Abstract
The carob moth (Ectomyelois ceratoniae) is the key pest of pomegranate, which causes a significant percentage of losses in pomegranate orchards and warehouses of Iran annually. The pest larvae are characterized by displaying a cryptic behavior within the fruit, which avoids most routine control techniques, especially chemical method. The low efficiency of traditional measurements and also the rich species diversity of natural enemies within the infested fruits highlight the necessity of exploring effective control methods, especially environmental friendly approaches. Entomopathogenic nematodes (EPNs) are a group of biological control agents that actively search for the host, including those in a cryptic habitat like the carob moth larvae within infested fruits. Here, we assumed that treatment of the infested and dropped fruits with EPNs may provide new insight into the management of the carob moth. Three species of EPNs, Steinernema feltiae, S. carpocapsae, and Heterorhabditis bacteriophora were selected and used in a series of in vitro and in vivo experiments. In preliminary assays, the EPNs species were used with different concentrations of infective juveniles (IJs) (0, 1, 5, 10, 25, and 50 IJ/larvae) in 2-cm diam. plates. The mortality rates of the laboratory tests were 79.75% and 76.5% for S. feltiae and S. carpocapsae, corresponded to LC50 value of 2.02 IJ/larva for S. feltiae and 2.05 IJ/larva for S. carpocapsae. On the contrary, H. bacteriophora demonstrated low virulence on the pest larvae in petri tests with a LC50 = 426.92 IJ/larva. Hence, both Steinernema species were selected for subsequent experiments. The penetration rate for S. feltiae and S. carpocapsae into the hemocoel of the pest was 43% and 31%, respectively, and the corresponding reproduction rate was 15,452 IJ/larva for S. feltiae and 18,456 IJ/larva for S. carpocapsae. The gathered data from those in vitro tests were used for a field assay. Different concentrations (5, 10, 50, 100, and 160 IJ/cm2 of the arena) of S. feltiae and S. carpocapsae were applied in the field test. The mean mortality results from the last test were 10.89% and 26.65% for S. feltiae and S. carpocapsae, respectively. Finally, we found that these low virulence rates of the nematodes were attributed to inhibitory/repellency effects of saprophytic fungi within the infested pomegranates, a usual status of the infested fruits in autumn or winter seasons. Future work on additional EPN populations more adapted to the extreme conditions of the pomegranate production area in Iran may provide sufficient evidence to continue the further investigation on the best EPN species populations and advanced formulations with high durability.
Keywords: biological control, entomopathogenic nematode, insect pathology, pomegranate moth, pathogenicity
Carob moth Ectomyelois ceratoniae Zeller (Lepidoptera: Pyralidae) is the most important and destructive insect pest of pomegranate, an important fruit in parts of Asia (Sarkhosh et al., 2006). This pest causes quantitative and qualitative damage up to 80% in orchards and storehouses of Iran, which is the world’s top pomegranate producer (Shakeri, 2004). The carob moth is a polyphagous pest and causes damage on fig, pistachio, dates, citrus, and almond (Gothilf, 1978; Mehrnejad, 2002; Nay and Perring, 2005). The larvae overwinter in fallen fruit or fruit left on trees and continue to develop inside the fruit. The first invasion of the pest occurs in early spring, concurrent with pomegranate flowering, and its activity is prolonged until the end of June or longer (Shakeri and Sadat Akhavi, 2003). The feeding larvae damage the pomegranates, providing a chance for saprophytic fungi penetration, such as Aspergillus spp. and Penicillium spp. The larvae feed in protected niches within the fruit, which is protected from the effects of insecticides. In order to control this pest, an integrated pest management approach, including alternative and environmentally friendly methods in pomegranate orchards, is needed (Shakeri, 2004). Several efforts have been conducted to control the pest including the use of sex pheromones for adult attraction (Kehat et al., 1995), oil extracts from Ferula asafoetida L. as a repellent (Shakeri, 2004), natural enemies (Kishani-Farahani et al., 2009), and sanitation (Nay and Perring, 2005). Different life stages of the carob moth have natural enemies from various groups of parasitoids, including Ichneumonoidea and Chalcidoidea (Kishani-Farahani et al., 2009), of which Trichogramma wasps have been released for pest control in pomegranate orchards (Nasrollahi et al., 1998). Among the insect pathogens, laboratory data are available about the potential of Bacillus subtilis (Ehrenberg) (Mnif et al., 2013) but the effect of other groups of insect pathogens and their pathogenicity against the carob moth have not yet been explored. Few studies have shown that entomopathogenic nematodes (EPN) have significant pathogenicity against different pest species from the Pyralidae family (Kaya and Gaugler, 1993; Grewal, 2002; Shapiro-Ilan and Gaugler, 2002; Lacey et al., 2015). Hence, we consider that EPNs have potential against the carob moth.
Nematode parasitism starts when an infective juvenile (IJ) enters the hemocoel of a host through natural openings such as the mouth, anus, and spiracles for Steinernematidae or via the integument for Heterorhabditis. They release their bacteria into the host body and kill the pest within 24 to 48 hr. The new generation of nematodes feeds on the bacterial symbiont and contents of the host body (Kaya and Gaugler, 1993; Gaugler, 2002). The EPNs have advantages such as movement ability, highly virulence, an ability to kill hosts quickly, easy mass rearing, high reproductive potential, broad host range, and safety to vertebrates, plants, and other nontarget organisms (Kaya and Gaugler, 1993). Because the carob moth overwinters as larvae in autumn and winter within the infested fruit that drop on the soil, our hypothesis is that it is possible to use EPN for their control. The main objective of the current research was addressing susceptibility of the overwintering stage of the carob moth to the EPNs. The second aim was identifying the penetration potential of EPN into the pest larvae and the host potential to support reproduction of the nematode. The third part of the study explored the efficacy of EPNs on the infested fruits under field conditions; the last test was designed to understand the migration behavior of the EPNs toward the pest larvae in heavily infested fruit and to identify effective species adapted to the pomegranate fruit.
Materials and Methods
Insect:
During 2013 to 2014, larval stages of carob moth were collected from infested orchards of Kashmar (Razavi Khorasan Province, Iran, 35°15′ N and 58°30′ E) and transferred to the laboratory under controlled conditions [25°C ± 1°C, 60% ± 5% relative humidity, and 16:8 hr (light:dark)]. We reared larvae on artificial diet (wheat bran 300 g, sugar 80 g, yeast 9 g, multivitamin 1.4 g, tetracycline antibiotics 0.6 g, sterile distilled water 120 ml, and glycerin 130 ml) and provided cohorts of different developmental stages. Infested fruits were placed in wooden cages (50 × 50 × 50 cm) and covered with ruche. The adult carob moths were collected daily using an aspirator and placed in mating glass containers (20 × 30 × 50 cm) whose bottoms were covered with cloth tissue; moths were fed with 5% hydromel solution. Tissues containing eggs were changed daily and transferred to the rearing containers. Hatched larvae were reared on artificial diets (Zolfaghariae et al., 2010) and kept under controlled conditions.
EPN cultures:
The nematode cultures were inoculated on last instar greater wax moth larvae Galleria mellonella L. (Lepidoptera: Pyralidae) with 100 ml suspension of the EPNs (Kaya and Stock, 1997). Three species of EPNs, including Heterorhabditis bacteriophora Poinar, Steinernema carpocapsae Weiser, and Steinernema feltiae Flipjev were selected for the tests. These species were commercial products provided by Koppert Co. (Berkel en Rodenrijs, The Netherlands). The emerging IJs were collected from White traps (White, 1927) and stored in tap water at 8°C ± 1°C for less than 4 wk.
Pathogenicity assay of EPNs on the carob moth (plate assay):
To determine appropriate doses for future assays on the last larval stage, preliminary tests evaluated the susceptibility of last instar larvae of the carob moth to different concentrations of H. bacteriophora, S. carpocapsae, and S. feltiae. The selected concentrations were 0, 1, 2, 5, 10, 25, 50, 100, 250, 500, and 1,000 IJs/larva. Based on those tests, five concentrations were finally selected (0, 1, 5, 10, 25, and 50 IJs) for S. carpocapsae and S. feltiae species, as well 100, 250, 500, and 1,000 IJs/larva for H. bacteriophora. The assays were carried out in 2.2-cm diam. plates, containing a filter paper (Whatman No. 1). One hour after application of the IJs, one larva was added to each plate. In the control treatment, just 50 µl distilled water was added to the plate. The plates were covered and incubated in controlled growth chamber [25°C ± 1°C, 65% ± 5% relative humidity, and 16:8 hr (light:dark)]. Insect mortality was checked after 48 hr, and dead larvae were dissected under the stereomicroscope to confirm that the mortality resulted from EPN infection. Each treatment had 14 replicates, and the whole experiment was conducted two times.
Nematode penetration and reproduction in the carob moth:
Last instar larvae of carob moth were placed in 2.2-cm diam. plates lined with a filter paper, individually. The EPN suspension from each species (S. carpocapsae and S. feltiae) was added at rates of 5 IJs per insect larva. The H. bacteriophora was excluded due to its low virulence and invasion rate. After 48 hr, larvae were rinsed and dissected to determine the number of IJs that penetrated the insect body. The treatment had 20 replicates and was conducted twice. To assess nematode reproduction, the dead larvae were rinsed and transferred to White traps (individually) and incubated at controlled conditions for 10 d, sufficient time for EPN reproduction. The total number of emerging IJs from each larva was determined. This test had 10 replications and was conducted twice.
Efficacy of EPNs on the carob moth in field conditions:
The aim of the field test was to determine the sensitivity of carob moths in their habitat to EPNs. For this experiment, infested fruits were collected from orchards. The infested pomegranates were placed on sterile soil from an orchard (10% moisture and 1 cm depth) inside containers with 24 cm diam. Two species of nematodes, S. carpocapsae and S. feltiae, were used with different concentrations (0, 5, 10, 50, 100, and 160 IJs/cm), and the suspension was spread using a pressurized hand sprayer. The H. bacteriophora was excluded from this test due to preliminary low performance. The treated pomegranates were dissected after a week to check mortality rates. The experiment was conducted with three replications and repeated twice.
Migration behavior of EPN in response to pomegranate and saprophytic fungi:
Visual inspection of the field data highlighted the co-occurrence of saprophytic fungi, and hence, we explored the impact of this fungus in the ability to find and kill the pest by EPN. This migration test was conducted to investigate the effect of pomegranate compounds and saprophytic fungi on the migration behavior of EPNs (S. carpocapsae, S. feltiae, and H. bacteriophora). The experimental container was 24 cm in diameter with three diametric lines of equal length created in the bottom of the container and filled with 1 cm layer of 0.01% water agar. The experiment was composed of three treatments including (i) inner contents of normal pomegranate; (ii) infested pomegranate with saprophytic fungi, or (iii) water agar alone as a control. Single larva of carob moth was placed into a perforated 1.5-ml microtube, and four microtubes were laid on each diametric line of the container. In the center of the container, a 6-cm-diam. round sponge containing 160 IJs/cm2 of nematodes within 500 µl sterile distilled water was placed.
The containers were covered and kept in controlled conditions. After 72 hr, the treatments were checked to count the dead larvae using dissection. Each treatment had three replicates and the experiment was repeated twice.
Statistical analysis:
The mortality ratio was corrected by control mortality with the Abbott’s formula (Abbotts, 1925). The data were square-root-transformed when necessary to meet assumptions of normality and homogeneity of variances. In the nematode penetration and reproduction assay, the data of larval mortality were subjected to one-way analysis of variance followed by Tukey multiple comparison test with significance level at P < 0.05. In the pathogenicity and migration assay of EPNs, mortality of larvae was analyzed with two-way analysis of variance and was followed by LSD test to compare larval mortality caused by interactive effects. The mortality data for EPNs against E. ceratoniae larvae within fruit was analyzed by two-way analyses of covariance in which EPN species and concentrations were considered main factors and the total number of E. ceratoniae larvae was entered as a covariate. Where covariate was significant (P < 0.05), differences among means were determined by comparisons of LSD test. Regression analysis was also used to determine how EPN concentration influenced the mortality rate of the carob moth larvae in relation to either EPN species (SAS Institute, 2002–2003).
Results
Pathogenicity assay of EPNs:
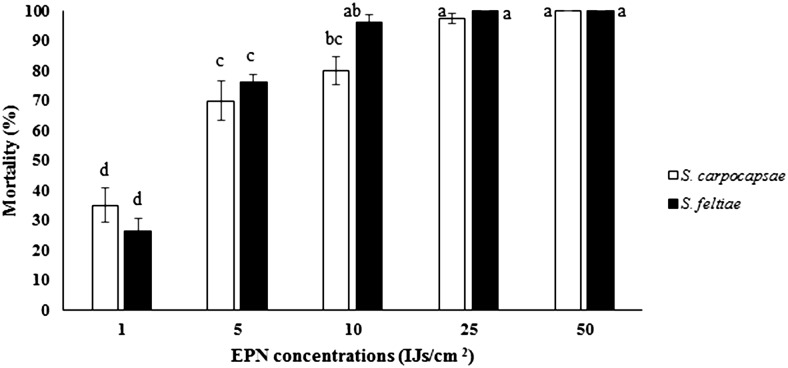
The calculated LC50 of EPNs on the carob moth larvae in laboratory conditions were 2 IJs for both steinernematids, whereas it was 426.9 IJs/larva for H. bacteriophora (Table 1). There was no significant difference between EPN species on larval mortality (F = 2.02; P = 0.1595; df = 70, 1), which indicates a similar mean mortality, 76.5% for S. carpocapsae and 79.7% for S. feltiae. Total mortality increased with higher EPN concentrations (F = 125.79; P < 0.0001; df = 70, 4) ranging from 30.62% to 100%, for the treatment with 1 to 50 IJs/cm2. The interactive effect of EPN species and IJ concentration on larval infection was significant (F = 3.19; P = 0.0183; df = 70, 4). The higher concentrations induced greater larval mortality of E. ceratoniae larvae by both S. feltiae and S. carpocapsae (Fig. 1).
Fig. 1.
Mortality (%) of last instar larvae of Ectomyelois ceratoniae infected by Steinernema carpocapsae and Steinernema feltiae in five different concentrations (5, 10, 50, 100, and 150 IJs/cm2). Different letters indicate significant differences between treatments. Data are presented as means (±SE).
Nematode penetration and reproduction:
The number of IJs that penetrated into the E. ceratoniae larvae was significantly different between both EPN species (F = 9.21; P = 0.0033; df = 78, 1) with 31% and 43% average rate for S. carpocapsae and S. feltiae, respectively, when applied in 5 IJs/larva after 48 hr. However, the total IJs production revealed that there was not any difference between both nematodes (F = 1.27; P = 0.2674; df = 38, 1), with values of 18,457 and 15,452 IJs per larva for S. carpocapsae and S. feltiae, respectively (Table 2).
Efficacy of EPNs against the Ectomyelois ceratoniae in field conditions:
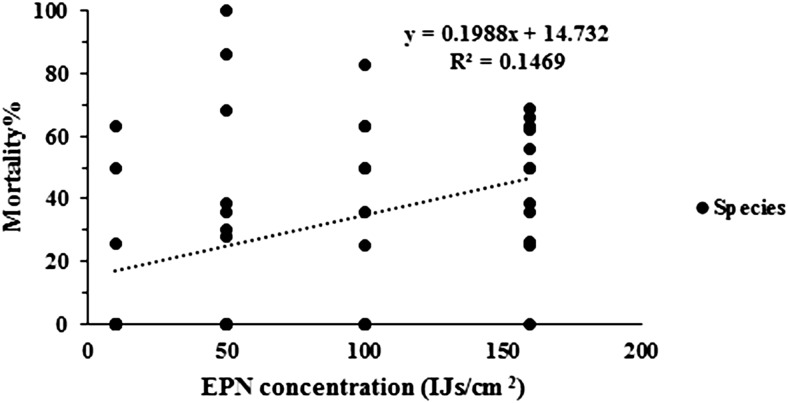
In this experiment, larval mortality was significantly influenced by the total numbers of E. ceratoniae larvae, which were analyzed as covariates (F = 8.09; P = 0.0071; df = 39, 1). There was also a significant effect of the EPN species on larval mortality (F = 59.96; P < 0.0001; df = 39, 1). The percentage of larval mortality caused by S. carpocapsae (49.32% ± 5.37%) was significantly higher than that produced with S. feltiae (10.50% ± 5.67%) The percentage of larval mortality increased significantly with IJ concentration (F = 10.90; P < 0.0001; df = 39, 3) (Fig. 2). The interactive effect of EPN species and IJ concentrations on larval infection was not significant (F = 1.12; P = 0.3536; df = 39, 3). The regression analysis of the data showed that mortality of E. ctomyelois ceratoniae larvae significantly increased with IJ concentration (R2 = 0.1469; P < 0.05). Maximum (45.09% ± 5.96%) and minimum mortality (11.55% ± 6.47%) was achieved when they were treated with 10 and 160 IJs/cm2, respectively (Fig. 2).
Fig. 2.
Linear regression of mean percentage mortality (%) of last instar larvae of Ectomyelois ceratoniae caused by the entomopathogenic nematode species Steinernema carpocapsae and Steinernema feltiae at five different concentrations (5, 10, 50, 100, and 150 IJs/cm2).
Migration behavior of EPNs:
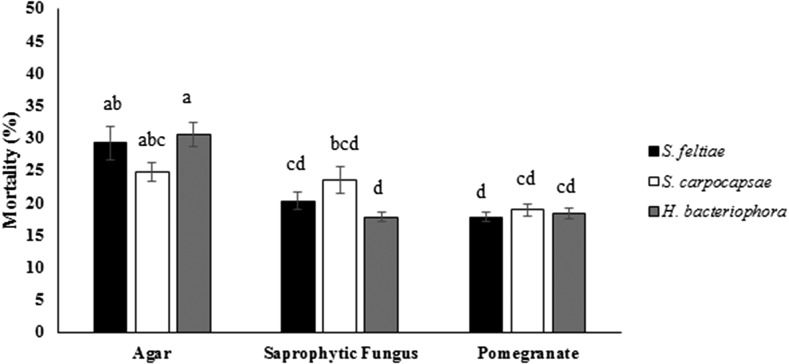
The result of the migration test showed that the substrate type (F = 44.89; P < 0.0001; df = 90, 2), distance between nematode and target (F = 23.99; P < 0.0001; df = 90, 1), and interactive effect of nematode species and substrate type significantly affected larval mortality (F = 4.99; P < 0.0011; df = 90, 4). The H. bacteriophora, S. feltiae, and S. carpocapsae induced greater larval mortality of E. ceratoniae in agar, whereas all EPN species had lower virulence in pomegranate (Fig. 3). The differences between EPN species efficacy were not significant (F = 0.02; P = 0.98; df = 90, 2). The interactive effects of nematode species × distance between nematodes and target (F = 2.61; P = 0.0791; df = 90, 2), substrate type × distance between nematodes and target (F = 1.99; P = 0.1430; df = 90, 2), and EPN species × substrate type × distance between nematodes and target were not significant (F = 1.98; P = 0.1042; df = 90, 4).
Fig. 3.
Mortality (%) of last instar larvae of Ectomyelois ceratoniae infected by Steinernema carpocapsae and Steinernema feltiae at a concentration of 5 IJs/cm2 in three types of substrate: agar, saprophytic fungus, and pomegranate. Different letters indicate significant differences between treatments. Data are presented as means (±SE).
Discussion
The current study aimed to determine the infectivity of EPN against the destructive life stage of the carob moth. It would be ideal to find agents that can search, find, and kill the pest larvae when applied in appropriate time and volume. Our hypothesis of using EPN as biological control agents was promising considering the cryptic niche and living habitat of the pest. These results showed that two species of the Steinernematidae family are more effective on the carob moth larvae in laboratory conditions [LC50 = 2 IJs/larva for S. carpocapsae and S. feltiae], when compared to H. bacteriophora, which indicated low virulence on the carob moth larvae [LC50 = 426.9 IJs/larva]. Two important factors in the pathogenesis of EPNs are host-seeking ability and efficacy to kill their host by symbiotic bacteria (Griffin et al., 2005). Although S. carpocapsae with ambusher behavior and S. feltiae with intermediate behavior are said to be more effective for ambulant pests (Campbell and Gaugler, 1993; Lewis et al., 1995; Campbell and Lewis, 2002), S. carpocapsae has the ability to find immobile insects (de Altube et al., 2008) and to change its behavior under different conditions. The success of those species can be attributed to the mobile behavior of the carob moth larvae in the trial arena that increases the distribution patterns of S. carpocapsae and S. feltiae, and this can have an effect on the pest’s mortality (Kaya and Koppenhöfer, 1996). This finding indicated that S. carpocapsae was more effective on horizontal surfaces while H. bacteriophora can move and search out hosts in vertical places. Additionally, some populations of EPN species have different host preferences (Griffin et al., 2005). The result showed that the carob moth larvae had the high susceptibility to both species of tested Steinernematidae. The strong ability of Steinernema species on the carob moth larvae were in agreement with the efficacy of Steinernema on other species of Pyralidae family. Our results are consistent with the finding of Legaspi et al. (2000) who showed S. riobravis caused 100% mortality at concentrations of 20 to 240 IJs/larva on another Pyralid species, the Mexican rice borer Eoreuma loftini (Dyar). Furthermore, the current data are in agreement with the results of Christos et al. (2007) who showed the potency of three strains of S. feltiae on Ephestia kuehniella Zeller larvae in stored wheat using three concentrations of 100, 300, and 900 IJs/larva. One of them caused 62% mortality after 14 d at the highest concentration. Siegel et al. (2004) investigated efficacy of S. carpocapsae and S. feltiae on navel orange worm Amyelois transitella (Walker) in fallen fruit and showed that S. carpocapsae was more effective than S. feltiae and caused >72% mortality at 50,000 to 1,000,000 IJs/m2, so announced it as a promising biological agent for postharvest control. Our finding is in agreement with the results of Siegel et al. (2004) on the navel orange worm; the similar biology of both insects may have potential for application of EPNs on fallen pomegranate in future studies.
After preliminary tests that included a concentration–response assay, the penetration and reproduction potential of EPN species against the carob moth larvae were addressed. The data indicated that H. bacteriophora did not have the potential to invade the target larvae with low concentrations. So while we calculated the LC50 for all three examined species, due to different concentrations used for determination of the LC50 of H. bacteriophora compared to S. carpocapsae and S. feltiae, we were unable to directly compare the LC50 and infectivity rates of three species. Based on concentration–response data, we excluded H. bacteriophora from the semi-field test. Also, we had the same event for penetration and reproduction potential of H. bacteriophora compared to S. carpocapsae and S. feltiae; so the presented data for invasion rate and offspring potential are calculated for S. carpocapsae and S. feltiae, excluding again the Heterorhabditis species. The rare effect of H. bacteriophora and great effect of S. carpocapsae and S. feltiae on the carob moth larvae might be related to differences in penetration ability among the species (Caroli et al., 1996).
The main purpose of biological control is to create a condition for lowering the pest density, permanently or temporarily. To achieve this aim, the biocontrol agent has to possess suitable traits. In the case of EPNs, their adaptation potential to host habitats, tolerance of extreme environmental conditions, and some other performances including reproduction potential in the host’s body are among the critical traits. The result of the current work showed that there were no significant differences among reproduction potential of two Steinernema species, but the number of IJs produced per infected host was higher in S. carpocapsae than in S. feltiae. This difference may be related to different factors including nematode body size because S. carpocapsae is slightly smaller than S. feltiae, so a greater number of IJs were produced per host body (Loya and Hower, 2003). In addition, other than body size, various factors are involved in the ability of EPNs to develop and reproduce inside a host. While the size may show some difference, other elements including temperature, humidity conditions, potential of the symbiotic bacteria, and nematode adaptation are crucial to the growth, development, reproduction, and effectiveness of the nematode, even the same insect defense system and the immune evasion by the nematode (Tomalak, 1994; Koppenhöfer et al., 1996; Shapiro-Ilan and Lewis, 1999; Stock, 2015).
For successful management of different pests, EPNs can be used more effectively with appropriate concentration and conditions (Shapiro-llan and Gaugler, 2002; Lacey, 2016), but some factors can have abundant effects on EPNs activity such as predatory nematodes, bacteria, arthropods, and fungi (Kaya and Gaugler, 1993; Kaya and Koppenhöfer, 1996; Stuart et al., 2006), which have the ability to regulate EPN populations in habitats (Strong et al., 1996; El-Borai et al., 2011). Balan and Gerber (1972) indicated Panagrellus redivivus L. was attracted and killed by Arthrobotrys dactyloides Drechsler. Also, Navarro et al. (2013) examined the effect of Fusarium oxysporum Schltdl on H. sonorensis Stock. Their result determined that this fungus can decrease IJ movement, virulence, progeny, penetration, and reproduction, and inhibit the growth of symbiotic bacteria. Further, abiotic factors also affect life history of EPNs at different levels. Considering this fact, the migration experiment was focused on showing the effect of saprophytic fungi within fruit and pomegranate components on EPNs virulence to select the most effective species under natural conditions. The result of those trials showed that H. bacteriophora in infected pomegranate with saprophytic fungi substrate caused the lowest mortality of the carob moth larvae (with an average mortality of 17.81%). This low mortality rate by H. bacteriophora can be attributed to the sensitivity of EPN species and its symbiont bacterium to saprophytic fungi in the trial environment while S. carpocapsae had higher mortality on the carob moth larvae (with an average mortality of 23.57%) followed by S. feltiae (with mean mortality of 20.42%).
There are diverse known and unknown compounds in pomegranates which can have an effect on EPNs and their activity. Ohri et al. (2010) stated that plant secondary metabolites such as phenolic substances produced in response to the invasion of plant pathogens have a defensive role against plant parasitic nematode attacks. In a similar work, Glazer et al. (2015) investigated the effect of various plant extracts containing tannin on H. bacteriophora. The nematodes were exposed to various concentrations of plant extracts (300, 900, 1,200, and 2,400 ppm). The most mortality recorded for Inula viscosa L. (strong-smelling inula) was 95% mortality of H. bacteriophora at the highest concentration. Their results were in agreement to our finding that the pomegranate fruit (including some compounds in the fruit) can kill EPNs. Several studies have shown that pomegranate has various components, including phenolic compounds (Tehranifar et al., 2010; Hajimahmoodi et al., 2013). The results showed that the sensitivity of the S. carpocapsae species was the lowest of the pomegranate fruit and it can kill more larvae of carob moths (with average mortality of 18.96%), whereas H. bacteriophora and S. feltiae were more sensitive to pomegranate fruit; it looked as if the fruit had repellent or inhibitory effects on EPNs (with mean mortality of 18.39% for H. bacteriophora and 17.81% for S. feltiae).
In the migration experiment, of the three tested EPN species, S. carpocapsae had the lowest sensitivity to used material including the pomegranate. This species caused the highest mortality compared to other examined species. We can explain these results by considering finding of Bal et al. (2014), who indicated that the ambusher S. carpocapsae disperse faster and farther comparing the cruiser, H. bacteriophora.
Among various elements that affect virulence/pathogenicity of EPNs against insects, two main factors, host-seeking ability and host killing potential by their bacterial symbiont, have high priority in determining potency of EPN species (Griffin et al., 2005). The current finding of higher efficacy of S. carpocapsae against the ambulant pest species like E. ceratoniae larva tends to support the population resilience of S. carpocapsae. This fact implies the behavior and host searching ability of S. carpocapsae, as ambusher–cruiser, is adjusted to the environmental condition and niche of the target insect pest (Wilson et al., 2012; Bal et al., 2014; Griffin, 2015).
The activity of the pomegranate moth, E. ceratoniae, causes the destruction of infested fruits and subsequent damage by secondary pathogens, mainly Aspergillus and Penicillium (Kashkouli and Eghtedar, 1975). These saprophytes provide an unsuitable niche for the tested EPN species. We assume that the unfavorable niche of the fruits is a limiting factor toward the successful movement and infection process of the EPN. Even the result of the semi-field trial indicated low-to-moderate total mortality of the larvae within the infested fruits, but those mortality rates are promising for future works, which required more research with more species/strains and also change in the application methods. It is clear that more attempts especially through testing more species/populations and also designing alternative application methods rather than direct spraying are among the key remaining questions to be addressed through the possible use of EPN within the integrated pest management of E. ceratoniae larvae. Those future explorations should gather sufficient data to judge on the actual potential of EPNs versus the carob moth.
Literature Cited
- Bal HK, Michael A, Grewal PS. Genetic selection of the ambush foraging entomopathogenic nematode Steinernema carpocapsae for enhanced dispersal and its associated trade-offs. Evolutionary Ecology. 2014;28:923–939. [Google Scholar]
- Balan J, Gerber NN. Attraction and killing of the nematode Panagrellus redivivus by the predaceous fungus Arthrobotrys dactyloides. Nematologica. 1972;18:163–173. [Google Scholar]
- Campbell JF, Gaugler R. Nictation behavior and its implications in the host search strategies of entomopathogenic nematodes behavior (Heterorhabditidae and Steinernematidae) Behavior. 1993;126:155–169. [Google Scholar]
- Campbell JF, Lewis EE. 2002. Entomopathogenic nematode host-search strategies. Pp.13–38 in E. E. Lewis, J. F. Campbell, and M. V. K. Sukhdeo, eds. The behavioral ecology of parasites. Wallingford, UK: CABI Publication.
- Caroli L, Glazer I, Gaugler R. Entomopathogenic nematode infectivity assay: Multi variable comparison of penetration into different hosts. Biocontrol Science and Technology. 1996;6:227–233. [Google Scholar]
- de Altube MDM, Strauch O, de Castro GF, Pena AM. Control of the flat headed root borer Capnodis tenebrionis (Linne) (Coleoptera: Buprestidae) with the entomopathogenic nematode Steinernema carpocapsae (Weiser) (Nematoda: Steinernematidae) in a chitosan formulation in apricot orchards. Biological Control. 2008;53:531–539. [Google Scholar]
- El-Borai FE, Campos-Herrera R, Stuart RJ, Duncan LW. Substrate modulation, group effects and the behavioral responses of entomopathogenic nematodes to nematophagous fungi. Journal of Invertebrate Pathology. 2011;106:347–356. doi: 10.1016/j.jip.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Glazer I, Salame L, Dvash L, Muklada H, Azaizeh H, Mreny R, Markovics A, Landau S. Effects of tannin-rich host plants on the infection and establishment of the entomopathogenic nematode Heterorhabditis bacteriophora. Journal of Invertebrate Pathology. 2015;128:31–36. doi: 10.1016/j.jip.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Gothilf S. Establishment of the imported parasite Pentalitomastix plethoricus (Hym.; Encyrtidae), on Ectomyelois ceratoniae (Lep.: Phycitidae) in Israel. Entomophaga. 1978;23:299–302. [Google Scholar]
- Grewal PS. 2002. Formulation and application technology. Pp. 265–288 in R. Gaugler, ed. Entomopathogenic nematology. Wallinford, UK: CABI Publishing.
- Griffin CT. 2015. Behaviour and population dynamics of entomopathogenic nematodes following application. Pp. 57–95 in R. Campos-Herrera, ed. Nematode pathogenesis of insects and other pests: Sustainability in plant and crop protection. Gewerbestrasse, Switzerland: Springer.
- Griffin CT, Boemare NE, Lewis EE. 2005. Biology and behavior. Pp. 47–64 in P. S. Grewal, R.-U. Ehler, and D. I. Shapiro-Ilan, eds. Nematodes as biocontrol agents. Wallingford, UK: CABI Publishing.
- Hajimahmodi M, Moghaddam G, Ranjbar AM, Khazani H, Sadeghi N, Oveisi MR, Jannat B. Total phenolic, flavonoids, tannin content and antioxidant power of pome Iranian pomegranate flower cultivar. American Journal of Plant Sciences. 2013;4:1815–1820. [Google Scholar]
- Kashkooli A, Eghtedar A. The study of pomegranate worm in Fars region. Applied Entomology and Phytopathology. 1975;41:21–32. [Google Scholar]
- Kaya HK, Gaugler R. Entomopathogenic nematodes. Annual Review of Entomology. 1993;38:181–206. [Google Scholar]
- Kaya HK, Koppenhöfer AM. Effects of microbial and other antagonistic organism and competition on entomopathogenic nematodes. Biocontrol Science and Technology. 1996;6:357–372. [Google Scholar]
- Kaya HK, Stock S. 1997. Techniques in insect nematology. Pp. 281–284 in L. A. Lacey, ed. Manual of techniques in insect pathology, vol. 1. San Diego, CA: Academic Press.
- Kehat M, Blumberg D, Dunkelblum E, Anshelevich L. Experiments with synthetic sex pheromones for the control of the raisin moth, and for monitoring the carob moth in date plantations. Alon Hanotea. 1995;49:284–290. [Google Scholar]
- Kishani-Farhani H, Goldansaz SH, Sabahi Q, Shakeri M. The study on parasitoid larvae of the carob moth in three regions, Varamin, Qom and Saveh. Journal of Iranian Plant Protection. 2009;2:337–344. (in Persian). [Google Scholar]
- Koppenhöfer AM, Fuzy AM, Kaya HK. Coexistence of two Steinernematid nematode species (Rhabditida: Steinernematidae) in the presence of two host species. Applied Soil Ecology. 1996;4:221–230. [Google Scholar]
- Lacey LA. 2016. Microbial control of insect and mite pests: From theory to practice. 1st ed. London: Elsevier.
- Lacey LA, Grzywacz D, Shapiro-Ilan DI, Frutos R, Brownbridge M, Goettel MS. Insect pathogens as biological control agents: Back to the future. Journal of Invertebrate Pathology. 2015;132:1–41. doi: 10.1016/j.jip.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Legaspi CA, Legaspi CB, Saldana RR. Evaluation of Steinernema riobravis (Nematoda: Steinernematidae) against the Mexican rice borer (Lepidoptera: Pyralidae) Journal of Entomology Science. 2000;35:141–149. [Google Scholar]
- Lewis EE, Selvan S, Campbell JF, Gaugler R. Changes in foraging behavior during the infective juvenile stage of entomopathogenic nematodes. Parasitology. 1995;110:583–590. [Google Scholar]
- Loya LJ, Hower AA. Infectivity and reproductive potential of the Oswego strain of Heterorhabditis bacteriophora associated with life stages of the clover root curculio, Sitona hispidulus. Journal Invertebrate Pathology. 2003;83:63–72. doi: 10.1016/s0022-2011(03)00044-2. [DOI] [PubMed] [Google Scholar]
- Mehrnejad M. Biology of carob moth, Ectomyelois ceratoniae new pest on pistachio in Rafsanjan. Applied Entomology and Phytopathology. 2002;60:1–11. [Google Scholar]
- Mnif I, Elleuch M, Chaabouni ES, Ghribi D. Bacillus subtilis SPB1 bio surfactant: Production optimization and insecticidal activity against the carob moth Ectomyelois ceratoniae. Crop Protection. 2013;50:66–72. [Google Scholar]
- Nasrollahi AA, Shojai M, Ziaii M. 1998. Large scale production and application of Trichogramma wasps for biological control of pomegranate moth Ectomyelois ceratoniae in Yazd province. Proceeding of 13th Iranian Plant Protection Congress, Junior College of Agriculture, Karaj, Iran. p. 167.
- Navarro PD, McMullen JG, II, Stock SP. Interactions between the entomopathogenic nematode Heterorhabditis sonorensis (Nematoda: Heterorhabditidae) and the saprobic fungus Fusarium oxysporum (Ascomycota: Hypocreales) Journal of Invertebrate Pathology. 2013;115:41–47. doi: 10.1016/j.jip.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Nay JE, Perring TM. Impact of ant predation and heat on carob moth (Lepidoptera: Pyralidae) mortality in California date gardens. Journal of Economic Entomology. 2005;98:72–731. doi: 10.1603/0022-0493-98.3.725. [DOI] [PubMed] [Google Scholar]
- Ohri P, Pannus SK. Effect of phenolic compounds on nematodes: A review. Journal of Applied and Natural Science. 2010;2:344–350. [Google Scholar]
- Sarkhosh A, Zamani Z, Fatahi R, Ebadi A. RAPD markers reveal polymorphism among some Iranian pomegranate (Punica granatum L.) genotypes. Scientia Horticulturae. 2006;111:24–29. [Google Scholar]
- Shakeri M. 2004. A review on investigations on pomegranate neck worm in Iran. Proceeding on evaluation of finding and current problems associated with Spectrobates ceratoniae management in pomegranate. Tehran, Iran: Ministry of Jihad-e-Agriculture, Organization of Research and Education. Pp. 20–45.
- Shakeri M, Sadat Akhavi Y. 2003. Pest and disease of pomegranate. Yazd, Iran: Tasbih Publication. p. 126.
- Shapiro-Ilan DI, Gaugler R. Production technology for entomopathogenic nematodes and their bacterial symbionts. Journal of Industrial Microbiology and Biotechnology. 2002;28:137–146. doi: 10.1038/sj.jim.7000230. [DOI] [PubMed] [Google Scholar]
- Shapiro-Ilan DI, Lewis EE. Infectivity of entomopathogenic nematodes from cadavers vs. aqueous applications. Environmental Entomology. 1999;28:907–911. [Google Scholar]
- Siegel J, Lacey LA, Fritts R, Jr, Higbee BS, Noble P. Use of steinernematid nematodes for post-harvest control of navel orange worm (Lepidoptera: Pyralidae) Amyelois transitella in fallen pistachios. Biological Control. 2004;30:410–417. [Google Scholar]
- Stock SP. 2015. Diversity, biology and evolutionary relationships. Pp. 3–27 in R. Campos-Herrera, ed. Nematode pathogenesis of insects and other pests. Gewerbestrasse, Switzerland: Springer.
- Strong DR, Whipple AV, Child AL, Kraig S, Bondonno M, Dyer K, Kaya HK, Maron JL. Entomopathogenic nematodes: Natural enemies of root-feeding caterpillars on bush lupine. Oecologia. 1996;104:85–92. doi: 10.1007/BF00333228. [DOI] [PubMed] [Google Scholar]
- Stuart RJ, Barbercheck ME, Grewal PS, Taylor RA, Hoy CW. Population biology of entomopathogenic nematodes: Concepts, issues and models. Biological Control. 2006;38:80–102. [Google Scholar]
- Tehranifar A, Zarei M, Nemati Z, Esfandiyari B, Vazifeshenas MR. Investigation of physico-chemical properties and antioxidant activity of twenty Iranian pomegranate (Punica granatum L.) cultivars. Scientia Horticulturae. 2010;126:180–185. [Google Scholar]
- Tomalak M. Genetic improvement of Steinernema feltiae for integrated control of the western flower thrips Frankliniella occidentalis. Bulletin OILB/SROP. 1994;17:17–20. (in French). [Google Scholar]
- White G. A method for obtaining infective nematode larvae from cultures. Science. 1927;66:302–303. doi: 10.1126/science.66.1709.302-a. [DOI] [PubMed] [Google Scholar]
- Wilson MJ, Ehlers R-U, Glazer I. Entomopathogenic nematode foraging strategies—is Steinernema carpocapsae really an ambush forager? Nematology. 2012;14:389–394. [Google Scholar]
- Zolfaghariae RH, Farazmand H, Vafaie shoshtari R, Babaie M, Tabatabaie SZ. Application of nuclear technology to practical control pomegranate moth. Journal of Nuclear Science and Technology. 2010;53:30–35. (in Persian). [Google Scholar]