Abstract
Early studies suggested that the androgen receptor (AR) might play important roles to promote the renal cell carcinoma (RCC) progression; however, the detailed mechanisms remain unclear. Here we demonstrated the higher YBX1 expression with lower C1QBP expression in human RCC clinical tissues, and the intensity of C1QBP was negatively correlated with the YBX1 nuclear expression. Mechanism dissection found C1QBP could interact with YBX1 to suppress the YBX1 activation via altering the YBX1 phosphorylation and nuclear translocation in RCC cells. The consequences of such suppression of YBX1 might then result in suppressing the RCC cell migration and invasion that involved altering the AR-modulated MMP9 signals. Interruption of this newly identified C1QBP → YBX1 → AR → MMP9-suppressed RCC cell invasion pathway via targeting YBX1 or AR partially reversed the RCC cell invasion. Importantly, results from in vivo mouse model with orthotopic implantation of RCC OSRC2 cells into the left renal capsule also confirmed in vitro cell line studies showing targeting YBX1 could suppress RCC cell invasion via regulation of AR/MMP9 signals. Collectively, these data suggest that C1QBP could regulate YBX1 to suppress the AR-enhanced RCC cell invasion. Targeting this newly identified C1QBP/YBX1/AR/MMP9 signal pathway may provide a new potential therapy to better suppress RCC metastasis.
Introduction
Renal cell carcinoma (RCC) is the most common kidney tumor arising from the cells in the lining of the kidney tubules [1]. RCC accounts for 3% of adult malignancies and approximately 90% to 95% of kidney neoplasms [2], [3]. Approximately 30% of RCC patients are at a later metastatic stage when they are first diagnosed. The molecular mechanisms of the metastasis of RCC have not been fully studied or understood. Immunotherapy has been the major therapeutic option for advanced RCC, yet the effect is limited. Although recently there have been targeted therapies developed for treating advanced RCC, the majority of advanced RCC patients remain refractory to these treatments [4], [5]. Thus, understanding the molecular mechanisms of RCC progression in order to identify new targets for future therapy is essential before we can better battle the advanced RCC.
The epidemiological studies indicated that a gender difference with male:female ratio in RCC incidence of 1.6:1.0 [6], [7], suggesting that sex hormones and/or their receptors may play important roles in the development of RCC. Zhu et al. found that AR could be detected in various stages of RCC [8], and He et al. found AR might play key roles in RCC progression [9]. However, which upstream signals may regulate AR to impact RCC remain unclear.
The nuclease-sensitive element-binding protein 1 (YBX1) is a member of the cold-shock protein superfamily that contains a highly conserved nucleic-acid-binding motif for binding to both DNA and RNA, and has been implicated in numerous cellular processes including regulation of transcription and translation, pre-mRNA splicing, DNA repair, and mRNA packaging [10], drug resistance and stress response to extracellular signals [12], [13]. YBX1 is also a component of messenger ribonucleoprotein (mRNP) complexes and may have a role in microRNA processing [11]. Interestingly, recent studies also indicated that YBX1 expression might be linked to tumor progression with abnormal expression in the cell nucleus of various tumors, including bladder, prostate, and breast [12], [13], [14], [15], [16], [17]. Moreover, in dialysis caused RCC, nuclear expressions of YBX-1 were higher than in sporadic RCC [18].
The complement component 1, q subcomponent binding protein (C1QBP) is a ubiquitously expressed and multi-compartmental cellular protein involved in various biological processes [19], [20]. Over-expressed C1QBP with a potential oncogene characteristic has been reported in various types of cancer including prostate, ovarian, liver, and breast [21], [22], [23], [24]. However, another study also indicated a lower expression of C1QBP in cervical cancer compared to normal tissues [26], suggesting the expression patterns of C1QBP in different tumors and its impacts on tumor progression may be cell-type dependent.
Here we demonstrate that C1QBP could regulate YBX1 to suppress the AR-enhanced RCC cell invasion.
Materials and Methods
Cell Culture and Transfection
The human RCC cell line, SW839 was purchased from Cell Resource Center for Biomedical Research, Tohoku University and OSRC2 was purchased from Riken Cell Bank (Tsukuba, Japan). Cells were cultured in DMEM supplemented with 10% fetal bovine serum and 1% glutamine and Pen-Strep solutions at 37°C and 5% CO2.
To generate AR, YBX1 and C1QBP overexpressed or knocked-down stable clones, OSRC2 and SW839 cells were transfected with lentiviral vectors (Promega, Madison, WI, USA), including pWPI-AR, pWPI-YBX1, pWPI-C1QBP, pWPI-Vec, pLKO1-sh-AR, pLKO1-sh-YBX1, pLKO1-sh-C1QBP, or pLKO1-scr, with the psAX2 packaging plasmid, and pMD2G envelope plasmid, then transfected into 293 T cell for 48 h to get the lentivirus supernatant. The lentivirus supernatant was collected and frozen at −80°C for later use. For stable clones, virally infected cells were cultured in media containing 2.5 μg/ml puromycin for 10 days and the puromycin-resistant clones were collected and expanded.
Clinical Specimens
Clear cell RCC primary tissue samples and corresponding para-carcinoma tissues were surgically removed and paraffin-embedded in the Tianjin Medical University Second Hospital between January 2005 and December 2005 with patients' consent and ethical committee approval. The age and gender of patients were noted and the tumor size, histological type, Fuhrman grade and presence or absence of invasion were evaluated. All patients had undergone radical nephrectomy with no preoperative or postoperative adjuvant therapy. All samples were analyzed by two experienced pathologists to ensure that they were correctly identified as RCC.
Immunohistochemical Staining
Paraffin-processed sections at 5 μm thickness were mounted on poly-D-lysine coated glass slides. Slides were dewaxed in 100% xylene, rehydrated by incubation in decreasing concentrations of alcohol, and incubated in 3% H2O2 to eliminate endogenous biotin. Briefly, sections blocked with horse serum were incubated with C1QBP antibody (Santa Cruz Biotechnologies, Santa Cruz, CA, USA) or YBX1 antibody (Abcam, Cambridge, UK) overnight at 4°C. After being washed with PBS, the immunoreactions were performed according to Max Vision HRP-Polymer anti-Rabbit IHC Kit (Miaxim.bio, Fuzhou, China). Sections were developed by peroxidase substrate DAB Detection Kit (Maxim.bio, China) and were counterstained by hematoxylin. Immunostaining for C1QBP and YBX1 expression was scored by 2 independent observers based on the relative color area and intensity of the brown DAB signals. For each section, at least 5 different fields were examined under the microscope. First, the immunostaining score was evaluated from 0 to 5: 0, (0-1)% of tumor cells positive; 1 point, (1-5)% of tumor cells positive; 2 point, (5-10)% of cells positive; 3 point, (10-20)% of cells positive; 4 point, (20-50)% of cells positive; and 5 point, >50% of cells positive. Second, the intensity of immunostaining level was determined by subjective visual scoring of the brown stain. Scoring levels were: 0 point, no staining; 1 point, yellow brown staining and 2 points, brown staining. The final score consists of addition of these two scores and the immunostaining for C1QBP was scored as – (0-3) and + (4-7).
Western Blot Analysis
Cells were lysed in RIPA buffer and protein concentration in the cell lystate solution was determined by BCA protein assay (Amresco, Cochran, Solon, OH, USA). 30 μg protein was separated on 10% SDS/PAGE gel and then transferred onto PVDF membranes (Millipore, Billerica, MA, USA). After blocking membranes, they were incubated with appropriate dilutions of specific primary antibodies: rabbit anti-AR (1:1000; Santa Cruz Biotechnology), mouse anti-GAPDH (1:1000; Santa Cruz Biotechnology), anti-YBX1 (1:1000; Abcam, Cambridge, UK), anti-p-YBX1 (1:1000; Abcam), anti-MMP9 (1:1000; Abcam) and anti-C1QBP (1:1000; Santa Cruz Biotechnology). The blots were incubated with HRP-conjugated secondary antibodies and visualized using ECL system (Thermo Fisher Scientific, Rochester, NY, USA).
Co-Immunoprecipitation
Cells were harvested and lysed with Triton X-100 buffer (40 mM Tris, 120 mM NaCl, 1% Triton X-100, 1 mM NaF, 1 mM Na3VO4). The cell lysates were then centrifuged at 20,000xg at 4 °C for 1 h and the pellets containing DNA and cell debris were removed. Their interacting proteins were enriched with 1 mg anti-C1QBP, anti-AR, and anti-YBX1 antibody. Normal mouse/rabbit immunoglobulin G was used as a control. The immunoprecipitated protein complexes were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and followed by Western blot analysis.
Immunofluorescence and Confocal Microscopy
Cells were plated in 12-well plates containing sterile glass coverslips, allowed to grow for 24 h and then starved in serum-free media for 6 h. After stimulation with 10 ng/mL EGF for 10 min at 37 °C, cells were fixed with 4% paraformaldehyde in PBS (pH 7.4) for 10 min at room temperature, permeabilized in 0.2% triton/PBS for 10 min and blocked in 3% BSA in PBS for 1 h at room temperature. The cells were incubated with primary antibodies at 4 °C overnight, followed by staining with Alexa Fluor 488- and 546-conjugated secondary antibodies for 1 h at room temperature. One ng/mL DAPI was used for nuclear staining. Coverslips were counter-stained with DAPI, mounted with ProLong Gold antifade reagents, and visualized with confocal laser scanning microscopy (Leica, Buffalo Grove, IL, USA).
Luciferase Reporter Assay
RCC cells were transfected with 0.5 mg of the various ARE plasmids (Promega, Madison WI, USA) and 0.05 mg TK (Promega) using Lipofectamine 3000, according to the manufacturer's protocol. Cells were also treated with 10 nM DHT. After 24 h, ARE activity was detected using a Dual-Luciferase Reporter Assay System (Promega). Light intensity was measured using a plate reader (ARVOTM MX, Perkin Elmer, Inc., Waltham, MA, USA).
Cell Invasion and Migration Assay
The invasion capability of RCC cells was determined by the transwell assay. The upper transwell chambers (Corning Inc., Corning, NY, USA) were pre-coated with diluted growth factor-reduced matrigel (1:5 serum free RPMI) and put into the incubator for 5 h. RCC cells were harvested and seeded at 1 × 105 cells/well with serum-free DMEM into the upper chamber and the lower chamber contained DMEM with 10% FBS. After 24 h incubation at 37 °C, the invaded cells attached to the lower surface of the membrane were fixed with paraformaldehyde and stained with crystal violet. Cell numbers were counted in five randomly chosen microscopic fields. For migration assays, RCC cells (5 × 104 cells/well) were harvested and seeded with serum-free DMEM into the upper chamber and the lower chamber contained DMEM with 10% FBS. After 6 h incubation at 37°C, cells were counted.
In Vivo Metastasis Studies
Nude mice 6–8 weeks old were purchased from NCI. Eighteen mice were divided into 3 groups (n = 6, Male:Female = 1:1): 1) control, 2) OSRC2-YBX1-siRNA and 3) OSRC2-YBX1-siRNA + AR-overexpression. We prepared xenografts by an incision in the back of mice and exposure of the left kidney. The mice were the injected with 1 × 106 cells (mixture with Matrigel, 1:1) into the sub-renal capsule and incision closed. Cells were also transduced with Luciferase so that metastasis in the mice could be measured using a Fluorescent Imager (IVIS Spectrum, Caliper Life Sciences, Hopkinton, MA) at 4 different time points (1, 4, 5, and 6 weeks after injection). After the last monitoring with the Imager, mice were sacrificed and tumors were further examined by IHC staining.
Statistical Analysis
Data values were presented as the mean ± SD. Differences in mean values between two groups were analyzed by t test. Chi-square and Fisher exact tests were used for statistical analysis of the correlations between C1QBP and YBX1 expression and clinicopathologic parameters. The correlation between the expression of C1QBP and nuclear localization of YBX1 was analyzed using Spearman tests. Differences with P values <0.05 were considered as statistically significant.
Results
The Expression of C1QBP and YBX1 Correlated With RCC Progression in Human Clinical RCC Samples
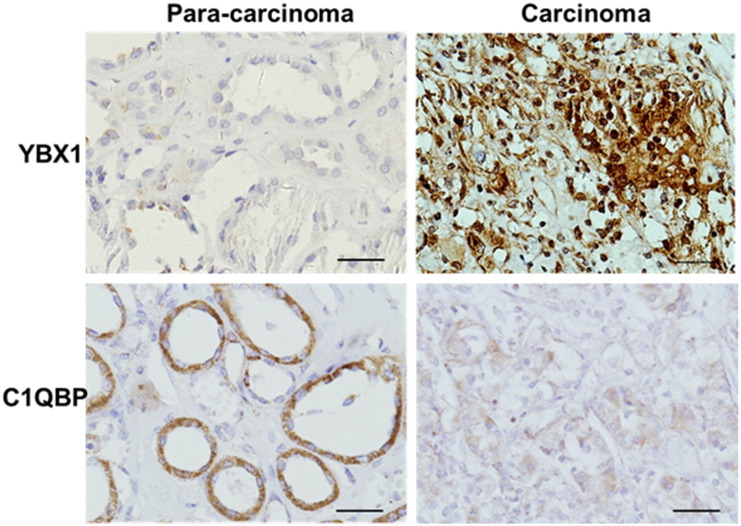
Immunohistochemistry (IHC) quantitation analysis of clear cell RCC clinical samples found higher expression of C1QBP in surrounding normal tissues (90% of 18/20), but in the RCC samples 57.7% (30/52) showed weak expression of C1QBP (P < 0.05) and 42.3% (22/52) were negative (Figure 1). Furthermore, pathological analysis revealed that the C1QBP staining scores in RCC were associated with T stage (P < 0.05) (Table 1). In contrast, IHC staining found the ratio of positive staining of YBX1 expression in the nuclei of RCC was significantly higher compared to those found in surrounding tissues (P < 0.01) (Figure 1), and this nuclear expression of YBX1 was correlated with T stage and the presence of metastases (P < 0.05) (Table 1).
Figure 1.
Expression of C1QBP and YBX1 in RCC clinical samples. Immunohistochemical analysis of C1QBP and YBX1 expression in the para-carcinoma tissues and carcinoma tissues. The scale bar was 20 μm.
Table 1.
The Expression of C1QBP and YBX1 in Renal Cell Carcinoma Tissues
| Parameter | N | C1QBP Expression |
P Value | Nuclear Expression of YBX1 |
P Value | |||
|---|---|---|---|---|---|---|---|---|
| - | + | - | + | |||||
| T stage | T1–2 | 34 | 14 | 20 | .012* | 22 | 12 | .011* |
| T3-4 | 18 | 14 | 4 | 5 | 13 | |||
| Metastasis | Positive | 10 | 6 | 4 | .736 | 2 | 8 | .036* |
| Negative | 42 | 22 | 20 | 25 | 17 | |||
| Tissue location | Tumor | 52 | 22 | 30 | .009* | 27 | 25 | .003* |
| Para-tumor | 20 | 2 | 18 | 18 | 2 | |||
Metastasis includes all types of metastasis.
Furthermore, the correlation between C1QBP and YBX1 nuclear expression was examined using the Spearman's correlation test. The results revealed that the intensity of C1QBP was negatively correlated with the YBX1 nuclear expression with the correlation coefficient of −0.35 (P = 0.011) (Table 2), which is in agreement with results from in vitro cell line studies showing the reduction of C1QBP expression is accompanied with the increase of nuclear translocation of YBX1 (Figure 2C).
Table 2.
Correlation Between C1QBP and YBX1 Nuclear Expression in RCC
| C1QBP | YBX1 |
Spearman's Rank Correlation Coefficient (P) | |
|---|---|---|---|
| Nnegative | Positive | ||
| Negative | 10 | 18 | −0.350 (P = 0.011) |
| Positive | 17 | 7 | |
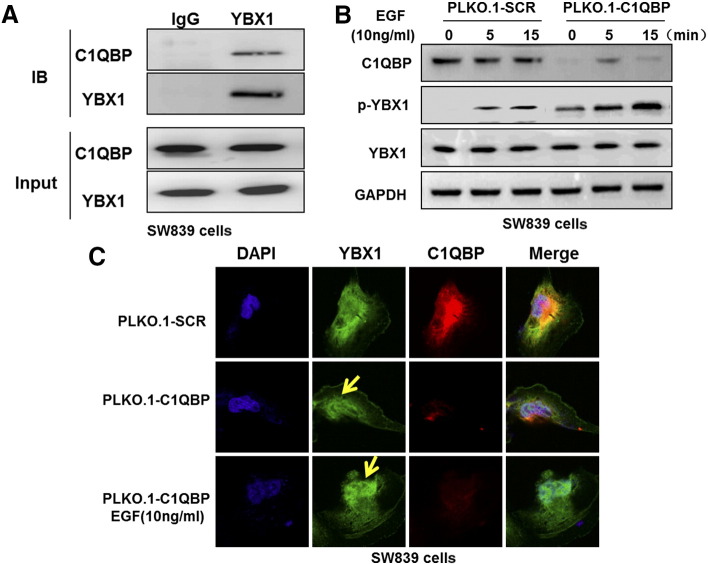
Figure 2.
The interaction between C1QBP and YBX1. (A) Reciprocal co-immunoprecipitation of endogenous C1QBP and YBX1 in SW839 cells. Immunoprecipitates of C1QBP and YBX1 were analyzed by Western blot. (B) Western blot analysis showed that the depletion of C1QBP in SW839 cells increased EGF-induced phosphorylation of YBX1. Cells were deprived of serum for 6 hours before stimulated with EGF at 10 ng/ml for 0, 5, and 15 min. Then the level of YBX1 and p-YBX1 in the cell lysates was analyzed by immunoblotting. (C) Confocal microscopy analysis of C1QBP and YBX1 in SW839 cells. Knockdown of C1QBP enhanced the EGF induced nuclear translocation of YBX1 (yellow arrows).
The Interaction Between C1QBP and YBX1
To dissect the mechanism linked to the expression of YBX1 and C1QBP, we applied the co-immunoprecipitation assay to examine if these 2 proteins could interact with each other in RCC cells. The results revealed that endogenous YBX1 and C1QBP could form a physical interaction in RCC SW839 cells (Figure 2A).
Early studies indicated that activation of YBX1 via EGF-induced phosphorylation could lead to its nuclear translocation [26]. Results from Figure 2B also confirmed that EGF could induce phosphorylation of YBX1 in the cold shock domain (S102) in a time-dependent fashion. Using confocal fluorescent microscopic analysis, we also found knockdown of C1QBP or treatment with EGF induced YBX1 phosphorylation and its nuclear translocation (Figure 2C).
Together, results from Figure 2, A–C suggest that C1QBP can interact with YBX1 and then suppress the activation of YBX1 via the YBX1 phosphorylation and nuclear translocation in RCC cells.
C1QBP Regulated RCC Cell Invasion and Migration via Interacting/Suppressing YBX1
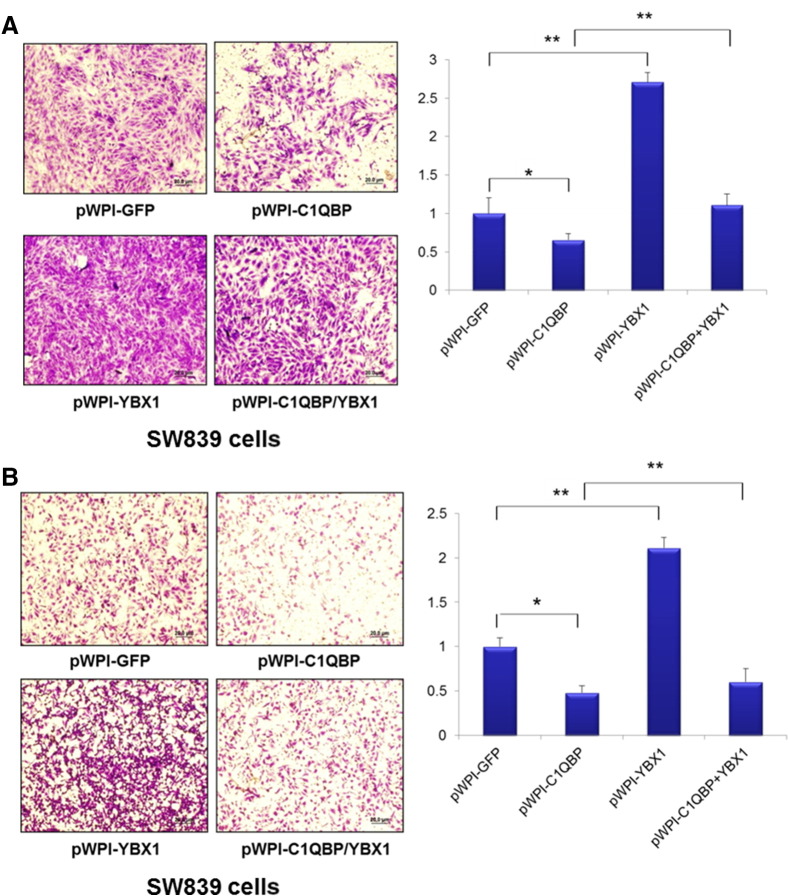
To further examine the consequences of C1QBP suppression of YBX1 activation and the impact of RCC progression, we overexpressed functional cDNAs of C1QBP and YBX1 into RCC SW839 and OSRC2 cells and found overexpression of C1QBP suppressed the invasion of SW839 and OSRC2 cells using Chamber co-culture invasion assay [9]. In contrast, overexpression of YBX1 enhanced the SW839 and OSRC2 cell invasion. Importantly, overexpression of YBX1 could reverse the C1QBP-suppressed SW839 cell invasion (Figure 3A and Supplementary Figure 1A).
Figure 3.
C1QBP regulated RCC cell invasion and migration via YBX1. SW839 cells were transfected with pWPI-GFP, pWPI-C1QBP, pWPI-YBX1, or pWPI-C1QBP together with pWPI-YBX1. (A) 1 × 105 cells were plated onto the upper matrigel coated transwell chambers with 8 μm pore polycarbonate membrane for cell invasion assays. (B) 5 × 104 cells were plated onto the upper chamber with 8 μm pore polycarbonate membrane for cell migration assays. Overexpression of YBX1 could reverse C1QBP inhibited cell invasion and migration of RCC SW839 cells. (*P < 0.05, **P < 0.01).
Similar results were also obtained when we replaced the invasion assay with the migration assay [9] showing overexpression of YBX1 could reverse the C1QBP-suppressed SW839 and OSRC2 cell migration (Figure 3B and Supplementary Figure 1B).
Together, results from Figure 3 and Supplementary Figure 1 suggest that C1QBP could interact with and modulate YBX1 activity that resulted in enhancing the migration and invasion of RCC cells.
C1QBP Regulated YBX1 to Alter the Expression and Transactivation of AR
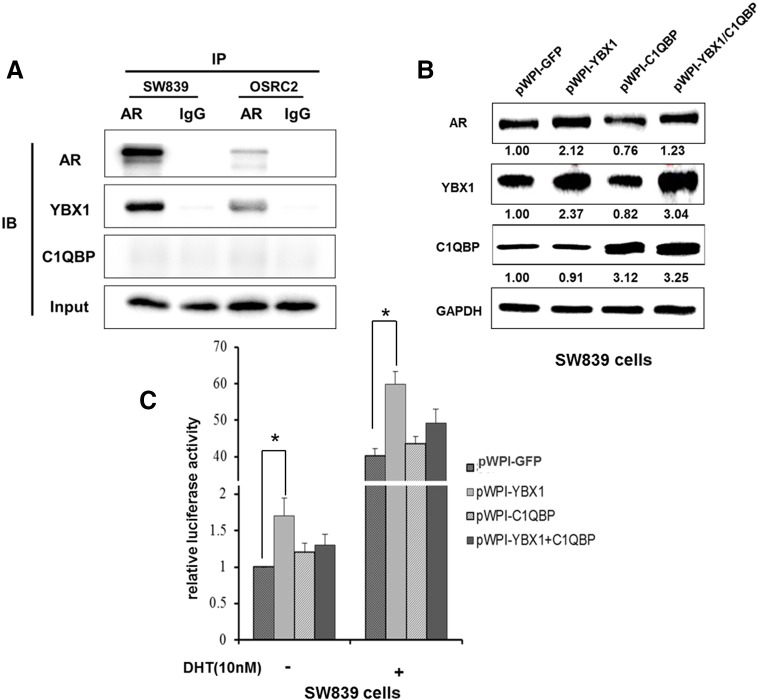
Next we determined the mechanism(s) how C1QBP-modulated YBX1 signals could suppress RCC cell invasion. As recent study indicated that AR might play key roles to promote the RCC metastasis [9], we were interested to see if C1QBP-modulated YBX1 signals may function through altering the AR signals to influence the RCC cell invasion. We applied the co-immunoprecipitation assay to examine the interaction of YBX1 and AR and results revealed they could interact with each other (Figure 4A).
Figure 4.
C1QBP regulated YBX1 to affect the expression and activity of AR. (A) YBX1 and AR could interact with each other in SW839 and OSRC2 cells. Co-immunoprecipitation experiment was performed on endogenous YBX1 and AR in RCC cells. (B) SW839 cells were transfected with pWPI-GFP, pWPI-YBX1, pWPI-C1QBP, or pWPI-YBX1/C1QBP expression plasmids. The protein expressions were tested. (C) SW839 cells were transfected with 0.5 mg of the various ARE plasmids and 0.05 mg TK and ARE activities were tested. Cells were also treated with/without DHT (10 nM). Overexpression of YBX1 enhanced the AR transactivation that could be partially reversed after overexpression of C1QBP (*P < 0.05).
We then applied the Western blot analysis to examine the potential impact after YBX1 interaction with AR in SW839 and OSRC2 cells, and results showed that overexpression of YBX1 enhanced the AR expression and overexpression of C1QBP suppressed the AR expression. Importantly, the C1QBP-suppressed AR expression was reversed by overexpression of YBX1 (Figure 4B and Supplementary Figure 2A), suggesting C1QBP might function through YBX1 to influence AR expression.
We also applied the Luciferase assay to examine the potential impact after YBX1 interaction with AR. The results revealed that in the presence of 10 nM DHT, addition of YBX1 enhanced the AR transactivation that could be reversed after overexpression of C1QBP in SW839 and OSRC2 cells (Figure 4C and Supplementary Figure 2B).
Together, results from Figure 4 and Supplementary Figure 2 suggest that C1QBP could modulate YBX1 activity that resulted in influencing the AR transactivation.
C1QBP Regulated YBX1 to Affect the AR-Enhanced RCC Cell Invasion
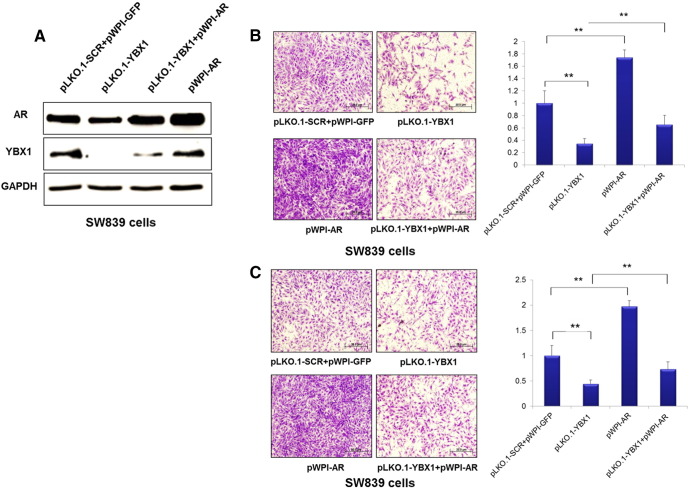
To determine if C1QBP-modulated YBX1 influenced AR transactivation which in turn altered RCC cell invasion, we overexpressed AR and knocked down YBX1 separately or in combination in SW839 and OSRC2 cells. YBX1 and AR expressions were examined by Western blot (Figure 5A and Supplementary Figure 3A). We first found that knocked-down YBX1 suppressed the SW839 and OSRC2 cell invasion and overexpression of AR enhanced the SW839 and OSRC2 cell invasion. Importantly, overexpressing AR also reversed the suppression of cell invasion by YBX1 knocking down in SW839 and OSRC2 cells (Figure 5B and Supplementary Figure 3B). Similar results were also obtained when we replaced invasion assay with migration assay (Figure 5C and Supplementary Figure 3C).
Figure 5.
YBX1 regulated RCC cell migration and invasion was mediated by AR. SW839 cells were transfected with pLK0.1-scr, pLK0.1-YBX1, pWPI-AR, or pLK0.1-YBX1 together with pWPI-AR plasmids. (A) YBX1 and AR expression in SW839 cell lines were tested by Western blot. (B) 1 × 105 SW839 cells were plated onto the upper matrigel coated transwell chambers with 8 μm pore polycarbonate membrane for cell invasion assay. (C) 5 × 104 SW839 cells were plated onto the upper chamber with 8 μm pore polycarbonate membrane for cell migration assay. (**P < 0.01).
Together, results from Figure 5 and Supplementary Figure 3 conclude that C1QBP suppressed YBX1 and AR transaction thus AR-enhanced RCC cell invasion.
C1QBP/YBX1/AR Signaling Regulated MMP9 Expression to Influence the RCC Cell Invasion
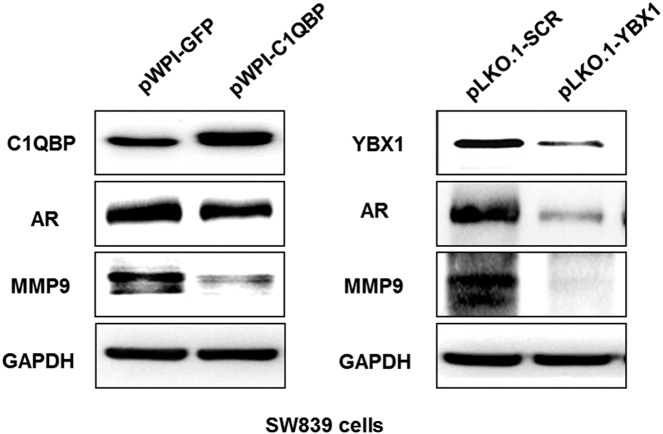
Next, we screened the expression of genes related to cell invasion and RCC metastasis and found YBX1 knockdown could decrease the expression of AR and MMP9. Importantly, AR knockdown could also decrease MMP9 expression (Supplementary Figure 4A). Western blot assay further confirmed that overexpressing C1QBP or knocking down YBX1 suppressed AR and MMP9 in SW839 and OSRC2 cell lines (Figure 6 and Supplementary Figure 4B), suggesting that C1QBP-YBX1 interaction regulated AR function which in turn influence the RCC cell invasion through MMP9.
Figure 6.
Signal pathway of C1QBP and YBX1 regulate invasion and migration of RCC through AR. SW839 cells were transfected with pWPI-GFP, pWPI-C1QBP, pLK0.1-scramble (SCR) or pLK0.1-YBX1 expression plasmids. The C1QBP, YBX1, AR, and MMP9 expressions were tested by western blot. Overexpression of C1QBP or knockdown of YBX1 suppressed AR and MMP9 protein expression in SW839 cell lines.
In Vivo Mouse Model to Prove the Effects of C1QBP-YBX1-AR Signals on RCC Cell Invasion
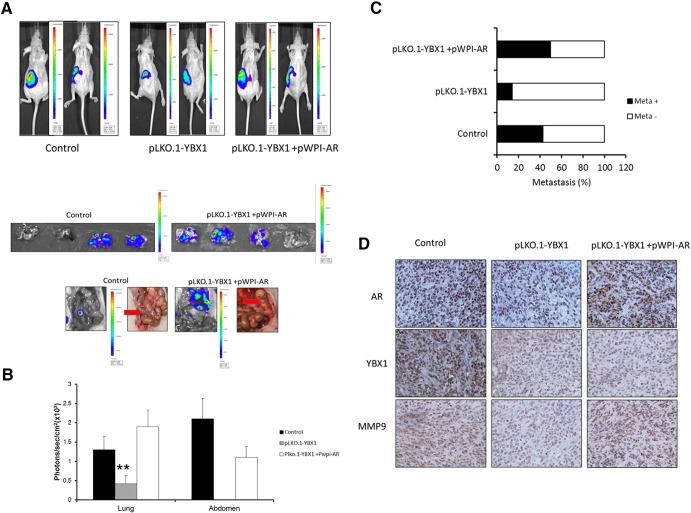
To confirm the above in vitro data in the in vivo mouse model, we performed the orthotopic implantation of RCC OSRC2 cells stably transfected with firefly luciferase reporter gene and infected with lentivirus for knocking down YBX1 with and without overexpression of AR into the renal capsule of mouse left kidney. The primary tumors and metastatic tumors in lung and enterocoelia were monitored through IVIS imaging (Figure 7A). The results revealed that mice with the YBX1 knocked down in OSRC2 cells had much fewer metastatic foci detected (Figure 7, B and C), and overexpression of AR could reverse the inhibition effect of YBX1 knockdown. These suggested that YBX1 could regulate AR to promote RCC cell invasion.
Figure 7.
In vivo mouse model to prove the effects of C1QBP/YBX1/AR signals on RCC cell invasion. Luc-OSRC2 (control), luc-OSRC2 + pLK0.1-YBX1 (pLKO.1YBX1) and luc-OSRC2 + pLK0.1-YBX1 + pWPI-AR (pLK0.1-YBX1 + pWPI-AR) cells were implanted into nude mice. The reporter gene signals were detected by IVIS imaging system. (A) The tumor growth (upper panels) and metastatic tumors in lung and enterocoelia (lower panels, red arrows) were detected by IVIS system. (B) Quantified data for bioluminescence in metastatic sites for mice of 3 groups. (C) Quantification of the tumor metastasis. (D) Mouse tumor samples were collected for IHC staining for AR, YBX1, and MMP9 (**P < 0.01).
We then analyzed the expression of YBX1-AR-MMP9 signals using IHC staining of the xenograft tissues and found that MMP9 expression is lower in the YBX1 knocked-down tumors (Figure 7D).
Together, results from Figure 7, A–C in vivo mouse model studies are in agreement with the above in vitro cell lines studies and demonstrate that targeting YBX1 could suppress RCC cell invasion via regulation of AR/MMP9 signals.
Discussion
Recent studies have shown that AR plays key roles in RCC progression [8], [9]. However, which upstream signals may regulate AR to impact RCC remained unclear. YBX1 functions as a transcription factor in the nucleus, which binds to the nucleotide sequence 5′-ATTGG-3′ denoted as the Y-box [27]. It is well known that YBX1 expression is closely associated with cell growth, drug resistance and clinical outcome in various cancers [12], [13], [14], [15], [16], [17], [18]. Using the ChIP-on-chip assay, Finkbeiner et al. found that YBX1 binds to the AR promoter in breast cancer cells, suggesting that AR is a direct gene target of YBX1 [28], and Shiota et al. [14] also found that YBX1 binds to the AR promoter in prostate cancer. Although it remains unclear whether YBX1 expression contributes to the RCC progression, here we found abnormal nuclear YBX1 expression in RCC suggesting it might function as an oncogene to influence the RCC progression.
Early studies suggested that DNA damaging agents, oxidative stress, and UV irradiation might influence the nuclear translocation of YBX1 to stimulate expression of genes related to cell viability [29], [30], [31], [32]. Our results revealed C1QBP as another factor that could interact with and influence the nuclear translocation of YBX1. This is consistent with their expression pattern showing the expression of C1QBP is negatively correlated with YBX1 nuclear localization in RCC samples [33]. Importantly, our finding that C1QBP might function through YBX1 to modulate AR expression provides a good model of how C1QBP/YBX1 might influence the RCC cell invasion. Other studies also linked the abnormal expression of C1QBP with the progression of various tumors, including prostate, breast and cervix [21], [22], [23], [24], [25].
Some studies in clinical samples have shown that AR expression in RCC is associated with low-stage, well-differentiated tumors and favorable prognosis [8], [34], [35]. These contrasting results of AR expression in different stages or grades of RCC raised the question whether AR expression is indeed linked to AR-mediated RCC progression, especially because early prostate cancer studies indicated that AR might have various mutations, and therefore AR expression alone might not directly link to prostate cancer stages [36], [37], [38]. Consistent with some studies in vitro, we found AR promoted RCC malignancy [9], [39]. So the expression of AR in clinical RCC samples might not equally reflect the function of AR in RCC patients. Expanding the previous researches in our laboratory, we found that both AR and YBX1, an inducer of AR, contribute to the metastasis of RCC in both in vitro assay as well as in vivo xenograft model.
One potential target by which AR increases the invasion and migration of cancer cells is the MMP family. It is well established that secretion of MMPs that can degrade ECM is a feature of metastatic cancer cells [40]. MMP9 is a well-characterized protease with strong proteolytic activity in the ECM [41]. Consistent with our previous studies, we found that MMP9 was a key molecule that mediated AR signals to enhance the metastatic potential of RCC, even though AR might interact with different co-regulators in different microenvironments and cancers [42], [43], [44].
In summary, the expression of C1QBP was negatively correlated with the YBX1 nuclear expression in RCC tissues. C1QBP low-expression and YBX1 nuclear expression may be used as independent prognostic markers to predict the progression of RCC. Interrupting this newly identified signal via targeting C1QBP, YBX1 or AR may allow us to develop new and better therapies to suppress RCC progression.
Acknowledgments
We thank K. Wolf (Department of Pathology, University of Rochester Medical Center, Rochester, New York) for help in editing the manuscript.
Footnotes
This work was supported by NIH Grants (CA127300 and CA156700), Taiwan Department of Health Clinical Trial and Research Center of Excellence GrantDOH99-TD-B-111-004 (China Medical University, Taichung, Taiwan), China 973 Grant (CB518304), National Natural Science Foundation of China (81202380 and 81402094), Natural Science Foundation of Tianjin (14JCQNJC11600), and China Postdoctoral Science Foundation (2013M541189).
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neo.2016.12.003.
Contributor Information
Yuanjie Niu, Email: niuyuanjie@gmail.com.
Chawnshang Chang, Email: chang@urmc.rochester.edu.
Appendix A. Supplementary data
Supplementary Figures
References
- 1.Vogelzang NJ, Stadler WM. Kidney cancer. Lancet. 1998;352:1691–1696. doi: 10.1016/S0140-6736(98)01041-1. [DOI] [PubMed] [Google Scholar]
- 2.Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34:193–205. doi: 10.1016/j.ctrv.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 4.Atkins MB, Ernstoff MS, Figlin RA, Flaherty KT, George DJ, Kaelin WG, Kwon ED, Libermann TA, Lineham WM, Mcdermott DF. Innovations and challenges in renal cell carcinoma: summary statement from the Second Cambridge Conference. Clin Cancer Res. 2007;13:667s–670s. doi: 10.1158/1078-0432.CCR-06-2231. [DOI] [PubMed] [Google Scholar]
- 5.Longo R, D'Andrea MR, Sarmiento R, Salerno F, Gasparini G. Integrated therapy of kidney cancer. Ann Oncol. 2007;18:vi141–vi148. doi: 10.1093/annonc/mdm244. [DOI] [PubMed] [Google Scholar]
- 6.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 7.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7:245–257. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu G, Liang L, Li L, Dang Q, Song W, Yeh S, He D, Chang C. The expression and evaluation of androgen receptor in human renal cell carcinoma. Urol. 2014;83:510.e19–510.e24. doi: 10.1016/j.urology.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 9.He D, Li L, Zhu G, Liang L, Guan Z, Chang L, Chen Y, Yeh S, Chang C. New Therapy via Targeting Androgen Receptor → HIF-2α → VEGF signals with ASC-J9® to Suppress Renal Cell Carcinoma Progression. Cancer Res. 2014 doi: 10.1158/0008-5472.CAN-13-2681. [pii: canres.2681.2013] [DOI] [PubMed] [Google Scholar]
- 10.Wolffe AP, Tafuri S, Ranjan M, Familari M. The Y-box factors: a family of nucleic acid binding proteins conserved from Escherichia coli to man. New Biol. 1992;4:290–298. [PubMed] [Google Scholar]
- 11.Nashchekin D, Zhao J, Visa N, Daneholt B. A novel Ded1-like RNA helicase interacts with the Y-box protein ctYB-1 in nuclear mRNP particles and in polysomes. J Biol Chem. 2006;281(20):14263–14272. doi: 10.1074/jbc.M600262200. [DOI] [PubMed] [Google Scholar]
- 12.Lasham A, Print CG, Woolley AG, Dunn SE, Braithwaite AW. YB-1: oncoprotein, prognostic marker and therapeutic target? Biochem J. 2013;449:11–23. doi: 10.1042/BJ20121323. [DOI] [PubMed] [Google Scholar]
- 13.Eliseeva IA, Kim ER, Guryanov SG, Ovchinnikov LP, Lyabin DN. Y-box-binding protein 1 (YB-1) and its functions. Biochemistry (Mosc) 2011;76:1402–1433. doi: 10.1134/S0006297911130049. [DOI] [PubMed] [Google Scholar]
- 14.Shiota M, Takeuchi A, Song Y, Yokomizo A, Kashiwagi E, Uchiumi T. Y-box binding protein-1 promotes castration-resistant prostate cancer growth via androgen receptor expression. Endocr Relat Cancer. 2011;18:505–517. doi: 10.1530/ERC-11-0017. [DOI] [PubMed] [Google Scholar]
- 15.Kolk A, Jubitz N, Mengele K, Mantwill K, Bissinger O, Schmitt M, Kremer M, Holm PS. Expression of Y-box-binding protein YB-1 allows stratification into long- and short-term survivors of head and neck cancer patients. Br J Cancer. 2011;105:1864–1873. doi: 10.1038/bjc.2011.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maciejczyk A, Szelachowska J, Ekiert M, Matkowski R, Hałoń A, Lage H, Surowiak P. Elevated nuclear YB1 expression is associated with poor survival of patients with early breast cancer. Anticancer Res. 2012;32:3177–3184. [PubMed] [Google Scholar]
- 17.Song YH, Shiota M, Yokomizo A, Uchiumi T, Kiyoshima K, Kuroiwa K, Oda Y, Naito S. Twist1 and Y-box-binding protein-1 are potential prognostic factors in bladder cancer. Urol Oncol. 2014;32:31.e1-7. doi: 10.1016/j.urolonc.2012.11.003. [pii: S1078-1439(12)00396-1] [DOI] [PubMed] [Google Scholar]
- 18.Fushimi F, Taguchi K, Izumi H, Kohno K, Kuwano M, Ono M, Nakashima Y, Takesue T, Naito S, Oda Y. Peroxiredoxins, thioredoxin, and Y-box-binding protein-1 are involved in the pathogenesis and progression of dialysis-associated renal cell carcinoma. Virchows Arch. 2013;463:553–562. doi: 10.1007/s00428-013-1460-y. [DOI] [PubMed] [Google Scholar]
- 19.Ghebrehiwet B, Peerschke EI. cC1q-R (calreticulin) and gC1q-R/p33: ubiquitously expressed multi-ligand binding cellular proteins involved in inflammation and infection. Mol Immunol. 2004;41:173–183. doi: 10.1016/j.molimm.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Ghiran I, Tyagi SR, Klickstein LB, Nicholson-Weller A. Expression and function of C1q receptors and C1q binding proteins at the cell surface. Immunobiology. 2002;205:407–420. doi: 10.1078/0171-2985-00142. [DOI] [PubMed] [Google Scholar]
- 21.Yu H, Liu Q, Xin T, Xing L, Dong G, Jiang Q, Lv Y, Song X, Teng C, Huang D. Elevated expression of hyaluronic acid binding protein 1 (HABP1)/P32/C1QBP is a novel indicator for lymph node and peritoneal metastasis of epithelial ovarian cancer patients. Tumour Biol. 2013;34:3981–3987. doi: 10.1007/s13277-013-0986-6. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Zhang F, Guo L, Wang Y, Zhang P, Wang R, Zhang N, Chen R. Interactome Analysis Reveals that C1QBP is Associated with Cancer Cell Chemotaxis and Metastasis. Mol Cell Proteomics. 2013;12:3199–3209. doi: 10.1074/mcp.M113.029413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amamoto R, Yagi M, Song Y, Oda Y, Tsuneyoshi M, Naito S, yokomizo A, kuroiwa K, Tokunaga S, Kato S. Mitochondrial p32/C1QBP is highly expressed in prostate cancer and is associated with shorterprostate-specific antigen relapse time after radical prostatectomy. Cancer Sci. 2011;102:639–647. doi: 10.1111/j.1349-7006.2010.01828.x. [DOI] [PubMed] [Google Scholar]
- 24.Dembitzer FR, Kinoshita Y, Burstein D, Phelps RG, Beasley MB, Garcia R, Harpaz N, Jaffer S, Thung SN, Unger PD. gC1qR expression in normal and pathologic human tissues: differential expression in tissues of epithelial and mesenchymal origin. J Histochem Cytochem. 2012;60:467–474. doi: 10.1369/0022155412440882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, Su YJ, Gu PQ, Ji ZY, Wang XG, Gao LJ. The role of the globular heads of C1q receptor (gC1qR) gene in regulating apoptosis of human cervical squamous cell carcinoma. Cell Physiol Biochem. 2012;30:1181–1190. doi: 10.1159/000343308. [DOI] [PubMed] [Google Scholar]
- 26.Stratford AL, Habibi G, Astanehe A, Jiang H, Hu K, Park E, Shadeo A, Buys TP, Lam W, Pugh T. Epidermal growth factor receptor (EGFR) is transcriptionally induced by the Y-box binding protein-1 (YB-1) and can be inhibited with Iressa in basal-like breast cancer, providing a potential target for therapy. Breast Cancer Res. 2007;9:R61. doi: 10.1186/bcr1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuwano M, Uchiumi T, Hayakawa H, Ono M, Wada M, Izumi H, Kohno K. The basic and clinical implications of ABC transporters, Y-box-binding protein-1 (YB-1) and angiogenesis-related factors in human malignancies. Cancer Sci. 2003;94(1):9–14. doi: 10.1111/j.1349-7006.2003.tb01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finkbeiner MR, Astanehe A, To K, Fotovati A, Davies AH, Zhao Y, Jiang H, Stratford AL, Shadeo A, Boccaccio C. Profiling YB-1 target genes uncovers a new mechanism for MET regulation in normal and malignant human mammary cells. Oncogene. 2009;28:1421–1431. doi: 10.1038/onc.2008.485. [DOI] [PubMed] [Google Scholar]
- 29.Stein U, Jürchott K, Walther W, Bergmann S, Schlag PM, Royer HD. Hyperthermia-induced nuclear translocation of transcription factor YB-1 leads to enhanced expression of multidrug resistance-related ABC transporters. J Biol Chem. 2001;276:28562–28569. doi: 10.1074/jbc.M100311200. [DOI] [PubMed] [Google Scholar]
- 30.Das S, Chattopadhyay R, Bhakat KK, Boldogh I, Kohno K, Prasad R, Wilson SH, Hazra TK. Stimulation of NEIL2-mediated oxidized base excision repair via YB-1 interaction during oxidative stress. J Biol Chem. 2007;282:28474–28484. doi: 10.1074/jbc.M704672200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koike K, Uchiumi T, Ohga T, Toh S, Wada M, Kohno K, Kuwano M. Nuclear translocation of the Y-box binding protein by ultraviolet irradiation. FEBS Lett. 1997;417:390–394. doi: 10.1016/s0014-5793(97)01296-9. [DOI] [PubMed] [Google Scholar]
- 32.Fujita T, Ito K, Izumi H, Kimura M, Sano M, Nakagomi H, Maeno K, Hama Y, Shingu K, Tsuchiya S. Increased nuclear localization of transcription factor Y-box binding protein 1 accompanied by up-regulation of P-glycoprotein in breast cancer pretreated with paclitaxel. Clin Cancer Res. 2005;11:8837–8844. doi: 10.1158/1078-0432.CCR-05-0945. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Yue D, Xiao M, Qi C, Chen Y, Sun D, Zhang N, Chen R. C1QBP negatively regulates the activation of oncoprotein YBX1 in the renal cell carcinoma as revealed by interactomics analysis. J Proteome Res. 2015;14(2):804–813. doi: 10.1021/pr500847p. [DOI] [PubMed] [Google Scholar]
- 34.LangRatschek M, Rehak P, Schips L, Zigeuner R. Steroid hormone receptor expression in renal cell carcinoma: an immunohistochemical analysis of 182 tumors. J Urol. 2004;171(2 Pt 1):611–614. doi: 10.1097/01.ju.0000108040.14303.c2. [ner C] [DOI] [PubMed] [Google Scholar]
- 35.Zhao H, Leppert JT, Peehl DM. A Protective Role for Androgen Receptor in Clear Cell Renal Cell Carcinoma Based on Mining TCGA Data. PLoS One. 2016;11(1):e0146505. doi: 10.1371/journal.pone.0146505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newmark JR, Hardy DO, Tonb DC, Carter BS, Epstein JI, Isaacs WB, Brown TR, Barrack ER. Androgen receptor gene mutations in human prostate cancer. Proc Natl Acad Sci U S A. 1992;89:6319–6323. doi: 10.1073/pnas.89.14.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Culig Z, Hobisch A, Cronauer MV, Cato AC, Hittmair A, Radmayr C, Eberle J, Bartsch G, Klocker H. Mutant androgen receptor detected in an advanced-stage prostatic carcinoma is activated by adrenal androgens and progesterone. Mol Endocrinol. 1993;7:1541–1550. doi: 10.1210/mend.7.12.8145761. [DOI] [PubMed] [Google Scholar]
- 38.Montgomery JS, Price DK, Figg WD. The androgen receptor gene and its influence on the development and progression of prostate cancer. J Pathol. 2001;195:138–146. doi: 10.1002/1096-9896(200109)195:2<138::AID-PATH961>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 39.Ha YS, Lee GT, Modi P, Kwon YS, Ahn H, Kim WJ, Kim IY. Increased Expression of Androgen Receptor mRNA in Human Renal Cell Carcinoma Cells is Associated with Poor Prognosis in Patients with Localized Renal Cell Carcinoma. J Urol. 2015;194(5):1441–1448. doi: 10.1016/j.juro.2015.03.078. [DOI] [PubMed] [Google Scholar]
- 40.Lee SJ, Kim WJ, Moon SK. Role of the p38 MAPK signaling pathway in mediating interleukin-28A-induced migration of UMUC-3 cells. Int J Mol Med. 2012;30(4):945–952. doi: 10.3892/ijmm.2012.1064. [DOI] [PubMed] [Google Scholar]
- 41.Rahman M, Miyamoto H, Chang C. Androgen receptor coregulators in prostate cancer: mechanisms and clinical implications. Clin Cancer Res. 2004;10(7):2208–2219. doi: 10.1158/1078-0432.ccr-0746-3. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Lee SO, Xia S, Jiang Q, Luo J, Li L, Yeh S, Chang C. Endothelial cells enhance prostate cancer metastasis via IL-6-- > androgen receptor-- > TGF-beta-- > MMP-9 signals. Mol Cancer Ther. 2013;12(6):1026–1037. doi: 10.1158/1535-7163.MCT-12-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Sun Y, Rao Q, Xu H, Li L, Chang C. Androgen receptor (AR) suppresses miRNA-145 to promote renal cell carcinoma (RCC) progression independent of VHL status. Oncotarget. 2015;6(31):31203–31215. doi: 10.18632/oncotarget.4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu S, Li L, Yeh S, Cui Y, Li X, Chang HC, Jin J, Chang C. Infiltrating T cells promote prostate cancer metastasis via modulation of FGF11 → miRNA-541 → androgen receptor (AR) → MMP9 signaling. Mol Oncol. 2015;9(1):44–57. doi: 10.1016/j.molonc.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures