends Cell Biol. 13, 229-237. [DOI] [PubMed] [Google Scholar]
- Cole, D.G. (2003). The intraflagellar transport machinery of Chlamydomonas reinhardtii. Traffic 4, 435-442. [DOI] [PubMed] [Google Scholar]
- Davies, C.R., Kaye, P.M., Croft, S.L., and Sundar, S. (2003). Leishmaniasis: new approaches to disease control. Br. Med. J. 326, 377-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny, P.W., Field, M.C., and Smith, D.F. (2001). GPI-anchored proteins and glycoconjugates segregate into lipid rafts in Kinetoplastida. FEBS Lett. 491, 148-153. [DOI] [PubMed] [Google Scholar]
- Denny, P.W., Gokool, S., Russell, D.G., Field, M.C., and Smith, D.F. (2000). Acylation-dependent protein export in Leishmania. J. Biol. Chem. 275, 11017-11025. [DOI] [PubMed] [Google Scholar]
- Denny, P.W., Goulding, D., Ferguson, M.A., and Smith, D.F. (2004). Sphingolipid-free Leishmania are defective in membrane trafficking, differentiation and infectivity. Mol. Microbiol. 52, 313-327. [DOI] [PubMed] [Google Scholar]
- Ginger, M.L., Chance, M.L., Sadler, I.H., and Goad, L.J. (2001). The biosynthetic incorporation of the intact leucine skeleton into sterol by the trypanosomatid Leishmania mexicana. J. Biol. Chem. 276, 11674-11682. [DOI] [PubMed] [Google Scholar]
- Godsel, L.M., and Engman, D.M. (1999). Flagellar protein localization mediated by a calcium-myristoyl/palmitoyl switch mechanism. EMBO J. 18, 2057-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gull, K. (1999). The cytoskeleton of trypanosomatid parasites. Annu. Rev. Microbiol. 53, 629-655. [DOI] [PubMed] [Google Scholar]
- Hertz-Fowler, C., Ersfeld, K., and Gull, K. (2001). CAP5.5, a life-cycle-regulated, cytoskeleton-associated protein is a member of a novel family of calpain-related proteins in Trypanosoma brucei. Mol. Biochem. Parasitol. 116, 25-34. [DOI] [PubMed] [Google Scholar]
- Hill, K.L. (2003). Biology and mechanism of trypanosome cell motility. Eukaryot. Cell 2, 200-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, J., and Hyman, A.A. (2003). Dynamics and mechanics of the microtubule plus end. Nature 422, 753-758. [DOI] [PubMed] [Google Scholar]
- Kozminski, K.G., Beech, P.L., and Rosenbaum, J.L. (1995). The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J. Cell Biol. 131, 1517-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski, K.G., Johnson, K.A., Forscher, P., and Rosenbaum, J.L. (1993). A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl. Acad. Sci. USA 90, 5519-5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfear, S.M., and Ignatushchenko, M. (2001). The flagellum and flagellar pocket of trypanosomatids. Mol. Biochem. Parasitol. 115, 1-17. [DOI] [PubMed] [Google Scholar]
- Marshall, W.F., and Rosenbaum, J.L. (2001). Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J. Cell Biol. 155, 405-414. Epub 2001 Oct 2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville, M.J., and Blackwell, J.M. (1991). Developmental changes in the glycosylated phosphatidylinositols of Leishmania donovani. Characterization of the promastigote and amastigote glycolipids. J. Biol. Chem. 266, 15170-15179. [PubMed] [Google Scholar]
- McConville, M.J., Mullin, K.A., Ilgoutz, S.C., and Teasdale, R.D. (2002). Secretory pathway of trypanosomatid parasites. Microbiol. Mol. Biol. Rev. 66, 122-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake, Y., Kozutsumi, Y., Nakamura, S., Fujita, T., and Kawasaki, T. (1995). Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochem. Biophys. Res. Commun. 211, 396-403. [DOI] [PubMed] [Google Scholar]
- Mullin, K.A., Foth, B.J., Ilgoutz, S.C., Callaghan, J.M., Zawadzki, J.L., McFadden, G.I., and McConville, M.J. (2001). Regulated degradation of an endoplasmic reticulum membrane protein in a tubular lysosome in Leishmania mexicana. Mol. Biol. Cell 12, 2364-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, E.N., Towler, D.A., and Glaser, L. (1985). Specificity of fatty acylation of cellular proteins. J. Biol. Chem. 260, 3784-3790. [PubMed] [Google Scholar]
- Pazour, G.J., San Agustin, J.T., Follit, J.A., Rosenbaum, J.L., and Witman, G.B. (2002). Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr. Biol. 12, R378-R380. [DOI] [PubMed] [Google Scholar]
- Pazour, G.J., Wilkerson, C.G., and Witman, G.B. (1998). A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT). J. Cell Biol. 141, 979-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper, R.C., Xu, X., Russell, D.G., Little, B.M., and Landfear, S.M. (1995). Differential targeting of two glucose transporters from Leishmania enriettii is mediated by an NH2-terminal domain. J. Cell Biol. 128, 499-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno, G., Siuda, E., Henderson, S., Segil, M., Vaananen, H., and Sassaroli, M. (1998). Distinct mutants of retrograde intraflagellar transport (IFT) share similar morphological and molecular defects. J. Cell Biol. 143, 1591-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, H.P., Menon, M.R., Panethymitaki, C., Goulding, D., McKean, P.G., and Smith, D.F. (2003). Myristoyl-CoA:protein N-myristoyltransferase, an essential enzyme and potential drug target in kinetoplastid parasites. J. Biol. Chem. 278, 7206-7214. [DOI] [PubMed] [Google Scholar]
- Ralton, J.E., and McConville, M.J. (1998). Delineation of three pathways of glycosylphosphatidylinositol biosynthesis in Leishmania mexicana. Precursors from different pathways are assembled on distinct pools of phosphatidylinositol and undergo fatty acid remodeling. J. Biol. Chem. 273, 4245-4257. [DOI] [PubMed] [Google Scholar]
- Ralton, J.E., Mullin, K.A., and McConville, M.J. (2002). Intracellular trafficking of glycosylphosphatidylinositol (GPI)-anchored proteins and free GPIs in Leishmania mexicana. Biochem. J. 363, 365-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum, J.L., and Witman, G.B. (2002). Intraflagellar transport. Nat. Rev. Mol. Cell. Biol. 3, 813-825. [DOI] [PubMed] [Google Scholar]
- Snapp, E.L., and Landfear, S.M. (1999). Characterization of a targeting motif for a flagellar membrane protein in Leishmania enriettii. J. Biol. Chem. 274, 29543-29548. [DOI] [PubMed] [Google Scholar]
- Swofford, D.L. (1997). PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sunderland, MA: Sinauer Associates.
- Tang, C., Loeliger, E., Luncsford, P., Kinde, I., Beckett, D., and Summers, M.F. (2004). Entropic switch regulates myristate exposure in the HIV-1 matrix protein. Proc. Natl. Acad. Sci. USA 101, 517-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J.D., Gibson, T.J., Plewniak, F., Jeanmouqin, F., and Higgins, D.G. (1997). The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24, 4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler, D.A., Eubanks, S.R., Towery, D.S., Adams, S.P., and Glaser, L. (1987). Amino-terminal processing of proteins by N-myristoylation. Substrate specificity of N-myristoyl transferase. J. Biol. Chem. 262, 1030-1036. [PubMed] [Google Scholar]
- Vaughan, S., and Gull, K. (2003). The trypanosome flagellum. J. Cell Sci. 116, 757-759. [DOI] [PubMed] [Google Scholar]
- Vedrenne, C., Giroud, C., Robinson, D.R., Besteiro, S., Bosc, C., Bringaud, F., and Baltz, T. (2002). Two related subpellicular cytoskeleton-associated protein in Trypanosoma brucei stabilize microtubules. Mol. Biol. Cell 13, 1058-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel, D., and Flugge, U.I. (1984). A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138, 141-143. [DOI] [PubMed] [Google Scholar]
- Wiese, M., Kuhn, D., and Grunfelder, C.G. (2003). Protein kinase involved in flagellar-length control. Eukaryot. Cell 2, 769-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, K., Showalter, M., Revollo, J., Hsu, F.F., Turk, J., and Beverley, S.M. (2003). Sphingolipids are essential for differentiation but not growth in Leishmania. EMBO J. 22, 6016-6026. [DOI] [PMC free article] [PubMed] [Google Scholar]