Abstract
Background
Osteoarthritis (OA) is the most common human joint disease. Recent studies suggest that an abnormal subchondral bone metabolism is intimately involved in the genesis of this disease. Bone remodelling is tightly regulated by a molecular triad composed of OPG/RANK/RANKL. RANKL exists as 3 isoforms: RANKL1, 2, and 3. RANKL1 and 2 enhance osteoclastogenesis whereas RANKL3 inhibits this phenomenon. We previously reported that human OA subchondral bone osteoblasts can be discriminated into two subgroups according to their level of PGE2 [low (L) or high (H)]. Moreover, we also showed that L-OA osteoblasts express higher levels of total RANKL compared to H-OA osteoblasts. In this study, we investigated the level of membranous RANKL, comparing L- and H-OA subchondral bone osteoblasts, as well as its modulation by osteotropic factors. The impact of the modulation of RANKL1 and 3 on the membranous RANKL level was also studied.
Methods
Gene expression was determined using real-time PCR for RANKL1 and semi-quantitative PCR for RANKL3. Membranous RANKL was measured by flow cytometry. The modulation of membranous RANKL and RANKL isoforms was monitored on the L- and H-OA osteoblasts and also following treatment with osteotropic factors, including vitamin D3 (50 nM), IL-1β (100 pg/ml), TNF-α (5 ng/ml), PGE2 (500 nM), PTH (100 nM), IL-6 (10 ng/ml) and IL-17 (10 ng/ml).
Results
Membranous RANKL levels were significantly increased in L-OA osteoblasts compared to normal (p<0.01) and H-OA (p<0.05). The gene expression level of the RANKL1 profile was reminiscent of the membranous RANKL level. Although RANKL3 gene expression was lower on the H-OA osteoblasts than on normal and L-OA osteoblasts (p<0.03), the overall outcome favoured RANKL1. Treatment with the tested factors showed a significant increase in membranous RANKL on the L-OA osteoblasts, with the exception of PTH and IL-17. Interestingly in this subpopulation, the RANKL3 gene expression level was significantly increased upon PTH and IL-17 treatment. No effect of the tested osteotropic factors was found on the H-OA.
Conclusion
Our findings showed that the normal, L- and H-OA subchondral bone osteoblasts differentially express membranous RANKL and RANKL isoforms, and that treatment with osteotropic factors generally favours increased membranous localization of RANKL on L-OA compared to H-OA osteoblasts. This phenomenon appears to take place through differential modulation of each RANKL isoform.
Keywords: Osteoarthritis, Osteoblasts, Subchondral bone, RANKL isoforms, Bone remodelling
Introduction
Osteoarthritis (OA) is characterized by the degradation and loss of articular cartilage and inflammation. Recent studies have provided the basis for the assertion that the subchondral bone is intimately involved in this disease [1]. The fate of articular cartilage is not determined exclusively by the stiffening of the subchondral bone, but rather by a remodelling process of this tissue [2–4]. Although controversial, some clinical studies in OA patients have suggested that the indices of bone resorption are increased early in the disease [5,6], while subchondral bone sclerosis is a relatively late phenomenon. Altogether, data suggest that abnormal subchondral bone metabolism occurs at an early stage of the disease and is the driving force behind cartilage degradation and loss.
The subchondral bone is composed of a specialized connective tissue formed by a mineralized matrix containing the specific collagen type I, proteoglycans and various growth factors and cytokines as well as the bone specific cell types, osteoblasts, osteoclasts and osteocytes [7–9]. Osteoblasts and osteoclasts contribute either alone or in combination to bone remodelling, and the disturbance between the activities of these two cell types is responsible for the development of an altered bone metabolism.
Human OA subchondral bone osteoblasts have demonstrated abnormal phenotypes, including elevated alkaline phosphatase activity and increased release of osteocalcin [10]. Other factors such as TGF-β, IGF-1 and uPA/plasmin system, to name a few, have been found at abnormal levels in this diseased tissue [11]. Moreover, our group has also reported that OA subchondral bone osteoblasts, which otherwise demonstrate no different phenotypic features, can be discriminated into two subgroups identified by their level (low or high) of endogenous prostaglandin E2 (PGE2) [12,13].
It is well known that bone metabolism is tightly controlled by some members of the tumour necrosis factor (TNF) family. In this context, a molecular triad composed of OPG/RANK/RANKL has been well established as an essential cytokine system for controlling the osteoclast biology [14].
Osteoprotegerin ligand or RANKL (Receptor Activator of Nuclear Factor κB Ligand) is synthesized mainly by the osteoblastic lineage cells, the immune cells and some cancer cells, and is essential for mediating bone resorption through mediating osteoclastogenesis and the activation of mature osteoclasts. RANKL stimulates osteoclastogenesis and osteoclast activity by binding to the cell surface receptor RANK, located on osteoclasts (precursor and mature) [14,15]. The binding of RANKL to the extracellular RANK domain leads to the activation of specific signalling pathways such as TRAF6, NF-κB and JNK, which are all involved during the formation of osteoclasts, osteoclast survival, and bone resorption [16,17]. Osteoprotegerin (OPG) is secreted by stromal cells and other cell types, including osteoblasts, and acts as a soluble decoy receptor for RANKL. OPG, by interacting with RANKL, inhibits the binding of RANKL to RANK, thereby preventing RANK activation and the subsequent osteoclastogenesis and, as a result, inhibits bone resorption [18].
The importance of these different factors has been demonstrated on RANKL-deficient mice, in which osteoclast formation was greatly reduced, and on overexpressing-OPG mice, in which a severe osteopetrosis with a reduced number of osteoclasts was observed [19,20]. Moreover, in animal models of arthritis, the administration of OPG as a therapeutic agent protects the bone from excessive resorption and the cartilage from being degraded [19,21,22].
RANKL thus appears to be of great importance in controlling the subchondral bone remodelling process. RANKL has been recently shown to exist as 3 isoforms: RANKL1, 2, and 3. RANKL1 and 2 encode for transmembrane forms with an absence of intracellular domain for RANKL2, whereas RANKL3 lacks the intracellular and transmembrane domain [23]. These different isoforms are able to differentially regulate osteoclastogenesis [23–25]. In cells, RANKL forms homo- or hetero-trimer structures between the isoforms [26,27], and the trimeric combination of the isoforms is crucial for orientating the membranous localization, thereby controlling the osteoclast formation and differentiation process. Hence, the trimeric structure of RANKL1 alone or associated with RANKL2 is translated into a membranous localization. However, when RANKL1 or 2 is co-transfected with RANKL3, a reduced level of membranous localization is observed, indicating that RANKL3, by preventing the membranous localization of RANKL, acts as an inhibitor of osteoclastogenesis [23–25].
Although the role of RANKL is well established, there is, to the authors’ knowledge, no data regarding the expression of this factor’s isoforms and their modulation either on human OA subchondral bone or in other diseases actively involving RANKL, such as post-menopausal osteoporosis, bone metastases and rheumatoid arthritis. Hence, this study aimed at investigating the level of membranous RANKL and the relationship between the expression of the different RANKL isoforms in human normal osteoblasts and in both of the OA subchondral bone osteoblast subgroups (low (L) and high (H) OA) as well as their modulation upon treatment with osteotropic factors. Data showed that the normal, L- and H-OA subchondral bone osteoblasts differentially express membranous RANKL as well as RANKL isoforms, and that treatment with osteotropic factors favours increased levels of membranous RANKL on L- compared to H-OA osteoblasts. This phenomenon occurs through differential modulation of each RANKL isoform.
Materials and methods
Specimen selection
Human subchondral bones were obtained from femoral condyles of normal individuals within 12 h of death (mean age±SD: 65±16) or from OA patients undergoing total knee arthroplasty (mean age±SD: 71±9). All patients were evaluated as having OA according to American College of Rheumatology clinical criteria [28]. At the time of surgery the patients had symptomatic disease requiring medical treatment in the form of acetaminophen, NSAIDs, or selective COX-2 inhibitors. None had received intra-articular steroid injections within 3 months prior to surgery, and none had received medication that would interfere with bone metabolism. The institutional Ethics Committee Board of the University of Montreal Hospital Centre approved the use of the human articular tissues.
Subchondral bone osteoblast culture
The subchondral bone osteoblast culture was prepared as previously described [10–13]. Briefly, bone samples were cut into small pieces and digested for 4 h with collagenase type I in BGJb medium (both from Sigma-Aldrich Canada, Oakville, ON, Canada) without serum at 37 °C in a humidified atmosphere of 5% CO2/95% air. After this period the bone pieces were cultured in BGJb medium containing 20% heat-inactivated fetal calf serum (FCS; Gibco-BRL, Burlington, ON, Canada) and an antibiotic mixture (100 U/ml penicillin base and 100 μg/ml streptomycin base; Gibco-BRL) at 37 °C in the humidified atmosphere. When cells were observed in the petri dishes, the culture medium was replaced with fresh medium containing 10% FCS until confluence. Osteoblasts passaged once were used.
The effects of the factors on RANKL were assessed by pre-incubating cells in DMEM (Gibco-BRL)/0.5% FCS for 24 h followed by 18 h (for mRNA determination) and 72 h (for protein determination) incubation with fresh DMEM/0.5% FCS containing the factors under study. The incubation period for RANKL expression was determined following preliminary experiments showing that factors demonstrated maximum effect at this time. The factors tested were vitamin D3 (50 nM; Sigma-Aldrich Canada), IL-1β (100 pg/ml; Genzyme, Cambridge, MA, USA), TNF-α (5 ng/ml; R&D Systems, Minneapolis, MN, USA), PGE2 (500 nM; R&D Systems), PTH (100 nM; Peninsula, Belmont, CA, USA), IL-6 (10 ng/ml; R&D Systems) and IL-17 (10 ng/ml; R&D Systems). The concentrations were chosen from previous works.
Identification of the human OA subchondral bone osteoblast subpopulations was performed according to previous publications [2,12,13]; OA osteoblasts producing low levels of PGE2 < 2000 pg/mg protein were classified as L-OA osteoblasts, and those producing high levels of PGE2 > 2000 pg/mg protein as H-OA osteoblasts.
RNA extraction, reverse transcription (RT) and polymerase chain reaction (PCR)
Total cellular RNA from human osteoblasts was extracted with the TRIzol™ reagent (Invitrogen, Burlington, ON, Canada) according to the manufacturer’s specifications. The RNA was quantitated using the RiboGreen RNA quantitation kit. The RT reactions were primed with random hexamers as described previously [29]. According to the published RANKL sequence (hRANKL1, gi: 18143618; hRANKL2, gi: 16610212; hRANKL3, gi: 21536432), primers were designed to amplify specifically RANKL1 and RANKL3. Of note, it was impossible to target RANKL2 in a specific manner, since our designed primers overlapping the remaining sequence of the 3′ and the 5′ at the deletion region were unable to adequately amplify RANKL2 (Fig. 1). The primer sequences were for RANKL1, 5′-GCCTGCGCCGCACCA (sense) and 5′-CTGCTCTGATGTGCTGTGATCC (antisense), RANKL2, 5′-CCCTGCACGCCCCAT (sense) and 5′-CTGCTCTGATGTGCTGTGATCC (anti-sense) and RANKL3, 5′-CGCCTGGCCTATTGAAGG (sense) and 5′-CTGCTCTGATGTGCTGTGATCC (antisense). For GAPDH, which served as housekeeping gene, the primers used were 5′-CAGAACATCATCCCTGCCTCT (sense) and 5′-GCTTGACAAAGTGGTCGTTGAG (antisense).
Fig. 1.
Schematic representation of the 3 human RANKL cDNA isoforms, namely RANKL1, RANKL2 and RANKL3. The start (ATG) and the end (TGA) of the ORF (Open Reading Frame) are indicated on each isoform. The location of the primer used for the specific amplification of each isoform is indicated by arrows: 1 and 4 indicate sense and antisense respectively for the amplification of RANKL1; 2 and 4 for RANKL2; and 3 and 4 for RANKL3.
Real-time quantitation of mRNA was performed as previously described [29] in the Rotor-Gene 6® RG-3000A (Corbett Research, Mortlake, NSW, Australia) with the 2× Quantitect SYBR Green PCR Master Mix (Qiagen, Mississauga, ON, Canada) according to the manufacturer’s specifications. The data were given as a threshold cycle (CT). Data were calculated as the ratio of the number of molecules of the target gene/number of molecules of GAPDH. The primer efficiencies for the test genes were the same as for the GAPDH gene. Of note, the RANKL1 arbitrary unit is represented as ×10−5.
Determination of RANKL3 expression level was not possible when using the quantitative PCR, as the quantity (50 ng of cDNA) was too low and resulted in non-reliable quantification. In order to use more RNA, we chose to quantitate this isoform using semi-quantitative PCR as follows. First-strand cDNA was synthesized by incubating 2 μg of total RNA in 20 μl of RT mixture containing 4 μl of MGCl2 25 mM (Invitrogen), 2 μl of PCR buffer 10X (Invitrogen), 1 μl random hexamers 50 μM (Invitrogen), 1 μl oligo-dT 50 μM (Roche Perkin-Elmer, Foster City, CA, USA), 1 μl of 10 mM dNTP (Invitrogen), 1 μl of 200 U/μl MMLV-RT (Invitrogen) and 0.5 μl of 32.9 U/μl RNAsine (Invitrogen) at 42 °C for 15 min, 5 min at 99 °C and then ice-chilled for 5 min. The RT reaction mixture was subjected to PCR using upstream and downstream RANKL3 and GAPDH primers (20 μM each), 5 μl of 10× PCR buffer (Invitrogen), 3 μl of 25 mM MgCl2 (Invitrogen), 2 μl of 10 mM dNTP (Invitrogen), 65.5 μl of sterile water and 0.5 μl of 5 U/μl Taq polymerase (Invitrogen). The RT-PCR with denaturation at 94 °C for 1 min and annealing at 60 °C for 1.5 min was performed for 45 and 20 cycles for RANKL3 and GAPDH respectively. PCR amplification products were resolved by electrophoresis on a 2% agarose gel. The relative amounts of PCR products were determined by quantifying the intensity of the bands using the TotalLab TL100 Software (Nonlinear Ltd, Newcastle, UK) and data were calculated as the ratio of the band intensity of the target gene over the band intensity of GAPDH. The amplification sequence obtained for RANKL3 was purified by using the Nucleospin Extract II (Macherey-Nagel, Bethlehem, PA, USA) and cloned by using the TA cloning kit Dual promoter, pCR II (Invitrogen). DH5α cells were used for amplifying the RANKL3 gene and then subjected to sequencing by using the M13 forward primers. The sequencing analysis confirmed the correct sequence of RANKL3 according to the literature and to its published on-line sequence as mentioned above.
PGE2 determination
PGE2 levels were as previously described [2,12,13] performed by an EIA assay from Cayman Chemicals (Ann Arbor, MI, USA) with a sensitivity of 7.8 pg/ml. All determinations were performed in duplicate for each cell culture.
Membranous RANKL determination
At the end of the incubation period (72 h), cells were washed once in 1% BSA/PBS, detached with the cell dissociation buffer enzyme-free (Invitrogen) at 37 °C, and centrifuged at 500 g for 5 min at 4 °C. The cells were re-suspended in 1% BSA/PBS and a 500 μl suspension was made, having a concentration of 1×106 cells/ml. The suspension was incubated for 30 min at room temperature and divided into tubes. One served as negative control to which mouse IgG (15 μg/ml; Chemicon International, Billerica, MA, USA) was added, and the other was labeled with the anti-human RANKL antibody (15 μg/ml; R&D Systems) for 30 min at 4 °C. After washing, a goat anti-mouse FITC-conjugated secondary antibody (7.5 μg/ml; R&D Systems) was added for another 30 min at 4 °C. Cells were then washed in PBS, re-suspended in PBS, and analyzed using flow cytometry (FACSCalibur, BD Biosciences, Mississauga, ON, Canada). The control sample was used to determine background fluorescence and compared to that of the sample incubated with the specific antibody. The level of fluorescence, measured by a FACScan using the CellQuest program (BD Biosciences), was calculated as the relative or mean fluorescent intensity of positive cells. For the experiments performed with the osteotropic factors, data are expressed over the control, which was assigned a value of 1.
Statistical analysis
Data are expressed as the mean±SEM. Statistical significance was assessed by the 2-tailed Student’s t-test, and p values <0.05 were considered significant.
Results
Levels of membranous RANKL and RANKL1 and 3 expression
We recently demonstrated [12,13] that the metabolic activity of PGE2 on human OA subchondral bone osteoblasts could be discriminated into two subgroups. In one subgroup, endogenous osteoblast PGE2 levels were comparable to normal and these cells were classified as low producers (L-OA) whereas cells in the other subgroup, in which a high production of PGE2 was observed, were classified as high producers (H-OA). In this study, we first evaluated the OA osteoblast levels of PGE2. As expected, data revealed two subgroups of osteoblasts: L-OA osteoblasts with PGE2 levels of 734±119 pg/mg protein, and H-OA osteoblasts with 4264±738 pg/mg protein. In general, the proportion of patients showing L OA osteoblasts consisted of about 60%.
The level of membranous RANKL and the gene expression level of the RANKL isoforms were then determined on normal osteoblasts and each of the OA subpopulations. According to the RANKL sequences, only the primers specific for the isoforms 1 and 3 could be employed. As mentioned above, specific expression of RANKL2 could not be adequately analyzed even by using a specific set of primers at the deletion region, as illustrated in Fig. 1. Since the cDNA sequence of human RANKL2 is similar to human RANKL1, except at the deletion region, specific primers could only be designed at that particular region, and the absence of reliable data could be due to a very low expression of human RANKL2. Hence, as RANKL1 is the major isoform and because expression of RANKL3 induces the opposite effect to that of RANKL1 and 2, this study was performed on the modulation of RANKL1 and RANKL3 isoforms.
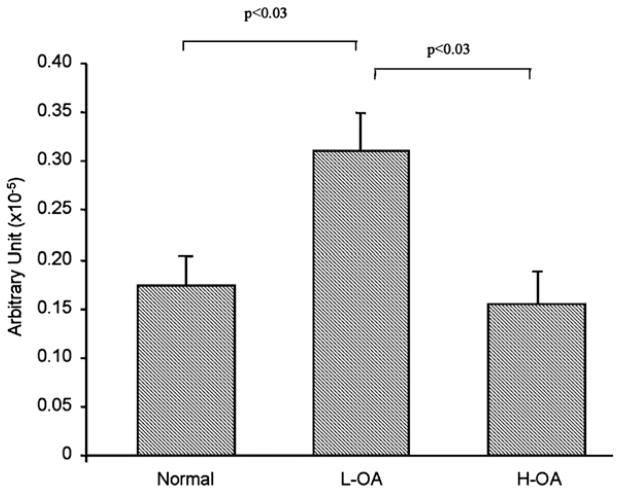
Data showed (Fig. 2) that human OA subchondral bone osteoblasts had higher levels of membranous RANKL compared to normal. A significant increase was found for the L-OA (n=5) compared to the normal (n=3) (p<0.01) and H-OA (n=6) osteoblasts (p<0.05). H-OA osteoblasts showed higher levels than normal; however, this increase did not reach statistical significance.
Fig. 2.
Membranous RANKL level in human normal (n=3), Low (L-, n=5) and High (H-, n=6) OA subchondral bone osteoblasts. The data are expressed as the mean±SEM fluorescence intensity. Statistical analysis was assessed by Student’s t-test and p values are as indicated.
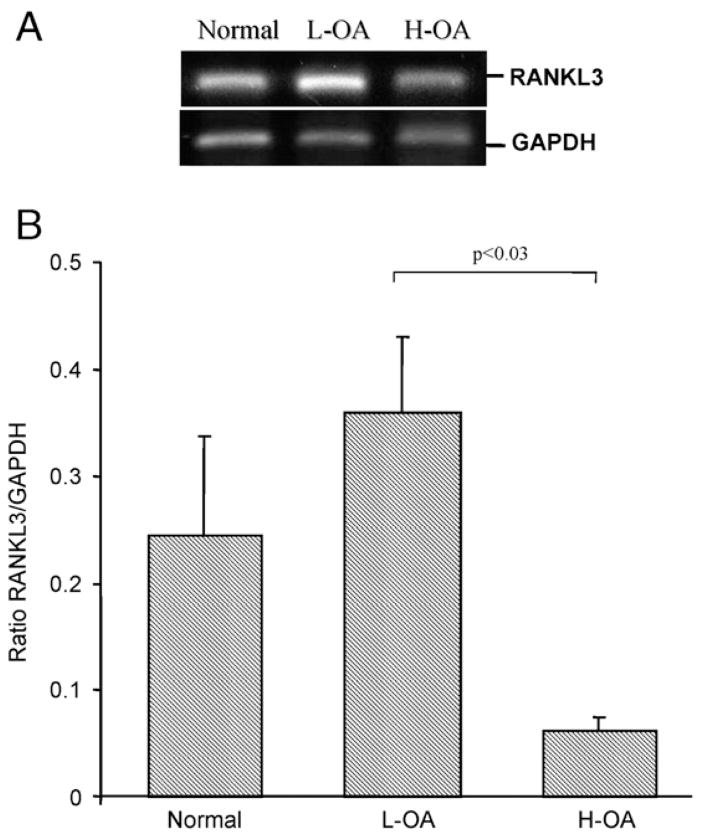
Differences between normal and the OA subgroups (L- and H-OA) were found for the isoforms RANKL1 and 3 (Figs. 3 and 4). L-OA osteoblasts (n=4) expressed significantly more RANKL1 (p<0.03) compared to normal osteoblasts (n=4) and the H-OA (n=4) (p<0.03) (Fig. 3). H-OA osteoblasts showed similar levels to normal. The RANKL3 gene expression (Fig. 4) was increased on the L-OA (n=3) compared to the normal (n=3), but this did not reach statistical significance. On the H-OA (n=3), RANKL3 was reduced by 4 fold compared to normal, and significantly reduced by 5.8 fold compared to the L-OA (p<0.03). Considering that in the L-OA osteoblasts RANKL3 is expressed at a much lower level than RANKL1 and that the expression level of RANKL1 over normal is significantly higher than RANKL3, the overall outcome thus favours RANKL1 and explains the significant increase in membranous RANKL.
Fig. 3.
Expression of RANKL1 isoform in human normal (n=4), Low (L-, n=4) or High (H-, n=4) OA subchondral bone osteoblasts. Total RNA was extracted and processed for real-time PCR, and the data are expressed as the mean±SEM of arbitrary unit as described in Materials and methods. Of note, the RANKL1 arbitrary unit is represented as ×10−5. Statistical analysis was assessed by the Student’s t-test and p values are as indicated.
Fig. 4.
A) A representative analysis of semi-quantitative RT-PCR of RANKL3 isoform and GAPDH expression performed on the normal, Low (L-) and High (H-) OA subchondral bone osteoblasts. B) Expression of RANKL3 in human normal (n=3), L- (n=3) and H(n=3) OA subchondral bone osteoblasts. The data are expressed as the mean ratio of RANKL3/GAPDH±SEM. Statistical analysis was assessed by Student’s t-test and p value is as indicated.
Modulation of membranous RANKL by remodelling factors
We further investigated on the two subgroups of human OA osteoblasts (L- and H-OA) the modulation of membranous RANKL levels upon treatment with factors known to affect osteoblast physiologic/pathophysiologic processes. These included inflammatory factors such as IL-1β, IL-6, IL-17, TNF-α and PGE2, as well as factors known to be involved in bone remodelling, vitamin D3 and PTH.
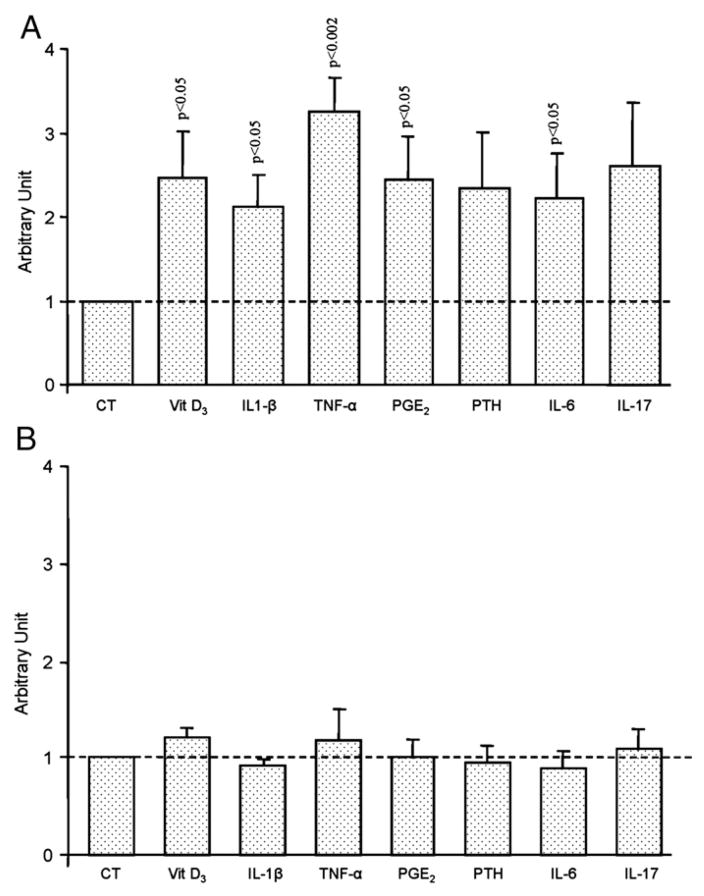
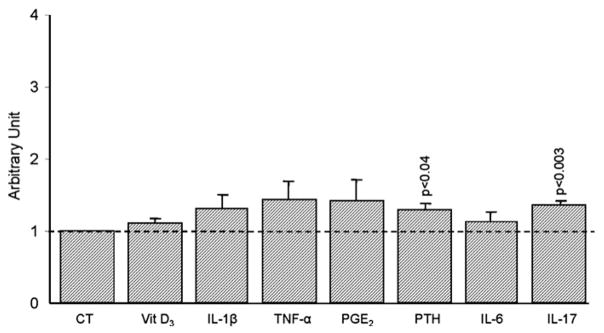
L- and H-OA osteoblasts responded completely differently to treatment with these factors. On L-OA osteoblasts (Fig. 5A), membranous RANKL levels (n=9) were significantly increased when incubated with vitamin D3 (p < 0.05), IL1-β (p<0.05), TNF-α (p<0.002), PGE2 (p<0.05) or IL-6 (0.05). An increase was also noted for PTH and IL-17, but this did not reach statistical significance. In contrast, no true effect following treatment with the remodelling factors was observed on the H-OA (n=4) osteoblasts (Fig. 5B).
Fig. 5.
Membranous RANKL levels of A) Low (n=9) and B) High (n=4) OA subchondral bone osteoblasts. Data are expressed and mean±SEM arbitrary unit calculated from fluorescence intensity over control which was attributed a value of 1. OA subchondral bone osteoblasts were incubated in the absence (CT) or presence of Vitamin D3 (Vit D3), 50 nM; IL-1β, 100 pg/ml, TNF-α, 5 ng/ml; PGE2, 500 nM; PTH, 100 nM; IL-6 (10 ng/ml) and IL-17 (10 ng/ml). Statistical analysis was assessed by Student’s t-test versus control.
Modulation of RANKL isoforms 1 and 3 upon treatment with osteotropic factors
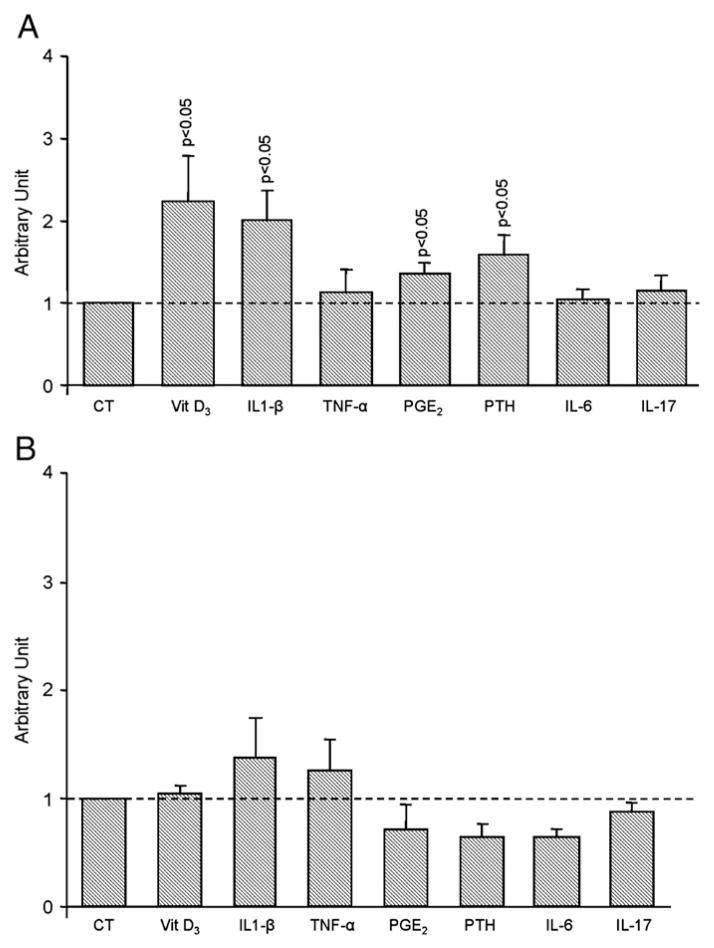
Treatment of L-OA osteoblasts (n=9) showed a similar effect to that of the membranous RANKL for vitamin D3 (2.2 fold), IL-1β (2 fold), PGE2 (1.3 fold) and PTH (1.6 fold), in which a significant (p<0.05) upregulation of RANKL1 was observed (Fig. 6A). Interestingly, TNF-α, IL-6 and IL-17 did not affect the expression level of this isoform. The H-OA osteoblasts (n=4), however, showed no significant effect on RANKL1 expression (Fig. 6B).
Fig. 6.
RANKL1 gene expression level of A) Low (n=9) and B) High (n=4) OA subchondral bone osteoblasts. Data are expressed as mean±SEM arbitrary unit over control which was attributed a value of 1. OA subchondral bone osteoblasts were incubated in the absence (CT) or presence of Vitamin D3 (Vit D3), 50 nM; IL-1β, 100 pg/ml, TNF-α, 5 ng/ml; PGE2, 500 nM; PTH, 100 nM; IL-6 (10 ng/ml) and IL-17 (10 ng/ml). Statistical analysis was assessed by Student’s t-test versus control.
Since no modulation of either membranous RANKL or RANKL1 levels was found on the H-OA osteoblasts, we further investigated the expression level of RANKL3 on the L-OA cells (n=5). Data showed that RANKL3 was significantly increased only upon treatment with PTH (p<0.04) and IL-17 (p<0.003) (Fig. 7), thus explaining the non-significant increase in membranous RANKL levels when L-OA osteoblasts were treated with these two factors.
Fig. 7.
RANKL3 gene expression level of Low OA subchondral bone osteoblasts (n=5). Data are expressed as mean±SEM arbitrary unit over control which was attributed a value of 1. OA subchondral bone osteoblasts were incubated in the absence (CT) or presence of Vitamin D3 (Vit D3), 50 nM; IL-1β, 100 pg/ml, TNF-α, 5 ng/ml; PGE2, 500 nM; PTH, 100 nM; IL-6 (10 ng/ml) and IL-17 (10 ng/ml). Statistical analysis was assessed by Student’s t-test versus control.
Although it was not possible to determine the RANKL2 expression level, we extrapolated from the RANKL1 and 3 data, the effect of the factors on RANKL2 pertaining to the membranous RANKL level. Table 1 summarizes the expected modulation of RANKL2 by the osteotropic factors. From this exercise, we expected the RANKL2 expression level to be highly upregulated upon TNF-α treatment and increased upon IL-6 and IL-17.
Table 1.
Summary of the data from the human Low OA subchondral bone osteoblast upon treatment with osteotropic factors and the expected modulation of RANKL2
| Cytokines | Membranous RANKL1 | RANKL1 | RANKL3 | Expected modulation of RANKL2 |
|---|---|---|---|---|
| Vit D3 | ↑ | ↑↑ | — | — |
| IL1-β | ↑ | ↑↑ | — | — |
| TNF-α | ↑↑ | — | — | ↑↑ |
| PGE2 | ↑ | ↑ | — | — |
| PTH | — | ↑ | ↑ | — |
| IL-6 | ↑ | — | — | ↑ |
| IL-17 | — | — | ↑ | ↑ |
↑ Refers to a significant increase, ↑↑ highly significant increase, or — no effect.
Discussion
Bone is continuously remodelled by a tightly balanced and coordinated action of bone-resorbing and bone-forming elements. During the pathogenesis of OA, the subchondral bone resorption/formation process has been shown to be abnormally controlled. However, a major area that remains to be studied in OA and other diseases is the modulation of membranous RANKL and the gene expression level of the different RANKL isoforms in the diseased tissues as well as upon treatment with bone remodelling factors. This information is necessary to better define and differentiate the pathological mechanisms that operate during OA and other diseases involving bone pathology, and to identify specific strategic treatments.
If the structure of RANKL1 alone or in association with RANKL2 is efficiently exported to the cell surface, the trimeric structure formed between these isoforms and RANKL3 remains intracellular, thus inhibiting the membranous localization of RANKL by disturbing the capacity to bind to the cell surface, modifying the efficiency of the transport of the protein, and/or the stability of the protein [23]. In this study, we investigated the expression level of two RANKL isoforms, namely RANKL1 and RANKL3, and their impact on the membranous RANKL levels.
Data from our study showed that RANKL gene expression and protein (membranous) levels differ according to human OA subchondral bone osteoblast metabolic state and strongly support the hypothesis that L-OA osteoblasts favour pro-resorptive activity [2]. Indeed, the membranous RANKL levels were significantly increased in the L-OA compared to normal and H-OA. These findings are of importance because in OA, the L-OA osteoblasts are generally found in a greater number of patients than the H-OA (60% and 40% respectively) and OA patients seem to have either an L-OA (low PGE2 level) or H-OA (high PGE2 level) phenotype. This indicates that OA patients undergoing surgery, although clinically similar, may show different patterns of expression of factors in the subchondral bone tissue indicative of the different subpopulations. A higher level of PGE2 and IL-6 has also been observed in periprosthetic trabecular bone osteoblasts from OA patients [30,31].
The differential RANKL expression appears to result from the modulation of RANKL isoforms, as the outcome of the expression levels of RANKL1 and RANKL3 in the L-OA osteoblasts favours RANKL1, which explains the upregulation of membranous RANKL in these cells. On the other hand, in the H-OA osteoblasts, RANKL1 showed similar levels to normal, yet RANKL3 was lower, but not statistically, than normal, thus somewhat favouring the translocalization of RANKL at the cell surface, as shown in this study. In turn, in the H-OA, membranous RANKL was significantly lower than in the L-OA osteoblasts, agreeing with previous data [2] which showed that the level of total RANKL gene expression in human H-OA osteoblasts is lower than in L-OA and normal. Interestingly, in the previous study [2] data also showed that in the H-OA osteoblasts, the OPG/RANKL ratio was significantly higher than in the L-OA osteoblasts, resulting from an increased level of OPG. The increased production of OPG in the H-OA compared to the L-OA could, in turn, contribute to a reduced expression of RANKL, as it has been demonstrated that OPG enhances RANKL degradation through an internalization process [32]. In contrast to H-OA, the OPG/RANKL ratio is much lower in the L-OA, due to a marked decrease in OPG and higher level of RANKL [2].
PGE2 has been demonstrated to exert both anabolic and catabolic effects depending on the activation of its specific receptor subtypes. In osteoblasts, three such receptors, EP1, EP2 and EP4, are present [33,34]. PGE2 signalling through the EP1 receptor enhances pro-anabolic effects such as bone formation, whereas signalling through the EP2 and EP4 receptors induces catabolic effects on bone [35–37]. Our data showed that in the L-OA osteoblasts (low PGE2 production), membranous RANKL is elevated. PGE2 treatment further increased membranous RANKL levels. According to the literature, RANKL modulation by PGE2 takes place through its specific receptors EP2 and EP4 [36]. Therefore, one could hypothesize that the L-OA osteoblasts preferentially express EP2 and EP4 receptors and that treatment with PGE2 enhances RANKL production through these receptors.
In contrast, in the H-OA osteoblasts (high level of PGE2), membranous RANKL was slightly increased compared to normal. Hence, the EP2 and EP4 receptors may be downregulated in favour of the EP1 receptor, thus explaining the pro-bone formation property of the H-OA cells. Alternatively, but not excluding the latter, the high level of PGE2 in these cells could contribute to a desensitization of the EP receptors, explaining the absence of effect on RANKL with exogenous PGE2. In addition, previous data [2] also showed that PGE2 strongly inhibits bone resorption. This concurs with the data of Take et al. [38] which demonstrate that PGE2 could act directly as an inhibitor of osteoclastogenesis by its interaction on osteoclast precursors, and of Raisz et al. [39] which showed that high concentrations of PGE2 favour bone deposition, as well as others showing that high levels of PGE2 induce collagen type I production in OA human osteoblasts [40] and IGF-1 [12] synthesis. However, although RANKL-independent mechanisms are involved in the control of bone remodelling, RANKL-dependent modulation remains the major pathway involved in such remodelling.
Since various in situ mediators are responsible for modulating the RANKL process, we investigated the effects of certain factors and cytokines believed to be involved in bone remodelling on RANKL protein and the isoforms, comparing L and H-OA. Emerging data from these experiments indicate that the factors tested have no effect on either membranous or RANKL1 expression levels on the H-OA osteoblasts, whereas some of the factors showed modulation on the L-OA osteoblasts. These data again strengthen the hypothesis that H-OA osteoblasts do not favour bone resorption and that the two cell subpopulations have different metabolic states.
On the L-OA human subchondral bone osteoblasts, TNF-α markedly increased the membranous RANKL without modulating RANKL1 and RANKL3 expression levels. On osteoblasts from small animals, TNF-α has been shown to potentiate osteoclastogenesis by increasing the levels of RANKL on osteoblasts [41,42]. It is then possible, as suggested in Table 1, that RANKL2 upon treatment with TNF-α might play a role in potentiating RANKL membranous localization. Alternatively, TNF-α could also play a role in the half-life of RANKL isoforms, the net effect of which would be an increase in the protein level. In this line of thought, it has been shown that some glycosaminoglycans (GAG) including heparan sulphate, dermatan sulphate and chondroitin sulfate strongly increase the membranous RANKL half-life, thereby increasing its level [43]. Hence, and although speculative, TNF-α may indirectly influence the half-life of membranous RANKL though the modulation of proteoglycans or short GAGs. Indeed, it has been shown that TNF-α is able to interact directly with some proteoglycans such as biglycan and decorin with a Kd of 0.81 and 1.23 μM respectively [44], thereby modulating their bioavailability in the micro-environment. Moreover, the production of biglycan on human fibroblasts, although a different cell type, has been shown to be induced by TNF-α, suggesting the possible involvement of this cytokine in the modulation of proteoglycan synthesis [45].
Our study suggests a close relationship between the membranous RANKL level and the modulation of the RANKL isoforms upon treatment with some of the osteotropic factors. Vitamin D3, IL-1β, and PGE2 all significantly upregulated membranous RANKL levels. These data agree with the literature reports that these factors increase osteoclastogenesis by inducing RANKL [42,46,47]. The stimulatory effect of these factors on the membranous RANKL level seems to occur via the modulation of the RANKL1 isoform, since a significant increase was found upon treatment, and the RANKL3 expression level was not modulated. PTH did not significantly increase the membranous RANKL level when compared to the control. This also concurs with the RANKL1 and 3 expression levels in which, although RANKL1 is upregulated by PTH, the induction of RANKL3 is expected to inhibit the translocation of RANKL at the cell surface, explaining the absence of modulation of membranous RANKL.
IL-6 is also known to potentiate RANKL levels as well as osteoclastogenesis [48]. As expected, the treatment of the L-OA osteoblasts with IL-6 increased the membranous RANKL. However, the absence of modulation of RANKL1 and 3 strongly suggests an upregulation of RANKL2 (Table 1). In regard to IL-17, the data showing that the membranous RANKL level was not statistically increased and the gene expression level of RANKL1 was unchanged and that of RANKL3 significantly increased, also suggest an increased RANKL2 expression level.
In conclusion, this study demonstrated that abnormal RANKL isoform expression and membranous levels in human OA subchondral osteoblasts are differentially regulated depending on their metabolic state. An explanation could be that the tissue micro-environment, which may be different in each OA osteoblast (L or H) subpopulation, acts on the cell metabolism, which in turn directs the cells toward a specific phase of activity: bone pro-resorption or pro-formation. Thus, the levels of some factors would be responsible for the differential expression of RANKL isoforms which lead to the localization of RANKL at the membrane. The present data showed that some osteotropic factors affect the OA osteoblast subpopulations differently. In addition, it could also be that events such as increased/decreased mechanistic properties of the tissue are responsible for the modulation of osteotropic factors leading to the regulation of one pathway over another. The differential expression/production of RANKL in the two human OA osteoblast subpopulations could indicate different stages of attempts to repair the damaged subchondral bone. This is in all probability part of the “typical” cycle of events that ensue during the evolution of this disease, and may explain the different anatomical/structural changes that take place in human OA subchondral bone.
Our data indicate that the differential modulation of the RANKL isoforms between the OA subpopulations leads to an increased membranous RANKL level in the L-OA. To our knowledge, no study has yet looked specifically at RANKL isoforms during the course of other bone pathologies. Osteolytic diseases such as post-menopausal osteoporosis, rheumatoid arthritis, multiple myeloma and breast cancer, have all shown bone remodelling as well as increased expression and production of RANKL [49,50], suggesting that this factor is responsible for bone resorption in these pathologies. Clinical trials targeting the inhibition of RANKL in some of these diseases have shown promising results against their pathological progression. In this context, the use of either recombinant OPG or a monoclonal antibody against RANKL has been very powerful at alleviating bone erosion in the above mentioned diseases [49,51–55]. Moreover, recent studies have also shown that the OPG/RANKL ratio plays an important role in controlling vascular calcification [56–59]: a low OPG/RANKL ratio was correlated with increased vascular smooth muscle cell calcification.
Thus, the modulation of a specific RANKL isoform could be useful to specifically target a particular disease without interfering with the isoform responsible for the homeostasis. Hence, targeting upstream factors responsible for the increased membranous localization of RANKL could not only lead to a specific therapeutic approach against OA, but also against other diseases involving RANKL modulation. In this perspective, the synthesis of small peptides mimicking the RANKL3 effect could be of significance for specific therapy.
Supplementary Material
Acknowledgments
The authors are grateful to Dr Marika Sarfati, Dr Guy Delespesse and Manuel Rubio from the Immunoregulation and Allergy Research Unit at the University of Montreal Research Centre for allowing us to use their apparatus and for their expert advice in flow cytometry, François-Cyril Jolicoeur and Virginia Wallis from the Osteoarthritis Research Unit at the University of Montreal Research Centre for the expert technical assistance and manuscript preparation, respectively.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bone.2008.04.006.
References
- 1.Martel-Pelletier J, Lajeunesse D, Pelletier JP. Subchondral bone and osteoarthritis progression: a very significant role. In: Buckwalter JA, Lotz M, Stoltz JF, editors. Osteoarthritis, inflammation and degradation: a continuum. Amsterdam: IOS Press; 2007. pp. 206–18. [Google Scholar]
- 2.Kwan Tat S, Pelletier JP, Lajeunesse D, Mineau F, Fahmi H, Jolicoeur FC, et al. The differential expression of OPG/RANKL in human osteoarthritic subchondral bone osteoblasts is an indicator of the metabolic state of these disease cells. Clin Exp Rheumatol. 2008;26:295–304. [PMC free article] [PubMed] [Google Scholar]
- 3.Pelletier JP, Boileau C, Brunet J, Boily M, Lajeunesse D, Reboul P, et al. The inhibition of subchondral bone resorption in the early phase of experimental dog osteoarthritis by licofelone is associated with a reduction in the synthesis of MMP-13 and cathepsin K. Bone. 2004;34:527–38. doi: 10.1016/j.bone.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 4.Brandt KD, Myers SL, Burr D, Albrecht M. Osteoarthritic changes in canine articular cartilage, subchondral bone, and synovium fifty-four months after transection of the anterior cruciate ligament. Arthritis Rheum. 1991;34:1560–70. doi: 10.1002/art.1780341214. [DOI] [PubMed] [Google Scholar]
- 5.Bettica P, Cline G, Hart DJ, Meyer J, Spector TD. Evidence for increased bone resorption in patients with progressive knee osteoarthritis: longitudinal results from the Chingford study. Arthritis Rheum. 2002;46:3178–84. doi: 10.1002/art.10630. [DOI] [PubMed] [Google Scholar]
- 6.Dieppe P, Cushnaghan J, Young P, Kirwan J. Prediction of the progression of joint space narrowing in osteoarthritis of the knee by bone scintigraphy. Ann Rheum Dis. 1993;52:557–63. doi: 10.1136/ard.52.8.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iqbal J, Zaidi M. Molecular regulation of mechanotransduction. Biochem Biophys Res Commun. 2005;328:751–5. doi: 10.1016/j.bbrc.2004.12.087. [DOI] [PubMed] [Google Scholar]
- 8.Karsenty G. Minireview: transcriptional control of osteoblast differentiation. Endocrinology. 2001;142:2731–3. doi: 10.1210/endo.142.7.8306. [DOI] [PubMed] [Google Scholar]
- 9.Karsenty G. The genetic transformation of bone biology. Genes Dev. 1999;13:3037–51. doi: 10.1101/gad.13.23.3037. [DOI] [PubMed] [Google Scholar]
- 10.Hilal G, Martel-Pelletier J, Pelletier JP, Ranger P, Lajeunesse D. Osteoblast-like cells from human subchondral osteoarthritic bone demonstrate an altered phenotype in vitro: possible role in subchondral bone sclerosis. Arthritis Rheum. 1998;41:891–9. doi: 10.1002/1529-0131(199805)41:5<891::AID-ART17>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 11.Hilal G, Martel-Pelletier J, Pelletier JP, Duval N, Lajeunesse D. Abnormal regulation of urokinase plasminogen activator by insulin-like growth factor 1 in human osteoarthritic subchondral osteoblasts. Arthritis Rheum. 1999;42:2112–22. doi: 10.1002/1529-0131(199910)42:10<2112::AID-ANR11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 12.Massicotte F, Fernandes JC, Martel-Pelletier J, Pelletier JP, Lajeunesse D. Modulation of insulin-like growth factor 1 levels in human osteoarthritic subchondral bone osteoblasts. Bone. 2006;38:333–41. doi: 10.1016/j.bone.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Massicotte F, Lajeunesse D, Benderdour M, Pelletier JP, Hilal G, Duval N, et al. Can altered production of interleukin 1β, interleukin-6, transforming growth factor-β and prostaglandin E2 by isolated human subchondral osteoblasts identify two subgroups of osteoarthritic patients. Osteoarthritis Cartilage. 2002;10:491–500. doi: 10.1053/joca.2002.0528. [DOI] [PubMed] [Google Scholar]
- 14.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 15.Kwan Tat S, Padrines M, Theoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15:49–60. doi: 10.1016/j.cytogfr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong AP, Tometsko ME, Glaccum M, Sutherland CL, Cosman D, Dougall WC. A RANK/TRAF6-dependent signal transduction pathway is essential for osteoclast cytoskeletal organization and resorptive function. J Biol Chem. 2002;277:44347–56. doi: 10.1074/jbc.M202009200. [DOI] [PubMed] [Google Scholar]
- 17.Darnay BG, Haridas V, Ni J, Moore PA, Aggarwal BB. Characterization of the intracellular domain of receptor activator of NF-kappaB (RANK). Interaction with tumor necrosis factor receptor-associated factors and activation of NF-kappaB and c-Jun N-terminal kinase. J Biol Chem. 1998;273:20551–5. doi: 10.1074/jbc.273.32.20551. [DOI] [PubMed] [Google Scholar]
- 18.Gravallese EM, Goldring SR. Cellular mechanisms and the role of cytokines in bone erosions in rheumatoid arthritis. Arthritis Rheum. 2000;43:2143–51. doi: 10.1002/1529-0131(200010)43:10<2143::AID-ANR1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 19.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–23. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 20.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–19. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 21.Redlich K, Hayer S, Maier A, Dunstan CR, Tohidast-Akrad M, Lang S, et al. Tumor necrosis factor alpha-mediated joint destruction is inhibited by targeting osteoclasts with osteoprotegerin. Arthritis Rheum. 2002;46:785–92. doi: 10.1002/art.10097. [DOI] [PubMed] [Google Scholar]
- 22.Romas E, Sims NA, Hards DK, Lindsay M, Quinn JW, Ryan PF, et al. Osteoprotegerin reduces osteoclast numbers and prevents bone erosion in collagen-induced arthritis. Am J Pathol. 2002;161:1419–27. doi: 10.1016/S0002-9440(10)64417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki J, Ikeda T, Kuroyama H, Seki S, Kasai M, Utsuyama M, et al. Regulation of osteoclastogenesis by three human RANKL isoforms expressed in NIH3T3 cells. Biochem Biophys Res Commun. 2004;314:1021–7. doi: 10.1016/j.bbrc.2003.12.191. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda T, Kasai M, Suzuki J, Kuroyama H, Seki S, Utsuyama M, et al. Multimerization of the receptor activator of nuclear factor-kappaB ligand (RANKL) isoforms and regulation of osteoclastogenesis. J Biol Chem. 2003;278:47217–22. doi: 10.1074/jbc.M304636200. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda T, Kasai M, Utsuyama M, Hirokawa K. Determination of three isoforms of the receptor activator of nuclear factor-kappaB ligand and their differential expression in bone and thymus. Endocrinology. 2001;142:1419–26. doi: 10.1210/endo.142.4.8070. [DOI] [PubMed] [Google Scholar]
- 26.Ito S, Wakabayashi K, Ubukata O, Hayashi S, Okada F, Hata T. Crystal structure of the extracellular domain of mouse RANK ligand at 2. 2-A resolution. J Biol Chem. 2002;277:6631–6. doi: 10.1074/jbc.M106525200. [DOI] [PubMed] [Google Scholar]
- 27.Lam J, Nelson CA, Ross FP, Teitelbaum SL, Fremont DH. Crystal structure of the TRANCE/RANKL cytokine reveals determinants of receptor–ligand specificity. J Clin Invest. 2001;108:971–9. doi: 10.1172/JCI13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altman RD, Asch E, Bloch DA, Bole G, Borenstein D, Brandt KD, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 29.Tardif G, Hum D, Pelletier JP, Boileau C, Ranger P, Martel-Pelletier J. Differential gene expression and regulation of the bone morphogenetic protein antagonists follistatin and gremlin in normal and osteoarthritic human chondrocytes and synovial fibroblasts. Arthritis Rheum. 2004;50:2521–30. doi: 10.1002/art.20441. [DOI] [PubMed] [Google Scholar]
- 30.Lavigne P, Shi Q, Lajeunesse D, Dehnade F, Fernandes JC. Metabolic activity of osteoblasts retrieved from osteoarthritic patients after stimulation with mediators involved in periprosthetic loosening. Bone Mar. 2004;34:478–86. doi: 10.1016/j.bone.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Shi Q, Lajeunesse D, Reboul P, Martel-Pelletier J, Pelletier JP, Dehnade F, et al. Metabolic activity of osteoblasts from periprosthetic trabecular bone in failed total hip arthroplasties and osteoarthritis as markers of osteolysis and loosening. J Rheumatol. 2002;29:1437–45. [PubMed] [Google Scholar]
- 32.Tat SK, Padrines M, Theoleyre S, Couillaud-Battaglia S, Heymann D, Redini F, et al. OPG/membranous-RANKL complex is internalized via the clathrin pathway before a lysosomal and a proteasomal degradation. Bone. 2006;39:706–15. doi: 10.1016/j.bone.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe M, Noda M, Nakajin S. Aromatase expression in a human osteoblastic cell line increases in response to prostaglandin E(2) in a dexamethasone-dependent fashion. Steroids. 2007;72:686–92. doi: 10.1016/j.steroids.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Sarrazin P, Bkaily G, Haché R, Patry C, Dumais R, Rocha FA, et al. Characterization of the prostaglandin receptors in human osteoblasts in culture. Prostaglandins Leukot Essent Fatty Acids. 2001;64:203–10. doi: 10.1054/plef.1999.0127. [DOI] [PubMed] [Google Scholar]
- 35.Tang CH, Yang RS, Fu WM. Prostaglandin E2 stimulates fibronectin expression through EP1 receptor, phospholipase C, protein kinase Calpha, and c-Src pathway in primary cultured rat osteoblasts. J Biol Chem. 2005;280:22907–16. doi: 10.1074/jbc.M500130200. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Pilbeam CC, Pan L, Breyer RM, Raisz LG. Effects of prostaglandin E2 on gene expression in primary osteoblastic cells from prostaglandin receptor knockout mice. Bone. 2002;30:567–73. doi: 10.1016/s8756-3282(02)00683-x. [DOI] [PubMed] [Google Scholar]
- 37.Suzawa T, Miyaura C, Inada M, Maruyama T, Sugimoto Y, Ushikubi F, et al. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: an analysis using specific agonists for the respective EPs. Endocrinology. 2000;141:1554–9. doi: 10.1210/endo.141.4.7405. [DOI] [PubMed] [Google Scholar]
- 38.Take I, Kobayashi Y, Yamamoto Y, Tsuboi H, Ochi T, Uematsu S, Okafuji N, et al. Prostaglandin E2 strongly inhibits human osteoclast formation. Endocrinology. 2005;146:5204–14. doi: 10.1210/en.2005-0451. [DOI] [PubMed] [Google Scholar]
- 39.Raisz LG. Physiologic and pathologic roles of prostaglandins and other eicosanoids in bone metabolism. J Nutr. 1995;125:2024S–7S. doi: 10.1093/jn/125.suppl_7.2024S. [DOI] [PubMed] [Google Scholar]
- 40.Shi Q, Vaillancourt F, Côté V, Fahmi H, Lavigne P, Afif H, Di Battista JA, et al. Alterations of metabolic activity in human osteoarthritic osteoblasts by lipid peroxidation end product 4-hydroxynonenal. Arthritis Res Ther. 2006;8:R159. doi: 10.1186/ar2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakashima T, Kobayashi Y, Yamasaki S, Kawakami A, Eguchi K, Sasaki H, et al. Protein expression and functional difference of membrane-bound and soluble receptor activator of NF-kappaB ligand: modulation of the expression by osteotropic factors and cytokines. Biochem Biophys Res Commun. 2000;275:768–75. doi: 10.1006/bbrc.2000.3379. [DOI] [PubMed] [Google Scholar]
- 42.Hofbauer LC, Lacey DL, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Interleukin-1beta and tumor necrosis factor-alpha, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone. 1999;25:255–9. doi: 10.1016/s8756-3282(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 43.Theoleyre S, Vusio P, Blanchard F, Gallagher J, Ricard-Blum S, et al. Characterization of osteoprotegerin binding to glycosaminoglycans by surface plasmon resonance: role in the interactions with receptor activator of nuclear factor kappaB ligand (RANKL) and RANK. Biochem Biophys Res Commun. 2006;347:460–7. doi: 10.1016/j.bbrc.2006.06.120. [DOI] [PubMed] [Google Scholar]
- 44.Tufvesson E, Westergren-Thorsson G. Tumour necrosis factor-alpha interacts with biglycan and decorin. FEBS Lett. 2002;530:124–8. doi: 10.1016/s0014-5793(02)03439-7. [DOI] [PubMed] [Google Scholar]
- 45.Tufvesson E, Westergren-Thorsson G. Alteration of proteoglycan synthesis in human lung fibroblasts induced by interleukin-1beta and tumor necrosis factor-alpha. J Cell Biochem. 2000;77:298–309. doi: 10.1002/(sici)1097-4644(20000501)77:2<298::aid-jcb12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 46.Liu XH, Kirschenbaum A, Yao S, Levine AC. Cross-talk between the interleukin-6 and prostaglandin E(2) signaling systems results in enhancement of osteoclastogenesis through effects on the osteoprotegerin/receptor activator of nuclear factor-{kappa}B (RANK) ligand/RANK system. Endocrinology. 2005;146:1991–8. doi: 10.1210/en.2004-1167. [DOI] [PubMed] [Google Scholar]
- 47.Kitazawa S, Kajimoto K, Kondo T, Kitazawa R. Vitamin D3 supports osteoclastogenesis via functional vitamin D response element of human RANKL gene promoter. J Cell Biochem. 2003;89:771–7. doi: 10.1002/jcb.10567. [DOI] [PubMed] [Google Scholar]
- 48.Palmqvist P, Persson E, Conaway HH, Lerner UH. IL-6, leukemia inhibitory factor, and oncostatin M stimulate bone resorption and regulate the expression of receptor activator of NF-kappa B ligand, osteoprotegerin, and receptor activator of NF-kappa B in mouse calvariae. J Immunol. 2002;169:3353–62. doi: 10.4049/jimmunol.169.6.3353. [DOI] [PubMed] [Google Scholar]
- 49.Kearns AE, Khosla S, Kostenuik P. RANKL and OPG Regulation of Bone Remodeling in Health and Disease. Endocr Rev. 2008;29:155–92. doi: 10.1210/er.2007-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Theoleyre S, Wittrant Y, Tat SK, Fortun Y, Redini F, Heymann D. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004;15:457–75. doi: 10.1016/j.cytogfr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Body JJ, Facon T, Coleman RE, Lipton A, Geurs F, Fan M, et al. A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res. 2006;12:1221–8. doi: 10.1158/1078-0432.CCR-05-1933. [DOI] [PubMed] [Google Scholar]
- 52.Cohen SB, Dore RK, Lane NE, Ory PA, Peterfy CG, Sharp JT, et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: A twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 2008;58:1299–309. doi: 10.1002/art.23417. [DOI] [PubMed] [Google Scholar]
- 53.McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821–31. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 54.Body JJ, Greipp P, Coleman RE, Facon T, Geurs F, Fermand JP, et al. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer. 2003;97:887–92. doi: 10.1002/cncr.11138. [DOI] [PubMed] [Google Scholar]
- 55.Bekker PJ, Holloway D, Nakanishi A, Arrighi M, Leese PT, Dunstan CR. The effect of a single dose of osteoprotegerin in postmenopausal women. J Bone Miner Res. 2001;16:348–60. doi: 10.1359/jbmr.2001.16.2.348. [DOI] [PubMed] [Google Scholar]
- 56.Boyce BF, Xing L. The RANKL/RANK/OPG pathway. Curr Osteoporos Rep. 2007;5:98–104. doi: 10.1007/s11914-007-0024-y. [DOI] [PubMed] [Google Scholar]
- 57.Cardus A, Panizo S, Parisi E, Fernandez E, Valdivielso JM. Differential effects of vitamin D analogs on vascular calcification. J Bone Miner Res. 2007;22:860–6. doi: 10.1359/jbmr.070305. [DOI] [PubMed] [Google Scholar]
- 58.Orita Y, Yamamoto H, Kohno N, Sugihara M, Honda H, Kawamata S, et al. Role of osteoprotegerin in arterial calcification: development of new animal model. Arterioscler Thromb Vasc Biol. 2007;27:2058–64. doi: 10.1161/ATVBAHA.107.147868. [DOI] [PubMed] [Google Scholar]
- 59.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–8. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.