Abstract
Immunity is a high-cost, high-benefit trait that defends against pathogens and noxious stimuli, but whose overactivation can result in immunopathologies and sometimes even death. Because many immune parameters oscillate rhythmically based on the time of day, the circadian clock has emerged as an important gatekeeper for reducing immunity-associated costs, which, in turn, enhances organismal fitness. This is mediated by interactions between the extrinsic environmental cues and the intrinsic oscillators of immune cells, which together optimize immune responses throughout the circadian cycle. The elucidation of these clock-controlled immunomodulatory mechanisms might uncover new approaches for treating infections and chronic inflammatory diseases.
Virtually all life on earth is exposed to regular 24-h environmental cycles generated by the Earth’s rotation. This in turn has led to the evolution of daily (circadian) rhythms, driven by cell-autonomous biological clocks, which enable organisms to anticipate and adapt to the temporal changes in their environment (1). The sleep-wake cycle is perhaps the most obvious output of the circadian system, but numerous other physiological systems are under the circadian control, including behavior and locomotor activity, body temperature; the cardiovascular, digestive, and endocrine systems; and metabolic and immune functions (2–7).
In mammals, the central circadian pacemaker is located in the suprachiasmatic nucleus (SCN), which entrains peripheral clocks found in nearly every cell of the body (2, 3). The SCN oscillator has two distinct properties. First, it is the only part of the circadian system that has retinal innervation, allowing it to be entrained by the solar cycle. Second, unlike the peripheral clocks which dampen over time, the inter-neuronal signalling pathways that establish communication between the SCN neurons endow it with an unlimited capacity to generate circadian outputs. At the organism level, circadian coherence in peripheral tissues is maintained by rhythmic generation of entrainment cues by the SCN, including circadian oscillations in body temperature, activity of the sympathetic nervous system (SNS), and circulating concentrations of glucocorticoids. The coherence between central and peripheral circadian clocks confers an adaptive advantage, and its disruption has been suggested to decrease organismal fitness. In support of this, lifestyles that disrupt inherent timing systems, such as exposure to abnormal lighting schedules in chronic shift work, are associated with an increased risk of cancer, metabolic disorders, and cardiovascular and cerebrovascular disease (4). Also, many human diseases exhibit circadian rhythmicity in their pathology, including myocardial infarction, asthma, and rheumatoid arthritis (4, 5).
Although the diurnal variation in host immune responses to lethal infection was demonstrated over 50 years ago (8, 9), only recently have studies started to uncover the multiple aspects of immune function that are under circadian control, such as host-pathogen interactions, trafficking of leukocytes, and the activation of innate and adaptive immunity (5–7). These observations together suggest that the circadian oscillators gate immune responses to anticipate environmental threats, such as those that might be encountered during foraging for food or looking for shelter, and to limit the costs of immune activation. Here, we highlight these recent advances in our understanding of how circadian oscillators limit immune and inflammatory responses to enhance organismal fitness. In particular, we discuss the circadian gating of immune responses, how oscillatory immune responses are generated and maintained, and the emerging cell autonomous functions of circadian oscillators in immune cells. A better understanding of how the cellular clock controls immune responses might lead to the development of chronotherapeutics to treat disorders of chronic inflammation and dysregulated immunity.
The clock of immune cells
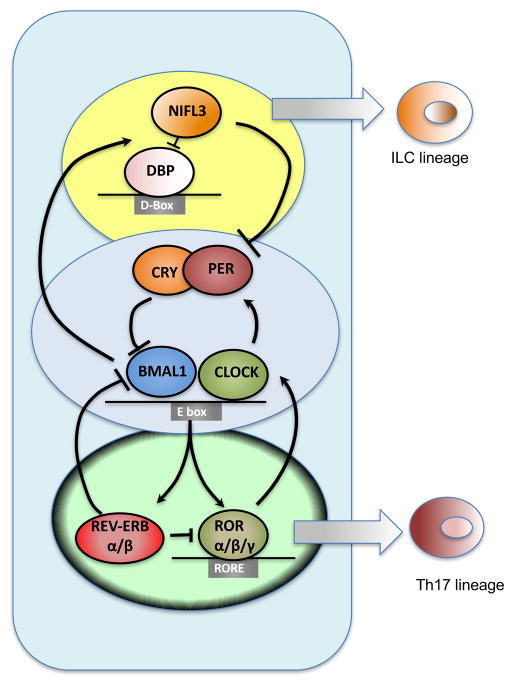
In mammals, circadian timekeeping arises from conserved transcription-translation feedback oscillator loops driven by a set of dedicated clock proteins (3) (1, 2). At its core, CLOCK and BMAL1 (also known as ARNTL) transcription factor heterodimers drive the expression of Periods, Cryptochromes (Cry), and Rev-erb nuclear receptors by binding to the E-box regulatory sequences in the promoters of these genes (Fig. 1). After a delay, the encoded proteins enter the nucleus and inhibit their own expression by modulating the transcriptional activity of CLOCK-BMAL1 heterodimers. Two additional circuits cooperate with the core clock to establish robust 24h rhythms (Fig. 1). First, the nuclear receptor RORα, which shares DNA binding sites with REV-ERBs, induces expression of Bmal1 in a feedforward loop, whereas REV-ERBs represses the transcription of Bmal1 by competing for the same site in a negative feedback loop. Second, albumin D-box binding protein (DBP) and the repressor nuclear factor interleukin 3 (NFIL3, also known as E4BP4) form an additional loop that regulates transcription of genes containing D-box sequences, including Period. These rhythmic feedback mechanisms generate oscillations in gene expression that convey circadian timing cues to cellular processes. The formation, trafficking, and degradation of different clock protein complexes throughout this transcriptional cycle establish the intrinsic 24 h period of the cellular clock.
Figure 1. Interlocking loops of the molecular clock drive immune responses.
The circadian pacemaker is controlled by 3 inter-locked transcription/translation feedback loops, involving rhythmic transcriptional repressors that act on E-Box, RORE, and D-Box sites. Genes driving the core clockwork also regulate multiple other non-circadian pathways. Two of the circadian oscillators, NFIL3 and RORα, also regulate development of ILCs and Th17 cells.
In addition to their functions in the cellular clock, circadian oscillators also participate in the development and specification of immune cell lineages. For example, NFIL3 is required for the development of a common precursor that gives rise to all innate lymphoid cell (ILC) lineages, including ILC1s, natural killer (NK) cells, ILC2s, and ILC3s (10–13). At least for ILC3s, NFIL3 is only required for their development through the common ILC precursor but not for their maintenance in the intestines or lymphoid tissues (12). This specific requirement of NFIL3 in the development of ILCs and NK cells might be independent of its functions in the cellular clock. If so, it will suggest that the transcriptional regulators that comprise the clock might cooperate with other factors to control non-rhythmic expression of target genes, as was recently demonstrated for BMAL1 and REV-ERBα (14, 15). For example, REV-ERBα regulates non-rhythmic functions in the liver by recruiting transcriptional repressor complex containing nuclear co-repressor (NCoR) and histone deacetylase 3 (HDAC3) to lipid metabolism genes (14). This mechanism might also be relevant to the development of interleukin-17 (IL-17)-producing CD4+ T helper (Th17) cells because their numbers are increased in Nfil3 null and decreased in RORα null mice (16, 17), whereas deletion of Bmal1 in T cells does not affect Th17 development in lymphoid organs and epithelial barriers (18). Also, rhythmic changes in the microbiota provide another mechanism by which alterations in Th17 cells and ILCs are maintained at barrier sites (19, 20). This is, in part, regulated by rhythmic expression of toll-like receptors in the intestinal epithelial cells by the cellular clock (21), which sense commensal derived molecular patterns to maintain intestinal homeostasis.
Circadian gating and host fitness
The immune system, which consists of innate and adaptive immunity, defends against noxious stimuli, such as infections and tissue injury (22). Although a controlled immune response is beneficial to the host (e.g. it confers protection against pathogens and promotes tissue repair), a dysregulated immune response can be harmful (e.g. it can cause septic shock). This double-edge sword of the immune or inflammatory response highlights the general principle that activation of immunity provides protection against noxious stimuli but also incurs costs. There are two types of costs that are incurred when immunity is activated: direct costs and vulnerabilities (23). Direct costs are unavoidable and incurred every time the immune response is activated, such as the metabolic costs of immune activation or the collateral damage to tissues. In contrast, costs from vulnerabilities are rare but when incurred are catastrophic, such as with septic shock. While direct costs can be reduced by decreasing the amplitude or duration of the immune response, reducing their frequency or probability minimizes costs associated with vulnerabilities. In this context, studies over the last decade suggest that the circadian oscillators function to minimize both direct costs and vulnerabilities associated with innate immune activation (Fig. 2A). Since natural selection operates to optimize the cost-benefit trade-offs of traits in a given environment (24), we suggest that the observed anticipatory immune responses are a consequence of this Darwinian selection process, in which the circadian clock minimizes costs and maximizes benefits of immunity to enhance organismal fitness.
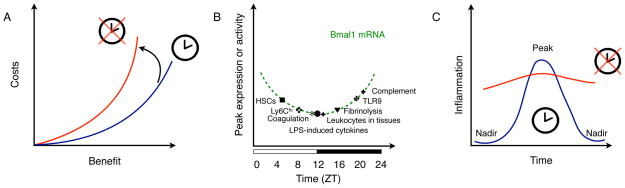
Figure 2. Gating of immune responses by the circadian clock.
(A) Cost-benefit trade-offs of immunity. The circadian clock functions to minimize the costs (direct costs and vulnerabilities) and maximize benefits of immunity. Loss of the circadian clock increases the costs of immunity for a given level of benefit. Disrupted clock is depicted in red, whereas normal clock is shown in blue.
(B) The circadian clock temporally gates various arms of the innate immune response. Peaks of innate immune parameters are plotted on a curve depicting the oscillation of Bmal1 mRNA during a circadian cycle. Zeitgeber time (ZT). ZT0 is the start of the light phase and ZT12 is the beginning of the dark phase, during a 24-hour light-dark cycle.
(C) Temporal gating of inflammatory responses by the circadian clock. The curve depicts the rhythmic changes in an inflammatory parameter, such as production of cytokines or chemokines, or trafficking of immune cells. The cellular clock generates the nadirs in this inflammatory parameter and its loss is associated with derepression of the nadirs without significant change in the peak, which increase the duration of inflammation. Disrupted clock is depicted in red, whereas normal clock is shown in blue.
There are three mechanisms by which the circadian oscillators might reduce direct costs and vulnerabilities of the inflammatory response. First, the circadian oscillators might temporally limit various aspects of innate immunity to distinct phases of the day-night cycle, thereby preventing their synchronous activation. For example, innate immunity, which comprises barrier defenses, antimicrobial peptides, complement and coagulation factors, cytokines and chemokines, and phagocytes (neutrophils, monocytes, macrophages, and dendritic cells) provides a multi-layered defense against pathogens. Although nearly all aspects of the innate immune system exhibit rhythmic oscillations, their peaks and nadirs occur at different phases of the circadian cycle (5, 25–32) (Fig. 2B). This temporal gating of innate responses to distinct circadian phases decreases vulnerability to septic pathology, because their synergistic activation contributes to the clinical manifestations of septic shock (33). Second, the circadian oscillator might control the duration of inflammatory response by limiting the expression of inflammatory genes to a particular phase of the circadian cycle. In support of this, deletion of BMAL1 or REV-ERBα does not significantly alter the peak of lipopolysaccharide (LPS)-induced inflammatory response in macrophages, but it diminishes its nadir (34). This loss of circadian gating has the net effect of prolonging the duration of the innate inflammatory response (Fig. 2C), which decreases fitness by increasing immunopathology and susceptibility to septic shock (35). A third mechanism for reducing the costs of innate immunity is to temporally gate the trafficking of innate immune cells to sites of inflammation. While the gating of neutrophils and monocytes is regulated by distinct mechanisms, the loss of these mechanisms results in increased susceptibility to endotoxin- or infection-induced tissue injury and sepsis, respectively (27, 28). These examples illustrate that temporal gating by the circadian oscillators reduces the costs of immunity, which is a high-cost, high-benefit trait (Fig. 2A). A corollary of this postulate is that severe infection might suppress this gating mechanism to maximize the inflammatory response. Indeed, during endotoxemia, the normal rhythmic outputs of circadian clock are disrupted, resulting in the appearance of new gene expression and metabolic rhythms that might function to support host immunity but also increase tissue damage and the probability of experiencing a catastrophic vulnerability (36).
Generation of oscillatory immune responses
As mentioned above, nearly every arm of the immune response (innate and adaptive) has been reported to oscillate in a circadian manner. For example, rhythmic oscillations occur in trafficking of innate and adaptive immune cells, susceptibility to bacterial infections and endotoxin-mediated septic shock, expression of pattern recognition receptors and their downstream signaling pathways, phagocytosis, secretion of complement and coagulation factors, and production of cytokines and chemokines (5, 25–32). In principle, these rhythmic oscillations in the immune system can be generated by two mechanisms: cell-extrinsic (cell non-autonomous to immune cells) or cell-intrinsic (cell autonomous to immune cells), which provide a framework for understanding hierarchical oscillatory behaviors and their entrainment by environmental cues (Fig. 3).
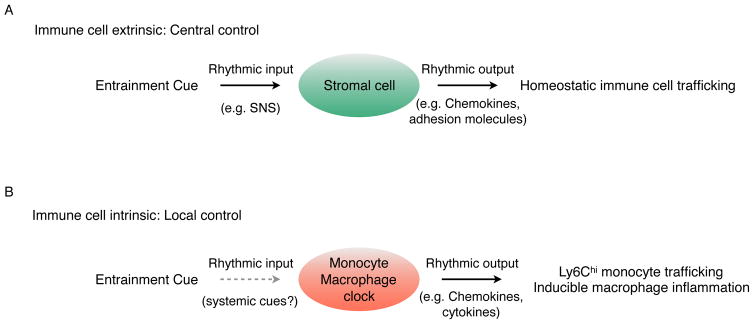
Figure 3. Cell-extrinsic and -intrinsic generation of oscillatory immune responses.
(A) Rhythmic oscillations in entrainment cues regulate homeostatic trafficking of hematopoietic stem cells and leukocytes. The SNS is the central entrainment cue that controls expression of chemokines and adhesion molecules in stromal cells, such as bone marrow stromal cells and endothelial cells, which imparts rhythmicity to trafficking of hematopoietic stem cells and leukocytes.
(B) Cell-intrinsic clocks regulate basal and inducible programs in myeloid cells for maintenance of local homeostasis.
Rhythmic trafficking and recruitment of immune cells under homeostasis is largely controlled by oscillators working in a cell-extrinsic manner. For example, the rhythmic release of hematopoietic stem cells under homeostasis is orchestrated by the central clock acting through the SNS. In this case, the oscillatory release of norepinephrine from the adrenergic nerves regulates the rhythmic expression and secretion of the chemokine CXCL12 (C-X-C Motif Chemokine Ligand 12) by bone marrow stromal cells (37). Because CXCL12 is required for retention of hematopoietic stem cells in their bone marrow niche, its diminished circadian secretion results in their rhythmic egress from the bone marrow into the circulation. This example illustrates the general mechanism by which extrinsic signals regulate the rhythmic trafficking of immune cells into tissues. In this case, extrinsic entrainment cues act upon tissue-specific stromal cells to generate oscillatory outputs, including the rhythmic expression of chemokines and adhesion molecules, which supports rhythmic trafficking of immune cells into tissues (27, 37) (Fig. 3A).
Although the rhythmic discharge by the SNS is the dominant entrainment cue for recruitment of innate immune and hematopoietic stem cells (6), homeostatic oscillations of the adaptive immune cells in the circulation might be entrained by glucocorticoids. For example, circulating numbers of T and B cells display an antiphasic relationship with serum corticosterone concentrations, implicating glucocorticoids as a potential entrainment cue for establishing the peak and nadir of T and B cells in the circulation (7, 38). The involvement of external cues for generation of oscillatory behaviors in T cells is also suggested by immunization experiments, which demonstrate that antigen-specific responses show a strong dependence on time of day (7, 39). This temporal gating of T cell activation and proliferation might be dependent on oscillatory input from the antigen presenting cells, because disruption of the master circadian oscillator Bmal1 in T and B cells did not significantly alter their development or responses to infectious or autoimmune challenges (18). These findings together indicate that cell autonomous clocks might be less important in generating oscillatory behaviors in adaptive immune cells, which operate on longer timescales and might rely on external cues from circulating hormones or antigen presenting cells to generate rhythmic outputs.
Cell autonomous circadian clocks provide a second mechanism for generating rhythmic oscillations in immune cells. Thus far, most studies have focused on cells of the myeloid lineage, including monocytes and macrophages. Independent of entrainment cues, rhythmic oscillations in the trafficking of inflammatory monocytes (designated Ly6Chi monocytes for high expression of Ly6C differentiation antigen) and the circadian gating of inflammatory responses in macrophages are primarily under the control of the cell autonomous clock (Fig. 3B). For example, the diurnal oscillations in abundance of Ly6Chi monocytes, which enhance organismal fitness during infection, are generated in a cell autonomous manner by BMAL1 (28). In this case, BMAL1 is required for the suppression of Ly6Chi monocytes in the circulation, which reduces the hosts vulnerability to septic shock. In support of this, myeloid cell disruption of Bmal1 abolishes the troughs of the Ly6Chi monocyte rhythm, rendering mice susceptible to death by sepsis. In an analogous manner, the oscillatory transcriptional outputs generated by BMAL1 regulate rhythmic expression of ~8% of the macrophage genome and establish the circadian phase during which signaling by endotoxin is most effective (29).
The cellular clock establishes the basal oscillations in gene expression and immune responses, but environmental stimuli can modulate these oscillatory immune functions in two important ways. First, external stimuli, such as LPS, can disrupt the phase, period, and amplitude of the cellular circadian clock, resulting in the loss of basal oscillatory rhythms and a shift from anticipatory to pathogen-associated responses (28, 36). Second, core components of the cellular clock can interact with signal-dependent transcription factors to exert rhythmic anti-inflammatory effects. This is perhaps best illustrated by the anti-inflammatory effects of glucocorticoids in the setting of endotoxin-induced lung inflammation. In this case, the rhythmic recruitment of neutrophils to the inflamed lung requires BMAL1-dependent oscillations in the expression of the chemokine gene Cxcl5 in lung epithelial cells (40). Loss of Bmal1 in lung epithelia, but not myeloid cells, disrupts both rhythmic neutrophil recruitment and the anti-inflammatory effects of glucocorticoids. This is likely caused by impaired recruitment of glucocorticoid receptor to the Cxcl5 enhancer in mice lacking Bmal1 in their lung epithelial cells. This example thus illustrates that loss of BMAL1 not only disrupts cellular rhythmic outputs but also alters the amplitude of inflammatory response by preventing their gating by anti-inflammatory factors, such as the glucocorticoid receptor.
Anti-inflammatory actions of cellular clocks
A defining feature of the cellular clock in innate immune cells is its ability to gate and repress inflammatory responses. Two mechanisms have been proposed by which the circadian oscillators rhythmically modulate basal inflammatory responses in cells. First, basal oscillations in chemokine gene expression is mediated by interactions between BMAL1 and the polycomb repressor complex 2 (PRC2) (28). The rhythmic binding of BMAL1-CLOCK to E-boxes in promoters of chemokine genes, such Ccl2, Ccl8 and S100a8, provides a direct mechanism for temporal gating of these genes by PRC2 during the circadian cycle (Fig. 4A). Second, loss of CRY proteins, which are potent transcriptional repressors and function in the negative feedback loop of the cellular clock, augments both basal and inducible inflammatory responses. This derepression of inflammation is a consequence of increased adenylyl cyclase activity in cells, resulting in protein kinase A-mediated phosphorylation of p65 subunit of NF-κB and its constitutive activation (41). However, it is not known whether these inhibitory functions of CRY proteins are rhythmic or constitutive.
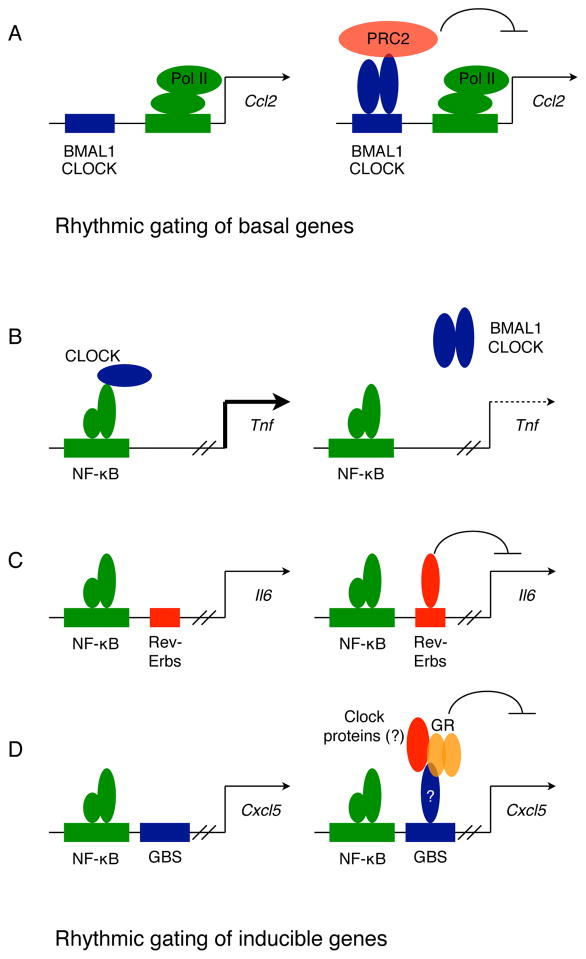
Figure 4. Models for anti-inflammatory actions of clock in myeloid cells.
(A) Rhythmic gating of basal genes. Interactions between CLOCK/BMAL1 heterodimers and the polycomb repressor complex 2 (PRC2) results in rhythmic repression of chemokine genes (such as Ccl2).
(B–D). Rhythmic gating of inducible genes. Several modes of action are proposed for rhythmic gating of LPS-induced inflammatory genes. The CLOCK protein can acetylate p65 subunit of NF-κB to induce expression of TNF, and its rhythmic sequestration by BMAL1 can drive oscillations in TNF expression (B). Recruitment of REV-ERB repressor complexes to inflammatory genes, such as Il6, can rhythmically repress their expression (C). Glucocorticoid- receptor mediated repressive effects on inflammatory chemokines requires a functional cellular clock, which may be essential for recruitment of glucocorticoid receptor (GR) complexes to glucocorticoid binding site (GBS) on the Cxcl5 gene (D).
In contrast, rhythmic modulation of inducible gene expression relies on interference with the transcription factor NF-κB, the major transcriptional activator of inflammation. On the basis of LPS-induced inflammatory responses, three different mechanisms have been proposed to rhythmically limit NF-κB activity on promoters and enhancers of inflammatory genes. First, CLOCK can directly interact with the p65 subunit of NF-κB to enhance its transcriptional activity on promoters of inflammatory genes. In this model, the sequestration of CLOCK by BMAL1 results in rhythmic repression of inflammatory genes (Fig. 4B), whereas loss of BMAL1 causes their derepression through constitutive recruitment of CLOCK to NF-κB-regulated promoters (42). However, it is unclear how specificity is achieved, because only a subset of NF-κB-induced genes are rhythmically expressed in LPS-stimulated cells. Second, REV-ERBs, which form the negative feedback loop of the cellular clock, can mediate repression through recruitment of NCoR complexes of HDAC3 (43). Although REV-ERBα and β have thousands of binding sites in macrophage-specific enhancer-like regions (44), they repress a subset of inflammatory genes in a signal-dependent manner by inhibiting enhancer-specific transcription (45). Thus, the circadian oscillations in REV-ERBs would dictate their ability to temporally gate inflammatory gene expression (Fig. 4C). In support of this, genetic deletion of Rev-Erbα derepresses a subset of inflammatory genes in macrophages without affecting the cellular rhythm, whereas its pharmacological agonism inhibits LPS-stimulated inflammation (34). The third mechanism for circadian gating of inflammation involves the glucocorticoid receptor, which participates in establishing synchrony between the peripheral clocks. Although serum concentrations of glucocorticoids oscillate in a circadian manner, they do not modulate the rhythmic inflammatory responses of macrophages, which are preserved in adrenealectomized mice (29). The rhythmic recruitment of the glucocorticoid receptor by BMAL1 to promoters of inflammatory genes, such as Cxcl5, might be necessary for their periodic repression (Fig. 4D), suggesting that the circadian clock gates the anti-inflammatory effects of glucocorticoids locally rather than systemically (40).
Conclusions and Perspectives
Much progress has been made on understanding how immune functions are regulated by biological clocks, but a number of questions still remain. How is specificity achieved in gating of immune responses? Is this primarily controlled by the master clock protein BMAL1 or by its downstream target proteins REV-ERBs and CRYs? Furthermore, what is the molecular code by which BMAL1, REV-ERBs and CRYs modulate expression of inflammatory genes? Because the circadian clock is a critical regulator of metabolism (4, 46), it will be important to determine whether oscillations in immunity are driven by clock-controlled changes in cellular metabolism. If so, it might indicate that metabolic priming by the cellular clock contributes to the time of day-dependence of immune responses. Although the immune system senses and responds to the external world, it is not known whether environmental signals can entrain adaptive and innate immunity. If so, the nature of the entrainment cues and the molecular basis by which they modulate immune functions in different environments should be investigated. A primordial function of the circadian oscillator is to temporally compartmentalize biochemically incompatible programs to distinct phases of the circadian cycle, such as photosynthesis during the day and nitrogen fixation at night in cyanobacteria (1). However, it is not known whether this feature of the oscillator is used to separate mutually incompatible programs in immune cells, such as those controlling cell fate specification or polarization. Finally, does the cellular oscillator contribute to observed heterogeneity in resident and recruited populations of immune cells or to cellular desynchrony during an immune response? Addressing these questions will enhance our basic understanding of how the circadian clock optimizes immune functions to anticipate changes in the environment, and provide mechanistic insights to facilitate the development of chronotherapies for treating inflammatory disorders.
Acknowledgments
We thank members of the Chawla laboratory for discussions, and A. Loh and R. D. Dockrell for comments on the manuscript, and B. Saer for assistance with artwork. Work in authors’ laboratories was supported by NIH grants DK094641, DK101064, DP1AR064158, and P30DK098722 (A.C.); BBSRC grants BB/K003097/1, BB/L00954/1, BB/N015584/1, Wellcome Trust 107851/Z/5/Z (A.L.). The authors apologize that, in the interests of brevity, papers by colleagues may not have been cited. The authors declare that they have no competing financial interests.
References and Notes
- 1.Bell-Pedersen D, et al. Nature reviews Genetics. 2005;6:44–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stratmann M, Schibler U. J Biol Rhythms. 2006;21:494–506. doi: 10.1177/0748730406293889. [DOI] [PubMed] [Google Scholar]
- 3.Mohawk JA, Green CB, Takahashi JS. Annual review of neuroscience. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bass J, Takahashi JS. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtis AM, Bellet MM, Sassone-Corsi P, O’Neill LA. Immunity. 2014;40:178–186. doi: 10.1016/j.immuni.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Scheiermann C, Kunisaki Y, Frenette PS. Nat Rev Immunol. 2013;13:190–198. doi: 10.1038/nri3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labrecque N, Cermakian N. J Biol Rhythms. 2015;30:277–290. doi: 10.1177/0748730415577723. [DOI] [PubMed] [Google Scholar]
- 8.Halberg F, Johnson EA, Brown BW, Bittner JJ. Proc Soc Exp Biol Med. 1960;103:142–144. doi: 10.3181/00379727-103-25439. [DOI] [PubMed] [Google Scholar]
- 9.Shackelford PG, Feigin RD. Science. 1973;182:285–287. doi: 10.1126/science.182.4109.285. [DOI] [PubMed] [Google Scholar]
- 10.Yu X, et al. Elife. 2014;3 [Google Scholar]
- 11.Xu W, et al. Cell reports. 2015;10:2043–2054. doi: 10.1016/j.celrep.2015.02.057. [DOI] [PubMed] [Google Scholar]
- 12.Geiger TL, et al. The Journal of experimental medicine. 2014;211:1723–1731. doi: 10.1084/jem.20140212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seillet C, et al. The Journal of experimental medicine. 2014;211:1733–1740. doi: 10.1084/jem.20140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, et al. Science. 2015;348:1488–1492. doi: 10.1126/science.aab3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang G, et al. Science translational medicine. 2016;8:324ra316. doi: 10.1126/scitranslmed.aad3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu X, et al. Science. 2013;342:727–730. doi: 10.1126/science.1243884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang XO, et al. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hemmers S, Rudensky AY. Cell reports. 2015;11:1339–1349. doi: 10.1016/j.celrep.2015.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gury-BenAri M, et al. Cell. 2016;166:1231–1246 e1213. doi: 10.1016/j.cell.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 20.Thaiss CA, et al. Cell. 2014;159:514–529. doi: 10.1016/j.cell.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 21.Mukherji A, Kobiita A, Ye T, Chambon P. Cell. 2013;153:812–827. doi: 10.1016/j.cell.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Medzhitov R. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 23.Okin D, Medzhitov R. Current biology: CB. 2012;22:R733–740. doi: 10.1016/j.cub.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stearns SC, Medzhitov R. Evolutionary medicine. Sinauer Associates, Inc., Publishers; Sunderland, Massachussetts: 2016. p. xix.p. 306. [Google Scholar]
- 25.Silver AC, Arjona A, Walker WE, Fikrig E. Immunity. 2012;36:251–261. doi: 10.1016/j.immuni.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mauvoisin D, et al. Proc Natl Acad Sci U S A. 2014;111:167–172. doi: 10.1073/pnas.1314066111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheiermann C, et al. Immunity. 2012;37:290–301. doi: 10.1016/j.immuni.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen KD, et al. Science. 2013;341:1483–1488. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keller M, et al. Proc Natl Acad Sci U S A. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellet MM, et al. Proc Natl Acad Sci U S A. 2013;110:9897–9902. doi: 10.1073/pnas.1120636110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashi M, Shimba S, Tezuka M. Biological & pharmaceutical bulletin. 2007;30:621–626. doi: 10.1248/bpb.30.621. [DOI] [PubMed] [Google Scholar]
- 32.Casanova-Acebes M, et al. Cell. 2013;153:1025–1035. doi: 10.1016/j.cell.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rittirsch D, Flierl MA, Ward PA. Nat Rev Immunol. 2008;8:776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibbs JE, et al. Proc Natl Acad Sci U S A. 2012;109:582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curtis AM, et al. Proc Natl Acad Sci U S A. 2015;112:7231–7236. doi: 10.1073/pnas.1501327112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haspel JA, et al. Nature communications. 2014;5:4753. doi: 10.1038/ncomms5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 38.Kawate T, Abo T, Hinuma S, Kumagai K. J Immunol. 1981;126:1364–1367. [PubMed] [Google Scholar]
- 39.Fortier EE, et al. J Immunol. 2011;187:6291–6300. doi: 10.4049/jimmunol.1004030. [DOI] [PubMed] [Google Scholar]
- 40.Gibbs J, et al. Nat Med. 2014;20:919–926. doi: 10.1038/nm.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Narasimamurthy R, et al. Proc Natl Acad Sci U S A. 2012;109:12662–12667. doi: 10.1073/pnas.1209965109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spengler ML, et al. Proc Natl Acad Sci U S A. 2012;109:E2457–2465. doi: 10.1073/pnas.1206274109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang B, Lazar MA. Cold Spring Harb Symp Quant Biol. 2015;80:233–238. doi: 10.1101/sqb.2015.80.027508. [DOI] [PubMed] [Google Scholar]
- 44.Lam MT, et al. Nature. 2013;498:511–515. doi: 10.1038/nature12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eichenfield DZ, et al. Elife. 2016;5 doi: 10.7554/eLife.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacobi D, et al. Cell Metab. 2015;22:709–720. doi: 10.1016/j.cmet.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]