Abstract
The use of natural compounds to control phytonematodes is significantly increasing, as most of the old synthetic pesticides have been banned due to their eco-hostile character. Plant secondary metabolites are now evaluated as biologically active molecules against Meloidogyne spp. but their target site in the nematode body is rarely specified. Herein, we report on the ultrastructure modifications of the Meloidogyne incognita J2 after treatment with nematicidal plant secondary metabolites, that is acetic acid, (E)-2-decenal, and 2-undecanone. The commercial nematicide fosthiazate acting on acetylcholinesterase was used as control. For this reason, scanning electron microscopy and transmission electron microscopy have been employed. The acetic acid mainly harmed the cuticle, degenerated the nuclei of pseudocoel cells, and vacuolised the cytoplasm. The (E)-2-decenal and 2-undecanone did neither harm to the cuticle nor the somatic muscles but they degenerated the pseudocoel cells. (E)-2-decenal caused malformation of somatic muscles. According to the above, the nematicidal compounds seem to enter the nematode body principally via the digestive system rather than the cuticle, since the main part of the damage is internal.
Keywords: botanical nematicidals, degeneration, vacuolization, (E)-2-decenal, electron microscopy, root-knot nematodes, ultrastructure
Future agricultural and rural development is, to a large extent, influenced by the projected food needs of the 2.5 billion people expected to swell the world population by 2020. Among agricultural pests that suppress crops, root-knot nematodes (RKN; Meloidogyne spp.) represent possibly the world’s most damaging one, due to their wide host range encompassing the majority of flowering plants, short generation period, high reproductive rate, and an ability to form disease complexes with other soilborne pathogens such as fungi (Trudgill and Block, 2001; Back et al., 2002). In the past, phytonematode control has been mainly based on chemical nematicides. In the recent years, the EU has employed a fundamental reform of the Common Agricultural Policy (CAP), highlighting respect for the environment, food safety and animal welfare standards, and imposing farmlands’ cross compliance with good agricultural and environmental conditions (Schillhorn van Veen, 1999). Additionally, due to environmental side effects and health concerns, many synthetic carbamate, organophosphate, and organophthalide nematicides have been banned (91/414/EEC) or are under evaluation (2009/128/EU). The most potent fumigant nematicide was methyl bromide, which has been banned according to the requirements of the Montreal Protocol due to its ozone depleting properties. On the other hand, industry does not easily sustain the economic cost of research and registration of new nematicides (Neale, 2000), even though in some cases like in the Netherlands, they represent more than 60% of the total pesticides used in agriculture (Chitwood, 2002). As a result, there are only few nematicides still in use, and their limited number makes repeated application of the same formulations inevitable. This fact has favored mechanisms of nematicides biodegradation in soil (Qui et al., 2004) as well as development of nematode resistance (Meher et al., 2009), with the end result expressed in the field as lack of efficacy. All the above facts necessitate the urge for new and alternative nematode control methods (Chitwood, 2002).
An interesting way of searching for biorational nematicides is screening naturally occurring compounds from plants (Isman, 2006, 2008). Plants, as long-lived stationary organisms, must resist attackers over their lifetime, so they produce and exude constituents of the secondary metabolism (PSMs) that play an important role in their defense mechanisms (Chitwood 2002). In fact, phytochemical research has its roots in allelochemistry, involving the complex chemical-mediated interactions between a plant and other organisms in its environment (Chitwood, 2002). Such PSMs can be developed for use as nematicides themselves, or they can serve as model compounds for the development of more potent chemically synthesized derivatives. The botanical pesticides pose less risk to nontarget organisms since many of them are part of the food chain; additionally, they can be used as pesticides to avoid the emergence of resistant races because they are often clusters of bioactive compounds with diverse mode of action. As a result, the plant-derived pesticides can be safely used in Integrated Pest Management (IPM) programs (Isman, 2006). The specific constituent compound(s) that are responsible for the biological activity of the botanical extract need to be identified in order to further delineate the biochemical mechanisms and to fully exploit the therapeutic potential (Akhtar and Mahmood, 1997).
The development of PSMs as tools in crop protection was initiated as they were involved in traditional agricultural practices and eventually by the identification of the active molecules. The OECD (Organization for Economic Cooperation and Development) defines the botanical substances as semiochemicals (pheromones, but also plant extracts, plant volatiles, and natural oils) with pest control activities; while recently, the term biocontrol agents has been preferred over that of biopesticides. The aldehydes, ketones, and acids belong to the PSMs and are bioactive against pests and nematodes (Ntalli and Caboni, 2012). In our previous work, we demonstrated the nematicidal potential of aldehydes, ketones, and acids against M. incognita in in vitro and pot experiments (Ntalli et al., 2010, 2011; Caboni et al., 2012). Especially, acetic acid (EC50/1d = 38.8 against M. incognita), 2-undecanone (EC50/1d = 20.6 and 22.5 against M. incognita and M. javanica, respectively), (E,E)-2,4-decadienal, and (E)-2-decenal (EC50/1d = 11.7 and 20.4, respectively against M. javanica) exhibited high activities on Meloidogyne spp. Recently, we published on (E)-2-decenal revealing malformations, in the form of constrictions, along the larvae body retained in treated eggs, and larvae hatched at less than 50% from eggs immersed in 1 μg⋅mL−1 solutions. (E)-2-decenal arrested the M. incognita life cycle in pot bioassays (EC50 = 114.47 mg⋅kg−1), and it additionally promoted tomato growth. Most interestingly, the binary mixture of (E)-2-decenal/(E,E)-2,4-decadienal exhibited strong synergy on paralysis activity of M. incognita, M. javanica, and M. arenaria (Ntalli et al., 2016).
Transmission electron microscopy (TEM) has been a useful technique to note alterations at the level of cells and tissues. It can reveal and explain changes observed on the external layers of the body as well as in the inside, in the case of xenobiotics that can diffuse through the cuticle or through feeding procedure. In fact, we have previously reported on the diffusion of nematicidal aldehydes in the nematode cuticle (Caboni et al., 2013; Ntalli et al., 2015). However, herein we studied the cytotoxicity of these substances at a cellular and subcellular level. In particular, the aim of this present work was to evaluate tissue and cell malformations caused by acetic acid, 2-undecanone, and (E)-2-decenal on M. incognita, through ultrastructural observations using scanning and transmission electron microscopes. Comparisons were made against the commercial nematicide fosthiazate.
Materials and Methods
Nematode rearing and chemicals:
Meloidogyne incognita specimens were collected from naturally infested tomato fields in Thessaloniki, Greece, and were reared on tomato (Solanum lycopersicum Mill.) cv. Belladonna. Freshly hatched (24 h) J2 and eggs at different growth stages were extracted from egg masses according to Hussey and Barker (1973), to be used for the bioassays.
Acetic acid, 2-undecanone, (E)-2-decenal, and fosthiazate (all ≥99%) were purchased from Life Science Chemilab S.A, Greece.
Exposure of M. incognita J2 to acetic acid, 2-undecanone, and (E)-2-decenal:
Substances were initially diluted in dimethylsulfoxide (DMSO) to prepare stock solutions and then they were brought to volume with water to double the treatment concentration levels (40 or 1,000 μg⋅mL−1). These treatment solutions were mixed in microcentrifuge tubes at a ratio of 1:1 (v/v) with nematode (J2s or eggs) suspensions, containing 100 individuals per tube, thus resulting to 20 and 500 μg⋅mL−1 of treatment concentrations. Distilled water as well as DMSO in water (1% v/v) served as controls. These two test concentration levels were selected so as to study concentration dependence of effects, with the lower one (20 μg⋅mL−1) being close to the EC50 value of acetic acid, 2-undecanone, and (E)-2-decenal against RKN. Microscopic malformations are rather qualitative, not quantitative; hence, the high test concentration (500 μg⋅mL−1) was needed so as to make differences evident. Microcentrifuge tubes were maintained in the dark at 20°C. After 24 h, the nematodes were washed four times in fresh distilled water and were used for ultrastructural observation by scanning and transmission electron microscopies. Bioassays were performed three times, and every treatment was replicated six times.
Scanning electron microscope (SEM) preparation:
Nematodes were fixed in 2% glutaraldehyde, buffered with 0.1 M sodium cacodylate (pH 7.2) at 4°C for 2 h, then postfixed in 1% osmium tetroxide, for 2 h and dehydrated through the series of ethanol increasing concentrations water solutions. Samples were critical point dried, coated with gold and observed in a Zeiss Evo 40 SEM.
TEM preparation:
One-day postexposure to treatment solutions (20 or 500 μg⋅mL−1), and earlier to 1 day of postlethal time, nematodes were fixed in 2% glutaraldehyde buffered in 0.175 M cacodylate, postfixed with 1% osmium tetroxide, dehydrated using a series of ethanol concentrations, and embedded in Spurr resin. Ultrathin sections (70 nm) were obtained using a Leica ultramicrotome, stained with uranyl acetate and lead citrate. The midpart of the body was observed under the JEOL 1200EX II JEM transmission electron microscope.
Results
SEM observations:
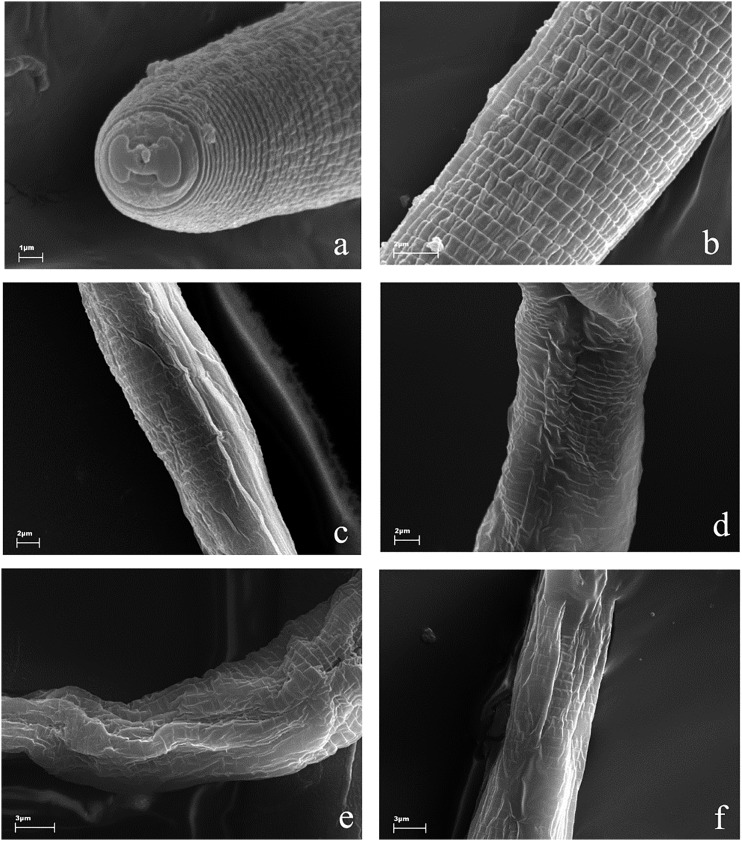
The cuticles of J2 larvae showed a typical pattern of annuli, which were thinner than 1 μm. They were separated by clear furrows. Control animals were round and turgid (Fig. 1A,B). Nematodes exposed to tested substances retained the pattern of annuli but they lost their turgidity and were characterized by regions of more or less intensively altered shape (Fig. 1C–F). J2s exposed to acetic acid, (E)-2-decenal, and fosthiazate showed invaginations and flattening of the body (Fig. 1C,E,F). On the other hand, 2-undecanone-treated larvae were still regular in shape, but their furrows were irregular and less deep than in the other cases (Fig. 1D).
Fig. 1.
Morphology of J2 larvae, scanning electron microscope observations. Control animals had a typical, round shape and were covered by regular annuli, separated by furrows (a, b). Nematodes exposed to acetic acid (c), 2-undecanone (d), (E)-2-decenal (e) and fosthiazate (f) showed irregular appearance of the cuticle, they were shrunken, wrinkled, and corrugated, with significantly lower turgidity and sunken structures along the body.
Ultrastructure of M. incognita J2, under TEM:
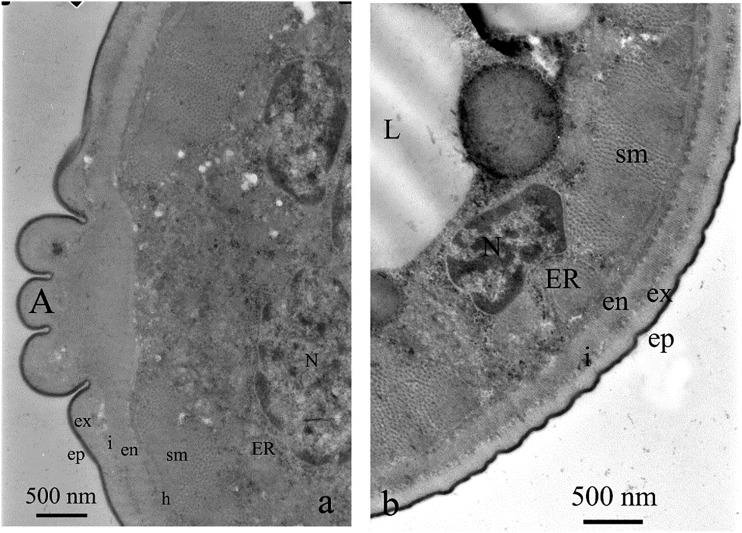
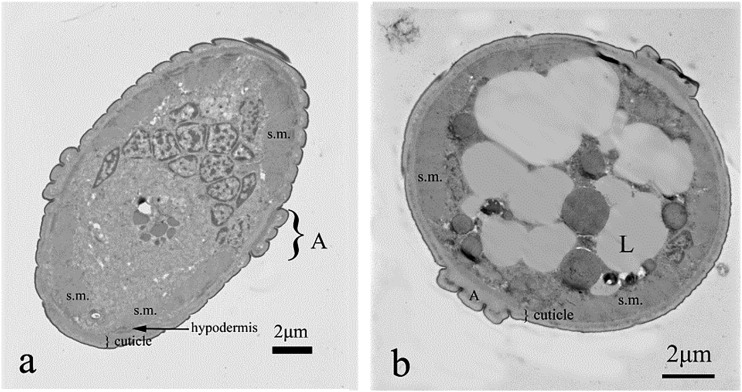
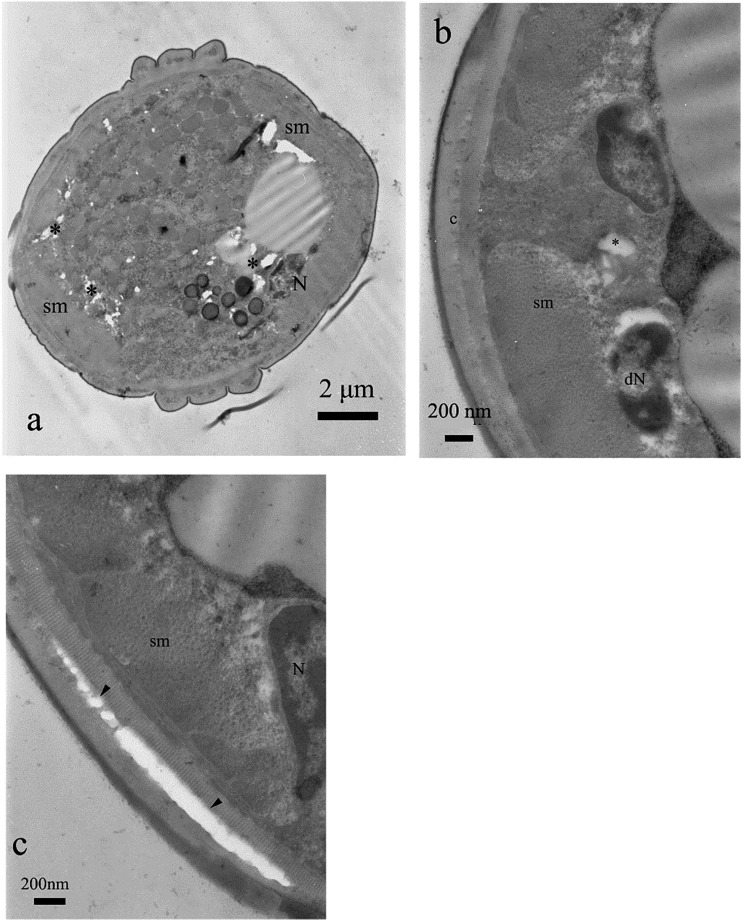
Body of M. incognita was covered with cuticle which thickness was lower than half of a micrometer, excluding alae, where it exceeded 1.5 µm. The cuticle was built of very thin, electron dense cortical layer, underlying electron lucent and homogenous basal cuticle layer. The most inner layer of cuticle—endocuticle—had similar electron density as exocuticle. However, it had a lamellar structure (Fig. 2A). Both layers were separated by an intermediate zone. Below, there was a thin layer of epidermis (i.e., hypodermis). This tissue was rather thin, located on electron dense basement membrane. Underlying layer of somatic muscles was clearly distinguishable from the neighboring tissues, due to their specific structure, with clearly seen myosin fibers (Fig. 2B). Pseudocoel occupied the center of the body. It was filled with syncytial gut cells, with no distinctive microvilli (Eisenback and Hunt, 2009), with relatively small electron lucent cavities (Fig. 3A). Some areas of the gut lumen were rich in lipid globules (Fig. 3B).
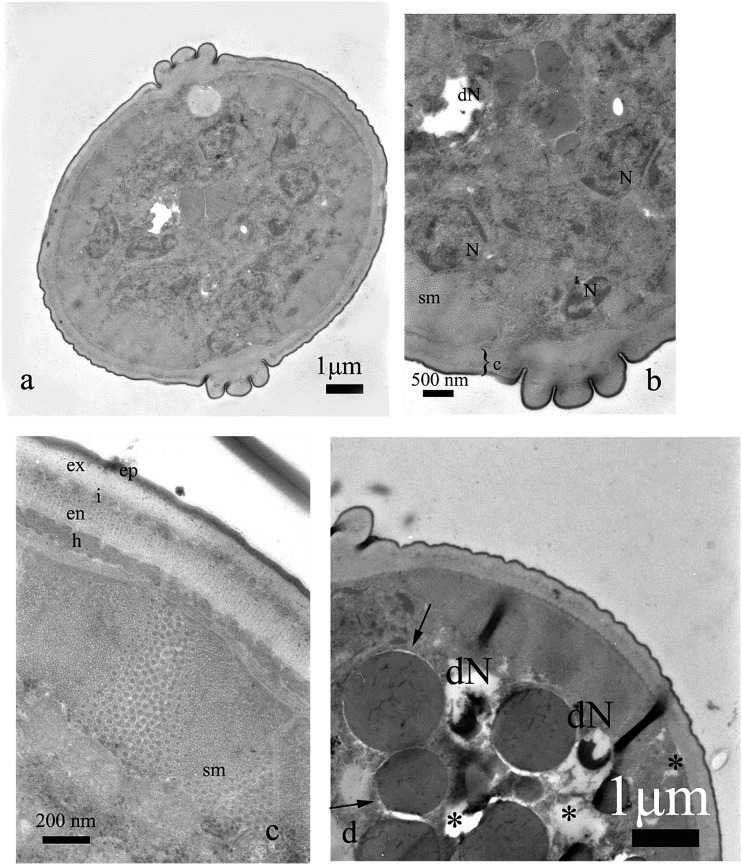
Fig. 2.
Control treatment and cross sections of M. incognita. Note cuticle with thin layer of hypodermis and one layer of somatic muscles (sm). Below the muscles there was a body cavity with syncytial gut epithelium and relatively few electron lucent pseudocoel cavities (a). In some areas of nematodes, gut lipid granules (L) were present (b).
Fig. 3.
Control treatment and cross section of the nematode body wall close to the lateral cord (a) with alae (A) and at the upper part of the body (b). Details of the cuticle were shown: ep = thin, electron dense, osmophilic epicuticle, ex = exocuticle, i = intermediate zone, en = lamellar endocuticle. Note thick layer of somatic muscles (sm) with prominent thick filaments and nuclei of cells surrounded by endoplasmic reticulum.
Ultrastructure of M. incognita J2 larvae suspended in acetic acid, 2-undecanone, and (E)-2-decenal, under TEM:
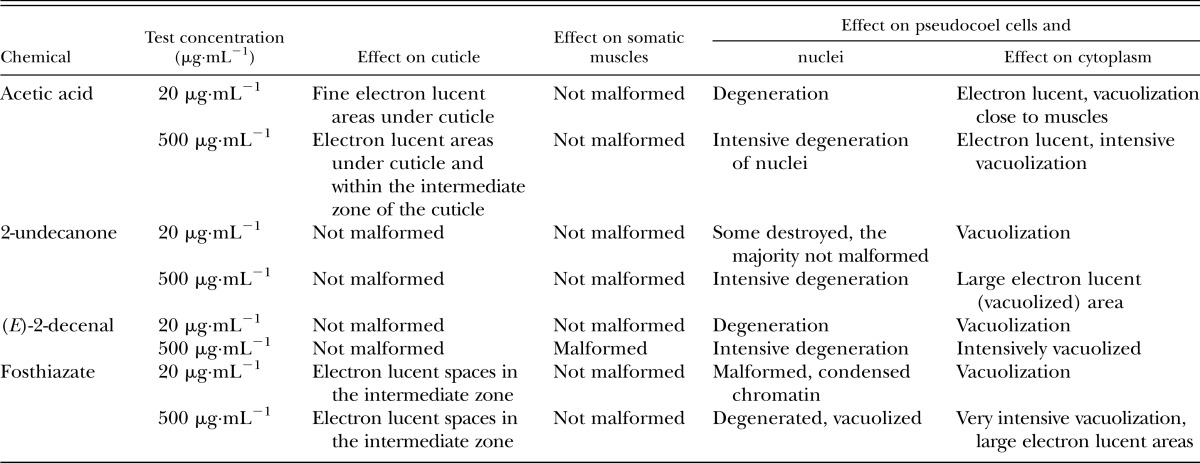
Acetic acid caused significant malformation of the subcuticular layers (Table 1). Cuticle itself was not significantly altered when 20 μg⋅mL−1 concentration of acetic acid was applied (Fig. 4A,B). However, within group exposed to 500 μg⋅mL−1 concentration of acetic acid, we observed vacuolization within hypodermis (Fig. 5A). The nuclei were seriously degenerated within both groups, with high ratio of electron dense chromatin (Fig. 5A,B). Intermediate zone was vacuolated, too (Fig. 5C). On the other hand, muscles were not damaged neither at 20 nor at 500 μg⋅mL−1, but the most prominent changes were seen under the muscles as areas of intensive cytoplasmic vacuolization (Fig. 5A,B).
Table 1.
Effects of acetic acid, 2-undecanone, (E)-2-decenal, and fosthiazate on ultrastructure of chosen tissues of Meloidogyne incognita J2 larvae.
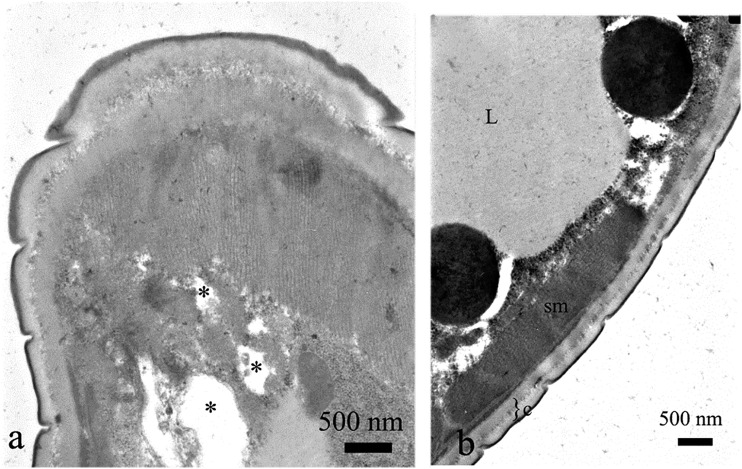
Fig. 4.
Cross section of the nematode treated with acetic acid, 20 μg⋅mL−1. Note A. electron-lucent regions close to the muscle layer (*), which were not present within B. control. sm = somatic muscles, c = cuticle, L = lipid granules.
Fig. 5.
Cross section of the nematode treated with acetic acid, 500 μg⋅mL−1. The electron-lucent regions close to the muscle layer (*, a) were much wider than those in case of 20 μg⋅mL−1, nuclei were destroyed (black arrowheads, a, b). Electron lucent areas were also visible within hypodermis (white arrowheads, a) and intermediate zone (^, c).
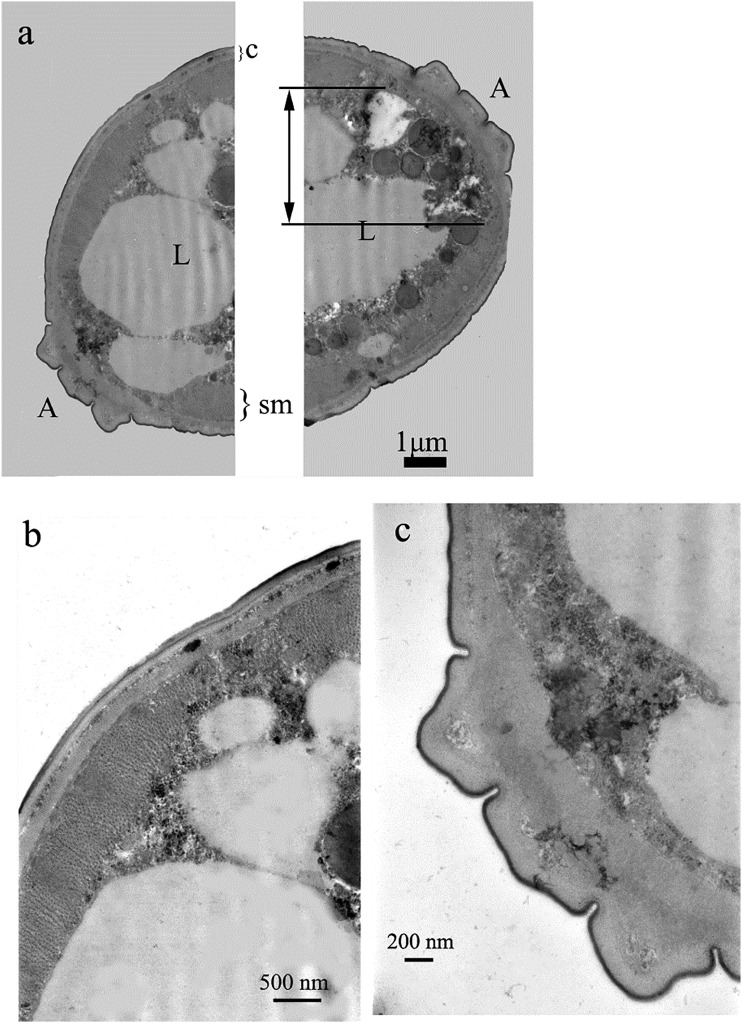
Cuticle was not malformed when larvae were exposed to 2-undecanone (Figs. 6A–C; 7C,D). Also musculature was not altered (Figs. 6C,7D). However, intensive vacuolization of cytoplasm and degeneration of nuclei was noted in the central part of the body, below the somatic musculature (Table 1). These malformations were concentration dependent in their intensity and area. Nuclear alterations resembled that observed for acetic acid. We could observe nuclei with high ratio of condensed chromatin, which began to degenerate, as well as those which seemed to be completely destroyed, with large electron lucent areas (Figs. 6B,D; 7A–C). Both types of malformations prevented nuclei from playing their roles. In the group exposed to higher concentration of 2-undecanone, irregularities of fat droplets were noted. Their shape was not round, as described for control animals (Fig. 7A,B). The droplets began to fuse (Fig. 7B).
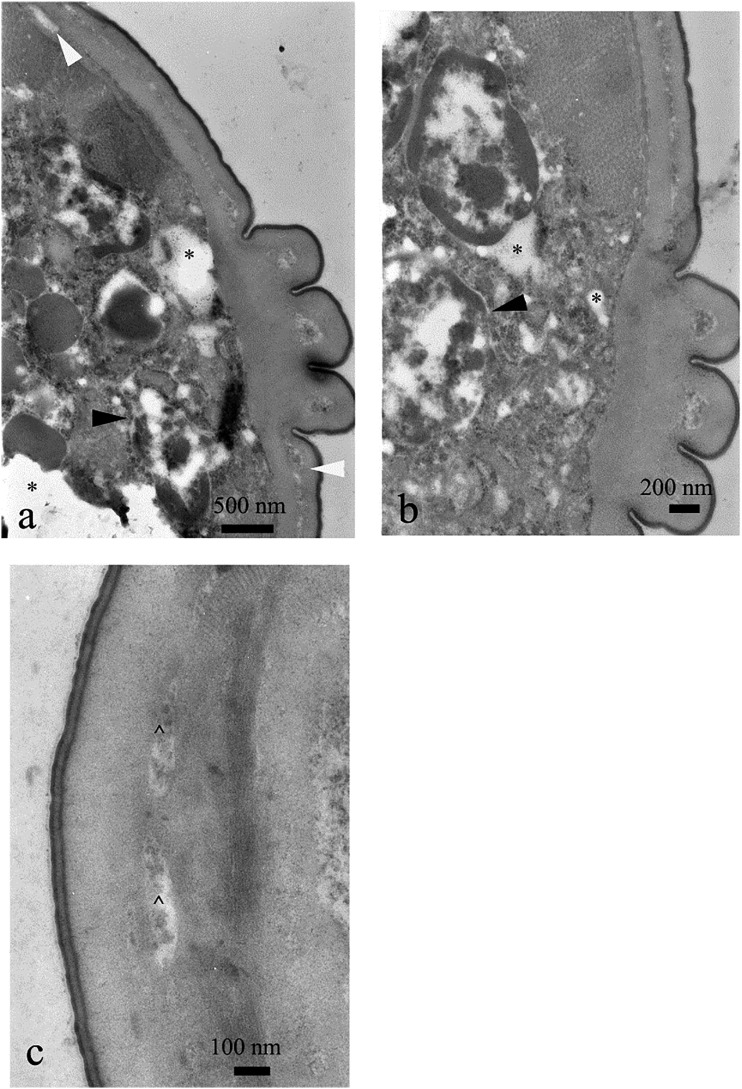
Fig. 6.
Cross section of the nematode treated with undecanone, 20 μg⋅mL−1. Some nuclei were destroyed (dN, b, d), whereas the majority of cells were not altered (a, b). Vacuolization (electron lucent areas) was present within cytoplasm (*, d). Cuticle, its sublayers (ep = epicuticle, ex = exocuticle, i = intermediate zone, en = endocuticle), hypodermis (h) and somatic muscles (sm) were not significantly altered, too (c, d).
Fig. 7.
Cross section of the nematode treated with undecanone, 500 μg⋅mL−1. Intensive vacuolization of syncytial gut (*, a–c), irregularities of lipid droplets and intensive degeneration of nuclei (dN) were noted (a–c). However, cuticle (c) and somatic muscles (sm) were properly developed (d).
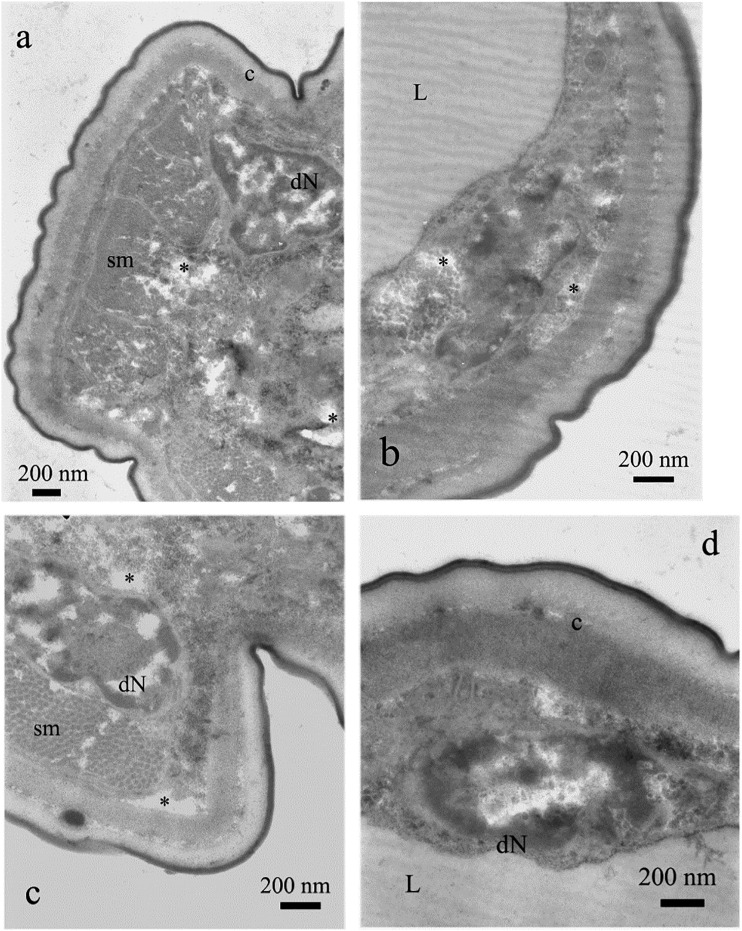
(E)-2-decenal did not cause visible alterations of cuticle itself (Figs. 8B,C; 9B). However, the higher concentration of this compound caused malformations of somatic muscles. Muscles were fragmented and did not form a continuous layer (Fig. 9A). Such changes were not observed for acetic acid and 2-undecanone. Also gut cells showed concentration-dependent degeneration of nuclei and cytoplasm (Table 1). Some areas were practically digested (Figs. 8A; 9A,C,D).
Fig. 8.
Cross section of the nematode treated with (E)-2-decenal, 20 μg⋅mL−1 (a). Note properly developed cuticle (c) and somatic muscles (sm) (b, c). Gut lumen is filled with lipid granules (L, a). The line with two arrowheads indicates region of intensive vacuolization (a).
Fig. 9.
Cross section of the nematode treated with (E)-decenal, 500 μg⋅mL−1. Note intensive vacuolization of gut cells (*, a–c) and degeneration of gut nuclei (dN, a, c, d). Also somatic muscles (sm) are malformed (a). Cuticle (c) and lipids (L) are not altered (b).
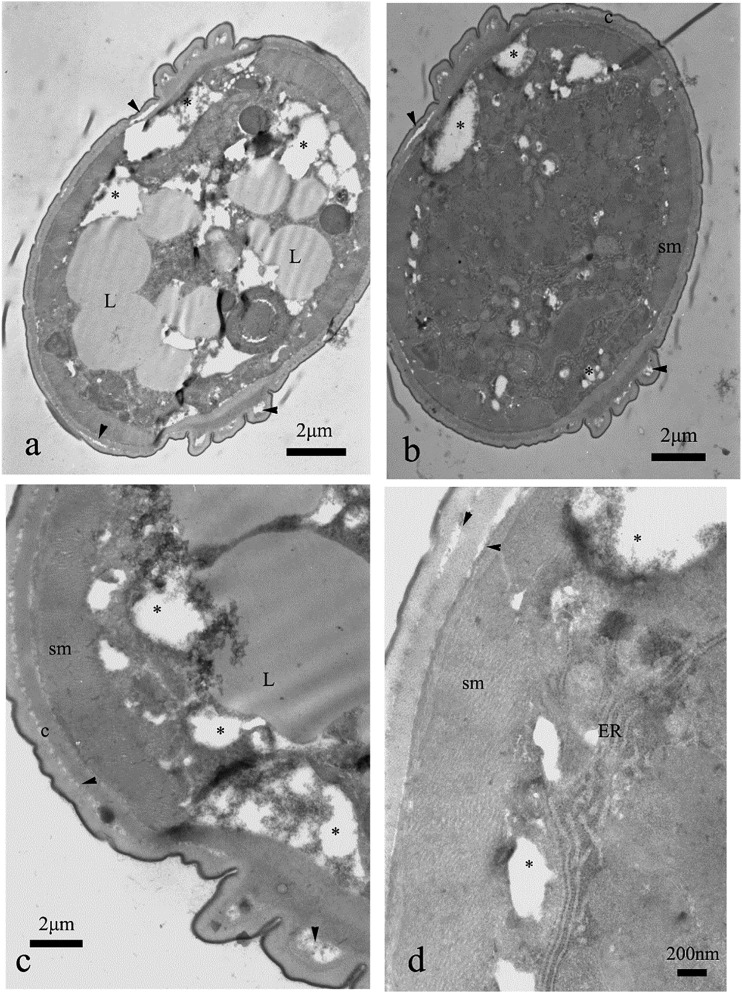
The last of the tested substances, the commercial nematicide fosthiazate, caused malformations within cuticle. Large, electron lucent areas were noted within intermediate zone, even within cuticle of nematodes exposed to the lower of the two tested concentrations (Fig. 10A–C). These changes were of the highest intensity among all the groups exposed to tested compounds (Figs. 10A,B; 11A–D). The somatic muscles were not harmed, but gut syncytium showed significant, drastic vacuolization, especially within the group exposed to 500 μg⋅mL−1 (Figs. 10A,B; 11A–D; Table 1). Lipid droplets were not regular and fused together (Fig. 11A,C). We noted nuclear condensation of nuclei within neural area (Figs. 10B,11C).
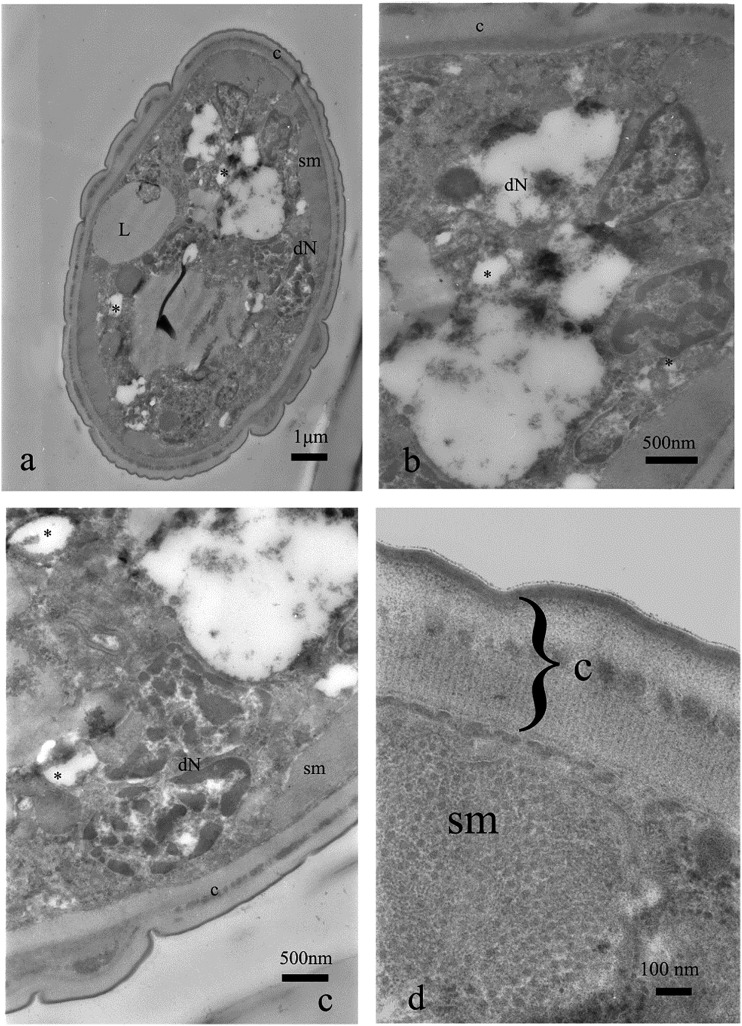
Fig. 10.
Cross section of the nematode treated with fosthiazate, 20 μg⋅mL−1. Note regions of vacuolization within gut cells (*, a, b), breaks between cuticle layers (arrowheads, c), degenerated nuclei (dN, b) and malformations of nerve area (b).
Fig. 11.
Cross section of the nematode treated with fosthiazate, 500 μg⋅mL−1. Cuticle contains areas where the layers are separated (arrowheads, a–d), gut syncytium is intensively vacuolized and destroyed in the majority of the cross sections (*, a–d). Lipid droplets (L) are fused together (a) or irregular (c).
In summary, the tested substances showed various types of malformations, of diverse intensity. Gut cells were much more harmed, than cuticle, hypodermis, or muscles. Ultrastructure of nuclei was altered by the majority of tested substances. Types of malformations and their intensities are summarized in Table 1.
Discussion
All four juvenile life stages (J1, J2, J3, and J4) of Meloidogyne spp, and the development into either adult males or females, are separated by molts. The J1 remains in the egg and molts into a J2 that is the infective stage. J2 invade plant tissue and initiate the formation of a feeding site, whereas the remaining life stages (except the adult male) are sedentary. The J3 and J4 stages do not feed because they remain ensheathed in the cuticles of the previous life stage and lose their stylets (Kyndt et al., 2013; Dinh et al., 2014). Therefore, the tested substances enter the J2 body via intestine or/and cuticle.
Acetic acid, 2-undecanone, (E)-2decenal, and (E,E)-(2,2)-decadienal are reported nematicidals (Ntalli et al., 2010, 2011; Caboni et al., 2012) but their effect on J2 ultrastructure is presented herein for the first time.
(E)-2-decenal and 2-undecanone mainly harmed pseudocoel cells rather than the cuticle, and somatic muscles. In specific, they provoked vacuolization of pseudocoel cells suggesting intensive processes of cellular digestion and altered osmotic conditions, as effects of intoxication. These findings differ from findings of Vardi et al. (2006) who reported significant activity of (E,E)-(2,2)-decadienal on diatom cells: increase of calcium within diatom cells and cell death, but did not note such activity caused by (E)-2-decenal. The unique finding was malformation of muscle tissue caused by (E)-2-decenal. Such activity was not reported for any other of the tested substances.
The nematode cuticle consists of lipids, carbohydrates, and more complex molecules like glycoproteins, lipoproteins or glycolipids and the surface coat of the nematode cuticle contains carbohydrates (Wharton et al., 2011). The chemical composition of the cuticle differs between gastrointestinal and soil-inhabiting plant parasites, as well as between life stages of same species (compare: Spiegel and McClure, 1995). Among proteins, collagen fibers are located within the whole cuticle (Hussey and Jansma, 1988) and are responsible for its flexibility and shape. Chemical changes of collagen within cuticle may result in morphological malformations of body surface. We previously reported on the cuticle detachment from internal organs in J2 treated with acetic acid (Aoudia et al., 2010). Changes of cuticle were observed in J2s exposed to acetic acid, which are in tune with other reports on altered arrangement of collagen network within tissues exposed to acetic acid (Davison et al., 1972). Moreover, acetic acid is used to extract collagen from various samples (Peck and Mashitah, 2013), thus revealing its dissolving properties. In our study, the effects on cuticle were evident in TEM observations as electron lucent areas within intermediate zone, filled with fluid. These areas might interfere with osmotic conditions within the body. The acetic acid is widely known as a precipitant of nucleoproteins, causing swelling of proteins through the absorption of water (Baker, 1958) and altering membrane integrity (Prudêncio et al., 1998). Such changes manifest in altered turgidity of the body, vacuolization of the internal structures and single cells. The acetic acid effect on nuclei was also evident under TEM. Similarly, chromatin condensation was described for nuclei of Zygosaccharomyces bailii yeast cells (Ludovico et al., 2003) and Bacillus sp. (Gomaa, 2012) exposed to acetic acid. The chromatin condensation maybe caused by fixation and denaturation of DNA induced by acetic acid (Shapiro et al., 1978; Meade, et al., 2010).
2-Undecanone caused malformations of cells in the pseudocoel, but not within epiderm or muscle layer. It probably reached the body mostly through the intestines. In fact, 2-undecanone is easily soluble in fats and slightly soluble in water (Chen et al., 2014). Therefore, rather than affecting the water-rich cytoplasm, 2-undecanone may cumulate in fat droplets and biological membranes and then possibly disturb their structure and functioning. Such changes were observed within animals exposed to higher concentration of this substance. Moreover, it may harm nuclear envelope and lead to nuclear malformations. In fact, 2-undecanone has been reported to affect the regulation of metabolic pathways (Chen et al., 2014), and to exhibit cytotoxic effects. Cytotoxicity was reported for 2-undecanone rich extract from Ruta chalepensis, against Leishmania protozooans (Ahmeda et al., 2011). 2-undecanone also exhibits activity against Caenorhabditis elegans, Drosophila melanogaster, and Rhizoctonia solani (Popova et al., 2014). It was also found to be cytotoxic against human carcinoma cells (Castaneda et al., 2007). 2-Undecanone-rich essential oil compositions from Zanthoxylum molle caused plasma membrane malformations and intensive vacuolation of cytoplasm in Aspergillus flavus (Tian et al., 2014). Such unspecific effects can be caused by the observed ultrastructural malformations, especially when nuclei are destroyed. In consequence, regulation of metabolism within cells is disturbed. That may lead to cell death. Hence, nuclear malformations may be possible by both: direct effect of ketones present in lipid-rich cell compartments or indirectly, by altered cellular/nuclear metabolic pathways and cell-lethal activity of tested compounds. Due to the relatively short time of exposure, the second possibility seems to be less probable. However, both hypotheses need to be supported by further studies on the cellular and subcellular level. SEM observations showed, that the turgidity of the whole body was not significantly altered after immersion to 2-undecanone. The nematodes were not regular in their shape, but the furrows were less deep than in the control animals. That suggests increased pressure of the hydroskeleton on the body surface. The shape of the whole-body TEM cross sections were regular, too. These findings suggest that the mode of action of undecanone must differ from the other three tested substances.
We observed intensive degeneration of syncytial cells when 2-decenal was used, too. The unique finding was malformation of muscle tissue. Such activity was not reported for any other of the tested substances. However, TEM analysis did not reveal cuticle malformations. Both 2-undecanone and (E)-2-decenal enter membrane body through the gut and then (E)-2-decenal might diffuse to the muscle layer. It might neither diffuse through the cuticle nor the diffusion is very fast thus not permitting reaction with biomolecules present within cuticle or hypodermis. Degeneration of tissues lowers turgidity of the body. Therefore, even though cuticle was not harmed, the body shape alters significantly as observed in SEM. Most probably, this was caused by destroyed muscles. Together with disturbed structure of internal tissues, it led to invaginations and flattening of the body shape.
Fosthiazate belongs to the organophosphorous group of pesticides. Therefore, it affects nervous tissue. This activity leads to death of the animal due to hyperactivity of the nervous tissue. Moreover, it induces oxidative stress that manifests in increased lipid peroxidation within tissues and affects S-adenosyl-L-methionine:phosphoethanolamine N-methyltransferase (Yin et al., 2012) which takes part in membrane phospholipid synthesis (Palavalli et al., 2006). Thus it may affect cell membranes and cause cellular toxicity. It can also alter structure and organization of lipid droplets in larvae. Such effects were observed in both tested concentrations of fosthiazate.
To the best of our knowledge, this is the first report on the ultrastuctural effects of acetic acid, (E)-2-decenal, and 2-undecanone on Meloidogyne incognita. Moreover, the data on the biochemical activity of tested substances is limited. Therefore, further studies on the suborganismal level are necessary, to explain the mode of toxic activity of aldehydes and ketones in cells and tissues. Our results suggest that natural substances may significantly decrease crop loss due to impaired nematode physiology. In particular, malfunctions of the gut may lead to decreased herbivory of the pests. Effects within musculature may affect mobility of J2 larvae and decrease root infestation. Cuticle malformations caused by acetic acid and fosthiazate may disturb molting and make the exposed nematodes more susceptible to other nematicides. Therefore, we think that even when lethality of natural substances is lower than lethality of chemical nematicides, they can act synergistically to synthetic pesticides.
Literature Cited
- Akhtar M, Mahmood I. Control of root-knot nematode Meloidogyne incognita in tomato plants by seed coating with Suneem and neem oil. Journal of Pesticide Science. 1997;22:37–38. [Google Scholar]
- Aoudia H, Ntalli N, Aissani N, Yahiaoui-Zaidi R, Caboni P. Nematotoxic phenolic compounds from Melia azedarach against Meloidogyne incognita. Journal of Agricultural and Food Chemistry. 2012;60:11675–11680. doi: 10.1021/jf3038874. [DOI] [PubMed] [Google Scholar]
- Back MA, Haydock PPJ, Jenkinson P. Disease complexes involving plant parasitic nematodes and soilborne pathogens. Plant Pathology. 2002;51:683–697. [Google Scholar]
- Baker JR. 1958. Principles of biological microtechnique. London: Methuen.
- Caboni P, Ntalli NG, Aissani N, Cavoski I, Angioni A. Nematicidal activity of (E,E)-2,4-(E,E)-2,4-decadienal and (E)-2-(E)-2-decenal from Ailanthus altissima against Meloidogyne javanica. Journal of Agricultural and Food Chemistry. 2012;60:1146–1151. doi: 10.1021/jf2044586. [DOI] [PubMed] [Google Scholar]
- Caboni P, Aissani N, Cabras T, Falqui A, Marotta R, Liori B, Ntalli N, Sarais G, Sasanelli N, Tocco G. 2013. Potent nematicidal activity of phthalaldehyde, salicylaldehyde, and cinnamic aldehyde against Meloidogyne incognita Journal of Agricultural and Food Chemistry 61:1794–803. [DOI] [PubMed]
- Castaneda F, Zimmermann D, Nolte J, Baumbach JI. Role of undecan-2-one on ethanol-induced apoptosis in HepG2 cells. Cell Biology and Toxicology. 2007;23:477–485. doi: 10.1007/s10565-007-9009-y. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang W, Shi C, Fang J. 2014. A comparative study of sodium houttuyfonate and 2-undecanone for their in vitro and in vivo anti-inflammatory activities and stabilities. International Journal of Molecular Sciences 15:22978–22994.
- Chitwood DJ. Phytochemical based strategies for nematode control. Annual Review of Phytopathology. 2002;40:221–249. doi: 10.1146/annurev.phyto.40.032602.130045. [DOI] [PubMed] [Google Scholar]
- Davison PF, Cannon DJ, Andersson LP. The effects of acetic acid on collagen cross-links. Connective Tissue Research. 1972;1:205–216. [Google Scholar]
- Dinh PTY, Knoblauch M, Elling AA. Nondestructive imaging of plant-parasitic nematode development and host response to nematode pathogenesis. Phytopathology. 2014;104:497–506. doi: 10.1094/PHYTO-08-13-0240-R. [DOI] [PubMed] [Google Scholar]
- Eisenback JD, Hunt DJ. 2009. General Morphology. Pp. 18–54 in R. N. Perry, M. Moens, and J. L. Starr. eds. Rootknot nematodes, Wallingford, UK: CABI.
- Gomaa OM. The involvement of acetic acid in programmed cell death for the elimination of Bacillus sp. used in bioremediation. Journal of Genetic Engineering and Biotechnology. 2012;10:185–192. [Google Scholar]
- Hussey RS, Jansma P. Immunogold localization of collagen in the cuticle of different life stages of Meloidogyne incognita. Journal of Nematology. 1988;20:641–642. [Google Scholar]
- Isman M. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annual Review of Entomology. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- Isman M. Botanical insecticides: for richer, for poorer. Pest Management Science. 2008;64:8–11. doi: 10.1002/ps.1470. [DOI] [PubMed] [Google Scholar]
- Kyndt T, Vieira P, Gheysen G, de Almeida-Engler J. Nematode feeding sites: unique organs in plant roots. Planta. 2013;238:807–818. doi: 10.1007/s00425-013-1923-z. [DOI] [PubMed] [Google Scholar]
- Ludovico P, Sansonetty F, Silva MT, Côrte-Real M. Acetic acid induces a programmed cell death process in the food spoilage yeast Zygosaccharomyces bailii. FEMS Yeast Research. 2003;3:91–96. doi: 10.1016/s1567-1356(02)00166-6. [DOI] [PubMed] [Google Scholar]
- Meade AD, Clarke C, Draux F, Sockalingum GD, Manfait M, Lyng FM, Byrne HJ. Studies of chemical fixation effects in human cell lines using Raman microspectroscopy. Analytical and Bioanalytical Chemistry. 2010;396:1781–1791. doi: 10.1007/s00216-009-3411-7. [DOI] [PubMed] [Google Scholar]
- Meher HC, Gajbhiye VT, Chawla G, Singh G. Virulence development and genetic polymorphism in Meloidogyne incognita (Kofoid & White) Chitwood after prolonged exposure to sublethal concentrations of nematicides and continuous growing of resistant tomato cultivars. Pest Management Science. 2009;65:1201–1207. doi: 10.1002/ps.1810. [DOI] [PubMed] [Google Scholar]
- Neale M. The regulation of natural products as crop-protection agents. Pest Management Science. 2000;56:677–680. [Google Scholar]
- Ntalli NG, Caboni P. Botanical Nematicides: A Review. Journal of Agricultural and Food Chemistry. 2012;60:9929–9940. doi: 10.1021/jf303107j. [DOI] [PubMed] [Google Scholar]
- Ntalli NG, Vargiu S, Menkissoglu-Spiroudi U, Caboni P. Nematicidal carboxylic acids and aldehydes from Melia azedarach fruits. Journal of Agricultural and Food Chemistry. 2010;58:11390–11394. doi: 10.1021/jf1025345. [DOI] [PubMed] [Google Scholar]
- Ntalli NG, Manconi F, Leonti M, Maxia A, Caboni P. Aliphatic ketones from Ruta chalepensis (Rutaceae) induce paralysis on root knot nematodes. Journal of Agricultural and Food Chemistry. 2011;59:7098–7103. doi: 10.1021/jf2013474. [DOI] [PubMed] [Google Scholar]
- Ntalli N, Oplos C, Michailidis M, Thanasenaris A, Kontea D, Caboni P, Tsiropoulos NG, Menkissoglu-Spiroudi U, Adamski Z. Strong synergistic activity and egg hatch inhibition by (E,E)-2,4-decadienal and (E)-2-decenal in Meloidogyne species. Journal of Pest Science. 2016;89:565–579. [Google Scholar]
- Palavalli LH, Brendza KM, Haakenson W, Cahoon RE, McLaird M, Hicks LM, McCarter JP, Williams DJ, Hresko MD, Jez JM. Defining the role of phosphomethylethanolamine N-methyltransferase from Caenorhabditis elegans in phosphocholine biosynthesis by biochemical and kinetic analysis. Biochemistry. 2006;45:6056–6065. doi: 10.1021/bi060199d. [DOI] [PubMed] [Google Scholar]
- Peck LK, Mashitah MD. The influence of acetic acid concentration on the extractability of collagen from the skin of hybrid Clarias sp. and its physicochemical properties: A preliminary study. Focusing on Modern Food Industry. 2013;2:123–128. [Google Scholar]
- Popova AA, Koksharova OA, Lipasova VA, Zaitseva JV, Katkova-Zhukotskaya OA, Eremina SI, Mironov AS, Chernin LS, Khmel IA. Inhibitory and toxic effects of volatiles emitted by strains of Pseudomonas and Serratia on growth and survival of selected microorganisms, Caenorhabditis elegans, and Drosophila melanogaster. BioMed Research International. 2014;2014:125704. doi: 10.1155/2014/125704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudêncio C, Sansonetty F, Côrte-Real M. Flow cytometric assessment of cell structural and functional changes induced by acetic acid in the yeasts Zygosaccharomyces bailii and Saccharomyces cerevisiae. Cytometry. 1998;31:307–313. doi: 10.1002/(sici)1097-0320(19980401)31:4<307::aid-cyto11>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Schillhorn van Veen TW. Agricultural policy and sustainable livestock development. International Journal of Parasitology. 1999;29:7–15. doi: 10.1016/s0020-7519(98)00174-x. [DOI] [PubMed] [Google Scholar]
- Shapiro IM, Moar MH, Ohno S, Klein G. Acetic acid treatment denatures DNA while preserving chromosomal morphology during the in situ hybridization procedure. Experimental Cell Research. 1978;115:411–414. doi: 10.1016/0014-4827(78)90296-3. [DOI] [PubMed] [Google Scholar]
- Spiegel Y, McClure MA. The surface coat of plant-parasitic nematodes: Chemical composition, origin, and biological role—A review. Journal of Nematology. 1995;27:127–134. [PMC free article] [PubMed] [Google Scholar]
- Tian J, Zeng X, Feng Z, Miaoa X, Peng X, Wang Y. Zanthoxylum molle Rehd. essential oil as a potential natural preservative in management of Aspergillus flavus. Industrial Crops and Products. 2014;60:151–159. [Google Scholar]
- Trudgill DL, Block VC. Apomictic, polyphagous root-knot nematodes: exceptionally successful and damaging biotrophic root pathogens. The Annula Review of Phytopathology. 2001;39:53–77. doi: 10.1146/annurev.phyto.39.1.53. [DOI] [PubMed] [Google Scholar]
- Vardi A, Formigginim F, Casotti R, De Martino A, Ribalet F, Miralto A, Bowler CA. A stress surveillance system based on calcium and nitric oxide in marine diatoms. PLoS Biology. 2006;4:0411–0419. doi: 10.1371/journal.pbio.0040060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton DA, Petrone L, Duncan A, McQuillan AJ. A surface lipid may control the permeability slump associated with entry into anhydrobiosis in the plant parasitic nematode Ditylenchus dipsaci. Journal of Experimenta Biology. 1999;211:2901–2908. doi: 10.1242/jeb.020743. [DOI] [PubMed] [Google Scholar]
- Yin C, Teng Y, Luo Y, Christie P. Proteomic response of wheat embryos to fosthiazate stress in a protected vegetable soil. Journal of Environmental Sciences. 2012;24:1843–1853. doi: 10.1016/s1001-0742(11)61013-9. [DOI] [PubMed] [Google Scholar]