Abstract
Temporal and spatial regulation of the actin cytoskeleton is vital for cell migration. Here, we show that an epithelial cell actin-binding protein, villin, plays a crucial role in this process. Overexpression of villin in doxycyline-regulated HeLa cells enhanced cell migration. Villin-induced cell migration was modestly augmented by growth factors. In contrast, tyrosine phosphorylation of villin and villin-induced cell migration was significantly inhibited by the src kinase inhibitor 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) as well as by overexpression of a dominant negative mutant of c-src. These data suggest that phosphorylation of villin by c-src is involved in the actin cytoskeleton remodeling necessary for cell migration. We have previously shown that villin is tyrosine phosphorylated at four major sites. To further investigate the role of tyrosine phosphorylated villin in cell migration, we used phosphorylation site mutants (tyrosine to phenylalanine or tyrosine to glutamic acid) in HeLa cells. We determined that tyrosine phosphorylation at residues 60, 81, and 256 of human villin played an essential role in cell migration as well as in the reorganization of the actin cytoskeleton. Collectively, these studies define how biophysical events such as cell migration are actuated by biochemical signaling pathways involving tyrosine phosphorylation of actin binding proteins, in this case villin.
INTRODUCTION
Villin, an epithelial cell-specific protein belongs to a family of actin-binding proteins that contain segments that display internal homology with each other (Arpin et al., 1988). The amino terminal core of villin retains the actin-capping and -severing functions of villin, whereas the carboxyl terminal headpiece enables villin to cross-link actin filaments. The actin-modifying properties of villin are regulated in vitro by calcium (Northrop et al., 1986), phosphoinositides (Janmey and Matsudaira, 1988; Kumar et al., 2004) and tyrosine phosphorylation (Zhai et al., 2001; Kumar and Khurana, 2004). It has been assumed for several years that villin's actin-bundling and not actin-severing functions are important because nonphysiologically high Ca2+ concentrations (200 μM) are required to activate villin's actin-severing activity. However, studies done with the villin knockout mice suggest that in the absence of villin, the actin-bundling properties associated with villin can be substituted by other proteins in the microvilli (Pinson et al., 1998); on the other hand, the actin-severing activity of the microvilli is lost (Ferrary et al., 1999). In recent years, we have demonstrated that villin's actin-modifying functions can be regulated in vitro by tyrosine phosphorylation and phosphatidylinositol bisphosphate (PIP2), suggesting that villin has the potential to function as a link between receptor activation and actin cytoskeleton reorganization even in the absence of high calcium (Arora and McCulloch, 1996; Zhai et al., 2001; Kumar and Khurana, 2004; Kumar et al., 2004). In addition, we have recently demonstrated that the autoinhibited conformation of villin can be released by tyrosine phosphorylation of villin (as opposed to high Ca2+), allowing it to sever actin at physiological Ca2+ concentrations (Kumar and Khurana, 2004). These results suggest that tyrosine phosphorylation rather than high calcium may be the mechanism by which villin severs actin in vivo. From our in vitro studies, we developed the hypothesis that in vivo villin may be a key factor integrating information from external stimuli that could affect the plasticity of the actin cytoskeleton from a more rigid to a dynamic network and that these spatial and temporal changes in the physical properties of the cytoskeleton could enhance cell motility (Panebra et al., 2001; Zhai et al., 2001; Kumar and Khurana, 2004; Kumar et al., 2004). Furthermore, that villin's ability to cycle between active (phosphorylated) and inactive (nonphosphorylated) forms may be a critical feature of its mechanism of action in cell migration.
The current study was designed to determine specifically the role of villin overexpression on cell morphology and cell migration. Overexpression of other proteins of the villin superfamily, including gelsolin and CapG have been demonstrated to increase the motile phenotypes of cells (Hartwig et al., 1989; Sun et al., 1995; Aizawa et al., 1996; Chen et al., 1996; Furnish et al., 2001). Conversely, the gelsolin-null mice and other mutant cell lines lacking these proteins have been shown to exhibit decreased rates of cell motility (Witke et al., 1995; Lu et al., 1997; Chellaiah et al., 2000). To confirm our hypothesis that villin and its ligand-binding activities described by in vitro experiments are mechanistically important to villin's relationship to cell motility, we used the villin-null HeLa cells to overexpress villin by using a tetracycline-regulated system. We demonstrate that villin participates in actin reorganization and cell migration. Furthermore, we determined that tyrosine phosphorylation of villin is essential to its role in cell migration. We have previously identified an area in the amino terminus of villin as the site of phosphorylation. Four major sites of phosphorylation were identified in this region by direct mutation of candidate tyrosines (Y) to phenylalanine (F), namely, Y-46, -60, -81, and -256 (Zhai et al., 2002). Mutation of any of these phosphorylation sites inhibits the ability of villin protein to polymerize actin filaments in vitro (Zhai et al., 2002). Based on our previous studies, phosphorylated villin could regulate cell motility by modifying actin assembly by distinct mechanisms, including lowering the binding affinity for F-actin, inhibiting polymerization of existing actin nuclei, and/or cutting preexisting filaments to generate new actin nuclei (Zhai et al., 2001, 2002). In this study, we investigated the role of each tyrosine phosphorylation site of villin in actin reorganization and cell migration by establishing HeLa cell lines stably expressing wild-type or each of the phosphorylation site mutants (tyrosine to phenylalanine) of villin. The ability of each mutant of villin (tyrosine to glutamic acid) to rescue actin organization and cell migration was analyzed to confirm the essential phosphorylation sites responsible for each of these biological processes. We demonstrate that villin participates in actin reorganization and cell migration by using more than one phosphorylation site. We propose that villin tyrosine phosphorylation is coordinated with actin remodeling, where it may serve to integrate and transduce signals involved in cell migration. Because there is significant homology between villin and other actin-capping and -severing proteins of its family that are also tyrosine phosphorylated (De Corte et al., 1997) and are known regulators of cell motility (Cunningham et al., 1991; Sun et al., 1995; Arora and McCulloch, 1996), the results of our study provide a structural basis for the mechanism of cell motility and help to understand the role of phosphorylation in this function.
MATERIALS AND METHODS
Materials
HeLa Tet-Off cells stably expressing the tTA tetracycline-controlled transactivator protein, G418, hygromycine, doxycycline, the eukaryotic expression vector pTRE-6 × HN, and the selection vector pTK-Hyg were purchased from BD Biosciences Clontech (Palo Alto, CA). Lipofectine was purchased from Invitrogen (Carlsbad, CA); monoclonal antibodies to phosphotyrosine (clone PY-20) were from MP Biomedicals (Irvine, CA); monoclonal antibodies to villin were from BD Transduction Laboratories (Lexington, KY); hepatocyte growth factor (HGF), epidermal growth factor (EGF), 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2), and 4-amino-7-phenyl-pyrazolo[3,4-d]pyrimidine (PP3) were purchased from Calbiochem (La Jolla, CA); Alexa Fluor 568-Phalloidin, and Alexa Fluor 488-conjugated DNase I were acquired from Molecular Probes (Eugene, OR); fluorescein isothiocyanate (FITC)-conjugated affinity-purified donkey anti-mouse IgG was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA); and QuikChange site-directed mutagenesis kit was purchased from Strategene San Diego, CA). c-Src cDNA (dominant negative); (K296R/Y528F mutation), and c-src monoclonal antibodies were purchased from Upstate Biotechnology (Lake Placid, NY). Cell culture reagents were purchased from Invitrogen. All other chemicals were from Sigma-Aldrich (St. Louis, MO) or Invitrogen.
Methods
Villin cDNA Construct. Human villin cDNA containing the entire coding sequence cloned in the prokaryotic expression vector pGEX2T (Panebra et al., 2001) was amplified by polymerase chain reaction (PCR) by using the primers GAL1 (5′GTTCCGCGGTCGACCATGACC3′) and GAL2 (5′TCAATTGGTCTAGAACTAATA3′). These primers contain sequences for the restriction enzymes SalI and XbaI, respectively. The SalI and XbaI digested amplicon was ligated into the plasmid pTRE-6 × HN that was digested with the same restriction enzymes. The ligation reaction was used to transform XL1-Blue competent cells and plasmids were isolated from several colonies. The plasmids were checked by sequential double digestion with SalI and XbaI, respectively. Plasmids that excised the insert were used for sequencing.
Phosphorylation Site Mutants of Villin. Human villin cloned in the eukaryotic expression vector pTRE-6 × HN was used as a template to mutate the known tyrosine phosphorylation sites in human villin. The identified phosphorylatable tyrosine (Y) residues in full-length human villin were changed to phenylalanine (F) or glutamic acid (E) by designing complementary primers. Tyrosine's at positions 46, 60, 81, and 256 were replaced with phenylalanine or glutamic acid by using the QuikChange site-directed mutagenesis kit to make single-base changes from TAT and TAC to TTT and TTC, or GAA and GAG, respectively. The mutation primers were as follows: Y46F, 5′-GATGGTGACTGCTTCATCATCCTGGC-3′ (forward) and 5′-GCCAGGATGATGA-AGCAGTCACCATC-3′ (reverse); Y60F, 5′-AGCAGCCTGTCCTTTGACATCCACT-AC-3′ (forward) and 5′-GTAGTGGATGTCAAAGGACAGGCTGCT-3′ (reverse); Y81F, 5′-GCAGCTGCCATCTTCACCACACAGATG-3′ 4 (forward) and 5′-CATCT-GTGTGGTGAAGATGGCAGCTGC-3′ (reverse); Y256F, 5′-TGCACTCAAACTGTT-CCATGTGTCTGAC-3′ (forward) and 5′-GTCAGACACATGGAACAGTTTGAGTG-CA-3′ (reverse). Y46E, 5′-TCGATGGTGACTGCGAAATCATCCTGGCTATC-3′ (forward) and 5′-GATAGCCAGGATGATTTCGCAGTCACCATCGA (reverse); Y60E, 5′-AGCAGCCTGTCCGAAGACATCCACTAC-3′ (forward) and 5′-GTAGT-GGATGTCTTCGGACAGGCTG CT-3′ (reverse); Y81E, 5′-GCAGCTGCCATCGAA-ACCACACAGATG-3′ (forward) and 5′-CATCTGTGTGGTTTCGATGGCAGCTGC-3′ (reverse); and Y256E, 5′-TGCACTCAAACTGGAACATGTGTCTGAC-3′ (forward) and 5′-GTCAGACACATGTTCCAGTTTGAGTGCA-3′ (reverse). The introduction of the desired codon was confirmed by sequencing.
Preparation of Recombinant Adenovirus Expressing Dominant Negative c-src Kinase. Replication-deficient recombinant type 5 adenovirus expressing dominant negative c-src was prepared using an adenoviral preparation kit developed by the University of Iowa (Anderson et al., 2000). Briefly, plasmid cDNA (containing dominant negative c-src) was digested with HindIII and BamHI and ligated into the pShuttle vector pacAd5 CMV K-N pA. Recombinant homogenous virus was generated in human embryonic kidney (HEK) 293 cells by homologous recombination. Nucleotide sequencing was used to confirm the construct. Adenoviral titers were determined by measuring their cytopathic effects in HEK 293 cells. HeLa cells were mock infected (with Ad-EGFP) or infected with the dominant negative c-src kinase at a multiplicity of infection of 100 by using standard protocol (White et al., 1984). Cell lysates were analyzed by Western blotting for c-src kinase expression.
Transfection of Tet-Off HeLa Cells with Full-Length and Mutant Villin cDNA. HeLa Tet-Off cells described by Gossen and Bujard (1992) and stably transfected with the tTA tetracycline-controlled transactivator were purchased from BD Biosciences Clontech. The pTRE-6 × HN full-length and mutant villin constructs were cotransfected with a selection plasmid carrying the hygromycine resistance gene by using Lipofectine. Transfected cells were selected by growing in media containing hygromycine (400 μg/ml) and G418 (100 μg/ml). Several clones were obtained, all expressing villin in a doxycycline-dependent manner. Clones expressing comparable amounts of wild-type and mutant villin proteins were selected for further studies. For all the experiments cells transfected with wild-type or mutant-villin proteins were grown in the absence or presence of doxycycline (1.0 μg/ml).
Cell Motility Assay. To measure cell motility, HeLa cells transfected with villin were seeded in six-well plates and cultured in the absence or presence of doxycycline. Cell migration was measured as described previously (Waters and Savla, 1999). Briefly, confluent monolayers were scraped with a plastic pipette tip across the diameter of the well to produce wounds of ∼800 μm in width. Cells were rinsed to remove cellular debris, and images were obtained at the initial time of wounding and at various times up to 12 h postwounding. Images of the wounds were collected with a Nikon TE300 inverted microscope equipped with a CoolSnap FX charge-coupled device camera (Roper Scientific, Trenton, NJ), an Optiscan ES102 motorized stage system (Prior Scientific, Rockland, MA), and a Pentium III computer with MetaMorph image analysis software (Universal Imaging, Dowingtown, PA). Images were collected by programming the X, Y, and Z coordinates of each wound location, allowing the stage to return to the precise location of the original wound. Data are expressed as a percentage of the original wound width to normalize variability in wounding from well to well, although similar size initial wounds were observed from experiment to experiment. Wound width measurements were averaged from two regions of the same well and the mean treated as a single data point. Comparisons between mean values were made using one-way repeated-measures analysis of variance and Turkey's modified t test (Bonferroni criteria) with p < 0.05 considered significant.
Phospho-Villin Antibodies. Recombinant villin that was tyrosine phosphorylated (VILT/WT) or not (VIL/WT) was prepared as described previously (Panebra et al., 2001). A phospho-villin antibody was raised using a commercial facility (BioSource International, Camarillo, CA). One of the antibodies, clone #VP-70782 was affinity purified and its specificity for the phospho-epitope was examined using Western analysis. Because the villin monoclonal antibody (mAb) cannot be used to immunoprecipitate villin, the phosphovillin antibody was used to immunoprecipitate villin from HeLa cells transfected with wild-type villin protein. Additional immunoprecipitation studies were done with a commercially available phospho-tyrosine antibody (clone PY-20).
Immunofluorescence Microscopy. HeLa Tet-Off cells expressing wild-type or mutant-villin proteins were cultured on coverslips and fixed in 3.7% formaldehyde in phosphate-buffered saline (PBS) for 15 min. The slides were washed twice with phosphate-buffered saline and permeabilized by incubation in PBS containing 0.2% Triton X-100 and 0.5% normal serum for 5 min at 4°C. The fixed and permeabilized cells were washed three times with PBS (10 min each) and blocked with 0.1% bovine serum albumin in 20 mM Tris, pH 7.2, 150 mM NaCl, and 0.1% Tween 20 for 30 min. Cells were incubated with villin mAb (1:100), and Alexa Fluor 568 was included to record distribution of F-actin. The cells were then incubated with a secondary antibody, FITC-conjugated affinity purified donkey anti-mouse IgG. The fluorescence was examined by confocal laser scanning microscopy (LSM 5 PASCAL; Carl Zeiss, Thornwood, NY).
F-Actin Content Measurements in HeLa Cells Expressing Wild-Type or Mutant Villin Proteins. The F-actin content of cells was determined as described by Cunningham (1995). Briefly, cells were fixed with 3.7% paraformaldehyde, permeabilized with 0.5% Triton X-100, and stained with 250 nM Alexa-Phalloidin at 37°C for 30 min. Ethidium bromide-binding DNA was used at a final concentration of 10 μM to label the DNA at room temperature for 5 min. The cells were washed with PBS, scraped in 500 μl of methanol, and the fluorescence was recorded. The Alexa-Phalloidin was quantitated at an excitation wavelength of 578 nm and emission of 600 nm, whereas the DNA was measured at excitation of 525 nm and emission of 605 nm. The relative F-actin content was quantified as the ratio of Alexa-Phalloidin fluorescence intensity to eithidium bromide fluorescence intensity.
Cell Proliferation Measurements. Cell proliferation was measured by bromodeoxyuridine (BrdU) labeling by quantitating BrdU incorporation into newly synthesized DNA of replicating cells by using the BrdU in situ detection kit according to the instructions of the manufacturer (BD Biosciences Pharmigen, San Diego, CA). Wounded monolayers were incubated with BrdU (10 mM) for 1 h, 10 h postwounding. The cells were fixed, washed, permeabilized, and then incubated with 0.3% H2O2 to block endogenous peroxidase. Immunodetection of incorporated BrdU label was done using a biotinylated anti-BrdU antibody that specifically recognizes 5-bromo-2′-deoxyuridine showing no cross-reactivity with thymidine or uridine. BrdU incorporation was detected by using the chromagen diaminobenzidine. Slides were counterstained with hematoxylin.
RESULTS
Cells Expressing Villin Migrate Faster than Villin-Null Cells
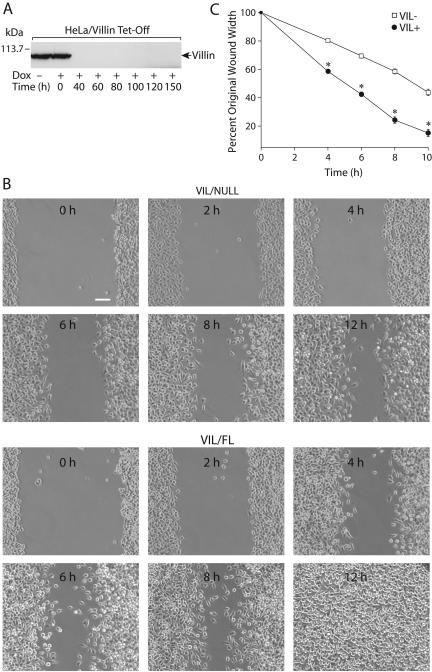
HeLa Tet-Off cells were cotransfected with full-length human villin cloned in pTRE-6 × HN and pTK-Hyg and subjected to selection with hygromycine B. Several stable transfectant lines were expanded and screened for efficient gene induction by removal of doxycycline. A representative transfectant of the villin gene was compared with parental cells for the gene expression in the absence or presence of doxycycline. Western blot analysis showed that the HeLa Tet-Off cells transfected with human villin cDNA can be induced to express significant amounts of full-length villin protein when cultured without doxycycline, whereas cells cultured in the presence of doxycycline (1.0 μg/ml) do not express villin (VIL/NULL) (Figure 1A). Untransfected HeLa cells as well as HeLa cells transfected with vector alone do not express villin (our unpublished data).
Figure 1.
HeLa Tet-Off cells expressing wild-type villin display significantly enhanced migration. (A) HeLa Tet-Off cells were stably transfected with full-length human villin cDNA. Cells cultured in the absence of doxycycline express villin (VIL/FL), whereas cells cultured in the presence of doxycycline (1.0 μg/ml) do not express villin (VIL/NULL). Expression of villin protein was followed 0–150 h after the addition of doxycycline. Western analysis was done using monoclonal villin antibodies. Data are representative of three experiments with similar results. (B) Equal numbers of wild-type (VIL/FL) and villin null (VIL/NULL) cells were cultured to confluence. Cells were denuded with a pipette tip and migration of the remaining cells into the wound is shown 0–12 h after the wound was introduced under low-serum condition (1.0% fetal bovine serum). Wound repair was measured as a percentage of initial wound area. Unlike villin-null cells, cells expressing villin seal the wound completely 12 h postwounding. Bar, 40 μm. (C) HeLa cells expressing villin migrate faster than villin-null cells at all time points. Migration distance was determined by taking two independent measurements from each well in a total of 24 wells. The error bars are the measured SEM and the asterisk denotes statistically significant values (p < 0.05, n = 24).
A wound-healing assay was used to determine the kinetics of migration of HeLa Tet-Off cells overexpressing villin both during basal conditions as well as chemotactic migration. Cell migration was recorded between time 0 and 24 h postwounding for cells transfected with wild-type villin cultured in the presence or absence of doxycycline under low serum (1.0% fetal bovine serum) conditions. Cell motility was judged from the reduction of the wound width 0–24 h after the wound was made. Migration of cells expressing villin (VIL/FL) was complete at 12 h postwounding (Figure 1B). In contrast a significant delay in the ability of the VIL/NULL cells (villin-transfected cells cultured in the presence of doxycycline) to migrate into the empty wound space was observed. Assessment of cell migration revealed that wild-type villin-expressing cells exhibited a dramatic increase in cell migration compared with villin-null cells (at 12 h, 4-fold, n = 24, p < 0.05; Figure 1B). Significant changes in the migratory capacity of villin-expressing cells also were observed at earlier time points (Figure 1C). Based on these data, all subsequent migration studies were done between 0 and 10 h postwounding. Untransfected HeLa cells as well as HeLa cells transfected with vector alone migrate at the same rate in the absence or presence of doxycycline (our unpublished data). Cell migration was studied at 10 h postwounding and in the presence of 1.0% serum, conditions that do not favor cell proliferation. Nevertheless, DNA synthesis with BrdU immunocytochemistry was measured and showed no significant difference in VIL/NULL and VIL/FL cells (Supplementary Figure S1). These studies demonstrate that overexpression of human villin increases cell migration.
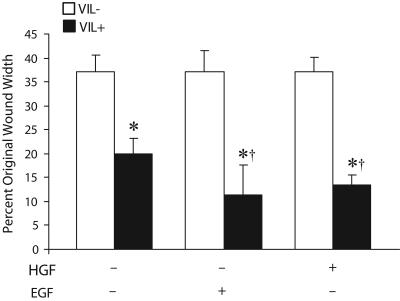
HGF is a multifunctional cytokine that can act as a motility-inducing factor for epithelial cells through the c-met tyrosine kinase (Sonnenberg et al., 1993). Similarly, EGF has been reported to enhance epithelial cell migration by mechanisms varied from HGF (Singh et al., 2004) as well as comparable with HGF (Muller et al., 2002). We measured the effects of both HGF and EGF on villin-induced cell migration. VIL/NULL and VIL/FL cells were cultured in low-serum (1.0%) medium for 24 h before wounding. Cells were treated with HGF (10 ng/ml) or EGF (10 nM) for 30 min and then wounded. The HGF and EGF treated cells were washed, and the media were replaced with DMEM containing 1.0% serum. As shown in Figure 2, at 6 h postwounding in the absence of any treatment VIL/FL migrated faster than VIL/NULL cells (n = 24, p < 0.05); treatment of these cells with HGF modestly enhanced the villin-induced cell migration (n = 24, p < 0.05). This is comparable with previous reports demonstrating a small increase in response to HGF (Athman et al., 2003). Similarly treatment of VIL/FL cells with EGF enhanced villin-induced cell migration by ∼9% (n = 24, p < 0.01). Together, these data show that expression of villin is sufficient to enhance cell migration and that HGF and EGF can modestly augment the villin-induced increase in cell migration. Furthermore, it suggests that overexpression of villin may have a direct effect on the signal transduction pathways that modify the actin cytoskeleton and enhance cell migration.
Figure 2.
Villin-induced cell migration is enhanced by HGF and EGF. VIL/FL cells were treated without or with HGF (10 ng/ml) or EGF (10 nM) for 30 min and cell migration followed over a period of 6 h. Wound repair is expressed as a percentage of the initial wound area after 6 h. VIL (-) refers to each clone cultured in the presence of doxycycline, whereas VIL (+) refers to the same clone cultured in the absence of doxycycline. The error bars are the measured SEM, and the asterisk (*) and cross (†) denote statistically significant values [p < 0.05, n = 24, compared with VIL (-) cells] and (p < 0.05, n = 24, compared with untreated cells) respectively.
Tyrosine Phosphorylation of Villin Is Essential for Villin-induced Cell Migration
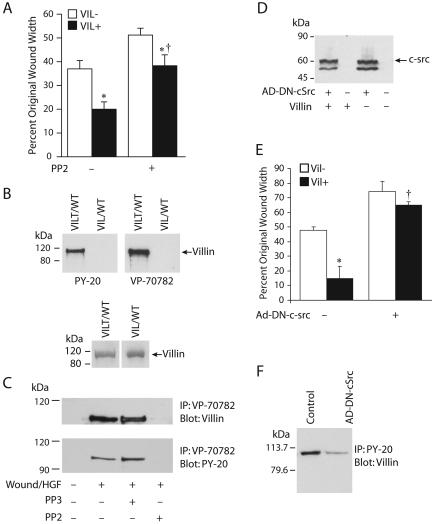
Previous studies from our laboratory have demonstrated that villin is a substrate in vitro for the src kinase(s) (Panebra et al., 2001; Zhai et al., 2002). In addition, our studies have suggested that villin phosphorylation and the resulting changes in the actin rearrangements might serve to promote the migratory phenotype of epithelial cells (Panebra et al., 2001; Zhai et al., 2002). To confirm this, we examined the migratory capacity of cells stably expressing wild-type villin in the absence or presence of the src kinase inhibitor PP2 and its inactive analog PP3. In agreement with our previous results, pretreatment of VIL/FL with the src kinase inhibitor PP2 inhibits the villin-induced cell migration (Figure 3A). Whereas PP2 inhibits both basal as well as EGF-induced cell migration, the effects of PP2 on villin-induced cell migration is significantly higher (11% in VIL/NULL cells vs. 20% in VIL/FL cells, n = 24, p < 0.05) (Figure 3A). Cells treated with the inactive analog PP3 behaved like untreated VIL/FL (our unpublished data). A phospho-villin antibody (VP-70782) that specifically recognizes recombinant tyrosine phosphorylated villin (VILT/WT) and does not cross-react with recombinant nonphosphorylated villin protein (VIL/WT) (Figure 3B, top) was used to immunoprecipitate tyrosine phosphorylated villin from HeLa cells. A commercially available anti-phosphotyrosine antibody (PY-20) confirms the specificity of the phospho-villin antibody (Figure 3B, top). The bottom panel in Figure 3B is an SDS-PAGE of VIL/WT and VILT/WT stained with GelCode Blue, showing the expression of the two recombinant proteins VILT/WT and VIL/WT. Consistent with our previous studies (Panebra et al., 2001; Zhai et al., 2002), tyrosine phosphorylation of villin (in response to wounding or treatment with HGF/EGF) is inhibited by PP2 but not PP3 (Figure 3C). By using this approach, no basal tyrosine phosphorylation of villin in the absence of wounding or EGF/HGF treatment could be detected in these cells with either phospho-villin antibody (VP-70782) or phospho-tyrosine antibody (PY-20). Either there is no basal phosphorylation of villin in the absence of wounding or growth factor treatment, or there is some basal tyrosine phosphorylated villin but the levels are too low to be detected by Western analysis. These data suggest that villin may be phosphorylated in vivo by src kinase(s) and that villin-induced motility may be mediated by activation of src kinase(s) and tyrosine phosphorylation of villin. To further confirm these observations, we used a dominant negative construct of c-src (Ad-DN-c-src). HeLa cells expressing full-length villin cultured in the absence or presence of doxycycline were infected with or without Ad-DN-c-src (Figure 3D), and we measured the effect of expression of dominant negative c-src on villin-induced cell motility. A lower band around 57 kDa was seen in these Western blots and was determined to be a degradation fragment of c-src (Figure 3D). As shown in Figure 3E, VIL/NULL and VIL/FL cells infected with Ad-DN-c-src migrate at very similar rates. Thus, expression of Ad-DN-c-src antagonized the capacity of villin to enhance cell motility, indicating that phosphorylation of villin by c-src is required for the villin-induced increase of cell migration. Infection with an unrelated vector (Ad-EGFP) had no effect on cell migration (Supplementary Figure S2). Overexpression of Ad-DN-c-src also inhibited the tyrosine phosphorylation levels of villin in VIL/FL cells (Figure 3F). Ad-DN-c-src did not completely inhibit the tyrosine phosphorylation of villin unlike PP2. One possibility is that PP2 inhibits tyrosine phosphorylation of villin completely because PP2 can inhibit not only c-src but also other members of the Src family, including c-yes and c-fyn, which also may be involved in the phosphorylation of villin. Nonetheless, the data suggest that tyrosine phosphorylation of villin by c-src plays a major role in villin-induced cell migration.
Figure 3.
Villin-induced cell migration is regulated by c-src kinase. (A) The src kinase inhibitor PP2 inhibits villin-induced cell migration. VIL/FL cells were treated without or with the src-kinase–specific inhibitor PP2 (10 nM), and cell migration was followed over a period of 6 h. Wound repair is expressed as a percentage of the initial wound area after 6 h. VIL (-) refers to each clone cultured in the presence of doxycycline, whereas VIL (+) refers to the same clone cultured in the absence of doxycycline. The error bars are the measured SEM, and the asterisk (*) and cross (†) denotes statistically significant values compared with VIL (-) cells and untreated cells (p < 0.05, n = 24) respectively. (B) Characterization of phospho-villin antibody. Recombinant phosphorylated (VILT/WT) and nonphosphorylated (VIL/WT) villin proteins were used to determine the specificity of the phospho-villin antibody. The top panels show that tyrosine phosphorylated villin is immunodetected by the phospho-villin antibody VP-70782 (at 1:3000 dilution) as well as a commercially available anti-phosphotyrosine antibody, PY-20 (at 1:1000 dilution). The bottom panel is a control gel showing phosphorylated (VILT/WT) and nonphosphorylated (VIL/WT) villin proteins separated by SDS-PAGE and stained with GelCode Blue. Data are representative of three experiments with similar results. (C) HeLa cells cultured in the presence or absence of doxycycline were wounded (20 wounds/dish) and allowed to migrate in the presence of HGF (10 ng/ml) with or without the src kinase inhibitor PP2 (10 nM) or its inactive analog PP3 (10 nM). Tyrosine phosphorylated villin was immunoprecipitated using the phospho-villin antibody (VP-70782; 1 μg), and the Western blot was probed with villin mAb or phosphotyrosine antibody (PY-20). Data are representative of three experiments with similar results. (D) Expression of dominant negative c-src in VIL/NULL and VIL/FL cells. This is a Western blot with c-src monoclonal antibodies. (E) Dominant negative c-src inhibits villin-induced cell migration. VIL/FL and VIL/NULL cells were infected with recombinant adenovirus [Ad-DN-c-src (dominant negative c-src)] for 4 h at a multiplicity of infection of 100. Virus-containing media were then removed and cells were cultured for an additional 18 h to allow for expression of transgenic proteins. This was followed by measurement of cell migration. The error bars are the measured SEM and the asterisk (*) and cross (†) denote statistically significant values [p < 0.05, n = 24, compared with VIL (-) cells and (p < 0.05, n = 24, compared with untreated cells], respectively. (F) Dominant negative c-src inhibits the tyrosine phosphorylation of villin. Tyrosine phosphorylated villin was immunoprecipitated from VIL/FL cells infected with or without Ad-DN-c-src by using anti-phosphotyrosine antibody (clone PY-20), and Western analysis was done using a villin mAb. This is a representative of three experiments with similar results.
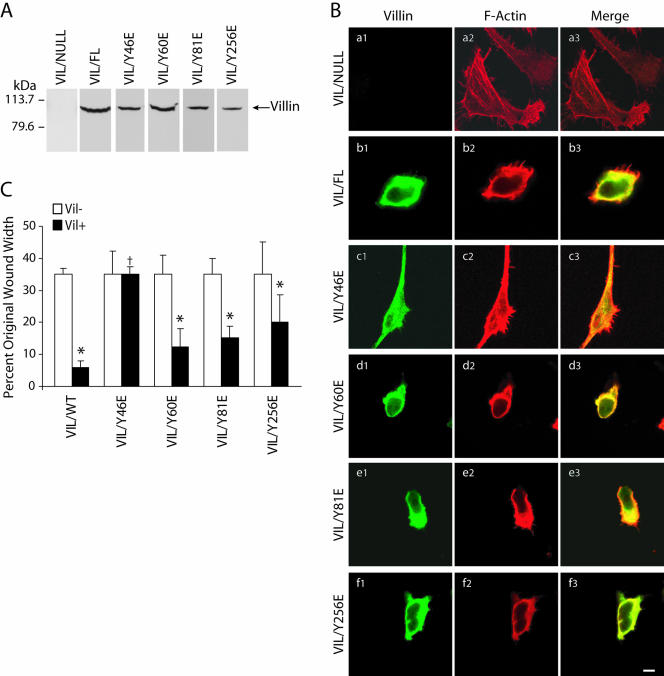
Tyrosine Phosphorylation at Y-60, Y-81, and Y-256 Is Essential for Villin-induced Cell Migration
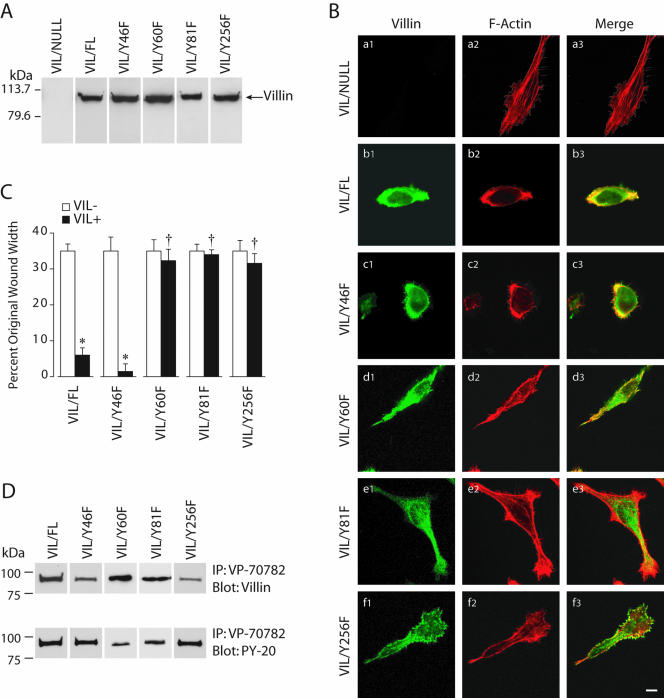
Previous studies from our laboratory have demonstrated that c-src phosphorylates human villin on tyrosine 46, 60, 81, and 256 (Zhai et al., 2002). To determine the role of tyrosine phosphorylated villin in cell migration, we examined the migratory capacity of cells stably expressing either wild-type or phosphorylation site mutants of villin, namely, VIL/Y46F, VIL/Y60F, VIL/Y81, and VIL/Y256F. Multiple clones expressing the villin proteins were selected by immunoblotting by using villin antibodies. Clones expressing similar amounts of villin proteins were selected for the migration studies (Figure 4A). We assessed the effect of overexpression of wild-type and mutant villin proteins in HeLa Tet-Off cells on the organization of the actin cytoskeleton. HeLa Tet-Off cells expressing wild-type and mutant-villin proteins were stained with Alexa-Phalloidin 568 (red) to examine the F-actin distribution and the localization of villin was examined using a villin mAb (green) (Figure 4B and Supplementary Figure S3). The overall morphology of HeLa cells reconstituted with villin or with empty vector seems strikingly different. Phalloidin staining showed that actin stress fibers were lost in villin-expressing HeLa cells (Figure 4B, a and b). The transfection of HeLa cells with wild-type villin results in cells that are less flat and develop membrane ruffles concurrent with the reorganization of F-actin (Figure 4B, b1–b3). Coincident with a virtual lack of stress fibers in these cells is the appearance of F-actin–rich membrane ruffles on the dorsal surface and filopodia-like structures, including microspikes on the ventral surface. All of these structures contained villin (our unpublished data). This is consistent with previous published reports (Friederich et al., 1989; Franck et al., 1990). The villin and F-actin in these cells localized at the cell perimeter where both proteins colocalized to peripheral ruffles, filopodia, and microspikes (Figure 4B, b1–b3). These results indicate that villin can direct the localization of F-actin to the cell cortex. The distribution of the villin mutant Y46F was similar to wild-type in that >90% of the villin protein as well as the F-actin colocalized to the cell margin (Figure 4B, c1–c3). The morphology of these cells was similar to VIL/FL cells with a disassembly of stress fibers and colocalization of the villin protein with F-actin in membrane ruffles, filopodia, and microspikes. In contrast, expression of villin phosphorylation mutants Y60F, Y81F, or Y256F resulted in redistribution of F-actin, suggesting that colocalization of villin and F-actin is dependent on tyrosine phosphorylation of villin at these sites (Figure 4B, d–f). In all these mutant cell lines, the villin mutant proteins were distributed diffusely in the cytoplasm as well as at the cell periphery. In addition, the distribution of F-actin was altered and was associated not only with the cell surface structures but also intracellularly, including what look like dorsal arcs (Figure 4B, e2 and f2). There was limited colocalization of villin and F-actin at the cell margins in these cells, and the majority of the villin and F-actin remained segregated between the cell margins and the intracellular compartment. These data suggest that phosphorylatable tyrosine residues at Y-60, -81, and -256 are required for both the colocalization of villin with F-actin as well as for the rearrangement of the actin cytoskeleton in the cell. Collectively, these results suggest that expression of villin is involved in reorganization of the actin cytoskeleton and further that villin-induced regulation of cell motility may be dependent on this redistribution of the actin cytoskeleton. There was no change in the total F-actin content in wild-type or mutant-villin cell lines (Supplementary Figure S3).
Figure 4.
Tyrosine phosphorylation of villin is required for villin-induced increase in cell migration. (A) HeLa cells were stably transfected with wild-type (VIL/FL) and phosphorylation site mutants of villin, namely, Y46F, Y60F, Y81F, and Y256F. This figure shows representative clones of each villin construct transfected in HeLa cells. Data are representative of six experiments with similar results. (B) Villin expression results in reorganization of the actin cytoskeleton. HeLa cells transfected with wild-type (VIL/FL), and mutant villin proteins were analyzed by confocal microscopy. Double staining of villin (a1–f1) and F-actin (a2–f2) was performed using villin monoclonal antibodies (1:100) and FITC-conjugated anti-mouse IgG (1:200) and Alexa-Phalloidin 568 (1 μg/ml), respectively. Composite images of villin (green) and F-actin staining (red) are shown. Merged images (a3–f3) show colocalization of villin and F-actin. Wild-type villin and VIL/Y46F colocalize with F-actin at the cell periphery. In contrast, phosphorylation mutants of villin VIL/Y60F, VIL/Y81F, and VIL/Y256F show intracellular distribution of villin and F-actin with minimal colocalization of villin and F-actin at the cell surface. Bars, 3 μm. (C) HeLa cells expressing equal amounts of wild-type and phosphorylation site mutants (Y to F) of villin were used in wound-healing experiments. Wounds ∼800 μm in width were made using a pipette tip, and migration of cells into the wound was followed between 0 and 10 h postwounding. Wound repair is expressed as a percentage of the original wound width after 10 h. The error bars are the measured SEM, and the asterisk (*) and cross (†) denote statistically significant values [p < 0.05, n = 24, compared with VIL (-) cells and p < 0.05, n = 24, compared with untreated cells], respectively. VIL (-) refers to each clone cultured in the presence of doxycycline, whereas VIL (+) refers to the same clone cultured in the absence of doxycycline. (D) Tyrosine phosphorylation of wild-type and mutant villin proteins. Cell extracts from VIL/FL and the mutant villin cell lines VIL/Y46F, VIL/Y60F, VIL/Y81F, and VIL/Y256F were immunoprecipitated with phospho-villin antibody (VP-70782) and Western analysis done with villin mAb or phospho-tyrosine antibody (PY-20). This is not a quantitative Western blot. The Western blot is representative of three other experiments with similar results.
Our earlier studies, including data presented in this study, suggest that villin phosphorylation and the resulting changes in the actin rearrangements in the cell might serve to promote the migratory potential of epithelial cells. To confirm this, we examined the migratory capacity of cells stably expressing wild-type or phosphorylation-site mutants of villin. As shown in Figure 4D, wild-type villin enhanced cell migration, whereas the villin phosphorylation site mutants VIL/Y60F, VIL/Y81F, and VIL/Y256F were able to completely reverse the migratory phenotype of HeLa cells, reducing their migration rates to VIL/NULL cells. In contrast, the villin mutant VIL/Y46F behaved like wild-type villin, suggesting that phosphorylation at this site is not essential for cell migration. To further quantify cell migration rate, we measured cell velocity. As shown in Table 1, the average velocity (0.13 μm/min) of VIL/FL and VIL/Y46F shows an ∼50% increase in cell velocity compared with the villin-null HeLa cells 10 h postwounding (n = 24, p < 0.05). In contrast, the villin mutants VIL/Y60F, VIL/Y81F, and VIL/Y256F moved at a lower velocity, speeds similar to the villin-null HeLa cells (0.09 μm/min, n = 48). There was no significant difference in cell proliferation between VIL/NULL, VIL/FL or cells expressing the phosphorylation site mutants of villin (Supplementary Figure S1). The villin protein is tyrosine phosphorylated in all these cell lines in response to wounding (Figure 4D). This is not a quantitative Western blot because comparison of phosphorylation levels in different cell lines with a point mutation in one of the three phosphorylation sites could not be done with a great deal of confidence. These data confirm that tyrosine phosphorylation of villin regulates effective cell migration and furthermore, they characterize the specific tyrosine residues that control this process.
Table 1.
Tyrosine phosphorylation of villin regulates the rate of cell migration
| Villin construct | Migration speed (μm/min) |
|---|---|
| VIL/NULL | 0.090 ± 0.002 |
| VIL/FL | 0.130 ± 0.002* |
| VIL/Y46F | 0.136 ± 0.003* |
| VIL/Y60F | 0.094 ± 0.002 |
| VIL/Y81F | 0.091 ± 0.001 |
| VIL/Y256F | 0.095 ± 0.001 |
Denotes statistically significant values (p < 0.05; n = 24)
To confirm the role of the specific tyrosine residues in the regulation of villin-induced cell migration, we used phosphomimetics, namely, tyrosine (Y) to gluatamic acid (E) phosphorylation site mutants of villin. Multiple clones expressing the phosphomimetic villin proteins were selected by immunoblotting by using villin monoclonal antibodies. Clones expressing comparable amounts of villin proteins were selected for the migration studies (Figure 5A). The intracellular distribution of villin as well as the F-actin localization also was examined using confocal microscopy (Figure 5B; Supplementary Figure S5). As shown in Figure 5B, d–f, Y60E, Y81E, and Y256E look like VIL/FL. In all these cell lines, villin and F-actin colocalize at the cell margin and in the membrane ruffles. In contrast, VIL/Y46E resembles the VIL/NULL cells (Figure 5B, c1–c3). As shown in Figure 5C, VIL/Y60E, VIL/Y81E, and VIL/Y256E migrated faster than the comparable Y to F mutants (Figure 4C). At 10 h postwounding, 95% (p < 0.01, n = 24) of the wound was closed in cells expressing VIL/FL compared with villin-null cells (65% closed, p < 0.05, n = 24). The phosphomimetics behaved like VIL/FL in demonstrating enhanced cell migration compared with the villin-null cells, with 80–88% of the wound closed at 10 h postwounding in VIL/Y256, VIL/Y81E, and VIL/Y60E, respectively (Figure 5C). The rates of cell migration in VIL/Y60E, VIL/Y81E, and VIL/Y256E were not identical to that of VIL/FL but were dose dependent, and densitometric analysis revealed that they paralleled the protein expression levels in these cell lines. The villin mutant VIL/Y46E in contrast inhibited cell migration, migrating at the same rate as villin-null cells, suggesting that this site must remain dephosphorylated for the motogenic effects of villin. Together, these results indicate that efficient cell migration from wounded cell monolayers requires tyrosine phosphorylation of villin and that tyrosine phosphorylation of villin at Y60, Y81, and Y256 is a specific mediator of villin-regulated cell migration.
Figure 5.
Phosphorylation of villin at Y-60, Y-81, and Y-256 is required for villin-induced cell migration. (A) HeLa cells were stably transfected with wild-type (VIL/FL) and phosphorylation site mutants of villin, namely, Y46E, Y60E, Y81E, and Y256E. This figure shows representative clones of each villin construct transfected in HeLa cells. Data are representative of six experiments with similar results. (B) Villin expression results in reorganization of the actin cytoskeleton. HeLa cells transfected with wild-type (VIL/FL) and mutant villin proteins were analyzed by confocal microscopy. Double staining of villin (a1–f1) and F-actin (a2–f2) was performed using villin monoclonal antibodies (1:100) and FITC conjugated anti-mouse IgG (1:200) and Alexa-Phalloidin 568 (1 μg/ml), respectively. Composite images of villin (green) and F-actin staining (red) are shown. Merged images show colocalization of villin and F-actin (a3–f3). Wild-type villin and VIL/Y60E, VIL/Y81E, and VIL/Y256E colocalize with F-actin at the cell periphery. In contrast, phosphorylation mutants of villin VIL/Y46E shows intracellular distribution of villin and F-actin with minimal colocalization of villin and F-actin at the cell surface. Bars, 3 μm. (C) HeLa cells expressing equal amounts of wild-type and phosphorylation site mutants (Y to E) of villin were used in wound-healing experiments. Wounds ∼800 μm in width were made using a pipette tip, and migration of cells into the wound was followed between 0 and 10 h postwounding. Wound repair is expressed as a percentage of the original wound width at 10 h. The error bars are the measured SEM, and the asterisk (*) and cross (†) denote statistically significant values [p < 0.05, n = 24, compared with VIL (-) cells and p < 0.05, n = 24, compared with untreated cells], respectively. VIL (-) refers to each clone cultured in the presence of doxycycline, whereas VIL (+) refers to the same clone cultured in the absence of doxycycline.
DISCUSSION
Actin structures are dynamic and are affected by extracellular signals. Although the organization of the actin cytoskeleton in a moving cell has been described reasonably well, it is still unclear how the cytoskeleton is directed by external signals to coordinate its activity to provide directional movement. In the present report, we provide evidence that tyrosine phosphorylation of villin, which could occur in response to receptor activation or during wound healing results in both rearrangement of the microfilament structure as well as regulation of cell migration. In this study, we further demonstrated that overexpression of villin enhances cell migration. This is consistent with previous observations made with proteins of the villin family, including cofilin (Aizawa et al., 1996), CapG, and gelsolin (Sun et al., 1995). Data presented in our study suggest that an epithelial cell also could use villin as an intrinsic signal to stimulate actin assembly and increase cell migration in the absence of an external signal. Furthermore, our studies demonstrate that this change in cell motility by villin can be enhanced in response to environmental cues such as chemoattractants. For our studies we used HGF, a cytokine that is known to coordinate changes in cell morphology associated with the induction of cell motility during epithelial-mesenchyme transition (Boyer et al., 1996) and EGF, another powerful motogen. Together, these studies suggest that villin and other members of its family may function in vivo to provide a signaling mechanism for translating cell surface receptor-mediated biochemical reactions into cell locomotion.
Tyrosine kinase activity has been shown to be necessary for intestinal epithelial cell migration (Calalb et al., 1995; Cary et al., 1996; Parsons and Parsons, 1997; Polk, 1998; Reiske et al., 1999). Activation of c-src kinase has been reported in preneoplastic colonic adenomas and in colon carcinomas. In addition overexpression of pp60c-src has been demonstrated to increase the invasive behavior of intestinal epithelial cells (Pories et al., 1998). Thus, both tyrosine phosphorylation and activation of c-src kinase have been associated with the motile properties of intestinal epithelial cells. We have previously demonstrated that villin is phosphorylated in vitro by c-src kinase (Zhai et al., 2002). In the present study we provide evidence that in migrating cells, villin is a substrate for c-src kinase as well. Further, the potent and specific src kinase inhibitor PP2 inhibits the villin-induced increase in cell migration. Overexpression of the dominant negative c-src likewise inhibits villin-induced cell migration. These data suggest that villin is a substrate for src kinases in vivo just as it is in vitro and furthermore that src kinase phosphorylation of villin is important for villin-mediated cell motility.
We have previously identified the tyrosine phosphorylation sites in villin and mapped phosphorylation at these sites with the actin-modifying activities of villin (Zhai et al., 2002). Human villin contain four major tyrosine phosphorylation sites, namely, Y-46, -60, -81, and -256. In the current study, we assessed the effects of tyrosine phosphorylation of villin as well as mapped the phosphorylation sites that regulate villin-induced increase in cell migration. Our studies revealed that phosphorylation at Y60, Y81, and Y256 is required for cell migration. Both Y46F and wild-type villin enhance cell migration and demonstrate similar intracellular distribution, including redistribution of actin to the cell perimeter, loss of stress fibers, and colocalization with F-actin, suggesting that actin cytoskeletal reorganization by villin is necessary for its role in cell migration. This is supported by the observation that the villin mutants VIL/Y60F, VIL/Y81F, and VIL/Y256F migrate like the VIL/NULL cells and demonstrate a different subcellular distribution of villin as well as microfilament organization pattern. The most obvious difference between villin-induced migrating and villin-null or phosphorylation site mutant-villin cells is that villin that can be tyrosine phosphorylated at residues 60, 81, and 256 as well as the phosphomimetics localize at or near the cell surface. In contrast villin that cannot be tyrosine phosphorylated at these sites, show an intracellular distribution and are not very well distributed in cell surface structures. One possibility is that by altering the ability of villin to be tyrosine phosphorylated, we may have modified the ligand-binding properties of villin that determine its intracellular distribution and localization.
Recombinant tyrosine phosphorylated villin does not associate with PIP2, whereas nonphosphorylated villin does (Panebra et al., 2001). Furthermore, we have demonstrated that tyrosine phosphorylation of villin decreases villin's binding affinity for F-actin (Zhai et al., 2001). This would suggest that within the cell at or near the leading edge, there could be two separate pools of villin: tyrosine-phosphorylated villin that does not associate with the plasma membrane and has decreased affinity for F-actin, and nonphosphorylated villin that could bind both PIP2, and F-actin. Consistent with this observation, we have previously reported that tyrosine-phosphorylated villin redistributes to a Triton X-100–soluble fraction of intestinal villus cells, whereas nonphosphorylated villin remains associated with the F-actin filaments in a Triton-insoluble fraction (Khurana et al., 1997). Because phosphorylated villin does not associate with PIP2, we speculate that this Triton-soluble pool may represent the cellular G-actin pool, whereas the Triton X-100–insoluble pool is the F-actin pool in live cells. This is a reasonable speculation because we also have reported that tyrosine phosphorylation of villin releases an autoinhibited conformation, allowing it to sever actin filaments at physiological Ca2+ concentrations (Kumar and Khurana, 2004). Furthermore, we have identified the biochemical properties of the individual phosphorylation sites in villin. Phosphorylation at Y60 enhances the actin-severing activity of villin, suggesting that this site could be involved in generating new barbed ends (Zhai et al., 2002). In contrast, phosphorylation at Y81 and Y256 inhibits the ability of villin to polymerize actin filaments, thus altering the F-actin dynamics in vivo. These sites may have additional biochemical properties such as to decrease the binding affinity of villin for F-actin, decrease the actin cross-linking activity of villin and/or induce conformational changes in villin, resulting in constitutively active villin that can sever actin filaments at nanomolar Ca2+. We have previously shown all these functions of villin to be regulated by tyrosine phosphorylation (Zhai et al., 2001; Kumar and Khurana, 2004). Alternatively, in vivo these tyrosine phosphorylation sites may be ligand-binding sites for second messengers that may regulate either tyrosine phosphorylation of villin such as c-src kinase or yet unidentified tyrosine phosphatases, as well as the SH2 domain of PLC-γ1 (Panebra et al., 2001), all of which would modify villin-induced cell migration.
The integrity of these tyrosine residues is required for actin nucleation and depolymerization by villin, because phosphorylation of any one site impairs villin's capacity to nucleate actin, and likewise mutation at Y60 to F severely impairs phosphorylated villin's ability to sever actin filaments (Zhai et al., 2002). In other words, villin's ability to regulate the actin dynamics is dependent on its phosphorylation. Inhibition of cell migration by villin mutants suggests that a decrease in the actin-severing activity of villin is a negative regulator of villin-induced increase in cell migration. In other words, increased actin depolymerization by villin could be the signal for the enhanced cell migration.
The phosphomimetics lend further support to the idea that phosphorylation of villin at these three sites is necessary for the regulation of villin-induced migration. Interestingly, the villin mutant Y46E inhibited cell migration. The significance of this observation is not clear at this point, except it suggests that although phosphorylation at Y46 is not required for cell migration (VIL/Y46F behaves like VIL/FL), maintaining this site in a dephosphorylated form may be necessary for the villin-induced effects on cell migration. This also suggests that regulation of cell migration by villin may require both kinases and phosphatases maintaining the Y-60, -81, and -256 sites in a phosphorylated state and the Y46 site in a dephosphorylated state for efficient cell migration.
Based on our observations, we propose the following model for villin-induced cell migration. We believe that spatially restricted accumulation of signaling molecules could determine the activation of one or more of villin's actin regulatory properties, thus establishing villin's function in regulating cell motility. One of these signals could be a tyrosine kinase and/or phosphatase that could determine the phosphorylation state of villin. In the unphosphorylated state, the actin cross-linking property of villin could be important. For instance, it is known that cross-linking of actin filaments is essential to convert the force of polymerization into forward movement of the membrane and the cell. Although Arp 2/3 is considered the major cross-linking component, it is possible that in epithelial cells villin plays a significant role in cross-linking the actin filaments and generating stable lamellae. The cross-linking functions of villin could determine the rate and extent of lamellipodia formation and/or regulate the amount of F-actin incorporated into newly formed lamellipodia. Unphosphorylated villin also could bind the membrane phospholipid PIP2 and the villin-PIP2 complex could favor persistent growth of the barbed ends by preventing capping of the barbed ends, thus favoring both filament growth as well as allowing actin to push the membrane forward.
Phosphorylated villin could have other functions. Phosphorylation of villin could increase actin depolymerization, which could replenish the actin monomer pool thereby allowing the cell to maintain high concentration of unpolymerized actin far from the equilibrium. Depolymerization of actin by villin also could produce new barbed ends that may be used for the formation of new filaments. Phosphorylation of villin and activation of villin's actin-capping function could help cap barbed ends. Capping of barbed ends could help maintain the length of actin filaments, thus resulting in short filaments that can generate propulsive force and effectively push the membrane forward. Tyrosine phosphorylation of villin could regulate directed cell movement. It is possible that some of the phosphorylation site mutants exhibit lower migration rates because of lack of directed and coordinated movement. Because phosphorylated villin also disassembles actin filament bundles, cytoskeletal disassembly associated with disruption of actin bundles by tyrosine phosphorylated villin could generate pulling forces that may even be involved in the rearward retraction of a moving cell. Examples of such solation-contraction include microtubules that segregate chromosomes during mitosis (Mogilner and Oster, 2003), and sperm of the nematode Ascaris suum (Miao et al., 2003). Cell migration is intimately linked to cytoskeleton dynamics and our study demonstrates that tyrosine phosphorylation that affects cytoskeleton dynamics also affects cell migration. Our studies suggest that filament turnover in cells may be defined by the regulated action of actin-binding proteins interacting with signaling molecules.
Supplementary Material
Acknowledgments
We thank Dr. R.K. Rao (University of Tennessee, Memphis, TN) for technical assistance with the confocal microscopy studies. This work was supported by grants from the American Digestive Health Foundation (Industry Research Scholar Award) and by National Institute of Diabetes and Digestive and Kidney Diseases grants DK-65006 and DK-54755 and from the National Institute of Health (to S.K.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–05–0431. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–05–0431.
The online version of this article contains supplementary material accessible through http://www.molbiolcell.org.
References
- Aizawa, H., Sutoh, K., and Yahara, I. (1996). Overexpression of cofilin stimulates bundling of actin filaments, membrane ruffling, and cell movement in Dictyostelium. J. Cell Biol. 132, 335-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, R.D., Haskell, R.E., Xia, H., Roessler, B.J., and Davidson, B.L. (2000). A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther. 7, 1034-1038. [DOI] [PubMed] [Google Scholar]
- Arora, P.D., and McCulloch, C.A. (1996). Dependence of fibroblast migration on actin severing activity of gelsolin. J. Biol. Chem. 271, 20516-20523. [DOI] [PubMed] [Google Scholar]
- Arpin, M., Pringault, E., Finidori, J., Garcia, A., Jeltsch, J.M., Vandekerckhove, J., and Louvard, D. (1988). Sequence of human villin: a large duplicated domain homologous with other actin-severing proteins and a unique small carboxy-terminal domain related to villin specificity. J. Cell Biol. 107, 1759-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athman, R., Louvard, D., and Robine, S. (2003). Villin enhances hepatocyte growth factor-induced actin cytoskeleton remodeling in epithelial cells. Mol. Biol. Cell 14, 4641-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer, B., Valles, A.M., and Thiery, J.P. (1996). Model systems of epitheliummesenchyme transitions. Acta Anat. 156, 227-239. [DOI] [PubMed] [Google Scholar]
- Calalb, M.B., Polte, T.R., and Hanks, S.K. (1995). Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol. Cell. Biol. 15, 954-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary, L.A., Chang, J.F., and Guan, J.L. (1996). Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J. Cell Sci. 109, 1787-1794. [DOI] [PubMed] [Google Scholar]
- Chellaiah, M., Kizer, N., Silva, M., Alvarez, U., Kwiatkowski, D., and Hruska, K.A. (2000). Gelsolin deficiency blocks podosome assembly and produces increased bone mass and strength. J. Cell Biol. 148, 665-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, P., Murphy-Ullrich, J.E., and Wells, A. (1996). A role for gelsolin in actuating epidermal growth factor receptor-mediated cell motility. J. Cell Biol. 134, 689-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, C.C. (1995). Actin polymerization and intracellular solvent flow in cell surface blebbing. J. Cell Biol. 129, 1589-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, C.C., Stossel, T.P., and Kwiatkowski, D.J. (1991). Enhanced motility in NIH 3T3 fibroblasts that overexpress gelsolin. Science 251, 1233-1236. [DOI] [PubMed] [Google Scholar]
- De Corte, V., Gettemans, J., and Vandekerckhove, J. (1997). Phosphatidylinositol 4,5-bisphosphate specifically stimulates PP60(c-src) catalyzed phosphorylation of gelsolin and related actin-binding proteins. FEBS Lett. 401, 191-196. [DOI] [PubMed] [Google Scholar]
- Ferrary, E., et al. (1999). In vivo, villin is required for Ca(2+)-dependent F-actin disruption in intestinal brush borders. J. Cell Biol. 146, 819-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck, Z., Footer, M., and Bretscher, A. (1990). Microinjection of villin into cultured cells induces rapid and long-lasting changes in cell morphology but does not inhibit cytokinesis, cell motility, or membrane ruffling. J. Cell Biol. 111, 2475-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich, E., Huet, C., Arpin, M., and Louvard, D. (1989). Villin induces microvilli growth and actin redistribution in transfected fibroblasts. Cell 59, 461-475. [DOI] [PubMed] [Google Scholar]
- Furnish, E.J., Zhou, W., Cunningham, C.C., Kas, J.A., and Schmidt, C.E. (2001). Gelsolin overexpression enhances neurite outgrowth in PC12 cells. FEBS Lett. 508, 282-286. [DOI] [PubMed] [Google Scholar]
- Gossen, M., and Bujard, H. (1992). Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 89, 5547-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig, J.H., Chambers, K.A., and Stossel, T.P. (1989). Association of gelsolin with actin filaments and cell membranes of macrophages and platelets. J. Cell Biol. 108, 467-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey, P.A., and Matsudaira, P.T. (1988). Functional comparison of villin and gelsolin. Effects of Ca2+, KCl, and polyphosphoinositides. J. Biol. Chem. 263, 16738-16743. [PubMed] [Google Scholar]
- Khurana, S., Arpin, M., Patterson, R., and Donowitz, M. (1997). Ileal microvillar protein villin is tyrosine-phosphorylated and associates with PLC-gamma1. Role of cytoskeletal rearrangement in the carbachol-induced inhibition of ileal NaCl absorption. J. Biol. Chem. 272, 30115-30121. [DOI] [PubMed] [Google Scholar]
- Kumar, N., and Khurana, S. (2004). Identification of a functional switch for actin severing by cytoskeletal proteins. J. Biol. Chem. 279, 24915-24918. [DOI] [PubMed] [Google Scholar]
- Kumar, N., Zhao, P., Tomar, A., Galea, C.A., and Khurana, S. (2004). Association of villin with phosphatidylinositol 4,5-bisphosphate regulates the actin cytoskeleton. J. Biol. Chem. 279, 3096-3110. [DOI] [PubMed] [Google Scholar]
- Lu, M., Witke, W., Kwiatkowski, D.J., and Kosik, K.S. (1997). Delayed retraction of filopodia in gelsolin null mice. J. Cell Biol. 138, 1279-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao, L., Vanderlinde, O., Stewart, M., and Roberts, T.M. (2003). Retraction in amoeboid cell motility powered by cytoskeletal dynamics. Science 302, 1405-1407. [DOI] [PubMed] [Google Scholar]
- Mogilner, A., and Oster, G. (2003). Polymer motors: pushing out the front and pulling up the back. Curr. Biol. 13, R721-733. [DOI] [PubMed] [Google Scholar]
- Muller, T., Bain, G., Wang, X., and Papkoff, J. (2002). Regulation of epithelial cell migration and tumor formation by beta-catenin signaling. Exp. Cell Res. 280, 119-133. [DOI] [PubMed] [Google Scholar]
- Northrop, J., Weber, A., Mooseker, M.S., Franzini-Armstrong, C., Bishop, M.F., Dubyak, G.R., Tucker, M., and Walsh, T.P. (1986). Different calcium dependence of the capping and cutting activities of villin. J. Biol. Chem. 261, 9274-9281. [PubMed] [Google Scholar]
- Panebra, A., Ma, S.X., Zhai, L.W., Wang, X.T., Rhee, S.G., and Khurana, S. (2001). Regulation of phospholipase C-gamma(1) by the actin-regulatory protein villin. Am. J. Physiol. 281, C1046-C1058. [DOI] [PubMed] [Google Scholar]
- Parsons, J.T., and Parsons, S.J. (1997). Src family protein tyrosine kinases: cooperating with growth factor and adhesion signaling pathways. Curr. Opin. Cell Biol. 9, 187-192. [DOI] [PubMed] [Google Scholar]
- Pinson, K.I., Dunbar, L., Samuelson, L., and Gumucio, D.L. (1998). Targeted disruption of the mouse villin gene does not impair the morphogenesis of microvilli. Dev. Dyn. 211, 109-121. [DOI] [PubMed] [Google Scholar]
- Polk, D.B. (1998). Epidermal growth factor receptor-stimulated intestinal epithelial cell migration requires phospholipase C activity. Gastroenterology 114, 493-502. [DOI] [PubMed] [Google Scholar]
- Pories, S.E., Hess, D.T., Swenson, K., Lotz, M., Moussa, R., Steele, G., Jr., Shibata, D., Rieger-Christ, K.M., and Summerhayes, C. (1998). Overexpression of pp60c-src elicits invasive behavior in rat colon epithelial cells. Gastroenterology 114, 1287-1295. [DOI] [PubMed] [Google Scholar]
- Reiske, H.R., Kao, S.C., Cary, L.A., Guan, J.L., Lai, J.F., and Chen, H.C. (1999). Requirement of phosphatidylinositol 3-kinase in focal adhesion kinase-promoted cell migration. J. Biol. Chem. 274, 12361-12366. [DOI] [PubMed] [Google Scholar]
- Singh, A.B., Tsukada, T., Zent, R., and Harris, R.C. (2004). Membrane-associated HB-EGF modulates HGF-induced cellular responses in MDCK cells. J. Cell Sci. 117, 1365-1379. [DOI] [PubMed] [Google Scholar]
- Sonnenberg, E., Meyer, D., Weidner, K.M., and Birchmeier, C. (1993). Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J. Cell Biol. 123, 223-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, H.Q., Kwiatkowska, K., Wooten, D.C., and Yin, H.L. (1995). Effects of CapG overexpression on agonist-induced motility and second messenger generation. J. Cell Biol. 129, 147-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters, C.M., and Savla, U. (1999). Keratinocyte growth factor accelerates wound closure in airway epithelium during cyclic mechanical strain. J. Cell. Physiol. 181, 424-432. [DOI] [PubMed] [Google Scholar]
- White, E., Blose, S.H., and Stillman, B.W. (1984). Nuclear envelope localization of an adenovirus tumor antigen maintains the integrity of cellular DNA. Mol. Cell. Biol. 4, 2865-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witke, W., Sharpe, A.H., Hartwig, J.H., Azuma, T., Stossel, T.P., and Kwiatkowski, D.J. (1995). Hemostatic, inflammatory, and fibroblast responses are blunted in mice lacking gelsolin. Cell 81, 41-51. [DOI] [PubMed] [Google Scholar]
- Zhai, L., Kumar, N., Panebra, A., Zhao, P., Parrill, A.L., and Khurana, S. (2002). Regulation of actin dynamics by tyrosine phosphorylation: identification of tyrosine phosphorylation sites within the actin-severing domain of villin. Biochemistry 41, 11750-11760. [DOI] [PubMed] [Google Scholar]
- Zhai, L., Zhao, P., Panebra, A., Guerrerio, A.L., and Khurana, S. (2001). Tyrosine phosphorylation of villin regulates the organization of the actin cytoskeleton. J. Biol. Chem. 276, 36163-36167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.