Abstract
Primary cardiac tumors are extremely rare and constitute only about 5% of all cardiac tumors. Cardiac myxomas are noncancerous primary tumors of the heart and constitute about of 50% of all primary heart tumors. Left-sided atrial myxomas are more common than right-sided atrial myxomas. Atrial myxomas can lead to a triad of complications. The most common symptoms are associated with obstruction due to the size and location of the tumor. The next most common symptoms are associated with pulmonary and systemic embolization. Patients may also present with constitutional symptoms. Diagnosis is made via means of transesophageal echocardiography and magnetic resonance imaging. Early diagnosis and surgical resection remain the treatment of choice to prevent complications. Patients usually have a good prognosis after resection.
Keywords: Atrial myxomas, Embolization, Extracardiac manifestations
Abbreviations
- TR
Tricuspid Regurgitation
- IL
Interleukin
- CT
Computed Tomography
- MRI
Magnetic Resonance Imaging
Introduction
Primary cardiac tumors are extremely rare and constitute only about 5% of all cardiac tumors [1], [2], [3]. Cardiac myxomas are noncancerous primary tumors of the heart and constitute about of 50% of all primary heart tumors [4]. Approximately 75% of them arise from the left atrium, 20% of them from the right atrium, and 5% in both atria and the ventricle [2]. Cardiac myxomas predominantly occur in women [5] with an average age of onset in the 6th decade of life [6]. Rare occurrences of atrial myxoma in a pediatric age group have also been reported [7]. Li et al. [8] evaluated the occurrence of cardiac myxomas in men and women. He found that the ratio of women to men for left atrial myxomas was 2.05:1, while that of right atrial myxomas was 0.75:1 [8]. A French study conducted by Pinede et al. [9] showed that myxomas can lead to a triad of complications, the first being obstruction (67%), followed by emboli (29%), with or without constitutional symptoms (34%).
Often myxomas mimic multiple cardiovascular diseases and hence demand a high degree of suspicion for diagnosis [10]. Apart from cardiac manifestations, there are many extracardiac manifestations associated with atrial myxomas which can be often misdiagnosed or confused with other medical conditions. Currently, there has been no published review regarding the extracardiac manifestations of atrial myxomas. The primary objective of our review is to outline and discuss all the extracardiac manifestations of atrial myxomas and their associated complications and prognosis.
Methodology
A systematic review of the MEDLINE database was conducted using the PubMed search engine. We included all articles published in the English language between January 1, 1950, and April 30, 2016. In PubMed, the combination of medical text terms used included “atrial myxomas,” “extracardiac manifestations,” and “extracardiac symptoms.” Primarily we selected publications within the last 25 years but did not exclude older publications that were highly regarded. We also searched the reference lists of the manuscripts by this strategy and selected those to be relevant. All pertinent reports and the relative reference lists were searched to identify any additional studies that could be included. All data were accessed between February 2016 and April 2016.
Myxomas
Left atrial myxomas
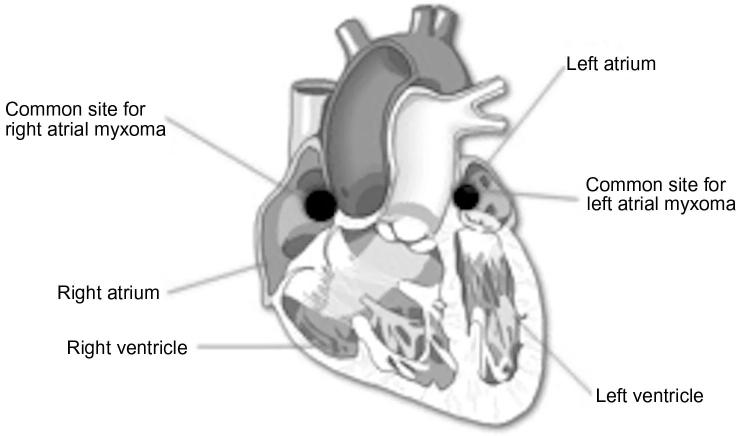
The most common location of myxomas is the left atrium, classically at the fossa ovalis border of the interatrial septum [6] or at the mitral annulus [8] (Fig. 1). They commonly present with systemic or constitutional symptoms. Cases have been reported where a myxoma was diagnosed as a large, asymptomatic incidental mass on echocardiogram. In a study conducted by Goswami et al. [11], 80% of patients with a cardiac myxoma presented with dyspnea. Other presenting symptoms included palpitations, syncope, pedal edema, chest pain, and constitutional symptoms. Around 30% of the patients presented with signs and symptoms associated with secondary embolization [11], [12]. These patients are often misdiagnosed as having mitral valve disease, tricuspid valve disease, ischemic heart disease, or cardiomyopathy.
Figure 1.
Illustration of the common sites of right and left atrial myxomas.
Right atrial myxomas
The second most common location for cardiac myxomas is the right atrium with the most common site of attachment being the interatrial septum, followed by the vena cava, at or near the coronary sinus [8] (Fig. 1). Right atrial myxomas are mostly asymptomatic [13]. Similar to left atrial myxomas, right atrial myxomas can also present with obstructive symptoms producing intracardiac flow disturbances, embolic symptoms—both pulmonary and systemic—(paradoxical emboli through patent foramen ovale) [14], and constitutional symptoms.
Histopathology
Different theories explain the origin of myxomas. Recent literature explains the origin of stromal cells by two hypotheses. One states that it is derived from endocardial neural cells [15], while other literature states that myxomas originate from the embryonic foregut and are thus derived from multipotent mesenchymal cells capable of neural and epithelial differentiation. Although the etiology is not well understood, the majority of cases are sporadic and approximately 10% of myxomas are of the autosomal dominant genetic pattern (Carney’s complex) [16]. On gross anatomy, cardiac myxomas are often pedunculated and soft in consistency with a smooth, villous, or friable surface. They usually weigh anywhere between 15 g and 180 g and their average size is between 2 cm and 6 cm [17]. The smooth myxomas are often large and are responsible for obstructive symptoms, while the villous and friable myxomas are usually responsible for the embolic phenomenon [9], [18]. Histologically, they are made of stellated, fusiform, and polygonal cells, within a mucopolysaccharide stroma. Immunohistochemically some are positive for CD31, CD34, and FVIIIAg. They can also stain positive for vimentin, desmin, smooth muscle myosin, CD56, S-100 protein, calretinin, α1 antitrypsin, and α1 antichymotrypsin [19]. Occasionally fibrocytes, smooth muscle cells, lymphocytes, and plasmacytes are also seen in the matrix [20]. Calcifications are present in approximately 10% of the myxomas [21]. Cardiac myxomas often synthesize vascular endothelial growth factor, which is responsible for significant angiogenesis [22], [23], [24]. The most important differential diagnosis for cardiac myxoma is mural thrombi with myxoid stroma. The similar histopathological appearance makes the diagnosis extremely challenging. Immunohistochemistry testing is of low value. In such cases, the calretinin marker, which is specific for myxomas, helps to differentiate it from mural myxoid thrombi [25]. Often, other cardiac tumors including malignant tumors can mimic myxomas and there have been few reports of a malignant transformation of an existing cardiac myxoma [26]. One report describes a patient developing primary cardiac sarcoma within a pre-existing atrial myxoma [27]. Another interesting histopathological report in cardiac myxoma is the presence of primary cardiac lymphoma within a cardiac myxoma. Epstein–Barr virus was associated with the development of CD30-positive large B-cell lymphoma with anaplastic morphology within the cardiac myxoma [28].
Cardiac and extracardiac manifestations
Obstructive symptoms
Obstructive symptoms usually depend on the site of the cardiac myxoma. Patients with left atrial myxomas may develop mitral valve regurgitation or stenosis depending upon the site of attachment [29]. When the tumor is located close to the mitral valve orifice, it results in obstruction and presents with symptoms of left heart failure symptoms and pulmonary congestion. Local invasion of the tumor can lead to conduction abnormalities and arrhythmias [30]. The most common presentation of atrial myxomas is dyspnea with exertion followed by orthopnea, paroxysmal nocturnal dyspnea, and pulmonary edema [11]. Careful physical examination including a good cardiac auscultation plays a vital role in the initial diagnosis. A retrospective study conducted by Aggarwal et al. [31] reported that approximately 89% of the patients had an auscultation abnormality and around 50% of them had a tumor plop. Echocardiogram usually demonstrates left atrial enlargement and cardiomegaly may be seen on ta chest x-ray [31].
Similar to the left side, the obstructive symptoms of right atrial myxomas depend on the site of attachment. The myxoma can be at the level of tricuspid valve causing obstructive symptoms, which mimics tricuspid stenosis. Rarely these patients can present with tricuspid regurgitation. A murmur may be appreciated over the tricuspid region with tricuspid regurgitation seen on the echocardiogram. Over time, symptoms of right-sided heart failure will develop such as pedal edema, hepatic congestion, exertional dyspnea, and ascites. Massive obstruction can lead to syncope and decompensated right-sided heart failure [32]. Similar to the left side, right-sided myxomas can also invade the myocardium causing conduction abnormalities and associated arrhythmias.
Embolic symptoms
Embolization commonly occurs in about 35% of all left atrial myxomas [6]. Villous and friable myxomas are the myxomas that are predominantly responsible [33]. It is actually the overlying thrombus on the surface of the tumor that embolizes more than the tumor itself [34]. A smaller tumor size of <4.5 cm poses an increased risk [35]. Owing to the high systolic left ventricular pressure, embolization due to left atrial myxomas commonly affects the central nervous system and retinal arteries. Embolization can also occur in the lower extremities (commonly iliac and femoropopliteal), viscera, spleen, adrenals, kidneys, and even the abdominal aorta [35]. The patient can present with dyspnea, transient ischemic attack, hemiplegia, syncope, loss of vision, arrhythmia, and chest pain. Symptoms may include Raynaud’s phenomenon, spotty skin pigmentation, abdominal pain, diarrhea, and other peripheral signs of embolization [15]. Approximately 10% of coronary embolizations are believed to be due to cardiac myxomas. In specific to patients with myxomas, studies report that around 20–35% of them experience neurological complications [9] and 9–22% of these myxoma cases suffer from an embolic stroke [16]. Left atrial myxoma should always be included in the differential diagnosis in case of multiple cerebral infarcts, especially in young individuals [16]. A delayed neurological complication of a myxoma includes the development of cerebral aneurysms and myxomatous metastasis. They tend to present with symptoms similar to central nervous system vasculitis and infective endocarditis [35]. Sakellaridis et al. [36] reported an uncommon case of left atrial myxoma presenting with Gerstmann’s syndrome. Embolic occlusion of the retinal arteries may lead to irreversible vision loss [37]. Cases of ophthalmic artery occlusion have also been reported [38]. Other ophthalmological manifestations secondary to cardiac myxomas includes nystagmus, homonymous hemianopia, and ocular motility disorders [37]. A rare catastrophic complication of cardiac myxoma is an abdominal aortic occlusion. Most of these cases tend to be infrarenal in nature [39].
Embolization occurs in about 10% of right atrial myxomas [6]. Embolization in right atrial myxomas can be of two types: (1) pulmonary artery embolism or systemic embolization if the patient has a patent foramen ovale or an atrial septal defect [14]; (2) pulmonary artery embolization is often life threatening and may require an immediate pulmonary embolectomy to prevent the development of pulmonary artery hypertension and consequent right ventricular dysfunction [40]. One of the delayed complications includes the development of a pulmonary artery aneurysm. The development of an aneurysm secondary to a myxoma is explained by two theories. One states that the embolic tumor cells cause inflammation and degrades the vascular wall leading to aneurysm formation [41]. The other states that the metastatic tumor cells penetrate the vessel wall and undergo subintimal proliferation leading to weakening of vascular integrity and thus aneurysm development [42]. A computer tomography pulmonary angiography provides valuable information regarding the pulmonary vasculature, lung parenchyma, and the cardiac mass in cases where aneurysms are suspected [30].
Constitutional symptoms
Constitutional symptoms are found to be reported more in women compared with men [9]. Constitutional symptoms are more common in cases of right-sided as opposed to left-sided cardiac myxomas for unknown reasons. It includes malaise, anorexia, fever, arthralgia, weight loss, and often mimics connective tissue disorders [6]. The symptoms are attributed to the release of cytokine interleukin-6 (IL-6). Laboratory abnormalities such as high erythrocyte sedimentation rate, anemia, leukocytosis, thrombocytopenia, and elevated gamma globulins can also be present [43]. Cultured myxoma cells from patients experiencing constitutional symptoms have shown elevated levels of messenger RNAs specific for IL-6 [18]. IL-6 facilitates the proliferation and differentiation of cells as well as the release of acute phase reactants during inflammation [18]. It also plays a significant role in the recurrence of atrial myxomas [18]. Prompt cardiac evaluation is extremely significant in patients presenting with nonspecific symptoms as early recognition of tumor aids in preventing complications and in improving quality of life (Table 1).
Table 1.
Extracardiac manifestations in atrial myxomas.
| Obstructive symptoms | Embolic symptoms | Constitutional symptoms | Other symptoms | |
|---|---|---|---|---|
| Left atrial myxomas | Mitral stenosis, mitral regurgitation, left sided heart failure, pulmonary congestion | CVA, retinal arteries, coronary arteries, cerebral arterial aneurysm, abdominal circulation, lower extremity circulation, other viscera, spleen, adrenals, kidneys | Malaise, anorexia, fever, arthralgia, weight loss, high erythrocyte sedimentation rate, anemia, leukocytosis, thrombocytopenia, elevated gamma globulin | Carneys complex, acute psychosis, coronary steal syndrome, infection, mononeuropathy |
| Right atrial myxomas | Tricuspid stenosis, right sided heart failure, pedal edema, hepatic congestion, ascites | Pulmonary embolism, pulmonary artery hypertension, pulmonary artery aneurysm, systemic embolization (similar to left atrial myxomas) in the presence patent foramen ovale/atrial septal defects | Malaise, anorexia, fever, arthralgia, weight loss, high erythrocyte sedimentation rate, anemia, leukocytosis, thrombocytopenia, elevated gamma globulin | Carneys complex, acute psychosis, coronary steal syndrome, infection, mononeuropathy |
CVA = cerebrovascular accident.
Other symptoms
Apart from the above-mentioned symptoms, other rare symptoms have been reported in patients with atrial myxoma. Atrial myxoma can present with symptoms of acute psychosis. Computer tomography scan and magnetic resonance imaging of the brain revealed multiple chronic infarcts which were hypothesized to have occurred from embolization of atrial myxomas, as the patient eventually got better after the removal of the myxoma [44]. Atrial myxomas can be part of a syndrome such as Carney complex. Carney complex is a rare autosomal dominant syndrome characterized by pigmented lesions of the skin along with endocrine, cutaneous, cardiac, and neural myxomatous tumors. Often these are young patients with widely distributed cutaneous lesions with a tendency to recur following excision [45]. Another author has also reported the association of acromegaly in a patient with Carney’s complex and left atrial myxoma. The patient described in this report had recurrent atrial myxomas requiring multiple surgeries who eventually presented with symptoms of acromegaly and magnetic resonance imaging of the brain confirmed the diagnosis [46]. Michaud et al. [47] reported the involvement of the peripheral nervous system with multiple mononeuropathies in patients with atrial myxoma. The patient described had symptoms of neurological pain, purpuric macules on the palms and soles, and multiple infarcts in the kidneys and spleen. These symptoms resolved after removal of the atrial myxoma. The pathophysiological mechanism includes multiple systemic infarctions from tumor fragmentations versus paraneoplastic and an autoimmune process [47]. Coronary steal syndrome has been reported in a patient with atrial myxoma. The patient described in this report had recurrent exertional chest discomfort and angiography revealed a large intracardiac left atrial mass supplied by two anomalous coronary arteries. After removal of the mass, which was identified as myxoma, the patient’s symptoms resolved [48]. Cardiac myxomas can also be a nidus for infection. Yuan [49] has described around 39 reports involving 39 patients with infected cardiac myxomas. The risk factors for infection of myxoma include dental procedures, infections, invasive procedures, and immunocompromised state. Common pathogens include streptococcus viridans, staphylococci, and Enterococcus faecalis. Patients had complications such as sepsis, disseminated intravascular coagulation, and embolic events. Prolonged antibiotics along with surgical resection were necessary for the treatment and death was reported in two patients [49].
Management
The diagnostic modality of choice is an echocardiography, which identifies the size, site, attachment, mobility, and also grossly differentiates the myxoma from vegetation or a thrombus [50]. Transesophageal echocardiography is preferred over transthoracic echocardiography [16]. Other diagnostic tests include a coronary angiography and magnetic resonance imaging. The treatment of choice is surgical excision which is aimed at complete resection and has excellent outcomes. Surgical excision is also followed by a pericardial patch placement for the construction of defects not amenable to primary closure. Histopathological analysis of the tumor is suggested to rule out a metastatic mass and to study the differentiation and growth rate of the tumor [51].
Apart from surgical excision of the myxoma, management of symptoms caused by the myxoma is also necessary. Left sided atrial myxoma can obstruct the functioning of the mitral valve leading to symptoms of congestive heart failure. Diuretics are necessary for treating the pulmonary vascular congestion secondary to diastolic heart failure. Since the left ventricular systolic function is within normal limits, drugs such as beta blockers, spironolactone, and angiotensin-converting enzyme inhibitors are usually not indicated in these patients [11], [30]. Local invasion of the tumor can cause cardiac arrhythmias requiring antiarrhythmic drugs and anticoagulation if indicated. Right-sided atrial myxomas can lead to symptoms of right-sided heart failure requiring diuretics for symptomatic relief [52]. Long-term continuation of these medications is not necessary as surgical excision of the tumor provides relief of these symptoms. Myxomas can also encroach upon the valves causing damage to them requiring surgical repair. Embolization of atrial myxomas or thrombus overlying the atrial myxoma can cause a myriad of symptoms depending on the location of the embolus. Currently, there is no clear literature or guideline commenting on anticoagulation in these patients. Often patients present to the hospital with symptoms secondary to embolization. Antiplatelet and anticoagulant agents are used for secondary prevention after an event of an ischemic stroke [53]. Surgical excision of the friable mass is considered therapeutic for all these patients [54]. Surgery in patients with a recent embolic stroke can be complicated due to the potential for hemorrhagic conversion upon the use of anticoagulation during surgery [34]. Constitutional symptoms are very nonspecific not requiring any specific treatment and they resolve after excision of the myxoma [52]. Other rare symptoms as mentioned above require specific symptom-oriented treatment. Despite the fact that the majority of myxomas are benign in nature, surgical resection is highly suggested to prevent devastating complications including sudden death [12], [29].
Prognosis
Recent literature states that cases of cardiac myxomas have low surgical mortality and show good long-term survival results. Recurrence rates are reported to be extremely low except for familial myxomas and multicentric cardiac myxomas [35]. The recurrence rates are noted to be 1–3% in sporadic forms, 12% in familial forms, and 22% in complex myxomas [9]. Interestingly, Wang et al. [35] found that more often than not, the site of recurrence is noted to be different from the original lesion. Minimal tumor manipulation, adequate exposure for complete tumor excision, and thorough inspection of the four chambers during surgery were put forth by Jones et al. [55] as steps essential to prevent recurrence. Regular follow-up with echocardiography is highly recommended after surgical excision [6].
Conclusion
Atrial myxomas are the most common type of primary cardiac tumors. Left atrial myxomas are more common than right atrial myxomas. They often cause a myriad of clinical signs and symptoms that can be classified as obstructive, embolic, or constitutional. Even though atrial myxomas are benign tumors, secondary complications due to their position and possibility of embolization can be devastating. A high clinical suspicion, prompt diagnosis with appropriate imaging modalities, and early surgical resection are important in preventing these complications.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Thiene G, Valente M, Lombardi M, Basso C. Tumors of the heart. In: Camm AJ, Lüscher TF, Serruys PW, editors. The ESC textbook of cardiovascular medicine. 2nd ed. New York, USA: Oxford University Press, Oxford; 2009, Chapter 20.
- 2.Burke A, Virmani R. Tumors of the heart and the great vessels. In: Rosai J, editors. Atlas of tumor pathology. 3rd ed. Fascicle 16. Washington, DC, USA: Armed Forces Institute of Pathology; 1996, p. 231.
- 3.Lam K.Y., Dickens P., Chan A.C. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med. 1993;117:1027–1031. [PubMed] [Google Scholar]
- 4.Ha J.W., Kang W.C., Chung N., Chang B.C., Rim S.J., Kwon J.W. Echocardiographic and morphologic characteristics of left atrial myxoma and their relation to systemic embolism. Am J Cardiol. 1999;83:1579–1582. doi: 10.1016/s0002-9149(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 5.Hi D., Yoon A., Roberts W.C. Sex distribution in cardiac myxomas. Am J Cardiol. 2002;90:563–565. doi: 10.1016/s0002-9149(02)02540-7. [DOI] [PubMed] [Google Scholar]
- 6.Lazaros G., Masoura C., Brili S., Stavropoulos G., Kafiri G., Stefanadis C. Large left atrial myxoma in an oligosymptomatic young woman. Hellenic J Cardiol. 2013;54:60–63. [PubMed] [Google Scholar]
- 7.Sernich S., Chauhan A., Singh D., Fuchs H., Caspi J. Left atrial myxoma in a child: a challenging diagnosis of a rare lesion. World J Pediatr Congenit Heart Surg. 2013;4:220–222. doi: 10.1177/2150135112473124. [DOI] [PubMed] [Google Scholar]
- 8.Li H., Guo H., Xiong H., Xu J., Wang W., Hu S. Clinical features and surgical results of right atrial myxoma. J Card Surg. 2016;31:15–17. doi: 10.1111/jocs.12663. [DOI] [PubMed] [Google Scholar]
- 9.Pinede L., Duhaut P., Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore) 2001;80:159–172. doi: 10.1097/00005792-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Fang B.R., Chiang C.W., Hung J.S., Lee Y.S., Chang C.S. Cardiac myxoma—clinical experience in 24 patients. Int J Cardiol. 1990;29:335–341. doi: 10.1016/0167-5273(90)90123-m. [DOI] [PubMed] [Google Scholar]
- 11.Goswami K.C., Shrivastava S., Bahl V.K., Saxena A., Manchanda S.C., Wasir H.S. Cardiac myxomas: clinical and echocardiographic profile. Int J Cardiol. 1998;63:251–259. doi: 10.1016/s0167-5273(97)00316-1. [DOI] [PubMed] [Google Scholar]
- 12.Vilela E.P., Moura L., Pepe D., Nunes E., Erthal F., Campana E. Giant atrial myxoma mimicking severe mitral stenosis in young patient. Arq Bras Cardiol. 2010;95:125–127. doi: 10.1590/s0066-782x2010001500023. [DOI] [PubMed] [Google Scholar]
- 13.Zairi I., Mzoughi K., Jnifene Z., Fennira S., Ben Moussa F., Kammoun S. A giant right atrial myxoma with pulmonary arterial hypertension. Pan Afr Med J. 2015;21:96. doi: 10.11604/pamj.2015.21.96.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surabhi S.K., Fasseas P., Vandecker W.A., Hanau C.A., Wolf N.M. Right atrial myxoma in a patient presenting with syncope. Tex Heart Inst J. 2001;28:2289. [PMC free article] [PubMed] [Google Scholar]
- 15.Pucci A., Gagliardotto P., Zanini C., Pansini S., di Summa M., Mollo F. Histopathologic and clinical characterization of cardiac myxoma: review of 53 cases from a single institution. Am Heart J. 2000;140:134–138. doi: 10.1067/mhj.2000.107176. [DOI] [PubMed] [Google Scholar]
- 16.Iyer P., Aung M.M., Awan M.U., Kososky C., Barn K. A case of large atrial myxoma presenting as an acute stroke. J Community Hosp Intern Med Perspect. 2016;6:29604. doi: 10.3402/jchimp.v6.29604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asai Y., Ichimura K., Kaneko M., Abe T. Treatment of life-threatening huge atrialmyxoma: report of two cases. Surg Today. 1999;29:813–816. doi: 10.1007/BF02482336. [DOI] [PubMed] [Google Scholar]
- 18.Gavrielatos G., Letsas K.P., Pappas L.K., Dedeilias P., Sioras E., Kardaras F. Large left atrial myxoma presented as fever of unknown origin: a challenging diagnosis and a review of the literature. Cardiovasc Pathol. 2007;16:365–367. doi: 10.1016/j.carpath.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Hernández-Bringas O., Ortiz-Hidalgo C. Histopathological and immunohistochemical features of cardiac myxomas. Arch Cardiol Mex. 2013;83:199–208. doi: 10.1016/j.acmx.2013.02.002. [Article in Spanish] [DOI] [PubMed] [Google Scholar]
- 20.Sato H., Tanaka T., Kasai K., Kita T., Tanaka N. Sudden death due to acute pulmonary embolism from asymptomatic right atrial myxoma. J Forensic Leg Med. 2008;15:454–456. doi: 10.1016/j.jflm.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Reynen K. Cardiac myxomas. N Engl J Med. 1995;333:1610–1617. doi: 10.1056/NEJM199512143332407. [DOI] [PubMed] [Google Scholar]
- 22.Amano J., Kono T., Wada Y., Zhang T., Koide N., Fujimori M. Cardiac myxoma: its origin and tumor characteristics. Ann Thorac Cardiovasc Surg. 2003;9:215–221. [PubMed] [Google Scholar]
- 23.Mallick S.R., Das P., Shukla B., Kothari S., Devagourou V., Ray R. Right atrial myxoma with glandular differentiation: a rare entity in pediatric age group. Ann Pediatr Cardiol. 2010;3:159–162. doi: 10.4103/0974-2069.74046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lloreta J., Juanpere N., Riverola A., Dallari D., Cañas M.A., Pijuan L. Cardiac myxoma with glandular differentiation: an immunohistochemical and ultrastructural study. Ultrastruct Pathol. 2013;37:77–82. doi: 10.3109/01913123.2011.584499. [DOI] [PubMed] [Google Scholar]
- 25.Terracciano L.M., Mhawech P., Suess K., D’Armiento M., Lehmann F.S., Jundt G. Calretinin as a marker for cardiac myxoma. Diagnostic and histogenetic considerations. Am J Clin Pathol. 2000;114:754–759. doi: 10.1309/NR6G-T872-F090-LBRW. [DOI] [PubMed] [Google Scholar]
- 26.Kasugai T., Sakurai M., Yutani C., Hirota S., Waki N., Adachi S. Sequential malignant transformation of cardiac myxoma. Acta Pathol Jpn. 1990;40:687–692. doi: 10.1111/j.1440-1827.1990.tb01617.x. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen A., Awad W.I. Cardiac sarcoma arising from malignant transformation of a preexisting atrial myxoma. Ann Thorac Surg. 2016;101:1571–1573. doi: 10.1016/j.athoracsur.2015.05.129. [DOI] [PubMed] [Google Scholar]
- 28.Aguilar C., Beltran B., Quiñones P., Carbajal T., Vilcapaza J., Yabar A. Large B-cell lymphoma arising in cardiac myxoma or intracardiac fibrinous mass: a localized lymphoma usually associated with Epstein-Barr virus? Cardiovasc Pathol. 2015;24:60–64. doi: 10.1016/j.carpath.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Kaya M., Ersoy B., Yeniterzi M. Mitral valve regurgitation due to annular dilatation caused by a huge and floating left atrial myxoma. Kardiochir Torakochirurgia Pol. 2015;12:248–250. doi: 10.5114/kitp.2015.54463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rokadia H.K., Heresi G.A., Tan C.D., Raymond D.P., Budd G.T., Farver C. A 33-year-old man with multiple bilateral pulmonary pseudoaneurysms. Chest. 2015;148:112–117. doi: 10.1378/chest.15-0624. [DOI] [PubMed] [Google Scholar]
- 31.Aggarwal S.K., Barik R., Sarma T.C., Iyer V.R., Sai V., Mishra J. Clinical presentation and investigation findings in cardiac myxomas: new insights from the developing world. Am Heart J. 2007;154:1102–1107. doi: 10.1016/j.ahj.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 32.Azevedo O., Almeida J., Nolasco T., Medeiros R., Casanova J., Bartosch C. Massive right atrial myxoma presenting as syncope and exertional dyspnea: case report. Cardiovasc Ultrasound. 2010;8:23. doi: 10.1186/1476-7120-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lazaros G., Latsios G., Tsalamandris S., Sfyras N., Toutouzas K., Tsiamis E. Cardiac myxoma and concomitant myocardial infarction. Embolism, atherosclerosis or combination. Int J Cardiol. 2016;205:124–126. doi: 10.1016/j.ijcard.2015.11.057. [DOI] [PubMed] [Google Scholar]
- 34.Hirose H., Youdelman B.A., Entwistle J.W. Stroke from a large left atrial myxoma. Open Cardiovasc Med J. 2008;2:115–117. doi: 10.2174/1874192400802010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z., Chen S., Zhu M., Zhang W., Zhang H., Li H. Risk prediction for emboli and recurrence of primary cardiac myxomas after resection. J Cardiothorac Surg. 2016;11:22. doi: 10.1186/s13019-016-0420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakellaridis T, Argiriou M., Koukis I., Panagiotakopoulos V., Spiliotopoulos C., Dimakopoulou A. Gerstmann's syndrome: can cardiac myxoma be the cause? Hellenic J Cardiol. 2008;49:52–54. [PubMed] [Google Scholar]
- 37.Yu Y., Zhu Y., Dong A., Su Z. Retinal artery occlusion as the manifestation of left atrial myxoma: a case report. BMC Ophthalmol. 2014;14:164. doi: 10.1186/1471-2415-14-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabater N., Alforja S., Rey A., Giralt J. Delayed diagnosis of ophthalmic artery obstruction due to atrial myxoma. Arch Soc Esp Oftalmol. 2013;88:313–315. doi: 10.1016/j.oftal.2012.02.008. [Article in Spanish] [DOI] [PubMed] [Google Scholar]
- 39.Huang C.Y., Chang Y.Y., Hsieh M.Y., Hsu C.P. Atrial myxoma presenting as total occlusion of the abdominal aorta and its major four branches. J Chin Med Assoc. 2012;75:349–352. doi: 10.1016/j.jcma.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Ikeda A., Tsukada T., Konishi T., Matsuzaki K., Jikuya T., Hiramatsu Y. Right atrial myxoma with a large tumor embolus in the left pulmonary artery. J Surg Case Rep. 2014;2014(10) doi: 10.1093/jscr/rju115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoane L., Allen J.H., Collins H.A. Radiologic observations in cerebral embolization from left heart myxomas. Radiology. 1966;87:262–266. doi: 10.1148/87.2.262. [DOI] [PubMed] [Google Scholar]
- 42.New P.F.J., Price D.L., Carter B. Cerebral angiography in cardiac myxoma. Radiology. 1970;96:335–345. doi: 10.1148/96.2.335. [DOI] [PubMed] [Google Scholar]
- 43.Jelic J., Milicić D., Alfirević I., Anić D., Baudoin Z., Bulat C. Cardiac myxoma: diagnostic approach, surgical treatment and follow-up. A twenty years experience. J Cardiovasc Surg (Torino) 1996;37:113–117. [PubMed] [Google Scholar]
- 44.Jain R.S., Nagpal K., Jain R., Prakash S., Handa R. Acute psychosis presenting as a sole manifestation of left atrial myxoma: a new paradigm. Am J Emerg Med. 2014;32(1556):e3–e5. doi: 10.1016/j.ajem.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Lanjewar D.N., Bhatia V.O., Lanjewar S.D., Carney J.A. Cutaneous myxoma: an important clue to Carney complex. Indian J Pathol Microbiol. 2014;57:460–462. doi: 10.4103/0377-4929.138771. [DOI] [PubMed] [Google Scholar]
- 46.Birla S., Aggarwal S., Sharma A., Tandon N. Rare association of acromegaly with left atrial myxoma in Carney’s complex due to novel PRKAR1A mutation. Endocrinol Diabetes Metab Case Rep. 2014;2014:140023. doi: 10.1530/EDM-14-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michaud M., Wolff V., Pelletier S., Evon P., Richard S. Painful multiple mononeuropathy as a first symptom of cardiac myxoma: an unusual clinical presentation. Cardiovasc Pathol. 2015;24:121–123. doi: 10.1016/j.carpath.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Stiver K., Bittenbender P., Whitson B.A., Bush C.A. Left atrial myxoma causing coronary steal: an atypical cause of angina. Tex Heart Inst J. 2015;42:270–272. doi: 10.14503/THIJ-14-4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan S.M. Infected cardiac myxoma: an updated review. Braz J Cardiovasc Surg. 2015;30:571–578. doi: 10.5935/1678-9741.20140112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swartz M.F., Lutz C.J., Chandan V.S., Landas S., Fink G.W. Atrial myxomas: pathologic types, tumor location, and presenting symptoms. J Card Surg. 2006;21:435–440. doi: 10.1111/j.1540-8191.2006.00265.x. [DOI] [PubMed] [Google Scholar]
- 51.Loire R. Myxoma of the left atrium, clinical outcome of 100 operated patients. Arch Mal Coeur Vaiss. 1996;89:1119–1125. [PubMed] [Google Scholar]
- 52.Lone R.A., Ahanger A.G., Singh S., Mehmood W., Shah S., Lone G.N. Atrial myxoma: trends in management. Int J Health Sci. 2008;2:141–151. [PMC free article] [PubMed] [Google Scholar]
- 53.Gee G.T., Bazan C., 3rd, Jinkins J.R. Imaging of cerebral infarction caused by atrial myxoma. Neuroradiology. 1994;36:271–272. doi: 10.1007/BF00593257. [DOI] [PubMed] [Google Scholar]
- 54.Yeh H.H., Yang C.C., Tung W.F., Wang H.F., Tung J.N. Young stroke, cardiac myxoma, and multiple emboli: a case report and literature review. Acta Neurol Taiwan. 2006;15:201–205. [PubMed] [Google Scholar]
- 55.Jones D.R., Warden H.E., Murray G.F., Hill R.C., Graeber G.M., Cruzzavala J.L. Biatrial approach to cardiac myxomas: a 3 year clinical experience. Ann Thorac Surg. 1995;59:851–855. doi: 10.1016/0003-4975(95)00064-r. [DOI] [PubMed] [Google Scholar]