Abstract
Takotsubo cardiomyopathy, also known as “takotsubo syndrome,” refers to transient apical ballooning syndrome, stress cardiomyopathy, or broken heart syndrome and is a recently recognized syndrome typically characterized by transient and reversible left ventricular dysfunction that develops in the setting of acute severe emotional or physical stress. Increased catecholamine levels have been proposed to play a central role in the pathogenesis of the disease, although the specific pathophysiology of this condition remains to be fully determined. At present, there have been very few reports of recurrent takotsubo cardiomyopathy. In this case report, we present a patient with multiple recurrences of takotsubo syndrome triggered by severe emotional stress that presented with recurrent loss of consciousness, QT prolongation, and polymorphic ventricular tachycardia (torsade de pointes) and left ventricular apical thrombus.
Keywords: Apical ballooning, QT prolongation, Recurrence, Takotsubo, Thrombus, Torsade de pointes, Ventricular dysfunction
Abbreviations
- TTS
Takotsubo syndrome
- LV
Left ventricular
- ACS
Acute coronary syndrome
- ECG
Electrocardiogram
- LOC
Loss of consciousness
- LQT
Long QT
- TdP
Torsade de pointes
- EF
Ejection fraction
- ICD
Internal cardiac defibrillator
- VF
Ventricular fibrillation
Introduction
Takotsubo cardiomyopathy with a recently recommended nomenclature of Takotsubo syndrome (TTS) is an increasingly recognized entity characterized by transient (reversible), mainly apical and mid left ventricular (LV) dysfunction, which less commonly involves other LV segments (sparing the apex) in the absence of significant coronary artery disease that is potentially triggered by emotional, physical stress (primary TTS), medical illness (acute exacerbations of multiple medical conditions such as asthma, pneumothorax, gastrointestinal bleeding, or hypoglycemia), or surgical intervention (secondary TTS) [1], [2], [3], [4], [5]. Increased catecholamine surges have been proposed to play a central role in the pathogenesis of this condition [6].
The takotsubo phenomenon was first reported in 1990 by Sato et al. [1], and fully described in 1991 by Dote et al. [2], who named the syndrome “takotsubo-like cardiomyopathy” because the appearance resembled a pot historically used in Japan to catch octopi. It is also known as stress-induced cardiomyopathy, apical ballooning syndrome, broken heart syndrome, and ampulla cardiomyopathy [1], [2], [3], [4], [5].
Typically, the LV function recovers and normalizes in few days to weeks. It may account for up to 2% of suspected acute coronary syndrome (ACS). Takotsubo syndrome is much more common in women than in men, particularly postmenopausal women [2], [3], [4], [7]. In a review of six prospective and four retrospective studies women accounted for 80–100% of cases, with a mean age of 61–76 years [7]. Triggering factors were bereavements of loved one, devastating financial losses, natural disasters, acute physical and critical illness, medical procedures or surgeries, and other catastrophic news.
Takotsubo cardiomyopathy (syndrome) is a diagnosis of exclusion [5], [8]. Researchers at the Mayo Clinic proposed diagnostic criteria in 2004, that include: (1) transient hypokinesis, akinesis, or dyskinesis in the LV midsegments with or without apical involvement; regional wall motion abnormalities that extend beyond a single epicardial vascular distribution, and frequently, but not always, a stressful trigger; (2) the absence of obstructive coronary disease or angiographic evidence of acute plaque rupture; (3) new electrocardiogram (ECG) abnormalities (ST-segment elevation and/or T-wave inversion) or modest elevation in cardiac troponin; and (4) the absence of pheochromocytoma and myocarditis [3]. In a recent position statement of the European Society of Cardiology, a new set of seven diagnostic criteria are proposed incorporating anatomical features, ECG changes, cardiac biomarkers, and reversibility of the myocardial dysfunction [5]. Numerous etiologies have been described, including catecholamine release during stress [3], [9], [10] and microvascular spasm or ischemia [11], [12].
Acute complications of TTS include tachyarrhythmias, bradyarrhythmias, pulmonary edema, cardiogenic shock, transient LV outflow tract obstruction, mitral valve dysfunction, acute thrombus formation, stroke, and death [5], [13].
Before this recent position statement [5], there were no established treatment algorithms for TTS; however, a new management algorithm is now proposed based on risk stratification pathways [5]. Most patients present with acute chest pain mimicking an ACS and are treated according to ACS guidelines. TTS was generally considered a benign condition; however, in-hospital mortality is 0–8% and death is much more common in the setting of LV-outflow obstruction and from noncardiac causes [13], [14], [15], [16], [17], [18]. The long-term outcome of TTS is not as benign as previously believed with recently reported 5-year mortality rates of 3–17% [5]. The results of a recent population-based registry showed that mortality rates in patients with TTS were worse than in control individuals without coronary artery disease and comparable to patients with ACS [19].
Recurrent TTS data are limited due to the relative short-term observation and only a few cases have been previously reported [16], [17]. Currently no evidence supports prophylactic treatment after the first presentation.
We present in this report a rare case of recurrent apical ballooning syndrome in a woman who presented with recurrent chest pain, loss of consciousness (LOC), long QT (LQT), torsade de pointes (TdP), and LV thrombus during severe emotional stress.
Case report
A 48-year-old woman with a history of postpartum depression (no medications) with no coronary risk factors was first hospitalized 7 years previously (2009) following an episode of LOC at home and chest pain. She was diagnosed with heart failure with abnormal wall motion on transthoracic ECG manifesting as severe hypokinesis involving the apex. She was given guideline-directed medical therapy for heart failure in the form of angiotensin converting enzyme inhibitors, β-blockers, aspirin, and diuretic therapy. Four months later following improvement of her condition, coronary angiogram and echocardiography were performed in a UK center and both were reported to be normal, therefore all medications were discontinued.
Over the past 6 years she had infrequent syncope always after emotional stress, in 2010 again after a heightened emotional situation she had recurrent chest pain, LOC, and dyspnea at another institution. Echocardiography reported low LV ejection fraction (EF)—40% with apical wall motion abnormalities—and hypokinesis. Medical therapy with angiotensin converting enzyme inhibitors, β-blockers, aspirin, and diuretics was introduced.
One year later (2011), she was seen in our cardiology clinic for follow-up and echocardiography once again revealed normal LV function with an EF of 55%.
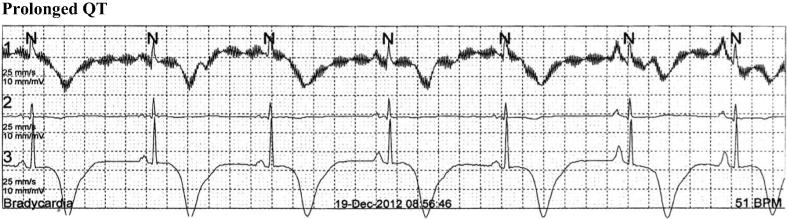
Further emotional distress 1 year later (2012) resulted in repeated LOC. ECG and rhythm strips upon hospitalization (Fig. 1) showed marked repolarization abnormalities with symmetrical deeply inverted T-wave in anterolateral leads, prolonged QT interval (corrected QT interval, 554 milliseconds) and sinus bradycardia (51 beats/min).
Figure 1.
Prolonged QT.
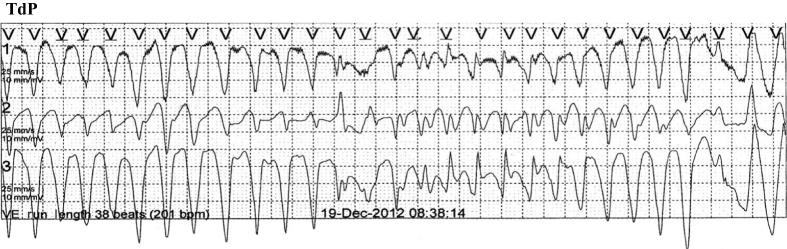
Telemetry on the coronary care unit showed several episodes of TdP which on five occasions rapidly degenerated to ventricular fibrillation (VF; Fig. 2). Serum electrolytes, inflammatory markers, and pulmonary hypertension were normal and she was not receiving any QT-prolonging drugs.
Figure 2.
Torsade de pointes.
She required electrical defibrillation and received magnesium sulfate and mexilitine. Additionally, β-blockers were held for bradycardia. Repeat coronary angiography again demonstrated normal epicardial vessels and no coronary spasm.
On reviewing her history, we noted that before this event, as well as all previous events, she was under the same severe emotionally stressful situations after which she always experienced central chest pain followed by LOC.
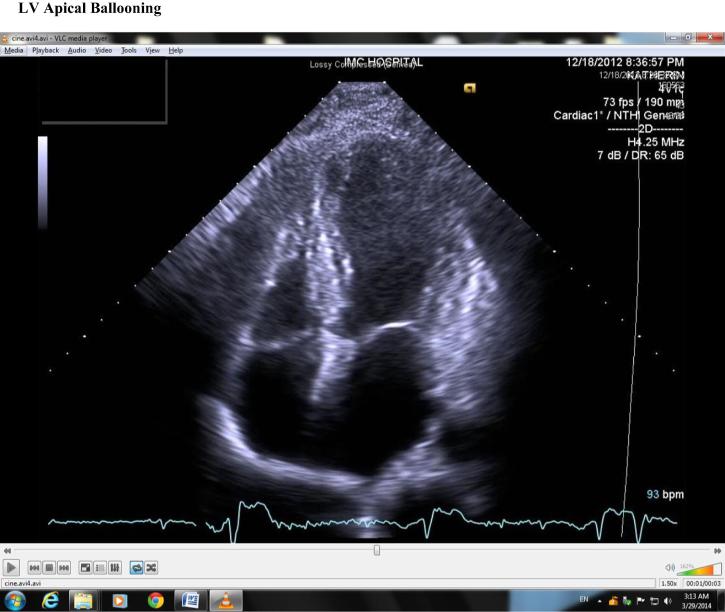
Echocardiography (Fig. 3) revealed ballooning of the LV apex, akinesia of the midanterior and midinferior wall with hyperkinesis of the basal segments, and an LV-EF of 30%.
Figure 3.
Left ventricular apical ballooning.
Internal cardiac defibrillator (ICD) implantation was decided for secondary prevention of sudden cardiac death.
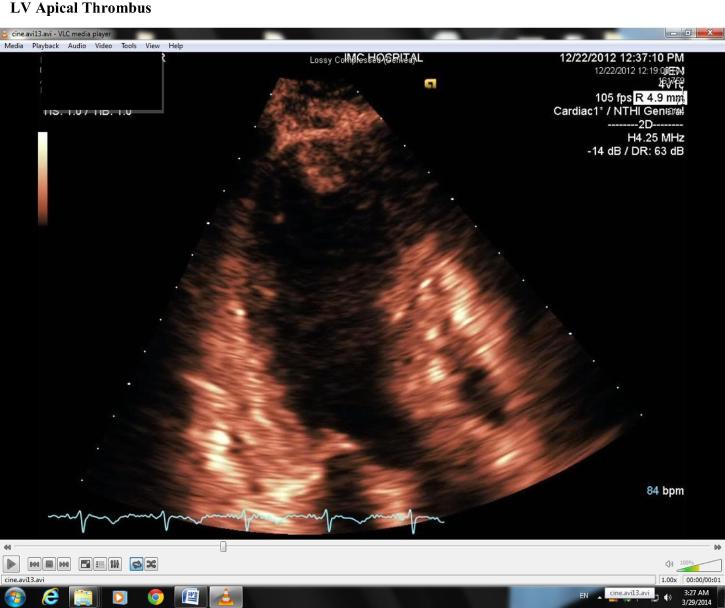
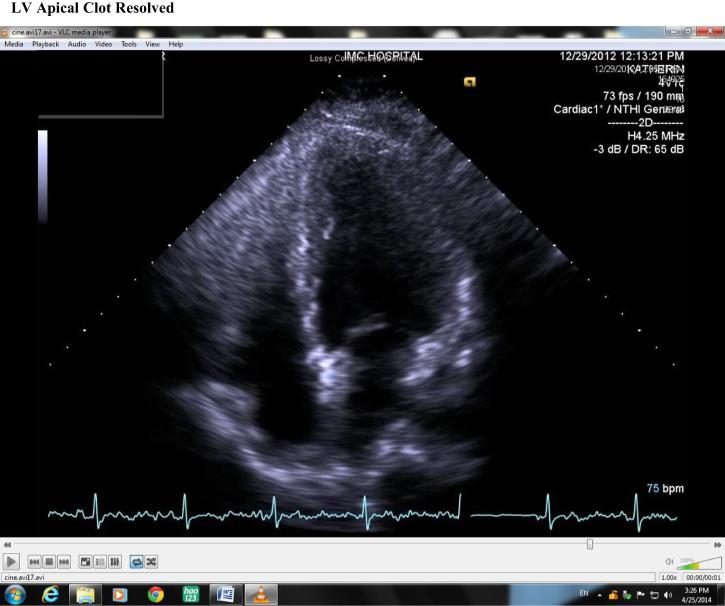
Four days later, while in hospital, repeat echocardiography revealed mild improvement of LV contractility with a rise in EF to 40%; however, there was a moderately sized LV apical thrombus (Fig. 4), hence anticoagulation was commenced. This soon resolved on repeat echocardiography 1 week later with marked improvement of LV contractility and once again normalization of LV function with an EF of 55% with resolution of the apical thrombus (Fig. 5).
Figure 4.
Left ventricular apical thrombus.
Figure 5.
Left ventricular apical clot resolved.
Serial ECGs for 3 consecutive days displayed marked repolarization abnormalities with fluctuating prolonged QT intervals that failed to normalize. After a long discussion among all treating physicians, the electrophysiologist, and the patient, an ICD was implanted; thereafter, she was completely stable.
The final diagnosis was as follows: recurrent TTS complicated by LV apical clot, acquired LQT, TdP, and VF cardiac arrest. She was discharged home on guideline-directed medical therapy for heart failure and she continues to have regular follow-ups. Echocardiogram repeated almost 1 year later revealed normal LV size and systolic function (EF, 55%) and there were no ICD therapies, heart block, or recorded arrhythmic events on device interrogation.
Discussion
TTS is a recently recognized syndrome. Many theories have been postulated for its possible pathophysiology. Studies have proposed the mechanism as an association with excessive sympathetic stimulation, microvascular dysfunction, and metabolic abnormalities [1], [2], [3], [4], [5], [6], [7], [20], [21].
Emotional and physical stresses often precipitate the clinical presentation. This suggests a relationship between cortical brain activity (a central catecholamine surge) and myocardial stunning [2], [3], [22], [23].
TTS is divided into two types: primary and secondary. In primary TTS, the acute cardiac symptoms are the primary reason for seeking care—such patients may or may not have clearly identifiable stressful triggers (often emotional). In secondary TTS, most cases occur in patients already hospitalized for another medical, surgical, obstetric, or psychiatric condition. In these patients, sudden activation of the sympathetic nervous system or a rise in catecholamines precipitates an acute TTS as a complication of the primary condition or its treatment.
Studies have found that patients with TTS have statistically significant higher levels of serum catecholamines (norepinephrine, epinephrine, and dopamine) than patients with myocardial infarctions [23], [24], [25], [26]. Increased beta-2-adrenoceptor activity in the setting of a high catecholaminergic state has been proposed as a possible reproducible model for this entity, inducing cardiac dysfunction and myocyte injury though calcium leakage due to hyperphosphorylation of ryanodine receptor 2 [27]. The apical portions of the LV have the highest concentration of sympathetic innervations found in the heart and increased beta-2 concentration gradient from the apex to the base could play an important role in apical myocardial dysfunction and ballooning commonly found in TTS cases [23], [24], [25], [26], [27]. Combining the results from multiple studies plasma norepinephrine levels were elevated in 74% of cases [15].
The pathogenesis of TTS may be multifactorial, similar to catecholamine-induced cardiomyopathy [24], pheochromocytoma [25], and subarachnoid hemorrhage [26]. The catecholamine hypothesis as a cause for reoccurring TTS, as in our case, can be further supported by observation of a similar reversible cardiomyopathy with global or focal dysfunction in patients with pheochromocytoma [25], in the setting of acute brain injury and Guillain–Barré autonomic neuropathy, which have also been postulated to be related to catecholamine excess. TTS has been reported as a novel association with catecholaminergic polymorphic VT particularly in young women with congenital LQT [28]. Catecholamine excess has reversible toxic effects on the myocardium, which has been documented in cases of pheochromocytoma [25], [26], [27], [28], [29], [30], [31]. Histological examination of biopsy samples from the affected LV of patients with TTS has shown intracellular accumulation of glycogen, many vacuoles, disorganized cytoskeleton and contractile structure, contraction band necrosis, and increased extracellular matrix proteins, which is associated with clinical states of catecholamine excess [32], [33], [34]. These alterations resolved nearly completely after functional recovery.
TTS is associated with minor release of cardiac enzymes, which suggests some microscopic damage to the myocytes. The absence of causative coronary artery disease on angiography and the diffuse rather than localized wall motion abnormalities point to an insult that is global but microscopic in nature.
In our report we present an interesting case with several important parts including repeated stress-induced syncope from acquired LQT and TdP, recurrent TTS, and development of apical thrombosis. Our patient did not have any significant family history of cardiac disease or sudden death and thus we did not test her for ryanodine receptor mutations.
Recurrent TTS data is limited due to the relative short-term observation and only a few cases have been previously reported [16], [17]. Five-year recurrence rates of 5–22% have been reported, with the second episode occurring from 3 months to 10 years after the index event [5]. Recurrence of a different anatomical variant has been reported. If a patient has a recurrence, long-term clinical follow-up should be considered. Currently no evidence supports prophylactic treatment after the first presentation.
There are no controlled data to define the optimal medical regimen to treat TTS, but it has been advocated that it is reasonable to treat these patients with standard medications for LV systolic dysfunction with guideline-directed medical therapy for heart failure. These include angiotensin converting enzyme inhibitors, beta-blockers, and diuretics, particularly for volume overload states [3]. Aspirin and statin therapy are also reasonable [8]. It was reported that thrombosis occurs in TTS cases, which might reflect vasoconstrictor, platelet activation, or prothrombotic effects of extremely high epinephrine levels [33], [34], [35], [36], [37]. In one study, 5% of patients with TTS developed LV thrombus, and all patients with LV thrombus were started on anticoagulation and one patient developed stroke [38]. This must be weighed against the hypothesized increased risk of cardiac rupture with apical ballooning and aspirin or heparin therapy [37]. Consequently, the role of anticoagulation is largely regarded on a case by case basis. It is reasonable to continue anticoagulation until the LV function returns and thrombus resolution [39]. In our case, heparin infusion was given for 7 days and was stopped after the disappearance of apical thrombus and oral anticoagulation was planned to start after ICD implantation, but after repeating the echo before the procedure, apical ballooning disappeared and LV contractility returned back to normal, therefore oral anticoagulation was abandoned.
Arrhythmia resulting from QT prolongation is commonly observed in patients with TTS. The prevalence of QT interval prolongation among TTS patients is high, ranging from 50% to 100% according to different case series [40], [41], [42]. QT interval prolongation might precipitate TdP, a polymorphic ventricular tachycardia that might lead to ventricular fibrillation and sudden death. This potentially fatal arrhythmia is associated with administration of QT prolonging agents, hypokalemia, hypomagnesemia, and congenital LQT syndrome, which were not applicable in our case.
Although QT interval prolongation is prevalent among TTS patients and might precede TdP, the latter has rarely been reported in TTS patients (it featured in 7 of 286 patients from the German registry data) [43]. Hypothesized ventricular arrhythmia mechanisms are not solely attributed to cathetcholamine excess and enhanced sympathetic activity and have also been proposed to occur from myocardial inhomogeneity resulting from myocardial edema in the LV dysfunctional segments and its predisposition to repolarization abnormalities including acquired LQT [43].
Literature reports the administration of magnesium sulfate as an effective therapy for ventricular tachycardia in the acute phase of TTS if the QT interval is prolonged [41], [43]. This reflected our acute phase management. However, we opted for ICD implantation given the degeneration to TdP to VF on multiple occasions, although at present, there is no clear cut recommendation for ICD implantation as a prophylaxis and some data have shown that pacemaker implantation is necessary and continues to be required even after resolution of the acute and subacute phases if the index dysrhythmia was heart block or sinus node suppression [44]. Contrarily, resolution of TdP was largely observed after resolution of the acute and subacute phases supporting transient measures like temporary pacing or wearable defibrillators rather than implantable ICDs. Similar to the reported literature, our patient at follow-up device interrogation did not suffer further ventricular arrhythmias.
β-Adrenoceptor blockers can prolong the QT interval and leave unopposed the potential adverse effects of high local concentrations of catecholamines at α-adrenoceptors. The use of β-blockers in the acute phase of TTS is still a matter of debate. Given the findings in an animal model, treatment with a combined α- and β-blocker seems rational, whereas treatment with a catecholamine as a pressor and cardiotonic seems contraindicated [43], [44], [45], [46], [47].
This case of recurrent TTS, LOC with acquired LQT, complicated by serious ventricular arrhythmias (TdP and VF) and acute LV apical thrombus is considered one of the rarest cases of TTS reported in literature. Chronic management of TTS is primarily empirical, and involves treatment of the underlying causes and situations. In patients who are hemodynamically stable, it is advantageous to prevent excessive sympathetic activation by combining α- and β-blockade. Administration of magnesium sulfate is the treatment of choice for ventricular tachycardia in the acute phase of TTS with LQT. In TTS complicated by LV apical thrombus, it is reasonable to continue anticoagulation until the LV function returns to normal.
We hope that our case with recurrent relapses and complications contributes to the understanding of this interesting rare condition and will further help in the management and follow-up of similar patients. We need to understand the pathogenesis and to advise rational treatment and prevention strategies, and to pay more attention not only to the myocardium and coronary arteries but also to the integration of central neural, autonomic, endocrine, and circulatory systems in emotional distress.
Lastly, we think more researches are needed to find a way to risk stratify TTS patients to detect those at risk for recurrence.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Sato H., Tateishi H., Uchida T., Dote K., Ishihara M. Takotsubo type cardiomyopathy due to multivessel spasm. In: Kodama K., Haze K., Hon M., editors. Clinical aspect of myocardial injury: from ischemia to heart failure. Kagakuhyouronsya; Tokyo: 1990. pp. 56–64. [In Japanese] [Google Scholar]
- 2.Dote K., Sato H., Tateishi H., Uchida T., Ishihara M. Myocardial stunning due to simultaneous multivessel coronary spasm: a review of 5 cases. J Cardiol. 1991;21:203–214. [PubMed] [Google Scholar]
- 3.Bybee K.A., Kara T., Prasad A., Lerman A., Barsness G.W., Wright R.S. Systematic review: transient LV apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141:858–865. doi: 10.7326/0003-4819-141-11-200412070-00010. [DOI] [PubMed] [Google Scholar]
- 4.Tsuchihashi K., Ueshima K., Uchida T., Oh-mura N., Kimura K., Owa M. Transient LV apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina pectoris-myocardial infarction investigations in Japan. J Am Coll Cardiol. 2001;38:11–18. doi: 10.1016/s0735-1097(01)01316-x. [DOI] [PubMed] [Google Scholar]
- 5.Lyon A.R., Bossone E., Schneider B., Sechtem U., Citro R., Underwood S.R. Current state of knowledge on Takotsubo syndrome: a position statement from the taskforce on Takotsubo syndrome of the Heart Failure Association of the European Society of Cardiology. Euro J Heart Fail. 2016;18:8–27. doi: 10.1002/ejhf.424. [DOI] [PubMed] [Google Scholar]
- 6.Abraham J., Mudd J.O., Kapur N.K., Klein K., Champion H.C., Wittstein I.S. Stress cardiomyopathy after intravenous administration of catecholamines and beta-receptor agonists. J Am Coll Cardiol. 2009;53:1320–1325. doi: 10.1016/j.jacc.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 7.Sharkey S.W., Lesser J.R., Zenovich A.G., Maron M.S., Lindberg J., Longe T.F. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111:472–479. doi: 10.1161/01.CIR.0000153801.51470.EB. [DOI] [PubMed] [Google Scholar]
- 8.Daniel Wachsman. Takotsubo cardiomyopathy: a little-known cardiomyopathy makes its debut. Cardiology. 2004;102:119–121. doi: 10.1159/000080480. [DOI] [PubMed] [Google Scholar]
- 9.Prasad A., Lerman A., Rihal C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408–417. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Hanna M., Finkelhor R.S., Shaw W.F., Bahler R.C. Extent of right and left ventricular focal wall-motion abnormalities in differentiating transient apical ballooning syndrome from apical dysfunction as a result of coronary artery disease. J Am Soc Echocardiogr. 2007;20:144–150. doi: 10.1016/j.echo.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Parsad S. Apical ballooning syndrome: an important differential diagnosis of acute myocardial infarction. Circulation. 2007;115:e56–e59. doi: 10.1161/CIRCULATIONAHA.106.669341. [DOI] [PubMed] [Google Scholar]
- 12.Meimoun P., Malaquin D., Sayah S., Benali T., Luycx-Bore A., Levy F. The coronary flow reserve is transiently impaired in tako-tsubo cardiomyopathy: a prospective study using serial Doppler transthoracic echocardiography. J Am Soc Echocardiogr. 2008;21:72–77. doi: 10.1016/j.echo.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Sharkey S.W., Windenburg D.C., Lesser J.R., Maron M.S., Hauser R.G., Lesser J.N. Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol. 2010;55:333. doi: 10.1016/j.jacc.2009.08.057. [DOI] [PubMed] [Google Scholar]
- 14.Gianni M., Dentali F., Grandi A.M., Sumner G., Hiralal R., Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27:1523–1529. doi: 10.1093/eurheartj/ehl032. [DOI] [PubMed] [Google Scholar]
- 15.Dorfman T.A., Iskandrian A.E., Aqel R. An unusual manifestation of Tako-tsubo cardiomyopathy. Clin Cardiol. 2008;31:194–200. doi: 10.1002/clc.20212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maroules C.D., Linz N.A., Boswell G.E. Recurrent Takotsubo cardiomyopathy. J Cardiovasc Comput Tomogr. 2009;3:187–189. doi: 10.1016/j.jcct.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Elesber A.A., Prasad A., Lennon R.J., Wright R.S., Lerman A., Rihal C.S. Four-year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol. 2007;50:448–452. doi: 10.1016/j.jacc.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 18.Bybee K.A., Murphy J., Prasad A., Wright R.S., Lerman A., Rihal C.S. Acute impairment of regional myocardial glucose uptake in the apical ballooning (takotsubo) syndrome. J Nucl Cardiol. 2006;13:244–250. doi: 10.1007/BF02971249. [DOI] [PubMed] [Google Scholar]
- 19.Tornvall P., Collste O., Ehrenborg E., Jarnbert-Petterson H. A case-control study of risk markers and mortality in takotsubo stress cardiomyopathy. J Am Coll Cardiol. 2016;67:1931–1936. doi: 10.1016/j.jacc.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 20.Wittstein I.S., Thiemann D.R., Lima J.A., Baughman K.L., Schulman S.P., Gerstenblith G. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 21.Singh N.K. Apical ballooning syndrome: the emerging evidence of a neurocardiogenic basis. Am Heart J. 2008;156:e33. doi: 10.1016/j.ahj.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Buchholz S., Rudan G. Tako-tsubo syndrome on the rise: a review of the current literature. Postgrad Med J. 2007;83:261–264. doi: 10.1136/pgmj.2006.052423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorfman T.A., Iskandrian A.E. Takotsubo cardiomyopathy: state-of-the-art review. J Nucl Cardiol. 2009;16:122–134. doi: 10.1007/s12350-008-9015-3. [DOI] [PubMed] [Google Scholar]
- 24.Ostadal B., Pelouch V., Ostadalova I., Novakova O. Structural and biochemical remodeling in catecholamine-induced cardiomyopathy: comparative and ontogenetic aspects. Mol Cell Biochem. 1995;147:83–88. doi: 10.1007/BF00944787. [DOI] [PubMed] [Google Scholar]
- 25.Nanda A.S., Feldman A., Liang C.S. Acute reversal of pheochromocytoma-induced catecholamine cardiomyopathy. Clin Cardiol. 1995;18:421–423. doi: 10.1002/clc.4960180712. [DOI] [PubMed] [Google Scholar]
- 26.Neil-Dwyer G., Walter P., Cruickshank J.M., Doshi B., O’Gorman P. Effect of propranolol and phentolamine on myocardial necrosis after subarachnoid hemorrhage. Br Med J. 1978;2:990–992. doi: 10.1136/bmj.2.6143.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellison G.M., Torella D., Karakikes I., Purushothaman S., Curcio A., Gasparri C. Acute beta-adrenergic overload produces myocyte damage through calcium leakage from the ryanodine receptor 2 but spares cardiac stem cells. J Biol Chem. 2007;282:11397–11409. doi: 10.1074/jbc.M607391200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schimpf R., Meinhardt J., Borggrefe M., Haghi D. Catecholaminergic polymorphic ventricular tachycardia and midventricular Takotsubo cardiomyopathy: a novel association? Herzschrittmacherther Elektrophysiol. 2013;24:63–66. doi: 10.1007/s00399-013-0248-8. [DOI] [PubMed] [Google Scholar]
- 29.Mori H., Ishikawa S., Kojima S., Hayashi J., Watanabe Y., Hoffman J.I. Increased responsiveness of left ventricular apical myocardium to adrenergic stimuli. Cardiovasc Res. 1993;27:192–198. doi: 10.1093/cvr/27.2.192. [DOI] [PubMed] [Google Scholar]
- 30.Kassim T.A., Clarke D.D., Mai V.Q., Shakir M.K.M. Catecholamine-induced cardiomyopathy. Endocr Pract. 2008;14:1137–1149. doi: 10.4158/EP.14.9.1137. [DOI] [PubMed] [Google Scholar]
- 31.Lenders J.W., Eisenhofer G., Mannelli M., Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–675. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 32.Nef H.M., Möllmann H., Kostin S., Troidl C., Voss S., Weber M. Tako-tsubo cardiomyopathy: Intraindividual structural analysis in the acute phase and after functional recovery. Eur Heart J. 2007;28:2456–2464. doi: 10.1093/eurheartj/ehl570. [DOI] [PubMed] [Google Scholar]
- 33.Abe Y., Kondo M., Matsuoka R., Araki M., Dohyama K., Tanio H. Assessment of clinical features in transient LV apical ballooning. J Am Coll Cardiol. 2003;41:737–742. doi: 10.1016/s0735-1097(02)02925-x. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida T., Hibino T., Kako N., Murai S., Oguri M., Kato K. A pathophysiologic study of tako-tsubo cardiomyopathy with F-18 fluorodeoxyglucose positron emission tomography. Eur Heart J. 2007;28:2598–2604. doi: 10.1093/eurheartj/ehm401. [DOI] [PubMed] [Google Scholar]
- 35.Akashi Y.J., Goldstein D.S., Barbaro G., Ueyama T. Takotsubo cardiomyopathy—A new form of acute reversible heart failure. Circulation. 2008;118:2754–2762. doi: 10.1161/CIRCULATIONAHA.108.767012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sasaki N., Kinugawa T., Yamawaki M., Furuse Y., Shimoyama M., Ogino K. Transient left ventricular apical ballooning in a patient with bicuspid aortic valve created a left ventricular thrombus leading to acute renal infarction. Circ J. 2004;68:1081–1083. doi: 10.1253/circj.68.1081. [DOI] [PubMed] [Google Scholar]
- 37.Kurowski V., Kaiser A., von Hof K., Killermann D.P., Mayer B., Hartmann F. Apical and midventricular transient LV dysfunction syndrome (tako-tsubo cardiomyopathy): frequency, mechanisms, and prognosis. Chest. 2007;132:809. doi: 10.1378/chest.07-0608. [DOI] [PubMed] [Google Scholar]
- 38.Kurisu S., Inoue I., Kawagoe T., Ishihara M., Shimatani Y., Nakama Y. Incidence and treatment of LV apical thrombosis in Tako-tsubo cardiomyopathy. Int J Cardiol. 2011;146:e58–e60. doi: 10.1016/j.ijcard.2008.12.208. [DOI] [PubMed] [Google Scholar]
- 39.Tobar R., Rotzak R., Rozenman Y. Apical thrombus associated with Takotsubo cardiomyopathy in a young woman. Echocardiography. 2009;26:575–580. doi: 10.1111/j.1540-8175.2008.00826.x. [DOI] [PubMed] [Google Scholar]
- 40.Fang C.C., Jao Y.T., Yi-Chen, Yu C.L., Chen C.L., Wang S.P. Transient left ventricular apical ballooning syndrome: the first series in Taiwanese patients. Angiology. 2008;59:185. doi: 10.1177/0003319707305463. [DOI] [PubMed] [Google Scholar]
- 41.Antzelevitch C. Ionic, molecular, and cellular bases of QT-interval prolongation and torsade de pointes. Europace. 2007;9(Suppl 4):iv4–iv15. doi: 10.1093/europace/eum166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torp-Pedersen C., Moller M., Bloch-Thomsen P.E., Kober L., Sandoe E., Egstrup K. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group. N Engl J Med. 1999;341:857. doi: 10.1056/NEJM199909163411201. [DOI] [PubMed] [Google Scholar]
- 43.Ueyama T. Emotional stress-induced Tako-tsubo cardiomyopathy: animal model and molecular mechanism. Ann N Y Acad Sci. 2004;1018:437–444. doi: 10.1196/annals.1296.054. [DOI] [PubMed] [Google Scholar]
- 44.Stiermaier T., Rommel K.P., Eitel C., Möller C., Graf T., Desch S. Management of arrhythmias in patients with Takotsubo cardiomyopathy: is the implantation of permanent devices necessary? Heart Rhythm. 2016 doi: 10.1016/j.hrthm.2016.06.013. in press. [DOI] [PubMed] [Google Scholar]
- 45.Yoshioka T., Hashimoto A., Tsuchihashi K., Nagao K., Kyuma M., Ooiwa H. Clinical implications of midventricular obstruction and intravenous propranolol use in transient LV apical ballooning (Tako-tsubo cardiomyopathy) Am Heart J. 2008;155(526):e1–e7. doi: 10.1016/j.ahj.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 46.Murugiah K., Wang Y., Desai N.R., Spatz E.S., Nuti S.V., Dreyer R.P. Trends in short- and long-term outcomes for takotsubo cardiomyopathy among Medicare fee-for-service beneficiaries, 2007 to 2012. JACC Heart Fail. 2016;4:197–205. doi: 10.1016/j.jchf.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palecek T., Kuchynka P., Linhart A. Treatment of Takotsubo cardiomyopathy. Curr Pharm Des. 2010;16:2905–2909. doi: 10.2174/138161210793176455. [DOI] [PubMed] [Google Scholar]