Abstract
In the southeastern United States, reniform nematode (Rotylenchulus reniformis) is a serious pest of upland cotton (Gossypium hirsutum), a species which has no naturally occurring resistance against this nematode. To identify sources of reniform nematode resistance in species closely related to upland cotton, 222 G. arboreum accessions from the U.S. germplasm collection were evaluated in repeated growth chamber experiments. In initial screenings, root infection was measured 4 wks after inoculation. The 15 accessions supporting the fewest infections (PI 529992, PI 615755, PI 615766, PI 615788, PI 615848, PI 615856, PI 615950, PI 615977, PI 615991, PI 616008, PI 616016, PI 616062, PI 616126, PI 616159, and A2 553) were evaluated again in confirmation tests lasting 8 wk. The combined totals of nematodes extracted from soil and eggs extracted from roots were analyzed. All 15 accessions tested supported significantly smaller reniform nematode populations than the susceptible controls (G. hirsutum cultivar Deltapine 16 and G. arboreum accession PI 529729). Nine accessions (PI 529992, PI 615755, PI 615766, PI 615788, PI 615856, PI 615950, PI 615991, PI 616008, and PI 616159) supported reniform nematode populations comparable to the resistant control (G. arboreum accession PI 615699), and accession PI 615848 had significantly fewer reniform nematodes than the resistant control. Cotton breeders would benefit from introgressing the newly identified resistance from these accessions into their upland cotton improvement programs.
Keywords: cotton, Gossypium hirsutum, reniform nematode, resistance
Cotton (Gossypium hirsutum L.) farmers from Texas to the Atlantic seaboard experience yield losses as a result of the reniform nematode (Rotylenchulus reniformis Linford and Oliveira) on an annual basis. Losses to reniform nematode for the 2013, 2014, and 2015 growing seasons averaged 3.3%, 6.1%, and 4.0% for cotton in Louisiana, Mississippi, and Alabama, respectively (Lawrence et al., 2014, 2015, 2016). A number of factors including lack of resistance within commercially available cultivars (Robinson et al., 1999; Usery et al., 2005; Starr et al., 2007), loss of effective soil-applied fumigants and nematicides from the market (Starr et al., 2007; Mueller, 2011), and grower preference for cotton monoculture over crop rotation (Robinson, 2007; Starr et al., 2007) allow nematode survival and reproduction resulting in population densities at or above damaging thresholds at planting and throughout the cropping season.
Host plant resistance would be highly advantageous to cotton growers because it is cost effective, environmentally friendly, simple to deploy, and it persists throughout the entire growing season. The primary reason for the lack of reniform nematode resistant cultivars is the lack of high levels of resistance to this nematode in G. hirsutum. Robinson et al. (2004) surveyed more than 1,800 primitive G. hirsutum accessions obtained from the U.S. National Plant Germplasm System (NPGS) cotton collection and found only six that were moderately resistant.
Germplasm lines have been released with resistance to reniform nematode derived from relatives of G. hirsutum. The tetraploid species Gossypium barbadense L. is the source of resistance in several germplasm lines released within the past decade. In 2010, two breeding lines of cotton, TAM RKRNR-9 (PI 662039) and TAM RKRNR-12 (PI 662040), with reniform nematode resistance derived from G. barbadense TX 110 (PI 163608) were released (Starr et al., 2011). Gossypium barbadense accession GB 713 (PI 608139) was the source of reniform nematode resistance in four other germplasm lines released in 2012. Three lines, M713 Ren1 (PI 665928), M713 Ren2 (PI 665929), and M713 Ren5 (PI 665930), were developed from a cross between G. barbadense GB 713 and the G. hirsutum cultivar SureGrow 747 (McCarty et al., 2013). The fourth germplasm line, BARBREN-713 (PI 671965), was developed by crossing G. barbadense GB 713 with the cultivar Acala NemX, followed by several backcrosses to G. hirsutum lines (Bell et al., 2015); this line has resistance to Meloidogyne incognita (Kofoid and White) Chitwood in addition to reniform nematode resistance. To date, no commercial cultivars have been released that have these germplasm lines in their pedigrees.
A greater research challenge is the exploitation of the reniform nematode resistance found in diploid Gossypium species. Transferring resistance from diploid Gossypium species into tetraploid cotton is difficult. Barriers to hybridization between the different species include mechanisms that prevent fertilization or inhibit development of viable seed from successful fertilizations (Brubaker et al., 1999; Mehetre et al., 2003; Mehetre and Aher, 2004; Ganesh Ram et al., 2008). Techniques such as bridging lines (Brubaker et al., 1999; Romano et al., 2009), induced polyploidy (Mehetre et al., 2003), in vitro interspecific fertilization (Liu et al., 1992), protoplast fusion (Sun et al., 2006), and ovule culture (Stewart and Hsu, 1977, 1978; Gill and Bajaj, 1984, 1987) have been used to overcome these breeding limitations.
Immunity to reniform nematode in G. longicalyx Hutch. & Lee (Yik and Birchfield, 1984; Stewart and Robbins, 1996); resistance in G. arboreum L. (Carter, 1981; Stewart and Robbins, 1995; Sacks and Robinson, 2009), G. somalense (Gurke) Hutch. (Yik and Birchfield, 1984), and G. stocksii Mast. Ex. Hook. (Yik and Birchfield, 1984); and moderate levels of resistance in G. aridum (Sacks and Robinson, 2009), G. herbaceum (Yik and Birchfield, 1984), and G. raimondii Ulbr. (Yik and Birchfield, 1984), have been reported. With the exception of G. longicalyx, in which all accessions tested to date have exhibited immunity, variability in resistance to reniform nematode exists within the diploid Gossypium species.
To date, the only germplasm lines released with resistance from a diploid species are LONREN-1 and LONREN-2, with resistance that had been introgressed from G. longicalyx (Bell et al., 2014). However, this resistance has been linked to intolerance (Sikkens et al., 2011; Weaver et al., 2013), with plants exhibiting stunting when challenged with high inoculum levels of the nematode. Because of this problem, nearly all breeding programs have stopped using this source of resistance. Gossypium hirsutum lines with reniform nematode resistance introgressed from G. arboreum accession A2-190 (PI 615699) (Sacks and Robinson, 2009) and G. arboreum accession A2-19 (PI 129723) (Avila et al., 2005) have been developed, though no germplasm lines from these programs have been released to date.
Because reniform nematode resistance has just recently become available in upland cotton, no data are available with respect to the durability of any one source of resistance. Variability within reniform nematode has been well documented on a genetic, morphological, and physiological basis (Dasgupta and Seshadri, 1971; Nakasono, 2004; Agudelo et al., 2005b; Arias et al., 2009; McGawley et al., 2010; Leach et al., 2012). Over time, reniform nematode may adapt to one or more resistance sources, as has been documented with development of races in pathogens such as Phytophthora infestans (Mont.) de Bary and Heterodera glycines Ichinohe. Use of a single source of resistance over time may result in development of nematode biotypes that can reproduce on the resistant cultivar (Young, 1998), so rotation among different resistance sources may be necessary to reduce selection pressure on the nematodes (Starr and Roberts, 2004). If different resistance genes can be identified, they could be combined (“pyramided”) into the same plant to make resistance more durable.
The objectives of this research were to evaluate a selection of Gossypium arboreum accessions for their reaction to the reniform nematode, and to identify sources of host plant resistance that could be introgressed into upland cotton and used to manage this pathogen.
Materials and Methods
Identification of resistant lines:
A total of 222 G. arboreum accessions were evaluated in growth chamber tests for resistance to infection by reniform nematode. The specific accessions tested are listed in Tables 1, 2, and 3. Seeds not already in the authors’ research collections were obtained from the NPGS (College Station, TX).
Table 1.
Infection of Gossypium roots by Rotylenchulus reniformis females 4 wk after inoculation in growth chamber Test 1. All accessions are Gossypium arboreum except for susceptible control Gossypium hirsutum cultivar Deltapine 16.
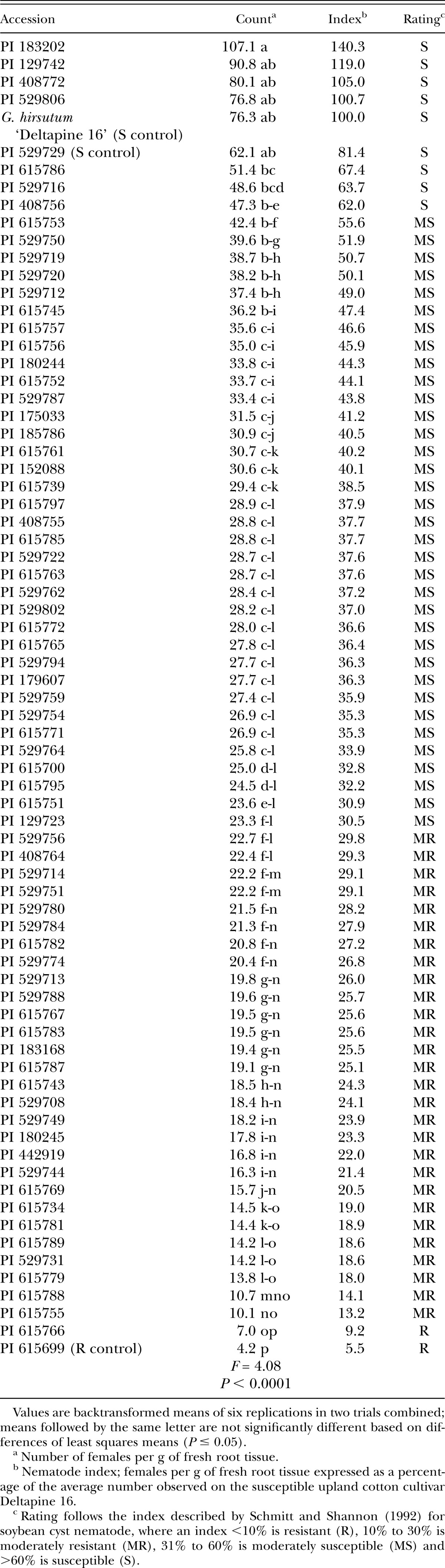
Table 2.
Infection of Gossypium roots by Rotylenchulus reniformis females 4 wk after inoculation in growth chamber Test 2. All accessions are Gossypium arboreum except for susceptible control Gossypium hirsutum cultivar Deltapine 16.
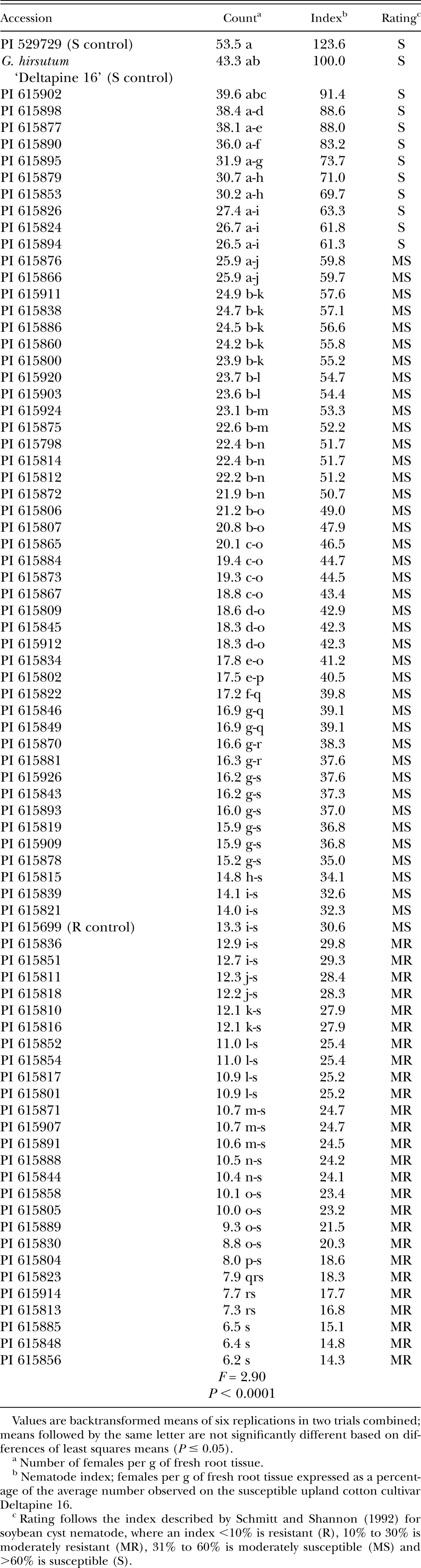
Table 3.
Infection of Gossypium roots by Rotylenchulus reniformis females 4 wk after inoculation in growth chamber Test 3. All accessions are Gossypium arboreum except for susceptible control Gossypium hirsutum cultivar Deltapine 16.
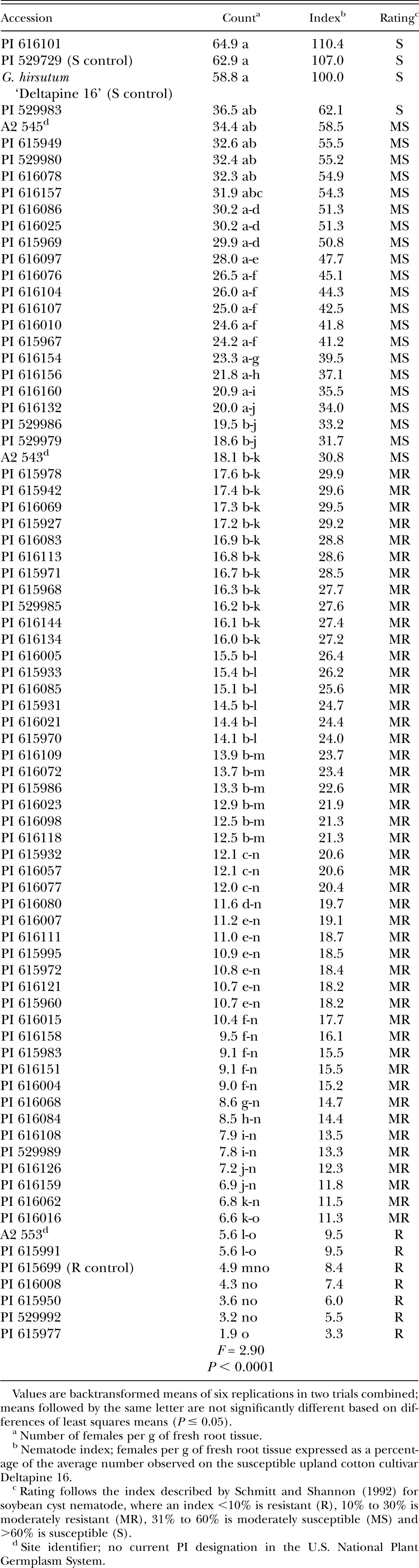
Accessions were arbitrarily divided into three screening tests of approximately 75 entries each due to growth chamber space limitations. The susceptible controls Gossypium hirsutum cultivar Deltapine 16 (Yik and Birchfield, 1984; Robinson and Percival, 1997) and G. arboreum accession PI 529729 (Sacks and Robinson, 2009; Erpelding and Stetina, 2013), and the resistant control G. arboreum accession PI 615699 (Sacks and Robinson, 2009) were included in each test. The experimental design for each screening was a completely randomized design with three replications, and each test was repeated. The growth chamber temperature was maintained at 28°C and the daylength was set at 16 hours. Soil moisture was maintained using an automated watering system, with the timing adjusted periodically during the experiment to supply additional water as plants grew.
Screening test protocols were similar to those described by Stetina et al. (2014). Briefly, single plants of each accession were established in conical plastic pots (Ray Leach SL-10 Cone-tainer, Stuewe & Sons, Inc., Tangent, OR) containing 120 cm3 of a steam-sterilized soil mixture consisting of one part sandy loam soil mixed with two parts sand. Approximately 7 days after planting, soil in each pot was infested with 1,000 reniform nematodes (mixed vermiform life stages) suspended in 1 ml water. Mississippi reniform nematode population MSRR04 (Arias et al., 2009), originally isolated from upland cotton and maintained in a greenhouse on tomato (Solanum lycopersicon cultivar Rutgers), was used for all experiments. Plants were harvested 4 wk after inoculation. Shoots were removed at the soil line and discarded. Roots were separated from soil, stained with red food coloring using standard protocols (Thies et al., 2002), and the number of swollen females attached to the roots were counted at ×50 magnification. After counting, roots were allowed to drain briefly on paper towels to remove excess water and fresh weights were recorded. Counts were expressed as females per gram of fresh root tissue to compensate for differences in root sizes.
In addition to statistically comparing root infection levels, accessions within each test were classified based on a nematode index, following that described by Schmitt and Shannon (1992) for soybean cyst nematode. Infection on an accession is expressed as a percentage of the average number of females that developed on susceptible G. hirsutum cultivar Deltapine 16. Based on the nematode index, accessions were classified as resistant (nematode index <10%), moderately resistant (10% to 30%), moderately susceptible (31% to 60%), or susceptible (>60%).
Confirmation of reaction to reniform nematode:
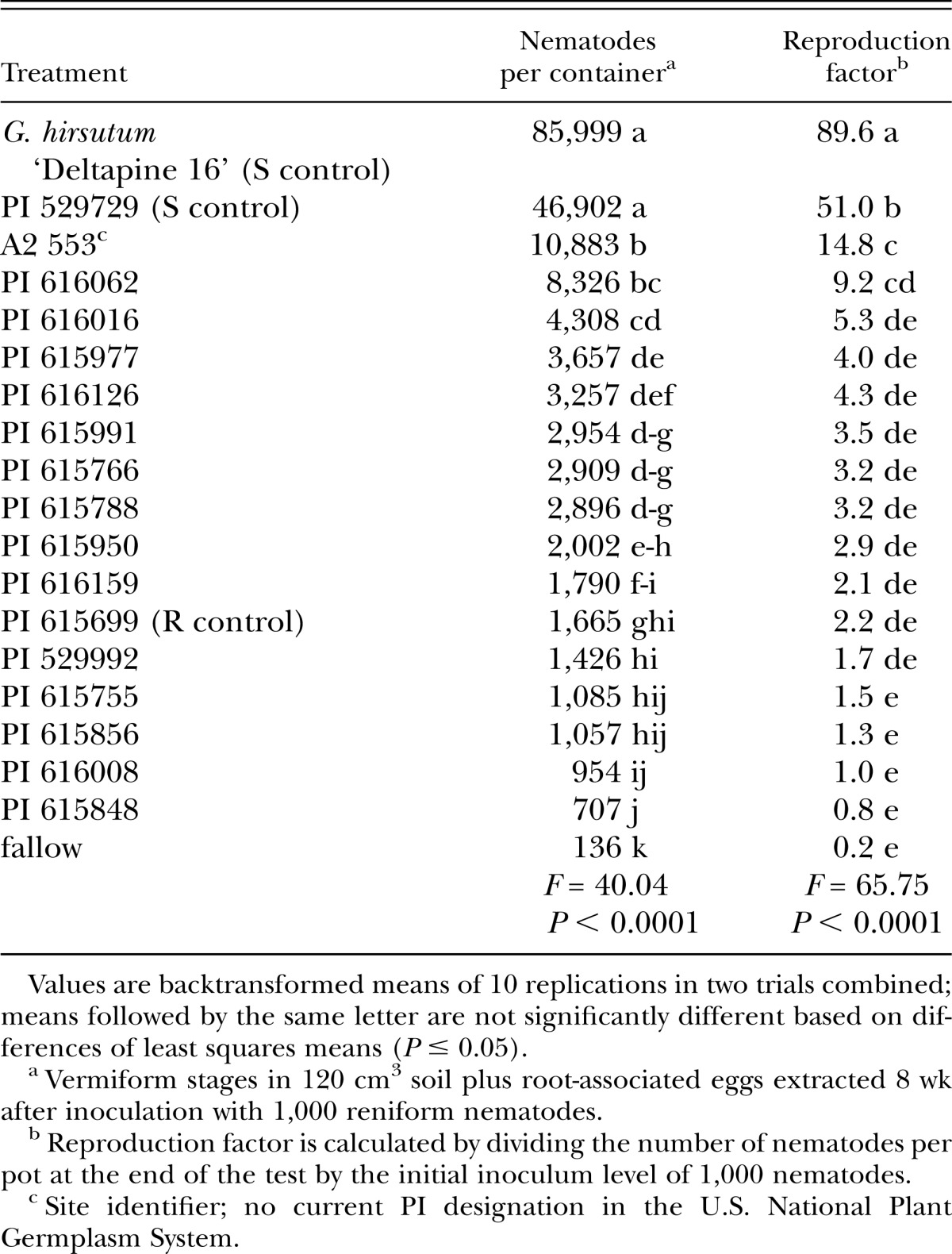
A subset consisting of 15 of the most resistant accessions identified in the initial screening tests was further evaluated in a longer-duration test that measured reniform nematode reproduction. As in the screening tests, the susceptible controls Gossypium hirsutum cultivar Deltapine 16 and G. arboreum accession PI 529729, and the resistant control G. arboreum accession PI 615699 were included. To monitor survival of the nematode with no roots present, a fallow treatment also was included.
Test establishment and inoculation procedures were the same as described for the initial screenings. The experimental design was a completely randomized design with five replications, and the test was repeated. The test duration was extended to 8 wk. At the end of the test, standard elutriation (Byrd et al., 1976) and sucrose centrifugation (Jenkins, 1964) protocols were used to extract vermiform stages of nematodes from all of the soil in each pot. In addition, eggs were extracted from the root system by cutting the roots into 2.5-cm segments, stirring for 10 min in a 0.6% NaOCl solution (Hussey and Barker, 1973), and collecting eggs on a standard 25-µm-pore sieve. Egg and vermiform counts were added together, and total numbers were analyzed.
In addition to statistically comparing reniform nematode population sizes, a reproduction factor was determined for each of the accessions. The reproduction factor is calculated by dividing the number of nematodes per pot at the end of test by the initial inoculum level of 1,000 nematodes. Reproduction factor values of 1.0 or more indicate that the plant is a good host for the nematode; poor hosts have values smaller than 1.0 (Walters et al., 1996).
Statistical analysis:
Prior to analysis of variance (ANOVA), nematode counts were subjected to log10(x+1) transformation to normalize data; backtransformed means are presented. Initial data analyses identified no significant differences between trials, and no significant interactions between trial and accession. Therefore, data from both trials of each identification and confirmation test were combined for final analysis, and trials and their interactions were modeled as random effects. Where significant differences among genotypes were found using ANOVA, differences of least squares means (P ≤ 0.05) were used to compare means. SAS statistical software (PROC MIXED; SAS Institute, Cary, NC) was used for analysis.
Results
The reactions to reniform nematode for all 222 G. arboreum accessions evaluated are presented in Tables 1, 2, and 3. The susceptible controls were significantly different from the resistant control in each of the three tests based on the number of females infecting the roots, although the number of infections on the resistant control was higher than expected in Test 2. These initial screening experiments identified 19 susceptible, 96 moderately susceptible, 100 moderately resistant, and 7 resistant accessions in total.
Though not statistically distinguishable from the control, four accessions classified as resistant had lower infection indices than the resistant control: PI 529992, PI 615950, PI 615977, and PI 616008 (Table 3). At the other end of the spectrum, five accessions classified as susceptible had higher infection indices than the susceptible controls: PI 183202, PI 129742, PI 408772, PI 529806 (Table 1); and PI 616101 (Table 3).
The 15 most resistant accessions identified in the initial screenings were tested again in longer experiments to confirm their reaction to the reniform nematode (Table 4). All accessions tested reduced reniform nematode populations compared to the susceptible controls. Nine accessions were comparable to the resistant control with respect to final population sizes, and accession PI 615848 supported significantly smaller reniform nematode populations than the resistant control. However, none of the accessions suppressed the populations to the same level as the fallow treatment. A comparison of the reproduction factors showed that 14 accessions and the fallow treatment were comparable to the resistant control, though only PI 615848 and the fallow treatment had reproduction factors less than 1.0, indicative of poor host status.
Table 4.
Comparison of reniform nematode population development on 17 Gossypium arboreum accessions, the susceptible control Gossypium hirsutum cultivar Deltapine 16, and one fallow treatment in a growth chamber.
Discussion
Ten G. arboreum accessions were identified as resistant to reniform nematode in both initial screening and subsequent confirmation tests. This conclusion was based on the number of females infecting the roots and on the nematode population development in growth chamber tests as compared to the resistant control G. arboreum accession PI 615699. The nine accessions that were comparable to the resistant control in supporting reniform nematode population development were PI 529992, PI 615755, PI 615766, PI 615788, PI 615856, PI 615950, PI 615991, PI 616008, and PI 616159. One accession, PI 615848, was more effective than the resistant control at suppressing reniform nematode population development, and had a reproduction factor of 0.8, indicative of poor host status. All of these sources supported 3% or less of the reniform nematode population development that was observed on the susceptible G. hirsutum control cultivar Deltapine 16. As such, any of them would be excellent candidates for inclusion in a germplasm improvement program.
Results from this study indicate that a reduced number of infections and smaller population sizes are associated with the 10 resistant accessions identified. However, specific mechanisms governing the successful establishment and maintenance of a feeding site, the rate of nematode development, and the number of eggs produced by each female were not evaluated (Agudelo et al., 2005a; Starr et al., 2011; Stetina, 2015), though any or all of these factors could be contributing to the observed resistance. Discerning the mechanism(s) behind the observed reniform nematode population suppression could be the subject of future research.
Within the subset of 222 accessions that were tested from the G. arboreum germplasm collection, the plants were divided fairly evenly between the resistant and susceptible ends of the reniform nematode resistance spectrum. Most of the accessions tested were classified as either moderately resistant or moderately susceptible based on root infection levels, with only a few lines initially identified as resistant. The subset of accessions tested represents only about 12% of the G. arboreum collection. A significant time investment will have to be made to screen the remainder of the accessions using the methods employed in this study. To facilitate discovery of new sources of resistance in this germplasm collection, molecular markers associated with the resistance already documented are needed. The markers could be used to rapidly evaluate the remaining accessions to identify accessions having similar DNA banding patterns as resistant accessions so that future screening efforts could be directed toward identifying putatively unique types of resistance.
In the screening experiments, 19 accessions susceptible to the reniform nematode were identified. Of these, PI 129742, PI 183202, PI 408772, PI 529806, and PI 616101 had higher female indices than the susceptible controls. While these accessions are not useful for developing cultivars resistant to reniform nematode, they do have utility in understanding how resistance is controlled. Populations from crosses between the susceptible and resistant accessions can be studied to determine how resistance is inherited, to identify molecular markers for resistance, and to map the location of the gene(s) conferring resistance.
A limitation of this study is that the accessions were screened using a single isolate of reniform nematode. There are reports in the literature documenting cotton (Agudelo et al., 2005b; Arias et al., 2009; McGawley et al., 2010) and soybean (Agudelo et al., 2005b; McGawley et al., 2011) lines responding differently to unique geographic populations of reniform nematode. Therefore, the accessions identified as resistant in this study could show a different level of resistance if challenged with different populations of the nematode.
In summary, this research provides new phenotypic information on 222 G. arboreum accessions, including the identification of 10 accessions with useful levels of reniform nematode resistance. Public and private cotton breeding programs could benefit from using these resistant accessions as parents, although there may be challenges related to the introgression of the resistance that were not evaluated in this study.
Literature Cited
- Agudelo PA, Robbins RT, Kim KS, Stewart JM. Histological changes in Gossypium hirsutum associated with reduced reproduction of Rotylenchulus reniformis. Journal of Nematology. 2005a;37:185–189. [PMC free article] [PubMed] [Google Scholar]
- Agudelo PA, Robbins RT, Stewart JM, Szalanski AL. Intraspecific variability of Rotylenchulus reniformis from cotton-growing regions in the United States. Journal of Nematology. 2005b;37:105–114. [PMC free article] [PubMed] [Google Scholar]
- Arias RS, Stetina SR, Tonos JL, Scheffler JA, Scheffler BA. Microsatellites reveal genetic diversity in Rotylenchulus reniformis populations. Journal of Nematology. 2009;41:146–156. [PMC free article] [PubMed] [Google Scholar]
- Avila CA, Stewart JM, Robbins RT. 2005. Transfer of reniform nematode resistance from diploid cotton species to tetraploid cultivated cotton. P. 182 in Proceedings of the Beltwide Cotton Conferences, New Orleans, LA. Cordova: National Cotton Council.(Abstr.). [Google Scholar]
- Bell AA, Robinson AF, Quintana J, Dighe ND, Menz MA, Stelly DM, Zheng X, Jones JE, Overstreet C, Burris E, Cantrell RG, Nichols RL. Registration of LONREN-1 and LONREN-2 germplasm lines of Upland cotton resistant to reniform nematode. Journal of Plant Registrations. 2014;8:187–190. [Google Scholar]
- Bell AA, Robinson AF, Quintana J, Duke SE, Starr JL, Stelly DM, Zheng X, Prom S, Saladino V, Gutiérrez OA, Stetina SR, Nichols RL. Registration of BARBREN-713 germplasm line of Upland cotton resistant to reniform and root-knot nematodes. Journal of Plant Registrations. 2015;9:89–93. [Google Scholar]
- Brubaker CL, Brown ADH, Stewart JM, Kilby MJ, Grace JP. Production of fertile hybrid germplasm with diploid Australian Gossypium species for cotton improvement. Euphytica. 1999;108:199–213. [Google Scholar]
- Byrd DW, Jr, Barker KR, Ferris H, Nusbaum CJ, Griffin WE, Small RH, Stone CA. Two semi-automatic elutriators for extracting nematodes and certain fungi from soil. Journal of Nematology. 1976;8:206–212. [PMC free article] [PubMed] [Google Scholar]
- Carter WW. Resistance and resistant reaction of Gossypium arboreum to the reniform nematode, Rotylenchulus reniformis. Journal of Nematology. 1981;13:368–374. [PMC free article] [PubMed] [Google Scholar]
- Dasgupta DR, Seshadri AR. Reproduction, hybridization, and host adaptation in physiological races of the reniform nematode, Rotylenchulus reniformis. Indian Journal of Nematology. 1971;1:128–144. [Google Scholar]
- Erpelding JE, Stetina SR. Genetics of reniform nematode resistance in Gossypium arboreum germplasm line PI 529728. World Journal of Agricultural Research. 2013;1:48–53. [Google Scholar]
- Ganesh Ram S, Hari Ramakrishnan S, Thiruvengadam V, Kannan Bapu JR. Prefertilization barriers to interspecific hybridization involving Gossypium hirsutum and four diploid wild species. Plant Breeding. 2008;127:295–300. [Google Scholar]
- Gill MS, Bajaj YPS. Interspecific hybridization in the genus Gossypium through embryo culture. Euphytica. 1984;33:305–311. [Google Scholar]
- Gill MS, Bajaj YPS. Hybridization between diploid (Gossypium arboreum) and tetraploid (Gossypium hirsutum) cotton through ovule culture. Euphytica. 1987;36:625–630. [Google Scholar]
- Hussey RS, Barker KR. A comparison of methods for collecting inocula for Meloidogyne spp., including a new technique. Plant Disease Reporter. 1973;57:1025–1028. [Google Scholar]
- Jenkins WR. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Disease Reporter. 1964;48:692. [Google Scholar]
- Lawrence K, Hagan A, Olsen M, Faske T, Hutmacher R, Mueller J, Wright D, Kemerait B, Overstreet C, Price P, Lawrence G, Allen T, Atwell S, Thomas S, Goldberg N, Edmisten K, Boman R, Young H, Woodward J, Mehl H. 2016. Cotton disease loss estimate committee report, 2015. Pp. 113–115 in Proceedings of the 2016 Beltwide Cotton Conferences, New Orleans, LA. Cordova: National Cotton Council. [Google Scholar]
- Lawrence K, Olsen M, Faske T, Hutmacher R, Muller J, Mario J, Kemerait R, Overstreet C, Sciumbato G, Lawrence G, Atwell S, Thomas S, Koenning S, Boman R, Young H, Woodward J, Mehl H. 2014. Cotton disease loss estimate committee report, 2013. Pp. 247–248 in Proceedings of the 2014 Beltwide Cotton Conferences, New Orleans, LA. Cordova: National Cotton Council. [Google Scholar]
- Lawrence KS, Olsen M, Faske T, Hutmacher R, Mueller J, Mario J, Kemerait B, Overstreet C, Price P, Sciumbato G, Lawrence G, Atwell S, Thomas S, Koenning S, Boman R, Young H, Woodward J, Mehl H. 2015. Cotton disease loss estimate committee report, 2014. Pp. 188–190 in Proceedings of the 2015 Beltwide Cotton Conferences, San Antonio, TX. Cordova: National Cotton Council. [Google Scholar]
- Leach M, Agudelo P, Lawton-Rauh A. Genetic variability of Rotylenchulus reniformis. Plant Disease. 2012;96:30–36. doi: 10.1094/PDIS-02-11-0132. [DOI] [PubMed] [Google Scholar]
- Liu C, Shun J, Liu J. In vitro interspecific fertilization, embryo development and formation of hybrid seedlings between Gossypium hirsutum and G. arboreum. Euphytica. 1992;60:79–88. [Google Scholar]
- McCarty JC, Jr, Jenkins JN, Wubben MJ, Gutiérrez OA, Hayes RW, Callahan FE, Deng D. Registration of three germplasm lines of cotton derived from Gossypium barbadense accession GB713 with resistance to the reniform nematode. Journal of Plant Registrations. 2013;7:220–223. [Google Scholar]
- McGawley EC, Overstreet C, Pontif MJ. Variation in reproduction and pathogenicity of geographic isolates of Rotylenchulus reniformis on soybean. Nematropica. 2011;41:12–22. [Google Scholar]
- McGawley EC, Pontif MJ, Overstreet C. Variation in reproduction and pathogenicity of geographic isolates of Rotylenchulus reniformis on cotton. Nematropica. 2010;40:275–288. [Google Scholar]
- Mehetre SS, Aher AR. Embryo rescue: a tool to overcome incompatible interspecific hybridization in Gossypium Linn.—a review. Indian Journal of Biotechnology. 2004;3:29–36. [Google Scholar]
- Mehetre SS, Aher AR, Gawande VL, Patil VR, Mokate AS. Induced polyploidy in Gossypium: A tool to overcome interspecific incompatibility of cultivated tetraploid and diploid cottons. Current Science. 2003;84:1510–1512. [Google Scholar]
- Mueller JD. 2011. The use of Temik® 15G on cotton and soybean in the southeast. Pp. 208–214 in Proceedings of the 2011 Beltwide Cotton Conferences, Atlanta, GA. Cordova: National Cotton Council. [Google Scholar]
- Nakasono K. Studies on morphological and physio-ecological variations of the reniform nematode, Rotylenchulus reniformis Linford and Oliveira, 1940 with an emphasis on differential geographic distribution of amphimictic and parthenogenetic populations in Japan. Journal of Nematology. 2004;36:356–420. [PMC free article] [PubMed] [Google Scholar]
- Robinson AF. Reniform in U.S. cotton: where, when why and some remedies. Annual Review of Phytopathology. 2007;45:11.1–11.25. doi: 10.1146/annurev.phyto.45.011107.143949. [DOI] [PubMed] [Google Scholar]
- Robinson AF, Bridges AC, Percival AE. New sources of resistance to the reniform (Rotylenchulus reniformis) and root-knot (Meloidogyne incognita) nematode in upland (Gossypium hirsutum) and sea island (G. barbadense) cotton. Journal of Cotton Science. 2004;8:191–197. [Google Scholar]
- Robinson AF, Cook CG, Percival AE. Resistance to Rotylenchulus reniformis and Meloidogyne incognita race 3 in the major cotton cultivars planted since 1950. Crop Science. 1999;39:850–858. [Google Scholar]
- Robinson AF, Percival AE. Resistance to Meloidogyne incognita race 3 and Rotylenchulus reniformis in wild accessions of Gossypium hirsutum and G. barbadense from Mexico. Supplement to the Journal of Nematology. 1997;29:746–755. [PMC free article] [PubMed] [Google Scholar]
- Romano GB, Sacks EJ, Stetina SR, Robinson AF, Fang DD, Gutiérrez OA, Scheffler JA. Identification and genomic location of a reniform nematode (Rotylenchulus reniformis) resistance locus (Renari) introgressed from Gossypium aridum into upland cotton (G. hirsutum) Theoretical and Applied Genetics. 2009;120:139–150. doi: 10.1007/s00122-009-1165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks EJ, Robinson AF. Introgression of resistance to reniform nematode (Rotylenchulus reniformis) into upland cotton (Gossypium hirsutum) from Gossypium arboreum and a G. hirsutum/Gossypium aridum bridging line. Field Crops Research. 2009;112:1–6. [Google Scholar]
- Schmitt DP, Shannon G. Differentiating soybean responses to Heterodera glycines races. Crop Science. 1992;32:275–277. [Google Scholar]
- Sikkens RB, Weaver DB, Lawrence KS, Moore SR, van Santen E. LONREN Upland cotton germplasm response to Rotylenchulus reniformis inoculum level. Nematropica. 2011;41:68–74. [Google Scholar]
- Starr JL, Koenning SR, Kirkpatrick TL, Robinson AF, Roberts PA, Nichols RL. The future of nematode management in cotton. Journal of Nematology. 2007;39:283–294. [PMC free article] [PubMed] [Google Scholar]
- Starr JL, Roberts PA. 2004. Resistance to plant-parasitic nematodes. Pp. 879–907 in Z. X. Chen, S. Y. Chen, and D. W. Dickson, eds. Nematology Advances and Perspectives, Volume 2: Nematode Management and Utilization. Wallingford: CABI Publishing.
- Starr JL, Smith CW, Ripple K, Zhou E, Nichols RL, Faske TR. Registration of TAM RKRNR-9 and TAM RKRNR-12 germplasm lines of upland cotton resistant to reniform and root-knot nematodes. Journal of Plant Registrations. 2011;5:393–396. [Google Scholar]
- Stetina SR. Postinfection development of Rotylenchulus reniformis on resistant Gossypium barbadense accessions. Journal of Nematology. 2015;47:302–309. [PMC free article] [PubMed] [Google Scholar]
- Stetina SR, Smith JR, Ray JD. Identification of Rotylenchulus reniformis resistant Glycine lines. Journal of Nematology. 2014;46:1–7. [PMC free article] [PubMed] [Google Scholar]
- Stewart JMcD, Hsu CL. In-ovulo embryo culture and seedling development of cotton (Gossypium hirsutum L.) Planta. 1977;137:113–117. doi: 10.1007/BF00387547. [DOI] [PubMed] [Google Scholar]
- Stewart JMcD, Hsu CL. Hybridization of diploid and tetraploid cottons through in-ovulo embryo culture. Journal of Heredity. 1978;69:404–408. [Google Scholar]
- Stewart JM, Robbins RT. 1995. Evaluation of Asiatic cottons for resistance to reniform nematode. Pp. 165–168 in D. M. Oosterhuis, ed. Proceedings of the 1994 Cotton Research Meeting and 1994 Summaries of Cotton Research in Progress. Special Report 166. Arkansas Agricultural Experiment Station, Fayetteville, AR.
- Stewart JM, Robbins RT. 1996. Identification and enhancement of resistance to reniform nematode in cotton germplasm. P. 225 in Proceedings of the Beltwide Cotton Conferences, Nashville, TN. Cordova: National Cotton Council. [Google Scholar]
- Sun Y, Nie Y, Guo X, Huang C, Zhang X. Somatic hybrids between Gossypium hirsutum L. (4x) and G. davidsonii Kellog (2x) produced by protoplast fusion. Euphytica. 2006;151:393–400. [Google Scholar]
- Thies JA, Merrill SB, Corley EL. Red food coloring stain: new safer procedures for staining nematodes in roots and egg masses on root surfaces. Journal of Nematology. 2002;34:121–133. [PMC free article] [PubMed] [Google Scholar]
- Usery SR, Jr, Lawrence KS, Lawrence GW, Burmester CH. Evaluation of cotton cultivars for resistance and tolerance to Rotylenchulus reniformis. Nematropica. 2005;35:121–133. [Google Scholar]
- Walters SA, Wehner TC, Barker KR. NC-42 and NC-43: Root-knot nematode-resistant cucumber germplasm. HortScience. 1996;31:1246–1247. [Google Scholar]
- Weaver DB, Sikkens RB, Lawrence KS, Sürmelioğlu Ç, van Santen E, Nichols RL. RENlon and its effects on agronomic and fiber traits in Upland cotton. Crop Science. 2013;53:913–920. [Google Scholar]
- Yik C-P, Birchfield W. Resistant germplasm in Gossypium species and related plants to Rotylenchulus reniformis. Journal of Nematology. 1984;16:146–153. [PMC free article] [PubMed] [Google Scholar]
- Young LD. 1998. Breeding for nematode resistance. Pp. 187–207 in K. R. Barker, G. A. Pederson, and G. L. Windham, eds. Plant Nematode Interactions. Madison: American Society of Agronomy.


