Abstract
Background
Excessive coronary calcification can lead to adverse outcomes after percutaneous coronary intervention (PCI). We therefore evaluated the impact of coronary calcium score (CCS) measured by multidetector computed tomography (MDCT) on immediate complications of PCI and rate of restenosis.
Methods
We performed a single-center retrospective analysis of 84 patients with coronary stenosis diagnosed by MDCT who underwent PCI. The Agatston method was used to measure total, target-vessel, and segmental (stent deployment site) CCS.
Results
In 108 PCI procedures, 32 lesions (29.5%) were American College of Cardiology/American Heart Association type A, 60 (55.5%) were type B, and 16 (15%) were type C. ANOVA showed significantly higher segmental CCS in type C than in type A lesions (29 ± 51 vs. 214 ± 162; p = 0.03). Six patients (7.1%) had periprocedural complications and seven (8.3%) had in-stent restenosis and angina. Mean total, target-vessel, and segmental CCS was significantly higher in complicated than in successful PCI (199 ± 325 vs. 816 ± 624, p = 0.001; 92 ± 207 vs. 337 ± 157, p = 0.001; and 79 ± 158 vs. 256 ± 142, p = 0.003, respectively), but there was no significant difference in CCS between successful PCI and PCI complicated by late restenosis.
Conclusions
CCS measured by MDCT has an important role in predicting early, but not late, complications from PCI.
Keywords: Coronary calcification, Percutaneous coronary intervention, Multidetector computed tomography, Periprocedural complications, In-stent restenosis
Abbreviations
- BVS
bioresorbable vascular scaffold
- CCA
conventional coronary angiography
- CCS
coronary calcium score
- MDCT
multidetector computed tomography
- PCI
percutaneous coronary intervention
- SYNTAX
synergy between PCI with TAXUS and cardiac surgery
Introduction
Percutaneous coronary intervention (PCI) is considered an effective treatment in selected patients with symptomatic coronary artery disease [1], [2], [3], [4], [5]. Many factors are associated with procedure outcome, such as lesion morphology, patient age, presence of multivessel disease, performance of an urgent or emergency procedure, and presence of congestive heart failure class III or IV [6], [7], [8], [9], [10]. Excess coronary calcium measured by electron-beam computed tomography is associated with a higher rate of unsuccessful percutaneous transluminal coronary angioplasty [11]. The presence of severe coronary calcification assessed by angiography in patients with acute coronary syndrome is associated with higher rates of cardiac death, ischemic target-lesion revascularization, and in-stent thrombosis at 1-year follow-up [12]. There are no clear data regarding the effect of coronary calcium score (CCS) measured by multidetector computed tomography (MDCT) on the outcome of PCI employing a drug-eluting stent. We hypothesized that increased CCS is associated with increased immediate and long-term PCI-related complications.
Materials and methods
Study settings and participants
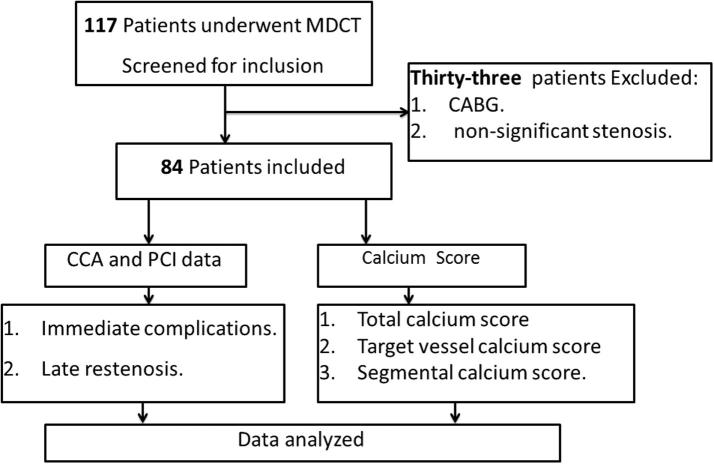
We conducted a retrospective review of 117 patients referred from an outpatient clinic with nonacute chest pain, who underwent MDCT between January 2011 and April 2014. These patients were referred for conventional coronary angiography (CCA) because of either high CCS or evidence of significant coronary artery stenosis by computed tomography angiography. Thirty-three patients did not undergo PCI because they had nonsignificant stenosis or coronary artery bypass graft surgery and were thus excluded; therefore, the remaining 84 patients, who underwent PCI for one or more coronary arteries, were included in our analysis (Fig. 1). Our study took place at Prince Sultan Cardiac Center Al Qassim and was approved by the center’s research ethics committee. Exclusion criteria were a history of PCI or coronary artery bypass grafting before MDCT.
Figure 1.
Study design. CABG = coronary artery bypasses graft; CCA = conventional coronary angiography; MDCT = multidetector computed tomography; PCI = percutaneous coronary intervention.
CT protocol
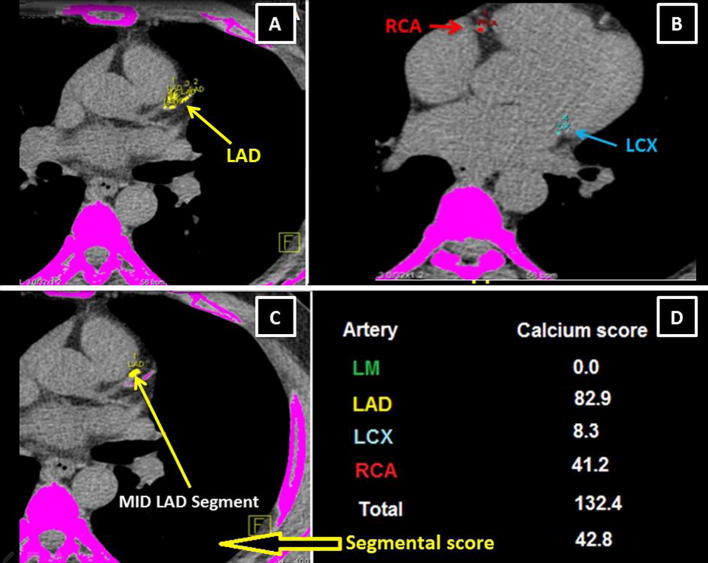
MDCT was performed using a Siemens Definition Flash scanner (Siemens Healthcare, Forchheim, Germany) with 0.28-second gantry rotation, 75-ms temporal resolution, and 0.6-mm collimation. Acquisition started with a scout image of the chest to define the field of view. Calcium-score imaging employed prospective electrocardiographic triggering at the best diastolic phase with 3-mm slice thickness. The images were reconstructed and analyzed using a multimodality workplace dedicated CT workstation with VE40A calcium score software (Siemens Medical Solutions, Forchheim, Germany) to calculate CCS using the Agatston method [13]. Calcium was defined as a lesion with a CT density of ⩾130 Hounsfield units and an area of ⩾1 mm2 [13]. The calcium score of each coronary artery, total coronary calcification, and segmental calcium score at the sites of stent deployment were measured (Fig. 2).
Figure 2.
Multidetector computed tomography noncontrast axial images show the measurement of calcium score of the coronary arteries. (A) Calcium at the LAD; (B) RCA calcium (red) and LCX (blue); (C) segmental calcification (middle LAD segment); (D) the calcium score of each coronary artery, total coronary and segmental calcium score. LAD = left anterior descending artery; LCX = left circumflex artery; RCA = right coronary artery.
Coronary angiography and percutaneous coronary intervention
Standard CCA techniques were used to assess the left and right coronary systems in multiple projections. All CCA procedures were performed using flat-detector technology (Philips Allura Xper FD10/10 biplane system; Philips, Eindhoven, the Netherlands). A drug-eluting stent was implanted in each patient during PCI. No anticoagulation was used prior to the procedure, although dual antiplatelet therapy was started immediately prior to the procedure and continued for >12 months. Patients post stent thrombosis were started on a double dose of clopidogrel (150 mg) for 1 week then a regular daily dose of clopidogrel (75 mg) in addition to aspirin (162 mg daily) for >1 year.
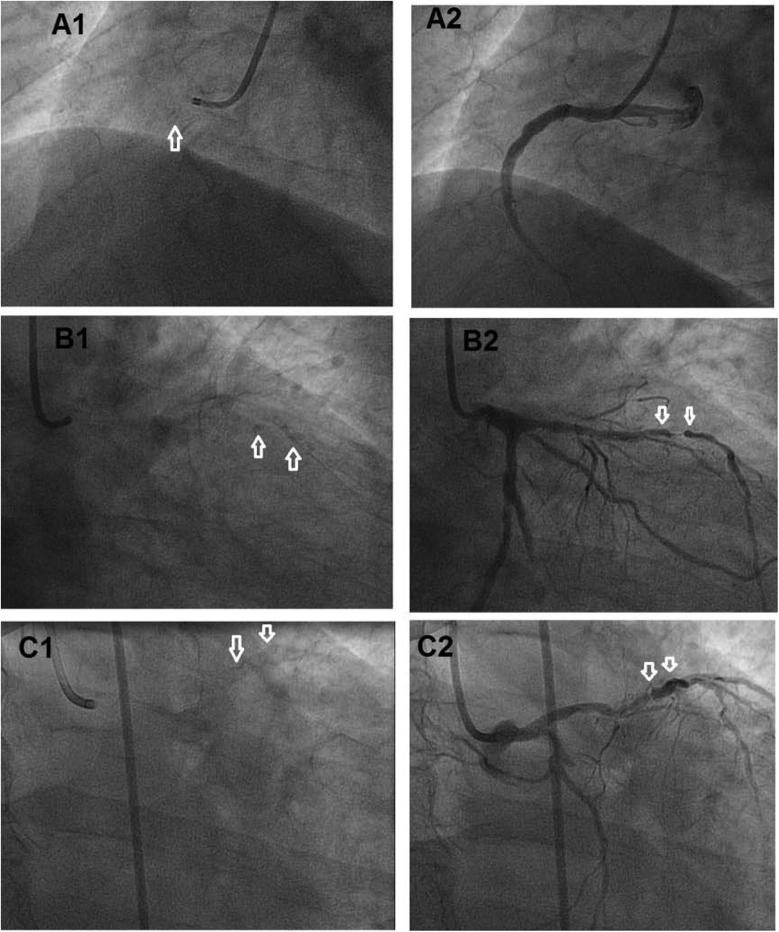
We analyzed percentage vessel stenosis, synergy between PCI with TAXUS and cardiac surgery (SYNTAX) score, American College of Cardiology/American Heart Association (ACC/AHA) classification of lesions (A, B, or C) [14], and angiographic calcium score [ANCS: 0, no calcification; 1, mild calcification (barely visible on close examination); 2, moderate calcification (radiopacity noted only during the cardiac cycle before contrast injection); and 3, severe calcification (radiopacity noted with contrast injection and generally involving both sides of the arterial wall)] [15], [16] (Fig. 3).
Figure 3.
Angiographic calcium score. (A1) Before and (A2) after contrast injection, showing mild calcification (barely visible on close examination); (B1 and B2) moderate calcification (radiopacity noted only during the cardiac cycle before contrast injection); (C1 and C2) severe calcification (radiopacity noted with contrast injection).
The interventionist reported the following parameters: procedure preparation categorized as easy (direct stenting, single balloon inflation) or difficult (multiple balloon inflations or use of Rotablator; Boston Scientific, Marlborough, MA, USA); durations of fluoroscopy and procedure; number and length of implanted stents, volume of contrast injected, final thrombolysis in myocardial infarction grade flow; and procedural complications such as no reflow, perforation, and dissection (defined as intimal tears in the arterial wall post stent deployment that required percutaneous management either by the deployment of further stents or prolonged balloon inflation for dissection sealing). Periprocedural myocardial infarction was defined as a more than five-fold increase above 99th percentile of upper normal limit in creatine kinase-myocardial band within 24 hours after PCI. Angiographic success was defined as a final thrombolysis in myocardial infarction grade flow of 3 and <10% stenosis with no procedural complications [17]. Late complications such as stent thrombosis, target-lesion revascularization, symptoms of angina, heart failure, and death were reported after 1-year follow-up.
Statistical analysis
Continuous data are presented as mean ± standard deviation and categorical variables are expressed as number and percentage. Student t test was used to analyze the differences between continuous variables and the chi-square test was used to compare categorical variables. ANOVA was used to compare the three different ACC/AHA lesion types with regard to segmental CCS and angiographic calcium score. All statistical analysis was performed using SPSS for Windows, Version 19.0 (SPSS Inc., Chicago, IL, USA). A p value of <0.05 was considered statistically significant for all tests.
Results
Table 1 shows patients’ clinical characteristics. ANOVA comparison of the differences among the three lesion types showed a significantly higher calcium score in type C than in type A lesions (29 ± 51 vs. 214 ± 162; p = 0.03), and a significantly higher ANCS with more advanced lesions (Table 2). Periprocedural complications were seen in six patients (7.1%): coronary dissection in four (4.8%; three dissections occurred after stent deployment and required an additional stent, and one coronary dissection was associated with stent delivery failure); and acute in-stent thrombosis in two (2.4%). Intravascular ultrasound was used during the procedure in three patients with coronary dissection to confirm the presence of a distal edge flap tear, while neither Rotablator nor optical coherence tomography were required.
Table 1.
Patient characteristics.
| Clinical characteristics | Patients (n = 84) |
|---|---|
| Age, mean ± SD (y) | 55 ± 12 |
| Male sex, n (%) | 55 (65) |
| Diabetes mellitus, n (%) | 40 (48) |
| Hypertension, n (%) | 42 (50) |
| Dyslipidemia, n (%) | 36 (43) |
| Family history of coronary artery disease, n (%) | 2 (2.4) |
| Current smoking, n (%) | 22 (26) |
| Body mass index, mean ± SD (kg/m2) | 29.2 ± 4.7 |
SD = standard deviation.
Table 2.
Multidetector computed tomography (MDCT) data with average total CCS and the percentage of vessel with detectable calcifications.
| MDCT data (84 patients) | ||||||
|---|---|---|---|---|---|---|
| CCS | Agatston score (mean ± SD) | Percentage of detected CCS, n (%) | ||||
| Total | 219 ± 337 | 80 (95.5) | ||||
| LM | 16 ± 32 | 36 (43) | ||||
| LAD | 81 ± 117 | 72 (86) | ||||
| LCX | 43 ± 81 | 62 (74) | ||||
| RCA | 80 ± 216 | 68 (81) | ||||
| CCA data | ||||||
| Lesion type | A | B | C |
p |
||
| A vs. B | A vs. C | B vs. C | ||||
| n (%) | 32 (29.5) | 60 (55.5) | 16 (15) | |||
| Segmental CCS (mean ± SD) | 29 ± 51 | 94 ± 197 | 214 ± 162 | 0.4 | 0.03 | 0.16 |
| Angiographic calcification | 0.7 ± 0.5 | 1.1 ± 0.6 | 1.9 ± 0.6 | 0.047 | 0.0001 | 0.007 |
| PCI data | ||||||
| Failed PCI, n (%) | 6 (7.1) | |||||
| Dissection | 4 | |||||
| In-stent thrombosis | 2 | |||||
| Late complications, n (%) | 7 (8.3) | |||||
| Angina | 5 | |||||
| In-stent restenosis | 2 | |||||
| Procedure time (min) | 38.5 ± 15 | |||||
| Fluoroscopy time (min) | 18 ± 7 | |||||
| Contrast (mL) | 97 ± 37 | |||||
| Stent number/vessel | 1.22 ± 0.46 | |||||
| Stent length (mm) | 25.8 ± 12.6 | |||||
CCA data show prevalence of different American College of Cardiology/American Heart Association type lesions and segmental CCS and angiographic score. PCI data shows the procedural success, times and contrast has been used.
CCA = conventional coronary angiography; CCS = coronary calcium score; LCX = left circumflex; LAD = left anterior descending; LM = left main; PCI = percutaneous coronary intervention; RCA = right coronary artery; SD = standard deviation.
Late complications occurred in eight patients (8.3%) during a follow-up of 14 ± 6 months: three (2.4%) had in-stent restenosis and underwent target-lesion revascularization; and five (5.9%) had symptoms of angina that were treated medically (2 patients had evidence of mild ischemia by myocardial perfusion imaging). Student t test analysis was used to compare PCIs with and without complications and with and without late complications. Results showed that the total, target-vessel, and segmental CCS were significantly higher when perioperative complications occurred (816 ± 624 vs.199 ± 325, p = 0.01; 337 ± 157 vs. 92 ± 207, p = 0.001; and 256 ± 142 vs. 79 ± 158, p = 0.003, respectively). In addition, longer procedure time seen with complicated PCI (48 ± 14 vs. 37 ± 14 minutes, p = 0.03). However, CCS did not differ significantly between patients with late complications and complication-free patients (Table 3, Table 4).
Table 3.
Comparisons between successful and periprocedure complicated percutaneous coronary intervention (PCI).
| Variables | Successful PCI (n = 102) | Complicated PCI (n = 6) | p |
|---|---|---|---|
| No. of patients | 78 | 6 | |
| Age, mean ± SD (y) | 54 ± 11 | 60 ± 16 | 0.2 |
| Male sex, n (%) | 50 (64) | 5 (83) | 0. |
| Diabetes mellitus, n (%) | 37 (47) | 3 (37.5) | 0.6 |
| Hypertension, n (%) | 40 (52) | 2 (16.6) | 0.3 |
| Dyslipidemia, n (%) | 32 (41) | 4 (66) | 0.2 |
| Family history of coronary artery disease, n (%) | 2 (2.5) | 0 (0) | 0.8 |
| Current smoking, n (%) | 19 (24) | 3 (50) | 0.18 |
| Total CCS, mean ± SD | 199 ± 325 | 816 ± 624 | 0.01 |
| Target vessel CCS, mean ± SD | 92 ± 207 | 337 ± 157 | 0.001 |
| Segmental CCS, mean ± SD | 79 ± 158 | 256 ± 142 | 0.003 |
| Stent length, mean ± SD (mm) | 24.6 ± 10.4 | 27 ± 15 | 0.1 |
| Number of stents used, mean ± SD | 1.2 ± 0.4 | 1.5 ± 0.85 | 0.28 |
| Procedure time, mean ± SD (min) | 37 ± 14 | 48 ± 14 | 0.03 |
| Fluoroscopy time, mean ± SD (min) | 17 ± 6 | 20 ± 7 | 0.19 |
| Contrast used, mean ± SD (mL) | 101 ± 38 | 93 ± 29 | 0.12 |
CCS = coronary calcium score; SD = standard deviation.
Table 4.
Comparisons between PCI with and without long-term complications.
| Variables | No restenosis (n = 101) | Late restenosis (n = 7) | p |
|---|---|---|---|
| No. of patients | 77 | 7 | |
| Age, mean ± SD (y) | 54 ± 11 | 56 ± 16 | 0.6 |
| Male sex, n (%) | 50 (65) | 5 (71) | 0.54 |
| Diabetes mellitus, n (%) | 36 (47) | 4 (57) | 0.44 |
| Hypertension, n (%) | 40 (52) | 2 (28.5) | 0.2 |
| Dyslipidemia, n (%) | 32 (41.5) | 4 (57) | 0.34 |
| Family history of coronary artery disease, n (%) | 2 (2.5) | 0 (0) | 0.8 |
| Current smoking, n (%) | 20 (26) | 2 (28.5) | 0.59 |
| Total CCS, mean ± SD | 200 ± 335 | 193 ± 190 | 0.9 |
| Target vessel CCS, mean ± SD | 78 ± 163 | 89 ± 89 | 0.78 |
| Segmental CCS, mean ± SD | 79 ± 158 | 256 ± 142 | 0.8 |
| Stent length, mean ± SD (mm) | 25 ± 10 | 23 ± 7 | 0.23 |
| Number of stents used, mean ± SD | 1.2 ± 0.5 | 1.3 ± 0.3 | 0.17 |
| Procedure time, mean ± SD (min) | 37 ± 14 | 36 ± 13 | 0.8 |
| Fluoroscopy time, mean ± SD (min) | 17 ± 6 | 15 ± 3 | 0.2 |
| Contrast used, mean ± SD (mL) | 98 ± 35 | 106 ± 37 | 0.2 |
CCS = coronary calcium score; SD = standard deviation.
Discussion
PCI is widely used to treat patients with significant coronary artery stenosis. Despite improvements in PCI technology and pharmacotherapy, the incidence of periprocedural myocardial infarction is about 3.5% and is due mostly to procedure complications such as stent thrombosis and side-branch occlusion [18]. The complexity of a lesion plays an important role in the prediction of PCI outcome; modified-ACC/AHA type C lesions are associated with lower procedure success rates and worse clinical outcomes [19]. In fact, the presence of calcium in an atheroma defines it as an advanced atherosclerotic lesion [20]. Furthermore, a lesion’s complexity by modified ACC/AHA classification and SYNTAX score, which is an anatomical scoring system that can predict major adverse cardiac events, is determined to a great degree by the presence of excessive calcification. In addition, a higher SYNTAX score is associated with a worse outcome in patients undergoing PCI [21], [22]. Rotational atherectomy can overcome extremely complex lesions with severe calcification by debulking atherosclerotic plaque before stent deployment during PCI, but there is conflicting evidence concerning its benefits [23]. Coronary calcification can be assessed qualitatively by invasive CCA using ANCS classification. In contrast, intravascular ultrasound is more sensitive and allows quantitative assessment of arterial calcification [24], and MDCT is a well-established, noninvasive tool that permits accurate measurement of CCS using the Agatston method [13]. Additionally, MDCT-based coronary angiography can provide valuable information that can assist coronary intervention by determining the severity of stenosis, the extent of calcification, the presence of multiple stenosis, and reliably estimate lesion lengths, vessel tortuosity, and the diameter of distal segments [25].
In the present analysis, we found that the amount of coronary calcium measured by total, target vessel, or segmental CCS can significantly predict periprocedural success and complications but not restenosis rate. Our results are consistent with those of a previous report by Wang et al. [26], which showed that heavy coronary calcification assessed by MDCT can predict periprocedural myocardial infarction in patients undergoing elective PCI, but which did not include data on long-term follow up. A similar finding by Sinitsyn et al. [11] was that the complication rates of percutaneous transluminal coronary angioplasty and PCI were higher with a higher CCS measured by electron-beam computed tomography. In that study, however, bare-metal stents were used and the complication rate higher than in the present study, in which patients received drug-eluting stents. Mehran et al. [27] reported that a higher calcification and plaque burden by preintervention intravascular ultrasound was associated with creatine kinase–myocardial band elevation after coronary intervention. In addition, Genereux et al. [12] demonstrated that severe calcification of the coronary target lesion assessed by CCA can independently predict major bleeding events after PCI in patients presenting with acute coronary syndrome.
Despite the standard dose of dual antiplatelet therapy used before the procedure, we observed a fairly high incidence of acute stent thrombosis (2.4%), which may be explained by the small number of patients in the study.
The results of the present study have clinical implication in patients who have MDCT before the CCA, the CCS data may help in management plan when the decision and outcome regarding PCI versus coronary artery bypass graft is indistinct.
With increasing use of bioresorbable vascular scaffold (BVS) during PCI, which requires optimal (1:1 balloon/vessel) predilatation to improves scaffold expansion, CCS will probably play valuable role in predicting optimal predilatation achievement with BVS. Therefore, we advise further studies to assess the role of CCS measured by MDCT before BVS deployment.
Our study had several limitations. First, our analysis is retrospective. Second, the number of patients included in the study and the number of complications was small. Finally, we did not investigate the effect of noncalcified plaque burden on the procedure outcome; therefore, prospective studies with larger numbers of patients should be conducted and other computed tomographic angiography data, such as noncalcified plaque type and burden, should be collected.
Conclusion
Our study showed that increased coronary calcification measured by MDCT is associated with an increase in immediate PCI complications but did not predict late complications such as in-stent restenosis.
Conflicts of interest
All authors have no conflicts of interest to declare.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Weintraub W.S., Spertus J.A., Kolm P., Maron D.J., Zhang Z., Jurkovitz C. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med. 2008;359:677–687. doi: 10.1056/NEJMoa072771. [DOI] [PubMed] [Google Scholar]
- 2.Boden W.E., O’Rourke R.A., Teo K.K., Hartigan P.M., Maron D.J., Kostuk W.J. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 3.Benzer W., Hofer S., Oldridge N.B. Health-related quality of life in patients with coronary artery disease after different treatments for angina in routine clinical practice. Herz. 2003;28:421–428. doi: 10.1007/s00059-003-2388-9. [DOI] [PubMed] [Google Scholar]
- 4.Wijeysundera H.C., Nallamothu B.K., Krumholz H.M., Tu J.V., Ko D.T. Metaanalysis: effects of percutaneous coronary intervention versus medical therapy on angina relief. Ann Intern Med. 2010;152:370–379. doi: 10.7326/0003-4819-152-6-201003160-00007. [DOI] [PubMed] [Google Scholar]
- 5.Abizaid A., Costa M.A., Centemero M., Abizaid A.S., Legrand V.M., Limet R.V. Clinical and economic impact of diabetes mellitus on percutaneous and surgical treatment of multivessel coronary disease patients: insights from the Arterial Revascularization Therapy Study (ARTS) trial. Circulation. 2001;104:533–538. doi: 10.1161/hc3101.093700. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi M.A., Safian R.D., Grines C.L., Goldstein J.A., Westveer D.C., Glazier S. Simplified scoring system for predicting mortality after percutaneous coronary intervention. J Am Coll Cardiol. 2003;42:1890–1895. doi: 10.1016/j.jacc.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Singh M., Lennon R.J., Holmes D.R., Jr, Bell M.R., Rihal C.S. Correlates of procedural complications and a simple integer risk score for percutaneous coronary intervention. J Am Coll Cardiol. 2002;40:387–393. doi: 10.1016/s0735-1097(02)01980-0. [DOI] [PubMed] [Google Scholar]
- 8.Hannan E.L., Arani D.T., Johnson L.W., Kemp H.G., Jr, Lukacik G. Percutaneous transluminal coronary angioplasty in New York State. Risk factors and outcomes. JAMA. 1992;268:3092–3097. [PubMed] [Google Scholar]
- 9.Shaw R.E., Anderson H.V., Brindis R.G., Krone R.J., Klein L.W., McKay C.R. Development of a risk adjustment mortality model using the American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR) experience: 1998–2000. J Am Coll Cardiol. 2002;39:1104–1112. doi: 10.1016/s0735-1097(02)01731-x. [DOI] [PubMed] [Google Scholar]
- 10.Singh M., Rihal C.S., Lennon R.J., Spertus J., Rumsfeld J.S., Holmes D.R., Jr. Bedside estimation of risk from percutaneous coronary intervention: the new Mayo Clinic risk scores. Mayo Clin Proc. 2007;82:701–708. doi: 10.4065/82.6.701. [DOI] [PubMed] [Google Scholar]
- 11.Sinitsyn V., Belkind M., Matchin Y., Lyakishev A., Naumov V., Ternovoy S. Relationships between coronary calcification detected at electron beam computed tomography and percutaneous transluminal coronary angioplasty results in coronary artery disease patients. Eur Radiol. 2003;13:62–67. doi: 10.1007/s00330-002-1399-x. [DOI] [PubMed] [Google Scholar]
- 12.Genereux P., Maehara A., Kirtane A., Brener S., Palmerini T., Lasalle L. Impact of coronary calcification on one-year outcomes after PCI in STEMI and NSTEMI: pooled analysis from horizons and ACUITY trials. J Am Coll Cardiol. 2013;61(10S) [Google Scholar]
- 13.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 14.Ryan T.J., Bauman W.B., Kennedy J.W., Kereiakes D.J., King S.B., 3rd, McCallister B.D. Guidelines for percutaneous transluminal coronary angioplasty. A report of the American Heart Association/American College of Cardiology Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Committee on Percutaneous Transluminal Coronary Angioplasty) Circulation. 1993;88:2987–3007. doi: 10.1161/01.cir.88.6.2987. [DOI] [PubMed] [Google Scholar]
- 15.Mintz G.S., Popma J.J., Pichard A.D., Kent K.M., Satler L.F., Chuang Y.C. Patterns of calcification in coronary artery disease. Circulation. 1995;91:1959–1965. doi: 10.1161/01.cir.91.7.1959. [DOI] [PubMed] [Google Scholar]
- 16.Tuzcu E.M., Berkalp B., De Franco A.C., Ellis S.G., Goormastic M., Whitlow P.L. The dilemma of diagnosing coronary calcification: angiography versus intravascular ultrasound. J Am Coll Cardiol. 1996;15(27):832–838. doi: 10.1016/0735-1097(95)00537-4. [DOI] [PubMed] [Google Scholar]
- 17.Smith S.C., Jr, Feldman T.E., Hirshfeld J.W., Jr, Jacobs A.K., Kern M.J., King S.B., 3rd ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention) Circulation. 2006;113:e166–e286. doi: 10.1161/CIRCULATIONAHA.106.173220. [DOI] [PubMed] [Google Scholar]
- 18.Muschart X., Slimani A., Jamart J., Chenu P., Dangoisse V., Gabriel L. The different mechanisms of periprocedural myocardial infarction and their impact on in-hospital outcome. J Invasive Cardiol. 2012;24:655–660. [PubMed] [Google Scholar]
- 19.Kastrati A., Schömig A., Elezi S., Dirschinger J., Mehilli J., Schühlen H. Prognostic value of the modified American college of Cardiology/American Heart Association stenosis morphology classification for long-term angiographic and clinical outcome after coronary stent placement. Circulation. 1999;100:1285–1290. doi: 10.1161/01.cir.100.12.1285. [DOI] [PubMed] [Google Scholar]
- 20.Stary H.C., Chandler A.B., Dinsmore R.E., Fuster V., Glagov S., Insull W., Jr A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis. American Heart Association. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 21.SYNTAX Score. <www.syntaxscore.com> [accessed 01.03.10].
- 22.Valgimigli M., Serruys P.W., Tsuchida K., Vaina S., Morel M.A., van den Brand M.J. Cyphering the complexity of coronary artery disease using the syntax score to predict clinical outcome in patients with three-vessel lumen obstruction undergoing percutaneous coronary intervention. Am J Cardiol. 2007;99:1072–1081. doi: 10.1016/j.amjcard.2006.11.062. [DOI] [PubMed] [Google Scholar]
- 23.Wasiak J., Law J., Watson P., Spinks A. Percutaneous transluminal rotational atherectomy for coronary artery disease. Cochrane Database Syst Rev. 2012;12:CD003334. doi: 10.1002/14651858.CD003334.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tobis J.M., Mallery J., Mahon D., Lehmann K., Zalesky P., Griffith J. Intravascular ultrasound imaging of human coronary arteries in vivo. Analysis of tissue characterizations with comparison to in vitro histological specimens. Circulation. 1991;83:913–926. doi: 10.1161/01.cir.83.3.913. [DOI] [PubMed] [Google Scholar]
- 25.Opolski M.P., Achenbach S. CT angiography for revascularization of CTO: crossing the borders of diagnosis and treatment. JACC Cardiovasc Imaging. 2015;8:846–858. doi: 10.1016/j.jcmg.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Wang X., Liu X., Ge H., Yang Q., Liu X., Shi D. Positive association of coronary calcium detected by computed tomography coronary angiography with periprocedural myocardial infarction. PLoS One. 2013;8:e82835. doi: 10.1371/journal.pone.0082835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehran R., Dangas G., Mintz G.S., Lansky A.J., Pichard A.D., Satler K.J. Atherosclerotic plaque burden and CK-MB enzyme elevation after coronary interventions: intravascular ultrasound study of 2256 patients. Circulation. 2000;101:604–610. doi: 10.1161/01.cir.101.6.604. [DOI] [PubMed] [Google Scholar]