Abstract
Extracting temporal periodicities and envelope shapes of sounds is important for listening within complex auditory scenes but declines behaviorally with age. Here we recorded local field potentials (LFPs) and spikes to investigate how aging affects the neural representations of different modulation rates and envelope shapes in the inferior colliculus of rats. We specifically aimed to explore the input-output (LFP-spike) response transformations of inferior colliculus neurons. Our results show that envelope shapes up to 256-Hz modulation rates are represented in the neural synchronization phase lags in younger and older animals. Critically, aging was associated with (1) an enhanced gain in onset response magnitude from LFPs to spikes; (2) an enhanced gain in neural synchronization strength from LFPs to spikes for a low modulation rate (45 Hz); (3) a decrease in LFP synchronization strength for higher modulation rates (128 and 256 Hz); and (4) changes in neural synchronization strength to different envelope shapes. The current age-related changes are discussed in the context of an altered excitation-inhibition balance accompanying aging.
Keywords: amplitude modulation, neural synchronization, inferior colliculus, rats, aging
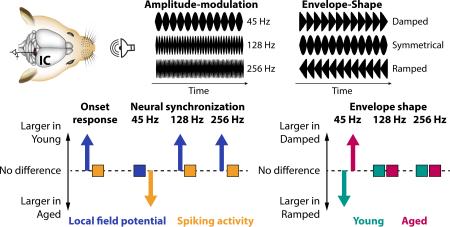
Graphical abstract
Amplitude-modulated sounds were presented to younger and older rats while local field potentials (LFPs) and spikes were recorded from inferior colliculus neurons. Aging was associated with an enhanced gain in onset response and neural synchronization strength (for a 45-Hz modulation rate) from LFPs to spikes, and with a decrease in LFP synchronization strength for higher modulation rates (128, 256 Hz). These changes might be due to an altered excitation-inhibition balance accompanying aging.
Introduction
Natural environmental sounds such as speech or music vary on multiple time scales. The temporal structure of sounds – for example, the rate of an amplitude modulation (AM) or the shape of an amplitude envelope – is a crucial acoustic feature on which basis different sounds are perceptually distinguishable (Patterson, 1994; Akeroyd & Patterson, 1995; Irino & Patterson, 1996; Furukawa & Moore, 1997). Neurophysiological studies in humans and animals show that neural activity synchronizes with a sound's amplitude modulation likely supporting perceptual discriminability (Picton et al., 2003; Joris et al., 2004). Neural synchronization has been observed at all stages of the auditory system ranging from auditory nerve fibers to auditory cortex (Vernier & Galambos, 1957; Epping & Eggermont, 1986; Preuß & Müller-Preuss, 1990; Joris & Yin, 1992; Palombi et al., 2001; Bibikov, 2002; Joris et al., 2004; Bartlett & Wang, 2005; 2007; Malone et al., 2007; Kadner & Berrebi, 2008; Kale & Heinz, 2010; Bartlett & Wang, 2011; Herrmann et al., 2013a; Parthasarathy et al., 2014). Furthermore, neural synchronization is influenced by the rate of the temporal modulation and the envelope shape of the modulation (Pressnitzer et al., 2000; Lu et al., 2001; Neuert et al., 2001; Parthasarathy & Bartlett, 2011).
Critically, hearing abilities, including perception of temporal aspects of sounds, decline with aging and hearing loss (Barsz et al., 2002; Pichora-Fuller, 2003; Walton, 2010). For example, gap detection as well as detection of temporal modulation is impaired in older listeners (Schneider et al., 1994; Moore & Skrodzka, 2002). Hearing difficulties in aging might be related to sensory degradation, alterations of the central auditory system, and/or cognitive decline (Canlon et al., 2010; Wayne & Johnsrude, 2015). In particular alterations of the central auditory system due to aging or hearing loss can be drastic, including reduced neuronal inhibition (Caspary et al., 1995; Burianova et al., 2009; Llano et al., 2012; Rabang et al., 2012; Takesian et al., 2012; for a review see Caspary et al., 2008) and increased neuronal responses (Popelár et al., 1987; Syka et al., 1994; Hughes et al., 2010; Manzoor et al., 2012; Stolzberg et al., 2012) along the auditory pathway. Furthermore, neural synchronization in aged animals appears to be enhanced for lower modulation rates but reduced for fast modulation rates (Palombi et al., 2001; Schatteman et al., 2008; for compatible work in humans see Purcell et al., 2004; Bidelman et al., 2014). Population responses (likely originating from the brainstem and midbrain) indicate age-related deficits in envelope-shape coding (Parthasarathy & Bartlett, 2011) and also suggest central gain enhancement of responses with aging (Parthasarathy et al., 2014).
Extracellular neuronal signals are commonly separated into spiking activity and slower potential fluctuations referred to as local field potentials (LFPs). Previous studies on sensory representations have focused on spiking activity (e.g., Palombi et al., 2001; Schatteman et al., 2008; Bartlett & Wang, 2011). More recently, the importance of field potentials in sensation has been emphasized (e.g., Lakatos et al., 2008; Whittingstall & Logothetis, 2009; Belitski et al., 2010; Haegens et al., 2011; Kajikawa & Schroeder, 2011; Kayser et al., 2015), but the extracellular LFP has remained largely unexplored in the study of aging animals (but see Gourévitch & Edeline, 2011). Yet, spiking activity primarily reflects the output of a neuron or neuronal population, whereas the LFP largely reflects the synaptic activity at the dendrites and soma, and thus contains information about the aggregate input to a neuron or neuronal population (but other non-synaptic activity might additionally contribute; Bullock, 1997; Logothetis et al., 2001; Logothetis & Wandell, 2004; Buzsáki et al., 2012). While the age- and hearing loss-related augmentation of neural firing along the ascending auditory pathway is well established (using neuronal spiking; Popelár et al., 1987; Hughes et al., 2010), the relative change from synaptic (input) activity to neuronal spiking output within a single brain structure and the age-related changes thereof are unknown. In particular the inferior colliculus in the midbrain is a prime candidate in which age-related changes in sensory transformation might occur due to the inferior colliculus’ relevance in integrating ascending sensory and descending neural signals (Adams, 1979; Pollak et al., 2003; Lee & Sherman, 2010) along with the age-related reduction of neural inhibition (Milbrandt et al., 1994; Raza et al., 1994; Caspary et al., 1995; Burianova et al., 2009; Rabang et al., 2012).
The current study investigates neuronal activity of inferior colliculus neurons in younger and older rats in response to amplitude-modulated sounds, varying in modulation rate and envelope shape. We specifically aimed to investigate whether aging affects the sensory transformation from synaptic neuronal signals (LFP) to spiking activity. Our main findings were (1) that onset-evoked LFPs were smaller for older compared to younger rats, whereas there was no difference in onset-evoked spiking activity; (2) that neural synchronization strength of LFPs was similar between age groups, whereas spike synchronization was increased for older rats at a low modulation rate; (3) that neural synchronization for fast modulation rates was larger for younger compared to older animals. We also observed age-related differences in neural synchronization specifically related to the envelope shape.
Methods and materials
Ethical approval
The experimental procedures described in the present investigation were approved by the Institutional Animal Care and Use Committee of Purdue University (PACUC #1111000167). The experiments included in this study comply with the policies and regulations described by Drummond (2009). Rats were housed one per cage in accredited facilities (Association for the Assessment and Accreditation of Laboratory Animal Care) with food and water provided ad libitum. The number of animals used was reduced to the minimum necessary to allow adequate statistical analyses.
Surgical procedures
Nine young (3–6 months, ~300 g) and eleven older (22–26 months, ~400–500 g) male Fischer-344 rats were used in this study. The aging Fisher-344 rat has been suggested to be a suitable model to study presbycusis in aging animals (Syka, 2010). Many of the animals in the study were tested for hearing threshold using the auditory brainstem response (ABR). The ABR responses showed clear age-related changes in hearing thresholds (all values dB SPL and standard deviation: 9 older animals: click 53.3 ±6.6, 8 kHz tone 36.7 ±2.5; 9 younger animals: click 36.4 ±3.8, 8 kHz tone 30.0 ±7.5), with a larger threshold discrepancy for clicks versus 8 kHz tones as reported previously (Parthasarathy & Bartlett, 2011). Methods for surgery, sound stimulation and recording are similar to those described in (Rabang et al., 2012; Herrmann et al., 2015). Surgeries and recordings were performed in a 9’×9’ double walled acoustic chamber (Industrial Acoustics Corporation). Animals were anesthetized using a mixture of ketamine (VetaKet, 80 mg/kg) and dexmedetomidine (Dexdomitor, 0.2 mg/Kg) administered intra-muscularly via injection. A constant physiological body temperature was maintained using a water-circulated heating pad (Gaymar) set at 37°C with the pump placed outside the recording chamber to eliminate audio and electrical interferences. The animals were maintained on oxygen through a manifold. The pulse rate and oxygen saturation were monitored using a pulse-oximeter to ensure they were within normal ranges during surgery. Supplementary doses of anesthesia (20mg/kg of ketamine, 0.05mg/kg of dexmedetomidine) were administered intra-muscularly as required to maintain areflexia and a surgical plane of anesthesia. An initial dose of dexamethasone and atropine was administered prior to incision to reduce swelling and mucosal secretions. A subdermal injection of Lidocaine (0.5 ml) was administered at the site prior to first incision. A central incision was made along the midline, and the calvaria exposed. A stainless steel headpost was secured anterior to bregma using an adhesive and three screws drilled into the skull to provide structural support for a head-cap, constructed of orthodontic resin (Dentsply). A craniotomy was performed from 9–13 mm posterior to bregma, which extended posterior to the lambda suture, and 3 mm wide extending from the midline. The dura mater was kept intact, and the site of recording was estimated stereotaxically using a rat atlas (Paxinos & Watson, 2006) as well as using internal vasculature landmarks and physiological measurements. At the completion of recordings, animals were euthanized with Beuthanasia (200 mg/kg IP). Once areflexive, they were perfused transcardially with 150-200 mL phosphate buffered saline with followed by 400–500 mL 4% paraformaldehyde. The brain was then removed and stored or processed further for Nissl or immunohistochemistry.
Stimulus generation
Sound stimuli were generated using SigGenRP (Tucker-Davis Technologies, TDT) at a 97.64 kHz sampling rate (standard TDT sampling rate) and presented through custom-written interfaces in OpenEx software (TDT). Sound waveforms were generated via a multichannel processor (RX6, TDT), amplified (SA1, TDT), and presented free-field through a Bowers and Wilkins DM601 speaker. The sounds were presented to the animal at azimuth 0° and elevation 0°, calibrated at a distance of 115 cm from speaker to ear, using a Bruel & Kjaer microphone and SigCal software (TDT). All recordings took place in an Industrial Acoustics booth lined with 1 inch (35 mm) Sonex foam with ~90% absorption for frequencies ≥1000 Hz, minimizing potential echoes or reverberations.
Acoustic stimulation
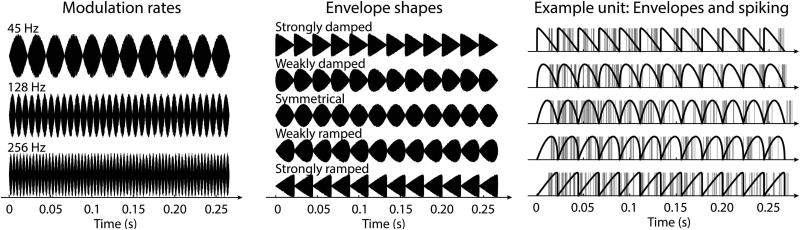
Stimuli consisted of white noise carriers that were modulated in amplitude at three different modulation rates using five different envelope shapes. Modulation rates were 45 Hz, 128 Hz, and 256 Hz (Figure 1, left). These rates were chosen to be comparable to our previous studies (Parthasarathy & Bartlett, 2011; Parthasarathy et al., 2014) and to test the limits of temporal precision, rather than testing slower amplitude-modulation rates. This set of modulation frequencies is also in accordance with human studies investigating age-related changes in neural synchronization to amplitude-modulated sounds (Boettcher et al., 2001; Purcell et al., 2004; Grose et al., 2009). The modulation frequencies in our study, though not dominant in terms of stimulus energy, are also critical for proper gender identification and are affected by aging (Schvartz & Chatterjee, 2012).
Figure 1. Stimulation conditions and example unit.
Left: Rats were stimulated with three different modulation rates: 45 Hz, 128 Hz, and 256 Hz (here the symmetrical envelope shape is displayed).Middle: Amplitude-modulated noise carrier. Five different envelope shapes ranging from strongly damped to strongly ramped amplitude modulations (with a 45-Hz modulation rate in the example). Right: Spiking responses of an example unit to different envelope shapes for a 45-Hz modulation rate. Envelopes are plotted in black solid lines. Gray vertical lines reflect spikes.
Envelope shapes ranged from strongly damped to strongly ramped generated by varying parameters of a beta function (Figure 1, middle). The beta function for one cycle (b) was defined as follows:
where t is a time vector ranging from 0 to 1 (covering a full cycle duration) and z the parameter determining the envelope shape. The parameter z could take on one of three values: 1.05, 1.4491, or 2; relating to strongly damped, weakly damped, and symmetrical envelopes, respectively. For the weakly and strongly ramped envelope shapes the beta functions generated for z values of 1.4491 and 1.05 were time-reversed, respectively (Figure 1, middle). Beta functions were peak-normalized. Sounds had a duration of approximately ~265 ms depending on modulation frequency, and sounds were presented every 1.25 seconds. Each of the 3 × 5 (modulation rate × envelope shape) sounds was randomly presented between 6–10 times (typically 10 times; consistent number within a unit).
Electrophysiological recordings
Single unit activity and multiunit activity in the inferior colliculus was recorded in vivo using a tungsten microelectrode (A-M Systems) encased in a glass capillary that was advanced using a hydraulic microdrive (Narishige). We recorded 93 units in younger rats and 90 units in older rats. The inferior colliculus was identified based on short-latency driven responses to tone stimuli. The central nucleus of the inferior colliculus was identified using the ascending tonotopy moving in a dorsoventral direction as well as narrowly tuned responses to pure tones with frequencies ranging from 0.5 to 40 kHz. Recordings were obtained from both the dorsal cortex and central nucleus. Although we cannot exclude that we recorded from lateral (or external) cortex, based on the recording depth, the presence of sustained responses to tones and amplitude-modulated stimuli, as well as clear frequency tuning in most cases, we estimate that most of our units were recorded from the central nucleus.
Neural signals were acquired using the tungsten microelectrode connected to a headstage (RA4, TDT) and were amplified (RA4PA preamplifier, TDT). The digitized waveforms and spike times were recorded with a multichannel recording and stimulation system (RZ-5, TDT) at a sampling rate of 24.41 kHz (standard TDT sampling rate). The interface for acquisition and spike sorting were custom made using the OpenEx and RPvdsEx software (TDT). The units acquired were filtered between 500 Hz (occasionally 300 Hz) and 5000 Hz. The acquired spikes were stored in a data tank and analyzed using custom written software in Matlab. Local field potentials were simultaneously recorded from the same electrode by sampling at 1525.88 Hz and bandpass filtering from 3 to 500 Hz. Line noise at 60 Hz was offline removed from the local field potential recordings using an elliptic notch filter (infinite-impulse response; zero-phase lag).
Data analysis: Onset and sustained responses
All offline data analyses were carried out in MATLAB (MathWorks Inc.). Response time courses were calculated for visualization purposes as the average across single-trial time courses (LFP) or peri-stimulus time histograms (spikes; 0.5 ms time steps, 4 ms moving rectangular window). Response magnitudes were assessed for an onset and a sustained time window. The onset time window ranged from 0 s to 0.05 s and the sustained time window ranged from 0.05 s to 0.275 s. For spikes, firing rates were calculated. For local field potentials, the response magnitude was calculated as the root-mean-squared amplitude.
Onset response latencies for the LFPs were extracted for the N1 (negative peak within 0–0.05 s). P1 latencies were not analyzed to avoid unreliable estimates for older rats. The latency of the first spike was extracted for trials eliciting at least one spike within the 0–0.05 s time window after stimulus onset (Heil, 2004).
Data analysis: Neural synchronization to amplitude-modulated sounds
Neural synchronization for local field potentials was calculated as follows. For each modulation rate and envelope shape condition separately, single-trial time courses were averaged, the 0.05–0.275 s time interval was extracted, and a fast Fourier transform (FFT) was calculated (zero-padding to obtain a frequency resolution of 0.25 Hz; 20–300 Hz). An amplitude spectrum and a phase spectrum was calculated as the magnitude and the angle, respectively, of the FFT complex values. Neural synchronization strength at the AM frequency was quantified as the mean amplitude across a 2-Hz frequency window centered on the AM frequency. The mean phase angle at the AM frequency, reflecting the phase delay/lag, was quantified as the circular mean across a 2-Hz frequency window centered on the AM frequency.
Neural synchronization for spiking activity was calculated as follows. For each modulation rate and envelope shape condition separately, spikes times were extracted from the 0.05–0.275 s time interval. Spike times were transformed to phase angles (p) using the following formula:
, where f is frequency and t a vector of spike times (of all trials of one condition). The minus sign in combination with the added π ensured that spiking phase angles and local field potential phase angles were comparable. Phase angles were wrapped to range from −π to π. Using the spikes of all trials of one condition, the empirical vector strength v, that is, the resultant vector length, was calculated as follows (Lachaux et al., 1999):
, where v is the vector strength, i the imaginary unit, p the vector of phase angles, and n the number of spikes (with j being the index). The vector strength can be biased by the number of spikes, with smaller v values for a higher number of spikes. For that reason, a normalized vector strength was calculated. To this end, n (number of spikes of a condition) random phase values between −π and π were generated and the vector strength (i.e., the resultant vector length) was calculated. Randomly drawing phase values and calculation of the vector strength was repeated 5000 times, which resulted in a distribution of random vector strengths given the number of spikes. The normalized vector strength was then calculated by subtracting the empirical vector strength from the mean of the random vector strength distribution and dividing the result by the standard deviation of the random vector strength distribution. The normalized vector strength was calculated for frequencies (f) ranging from 20 Hz to 300 Hz with a frequency resolution of 1 Hz, resulting in a vector strength spectrum. A phase spectrum was calculated as the circular mean across single-spike phase values (p) for each frequency. Neural synchronization strength at the AM frequency was quantified as the mean normalized vector strength across a 2-Hz frequency window centered on the AM frequency. The mean phase angle at the AM frequency, reflecting the phase delay, was quantified as the circular mean across a 2-Hz frequency window centered on the AM frequency.
We also calculated the percentage of units that could be considered highly sensitive to the specific modulation rates. For spikes synchronization, the normalized vector strength reflects a statistical measure – a z-score – and we considered a unit to be sensitive to a modulation rate if its normalized vector strength was equal or larger than 1.645 (i.e., one-tailed P ≤ 0.05). For LFP synchronization, a 1/f pattern was observed for the amplitude spectra (Figure 4) requiring a different approach for the LFPs. Here we made use of a 95% confidence interval (i.e., one-tailed P ≤ 0.05; Gardner & Altman, 1986). In detail, the confidence interval for a given stimulus modulation rate was calculated by making use of the amplitudes of the other two stimulus modulation rates. For example, the confidence interval for the 45-Hz modulation rate condition was calculated using the amplitudes at 45 Hz of the 128-Hz and 256-Hz modulation rate conditions (for 128 Hz the 45-Hz and 256-Hz modulation rates were used; for 256 Hz only the 45-Hz modulation rate was used because 128 Hz produced a harmonic response at 256 Hz). Finally, we considered a unit to be sensitive to a modulation rate if its amplitude at the modulation frequency was equal or greater than the respective 95% confidence interval.
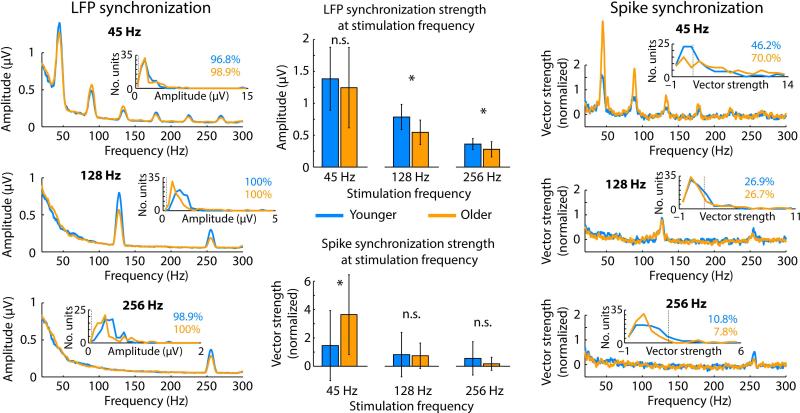
Figure 4. Neural synchronization of local field potentials (left) and spikes (right) for each modulation rate and age group.
Small insets show unit-histograms for synchronization strength and percentage of modulation-rate sensitive units. The thin dashed lines in the histograms indicate the significance criterion (averaged across age groups for LFPs). Bar graphs (middle) show median neural synchronization strength. Error bars reflect the semi-interquartile range. *PFDR ≤ 0.05, n.s. – not significant.
Statistical analyses
For the statistical analyses, we utilized the Wilcoxon rank sum test to compare different age groups and the Wilcoxon signed rank test for within-unit (repeated-measures) comparisons using the Matlab inbuilt functions (ranksum, signrank; respectively). Effect sizes are reported as requivalent (Rosenthal & Rubin, 2003; hereafter referred to simply as r) for statistical tests of linear dependent measures. requivalent is equivalent to a Pearson product-moment correlation for two continuous variables, to a point-biserial correlation for one continuous and one dichotomous variable, and to the square root of partial η2 (eta-squared) for ANOVAs. Where appropriate, false discovery rate (FDR) was applied in order to control the proportion of false positives among significant comparisons (Benjamini & Hochberg, 1995; Genovese et al., 2002).
Results
Response magnitudes of local field potential and spiking activity
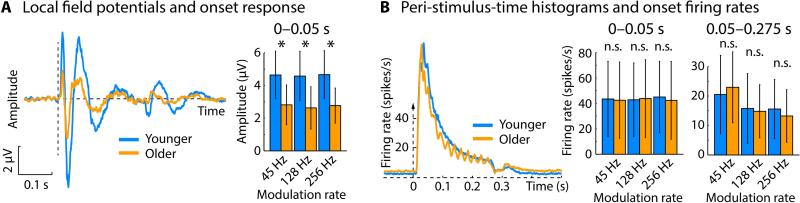
First we investigated responses to the onset of sounds independent of envelope shape, focusing on the 0–0.05 s time interval. For the local field potentials, responses were significantly larger for younger compared to older rats for all three modulation rates (root-mean-squared amplitudes; 45 Hz: PFDR = 0.001, r = 0.401; 128 Hz: PFDR < 0.001, r = 0.419; 256 Hz: PFDR < 0.001, r = 0.412; Figure 2A). In contrast, firing rates were not different between age groups (45 Hz: PFDR > 0.05, r = 0.010; 128 Hz: PFDR > 0.05, r = 0.044; 256 Hz: PFDR > 0.05, r = 0.073; Figure 2B). Firing rates were additionally examined for the sustained time interval ranging from 0.05 s to 0.275 s. Again, no significant difference between age groups was observed (45 Hz: PFDR > 0.05, r = 0.015; 128 Hz: PFDR > 0.05, r = 0.094; 256 Hz: PFDR > 0.05, r = 0.146; Figure 2B).
Figure 2. Response magnitude of local field potentials and spiking activity.
A) Time courses of local field potentials averaged across all conditions (modulation rate; envelope shape). The late bump for young rats is due to the stimulus offset. Bar graphs show median root-mean-squared amplitudes (0–0.05 s). Error bars reflect the semi-interquartile range. B) Peri-stimulus-time histograms (across all conditions). Bar graphs show median firing rates (0–0.05 s; 0.05–0.275 s) and error bars reflect the semi-interquartile range. *PFDR < 0.05, n.s. – not significant.
In order to directly compare whether aging affects the response-magnitude relationship between local field potentials and spikes, we calculated for each unit the ratio between the root-mean-squared local field potential amplitude and the firing rates for the two time windows of interest. For the onset time window (0–0.05 s), the ratio was significantly smaller for older compared to younger rats for all three modulation rates (45 Hz: PFDR < 0.01, r = 0.227; 128 Hz: PFDR = 0.01, r = 0.219; 256 Hz: PFDR = 0.05, r = 0.179), suggesting that a relatively smaller synaptic (input) activity is sufficient to elicit spiking (output) activity in older rats. No difference between age groups was found for the sustained time window (0.05–0.275 s; for all PFDR > 0.05, r < 0.13). An analysis contrasting firing rates for damped versus ramped envelopes is provided in the Supplementary Materials and Figure S1.
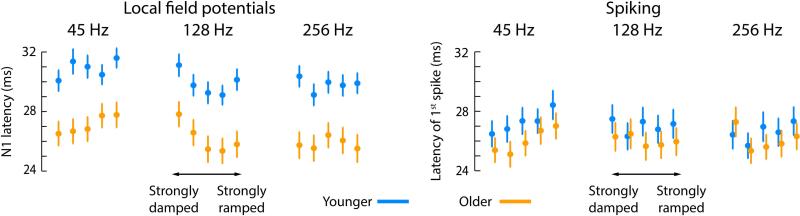
Response latencies of local field potential and spiking activity
Figure 3 shows responses latencies for local field potentials and spikes. Median N1 latencies were significantly smaller for older than younger rats (45 Hz: PFDR < 0.01, r = 0.318; 128 Hz: PFDR = 0.01, r = 0.306; 256 Hz: PFDR < 0.01, r = 0.322). In addition, N1 latencies were smaller for damped compared to ramped envelope shapes (collapsed across weakly and strongly damped/ramped) for the 45-Hz modulation rate (younger: PFDR = 0.05, r = 0.240; older: PFDR < 0.05, r = 0.299; no age group difference: PFDR > 0.05, r = 0.088). There was no difference in N1 latencies between damped and ramped envelope shapes for the 128-Hz and the 256-Hz modulation rate (for all, PFDR > 0.05, r < 0.25).
Figure 3. Condition-specific onset latencies for N1 and first spike.
Circles reflect the mean across units. The error bar reflects the standard error or the mean.
The median latencies of the first spike did not differ between age groups (45 Hz: PFDR > 0.05, r = 0.080; 128 Hz: PFDR > 0.05, r = 0.061; 256 Hz: PFDR > 0.05, r = 0.028). The spike latencies were smaller for damped compared to ramped envelope shapes (collapsed across weakly and strongly damped/ramped) for the 45-Hz modulation rate (younger: PFDR < 0.05, r = 0.465; older: PFDR < 0.05, r = 0.474; no age group difference: PFDR > 0.05, r = 0.094) and for younger rats for the 256-Hz modulation rate (younger: PFDR < 0.05, r = 0.369; older: PFDR > 0.05, r = 0.065; and a larger increase for younger rats: PFDR = 0.05, r = 0.234). There were no effects of latency as a function of envelope shape for the 128-Hz modulation rate (for all, PFDR > 0.05, r < 0.10).
Neural synchronization strength of local field potentials and spiking activity
Figure 4 shows neural synchronization strength of local field potentials and spiking activity. We observed clear peaks in the LFP amplitude spectrum at each AM frequency. Similarly, clear peaks were observed for spike synchronization at the AM frequencies.
For local field potentials, synchronization strength at the AM frequency was significantly larger for younger compared to older rats for 128 Hz (PFDR = 0.001, r = 0.300) and 256 Hz (PFDR = 0.01, r = 0.236), whereas there was no difference between age groups at 45 Hz (PFDR > 0.10, r = 0.010; Figure 4). For spiking activity, synchronization strength at the AM frequency was significantly larger for older compared to younger rats for 45 Hz (PFDR < 0.01, r = 0.262). No difference between age groups was observed for 128 Hz (PFDR > 0.05, r = 0.022; Figure 4) and 256 Hz (PFDR > 0.05, r = 0.160, although the effect was significant for an uncorrected P-value). The percentages of modulation-rate sensitive neurons are provided in the small insets in Figure 4. In sum, neural synchronization in older rats was systematically reduced (compared to younger rats) for fast modulation rates (although not statistically significant in some cases). In contrast, spike synchronization to the low modulation rate (45 Hz) was enhanced for older animals.
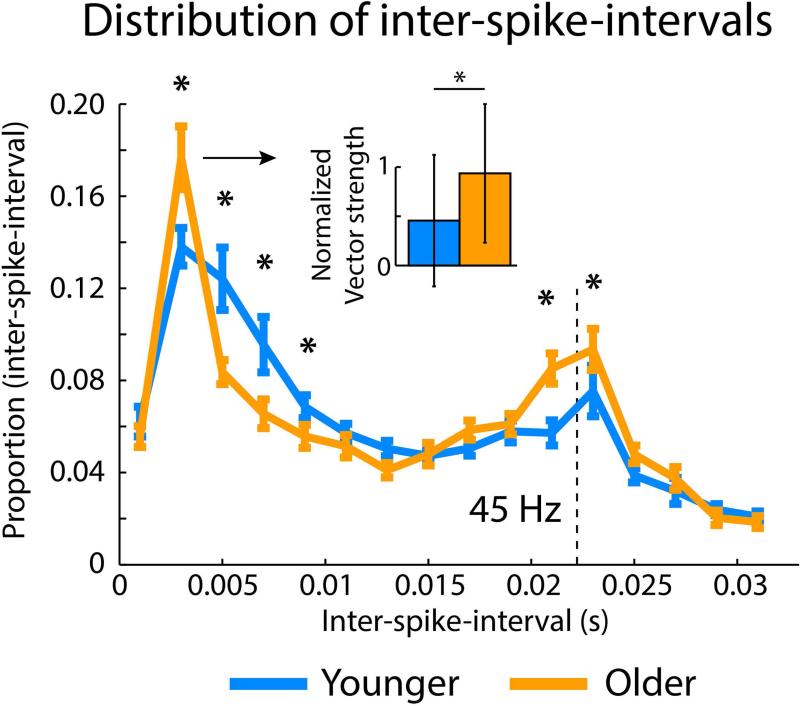
Figure 5 further displays the age difference in neural synchronization for the 45-Hz modulation rate using inter-spike-intervals. That is, for each trial with 2 or more spikes within the 0.05–0.275 s time interval, the inter-spike-intervals were calculated. Subsequently, the relative number of inter-spike-intervals was calculated for each unit (16 non-overlapping bins of 2-ms width). This analysis revealed an increase in the relative number of short inter-spike-intervals (~3 ms; PFDR < 0.05, r = 0.162) and an increase for inter-spike intervals at around the AM frequency (45 Hz, ~22.2 ms; PFDR = 0.01, r = 0.217) for older compared to younger rats. In contrast, the relative number of intermediate inter-spike-intervals was increased for younger rats (~5–9 ms; PFDR < 0.01, r = 0.331; Figure 5). We further tested whether the relative increase of ~3 ms inter-spike intervals for older rats reflects an increase relative to the 45-Hz stimulation cycle or whether it reflects a non-synchronized increase in ~3 ms inter-spike intervals. To this end, the normalized vector strength was calculated (f = 45 Hz) based on the phase of the first spike of the ~3 ms inter-spike-intervals (2–4 ms bin). The normalized vector strength for ~3 ms inter-spike-intervals was larger for older compared to younger rats (PFDR = 0.05, r = 0.160; Figure 5 inset). Taken together, the data indicate that there were short bursts of spikes tightly linked to the 45-Hz AM rate for older rats, whereas spikes occurred more distributed across the 45-Hz modulation cycle for younger rats.
Figure 5. Distribution of inter-spike-intervals.
Distribution of inter-spike-intervals (mean across units) for the 45-Hz modulation rate stimuli. Error bars reflect the standard error of the mean. Asterisks reflect a significant difference between age groups (*P < 0.05). The small inset reflects the median normalized vector strength of ~3 ms inter-spike-intervals (the error bars are the semi-interquartile range), showing that the relative increase in ~3 ms inter-spike-intervals reflect tightly synchronized bursts of spikes in older rats.
Effects of envelope shape on neural synchronization strength
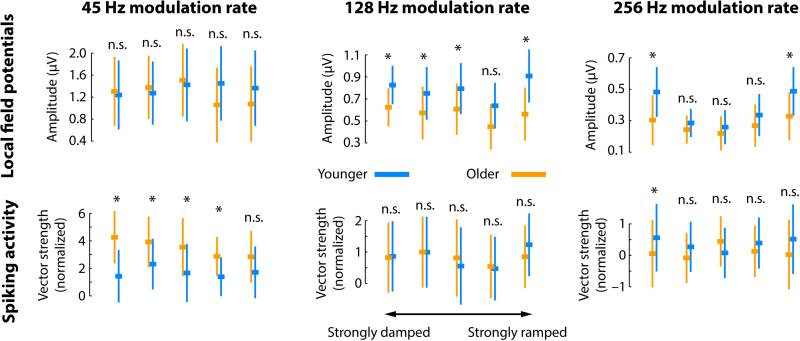
Age-related differences in neural synchronization strength for individual envelope shapes and corresponding statistical significances are depicted in Figure 6. In order to statistically assess the effect of envelope shape on neural synchronization, we averaged the synchronization strength for weakly and strongly damped conditions and for weakly and strongly ramped conditions. We contrasted damped versus ramped envelope shapes independently for younger and older rats, followed by comparison of the damped-ramped difference between age groups.
Figure 6. Neural synchronization strength for the three modulation-rate and five envelope-shape conditions.
Top row: LFP synchronization strength (amplitude). Bottom row: Spike synchronization strength (normalized vector strength). The squares reflect the median across units. The error bar is the semi-interquartile range. Statistical tests reflect the age-group comparison. *PFDR ≤ 0.05, n.s. – not significant. Note that the y-axis is not uniform for the different modulation rates. Instead, age differences and magnitude differences as a function of envelope shape are displayed in more detail.
The results for LFPs were as follows (Figure 6, top row). For the 45 Hz-modulation rate, synchronization strength was smaller for damped compared to ramped envelopes for younger rats (PFDR < 0.05, r = 0.254), whereas the opposite (i.e., damped > ramped) was found for older rats (PFDR = 0.01, r = 0.313). Furthermore, the damped-ramped difference was larger for older compared to younger rats (PFDR < 0.001, r = 0.279). Effects for the 128-Hz modulation rate were weaker, with a stronger synchronization strength for damped than ramped envelopes in older rats (PFDR < 0.05, r = 0.292; no effect for younger rats and no age group difference was found, for both PFDR > 0.05, r < 0.25). For the 256-Hz modulation rate, there was no difference between damped and ramped envelope shapes (younger: PFDR > 0.05, r = 0.238; older: PFDR > 0.05, r = 0.184; age group difference: PFDR > 0.05, r = 0.081). However, as depicted in Figure 6, synchronization strength followed a quadratic trend (younger: PFDR < 0.001, r = 0.678; older: PFDR < 0.001, r = 0.586) were damped as well as ramped envelopes led to strongest synchronization. The quadratic trend was larger for younger than older rats (PFDR < 0.001, r = 0.338).
The results for spike synchronization were as follows (Figure 6, bottom row). No effects of envelope shape were observed for the 45-Hz modulation rate when synchronization strength was collapsed across weakly and strongly damped/ramped shapes. However, synchronization strength for the strongly damped shape was larger than for the strongly ramped shape for older rats (PFDR < 0.05, r = 0.282; younger rats: PFDR > 0.05, r < 0.01), but this difference did not differ between age groups when FDR-corrected (PFDR > 0.05, r = 0.164). There were no effects of envelope shape for the 128-Hz modulation rate (for all PFDR > 0.05, r < 0.15). For the 256-Hz modulation rate, there was no difference between damped and ramped envelope shapes (younger: PFDR > 0.05, r = 0.032; older: PFDR > 0.05, r = 0.044; age group difference: PFDR > 0.05, r = 0.008). However, similar to LFP synchronization, spike synchronization strength followed a quadratic trend for younger rats (PFDR < 0.001, r = 0. 386), but not for older rats (PFDR > 0.05, r = 0.016), and the quadratic trend was larger for younger than older rats (PFDR < 0.01, r = 0.232).
Relative phase delay differences between envelope shapes
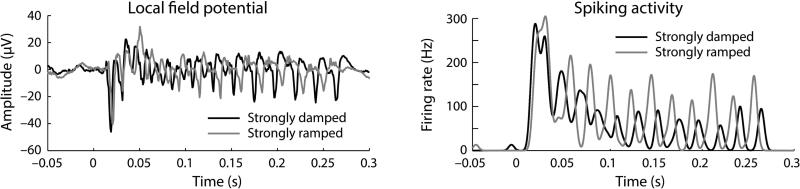
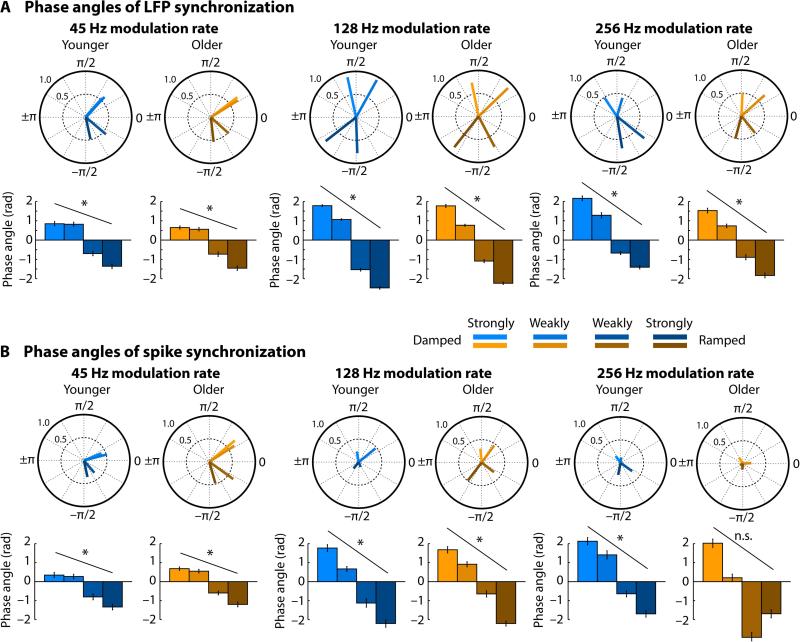
Next we examined the relative phase delay (i.e., the phase angle) of neural synchronization for sounds modulated by different envelope shapes. Figure 7 shows the local field potential and spiking activity time courses for a sample unit responding to a 45-Hz modulation rate of a strongly damped and a strongly ramped envelope shape. The recorded units expressed some variability in their specific phase delays, and normalized phase delays were thus calculated for each unit to account for this (for an analysis of the phase consistency/variability across units see Supplemental Materials and Figure S2). To this end, the phase angle corresponding to the symmetrical envelope shape was subtracted (circular subtraction) from the two damped and the two ramped envelope shapes (for an analysis of non-normalized phase delays see Supplemental Materials and Figure S3). Modulation of phase delays by envelope shapes was analyzed by fitting a linear function to the normalized phase delays, separately for each unit and modulation rate. The estimated linear coefficient were used as the dependent measure.
Figure 7. Sample unit responses showing phase lag differences in neural synchronization for different envelope shapes.
Local field potentials (left) and spiking activity (right) for a strongly damped and a strongly ramped envelope shape for a 45-Hz modulation rate. Spiking activity time courses were calculated by convolving spikes with a Gaussian function (SD = 0.003 s).
For local field potentials, phase delays (angles) were systematically modulated for all modulation rates and both age groups (testing the linear coefficient against zero; for all PFDR < 0.001, r > 0.54); phase delays for damped envelopes were leading (larger than zero) and phase angles for ramped envelopes were lagging (smaller than zero) compared to the symmetrical envelope (Figure 8). Modulation of phase delays by envelope shapes was smaller for the 45-Hz modulation rate than the 128-Hz modulation rate (testing linear coefficients against each other; PFDR < 0.001, r > 0.6) and the 256-Hz modulation rate (PFDR < 0.001, r = 0.264). Modulation of phase delays was also larger for the 128-Hz modulation rate compared to the 256-Hz modulation rate (PFDR < 0.001, r = 0.433). The phase delays for spike synchronization showed a similar pattern (Figure 8). Phase delays were systematically modulated for all modulation rates and both age groups (for all PFDR < 0.05, r > 0.3) with the exception of the 256-Hz modulation rate for older rats (PFDR > 0.05, r = 0.216). Modulation of phase delays did not significantly differ between modulation rates (for all PFDR > 0.05, r < 0.12), although the phase delay patterns were very similar to the LFP phase delays (Figure 8).
Figure 8. Phase angles (delays) of local field potential and spike synchronization.
Circle plots show the resultant vector reflecting the phase angle consistency across units (i.e., a longer vector reflects higher consistency) and the mean phase angle. Phase angles of the four conditions (strongly damped, weakly damped, weakly ramped, strongly ramped) were normalized for each unit individually to their symmetrical condition (i.e., circular subtraction). Bar graphs show the mean phase angle and error bars reflect the circular standard error. Asterisks indicate a significant modulation of phase angles by envelope shapes. *PFDR < 0.05, n.s. – not significant
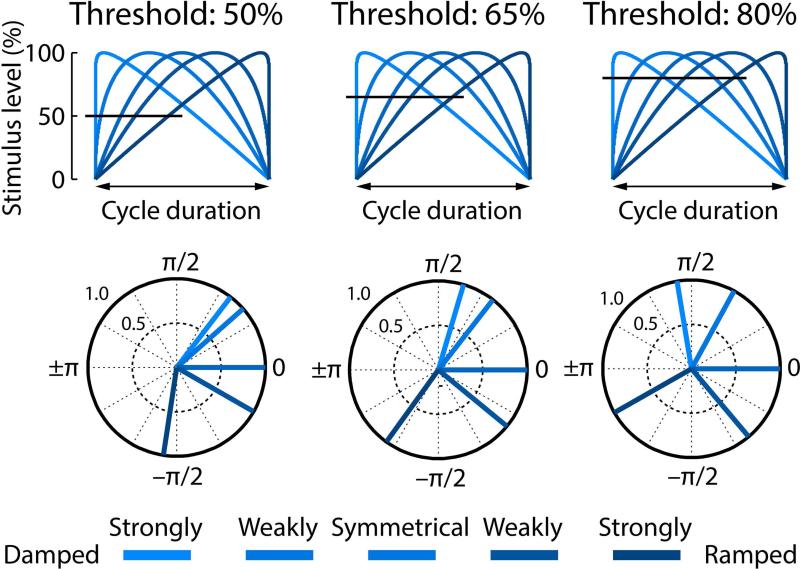
The degree to which phase delays were modulated by the envelope shapes appears to differ between modulation rate conditions; the 45-Hz modulation rate conditions seems less modulated, in particular for the two damped conditions. In order to obtain a better understanding of the relationship between the stimulus envelope shapes and the phase delays of neural synchronization, we calculated hypothetical phase angles by thresholding the AM envelopes at 50%, 65%, and 80% stimulus level (Figure 9).
Figure 9. Hypothetical relationship between stimulus envelope shape and synchronization phase angle.
The top row shows the five envelope shapes for one cycle. The horizontal line marks a hypothetical response threshold for three different example thresholds (50%, 65%, 80%). The bottom row reflect the phase angles (normalized to the symmetrical condition, similar to Figure 8) corresponding to the point of threshold.
From visual comparison between the phase angles displayed in Figure 8 and Figure 9 it appears that neural synchronization at 45-Hz modulation rates is associated with a lower response threshold (resemblance to 50% threshold), while 128-Hz and 256-Hz modulation rates are associated with a higher response threshold (resemblance to 80% threshold; Figure 9).
Discussion
The current study investigated onset responses and neural synchronization of LFP and spiking activity in the inferior colliculus of younger and older rats. We were specifically interested in the ability of neurons to encode both periodicity and envelope shape and the age-related changes from a neuron's synaptic activity to the spiking output. We observed a relative increase in sound onset-evoked neural activity from the LFP to spikes for older rats and a relative increase in neural synchronization strength from LFPs to spikes for older rats for the 45-Hz modulation rate. Furthermore, neural synchronization at fast modulation rates was reduced for older compared to younger rats.
Neural synchronization to amplitude-modulated sounds
We observed clear peaks in the frequency spectrum in response to amplitude-modulated noises at 45 Hz, 128 Hz, and 256 Hz for LFPs and spikes (although spiking activity was not commonly synchronized with the 256-Hz modulation rate), with an overall decreasing synchronization strength for higher modulation frequencies (Figure 4). Our findings are consistent with previous studies on neural synchronization in the inferior colliculus (Langner & Schreiner, 1988; Krishna & Semple, 2000; Palombi et al., 2001; Walton et al., 2002; Ter-Mikaelian et al., 2007), which, however, exclusively focused on spiking activity. The current study critically shows that neural synchronization changes from LFP to spikes as evidenced by the different age-related patterns particularly at low modulation rates (i.e., 45 Hz; see below).
Neural synchronization was not only affected by the modulation rate of the sounds, but in addition by the shape of the amplitude envelope. In particular, for the 45-Hz modulation rate, synchronization strength was larger for damped compared to ramped envelope shapes for older rats, while the synchronization strength was larger for ramped compared to damped shapes for younger rats (at least for the LFP; Figure 6). The other intriguing finding was the quadratic trend observed for the 256-Hz modulation rate, such that neural activity synchronized more strongly with the damped and ramped envelope shapes than with the symmetrical envelope, and this quadratic trend was more pronounced in younger rats. Previous investigations of neural synchronization to different envelope shapes have been sparse (Pressnitzer et al., 2000; Lu et al., 2001; Neuert et al., 2001; Parthasarathy & Bartlett, 2011; for comparisons between click or noise burst sequences and amplitude-modulated sounds see Epping & Eggermont, 1986; Zheng & Escabi, 2013). One study focused on neural population responses measured at the scalp and observed larger neural synchronization for damped than ramped envelope shapes for 128-Hz and 256-Hz modulation rates (Parthasarathy & Bartlett, 2011). Others focused strongly on differences in firing rate magnitude between envelope shapes and only to some extent on synchronization, observing, in contrast to the current study (see Figure S1), greater firing rates for ramped compared to damped envelope shapes (Pressnitzer et al., 2000; Neuert et al., 2001), although others have observed a more diverse pattern (Lu et al., 2001). Based on the current data in combination with previous results, we suggest to remain cautious regarding functional interpretations of modulations in neural synchronization strength or overall firing rates attributed to effects of envelope shape. Although there have only been a few studies investigating neural representations of envelope shapes (Pressnitzer et al., 2000; Lu et al., 2001; Neuert et al., 2001; Parthasarathy & Bartlett, 2011), observations across studies showed considerable variability which, in our opinion, requires further research before clear conclusions are warranted.
We suggest that a more dominant coding scheme distinguishing between envelope shapes might be the timing or phase delay of neural synchronization. We observed large changes in the phase delay of neural synchronization for different envelope shapes (Figure 8). For all modulation rates, synchronization was systematically delayed (with respect to an AM cycle) from damped to ramped envelope shapes. However, for the 45-Hz modulation rate, almost no change in phase delay was observed between the strongly and weakly damped envelopes, but for all other envelope shapes (Figure 8). A simple simulation (Figure 9) suggests that the response threshold was lower for 45-Hz modulation rates than 128-Hz and 256-Hz modulation rates. Critically, our results show that the phase delay (i.e., timing) of neural synchronization represents envelope shapes at least up to 256 Hz, providing an important coding mechanism in addition to rate coding and coding by synchronization strength which have previously been emphasized (Epping & Eggermont, 1986; Pressnitzer et al., 2000; Lu et al., 2001; Walton et al., 2002; Gao & Wehr, 2015; for an emphasis on spike timing precision see also Zheng & Escabí, 2008; Zheng & Escabi, 2013). The observed timing differences in neural synchronization might be important to perceptually distinguish (potentially superimposed) sounds that exhibit different envelope shapes. Yet, the timing differences observed here are likely inherited from neural circuitries preceding the inferior colliculus within the auditory pathway as suggested by the absence of phase delay changes as a function of envelope shapes in LFP-spike synchronization (see Supplementary Materials and Figures S4 and S5).
Effects of aging on response magnitude and neural synchronization strength
The current study aimed to investigate the age-related changes of neural signal transduction from the synaptic (input; LFP) activity to spiking output. We observed a relative increase in sound onset-evoked neural activity from the LFP to spikes for older rats (Figure 2) and a relative increase in neural synchronization strength from LFPs to spikes for older rats at 45-Hz modulation rates, whereas synchronization strength was reduced for 256-Hz modulation rates (Figure 4).
Previous work in animals showed enhanced firing rates along the ascending auditory pathway following hearing loss and accompanying aging (Popelár et al., 1987; Syka et al., 1994; Hughes et al., 2010; Manzoor et al., 2012). In particular auditory cortex neural activity appears to be enhanced in animals with hearing loss (Stolzberg et al., 2012) as well as in aged animals and humans (Laffont et al., 1989; Hughes et al., 2010; Herrmann et al., 2013b; Bidelman et al., 2014; Herrmann et al., 2016). Assuming that synaptic neuronal activity at the soma and dendrites (input to a neuron or neuronal population) strongly contributes to the LFP and spiking reflects the output of a neuron or neuronal population (Bullock, 1997; Logothetis et al., 2001; Logothetis & Wandell, 2004; Buzsáki et al., 2012), the current data show that within a single midbrain structure (inferior colliculus) neuronal response magnitudes become relatively enhanced from synaptic to spiking activity in aged animals.
Previous studies investigating spike synchronization in aging animals reported that temporal modulation transfer functions shift from a band-pass to a low-pass shape (Palombi et al., 2001; Schatteman et al., 2008). That is, synchronization strength increases at slower modulation rates and decreases at faster modulation rates in older animals. Consistently, the current data reveal an age-related increase in spike synchronization at a slow modulation rate (45 Hz) and a decrease in LFP and spike synchronization at faster modulation rates (128 Hz and 256 Hz; although not statistically significant in some cases). Scalp recordings in humans and animals have shown similar age-related increases in neural synchronization for slower modulation rates (Boettcher et al., 2002; Purcell et al., 2004) and a decrease in synchronization strength for fast stimulus modulation rates (Grose et al., 2009; Clinard et al., 2010; Anderson et al., 2012; Parthasarathy & Bartlett, 2012; Bidelman et al., 2014; Parthasarathy et al., 2014), although in particular responses to slow rates may also have significant contributions from auditory cortex in such scalp recordings (Herdman et al., 2002; Coffey et al., 2016).
In particular our LFP synchronization data critically add to the existing body of work: (1) The data show that neural activity within the aging inferior colliculus synchronizes with the fast temporal modulation of sounds (at least up to 256 Hz), precisely capturing different stimulus envelope shapes in the neural phase delay. (2) We show that neural synchronization to a slow modulation rate (here 45 Hz) relatively increases from the synaptic activity (LFP) to spiking output in older animals. That is, no age difference in neural synchronization was observed for the LFPs. In contrast, neurons in the aged inferior colliculus elicited precisely phase-locked bursts of spikes, whereas in younger animals spikes were more distributed over an AM cycle and thus less synchronized (see Figures 4 and 5). (3) Neural synchronization strength decreased from damped to ramped envelope shapes (45-Hz modulation rate, LFP) for older rats whereas it increased in younger rats. (4) Synchronization strength in older animals was reduced for modulation rates greater than 100 Hz.
One of the most consistent changes within the central auditory system following hearing loss and accompanying aging is the reduction of neural inhibition along the ascending auditory pathway (Raza et al., 1994; Caspary et al., 1995; Vale et al., 2004; Caspary et al., 2008; Burianova et al., 2009; Takesian et al., 2009; Llano et al., 2012; Rabang et al., 2012; Takesian et al., 2012; Gao et al., 2015). Accordingly, recent observations of enhanced neural responses (excitability) due to hearing loss and aging have been discussed in the context of reduced neural inhibition (Hughes et al., 2010; Herrmann et al., 2016). Neural inhibition modulates sound-evoked neural activity levels (Faingold et al., 1989; Pollak & Park, 1993), which is consistent with our observation of a relatively enhanced onset response from LFP to spiking activity and reduced N1 latencies for older compared to younger rats (Figures 2 and 3).
Neural inhibition has also been shown to be crucial for shaping neural activity elicited by amplitude-modulated sounds (Yang & Pollak, 1997; Backoff et al., 1999; Cai & Caspary, 2015; but see also Caspary et al., 2002; Zhang & Kelly, 2003). Yet, the direction in which a reduction in neural inhibition affects neural synchronization is not clear. For example, reduced neural inhibition in the cochlear nucleus led to decreased neural synchronization to amplitude-modulated sounds (Backoff et al., 1999). A different study shows for neurons in the lateral lemniscus that a reduction of neural inhibition leads to an increase in synchronization strength for neurons that did not synchronize strongly before blockage of neural inhibition and to a decrease in synchronization strength for neurons that strongly synchronized before the blockage of inhibition (Yang & Pollak, 1997). In the inferior colliculus, synchronization was mostly unaffected by reduced inhibition (Caspary et al., 2002). These differences are in line with computational modelling suggesting that the influence of neural inhibition on neural synchronization is rather complex (Rabang et al., 2012).
A tentative interpretation of the current findings is that the age-related increase in synchronization strength for the low modulation frequency (45 Hz) and the age-related decrease in synchronization strength for the high modulation frequency (e.g., 256 Hz) are both the result of a reduction in neural inhibition in the auditory system of aging rats. Although there are other potential mechanisms, such as changes in ion channel (K, Cl, HCN) distribution, changes in short-term plasticity or changes in excitatory receptors (Kotak et al., 2005), reduction in inhibition is well supported by prior studies (Caspary et al., 2008; Rabang et al., 2012) and could potentially show modulation-frequency dependent changes in net excitability. Reduced neural inhibition leads to increased firing rates (assuming no concurrent decreases in excitation) which in turn might lead to a larger modulation of firing rate induced by the amplitude modulation, that is, increased synchronization strength. Another possibility is that an age-related reduction of neural inhibition is accompanied by reduced excitation, similar to hearing loss during development (Kotak & Sanes, 1997; Vale & Sanes, 2002). This would lead to unchanged or decreased firing rates, but might still lead to increased synchronization strength because the net excitation becomes restricted to near the peak phase of the AM cycle. Critically, reduced neural inhibition might also result in an increase in the amount of temporal jitter (by changing the excitation-inhibition balance) either within the input from a single neuron or across inputs from multiple neurons, potentially reducing temporal selectivity (Wehr & Zador, 2003; Isaacson & Scanziani, 2011). For low modulation rates, a mild increase in jitter could be offset by a relative increase in firing rate. In contrast, a mild increase in temporal jitter would decrease neural synchronization for high modulation rates because the duration of an AM cycle would likely be comparable to or shorter than the jitter. In addition, the input rate and/or synaptic depression might systematically vary depending on the AM rate of the stimulus (Joris et al., 2004; Rabang & Bartlett, 2011). For example, if the input rate were to decrease or synaptic depression to increase with AM rate, this would likely lead to inputs near or below the threshold for high AM rates (even with reduced inhibition) and thus to a reduction in synchronization strength as assessed by spiking activity. Future studies combing empirical and computation approaches are needed to shed more light on the role of the excitation-inhibition balance in age-related changes in neural synchronization. Furthermore, additional work is necessary to explore other mechanisms for increasing neuronal excitability, such as mechanisms that alter resting membrane potential, input resistance, or time constants.
Conclusions
The current study investigated the age-related changes in neural response magnitudes and neural synchronization in inferior colliculus neurons focusing on local field potentials and spiking. Activity in the inferior colliculus allows distinguishing stimulus envelope shapes for modulation rates higher than 250 Hz. Aging was accompanied by a relative increase in response magnitude to sound onsets and a relative increase in neural synchronization strength from the local field potential (synaptic activity) to spiking output for older rats. Aging was also associated with decreased neural synchronization strength at higher amplitude-modulation rates. The current data show that signal transformation within a single midbrain structure (inferior colliculus) is altered in aged animals.
Supplementary Material
Acknowledgements
This study was supported by NIDCD DC-011580 to ELB and the Brain and Mind Institute at the University of Western Ontario (postdoctoral fellowship award to BH).
Footnotes
Author contributions
BH analyzed data and drafted the manuscript. AP designed the study, collected data, and drafted the manuscript. ELB designed the study, collected data, and drafted the manuscript.
Disclosure
The authors claim no actual or potential conflicts of interest.
Data Accessibility
The article's data are available on the dryad data repository (https://datadryad.org/).
References
- Adams J. Ascending projections to the inferior colliculus. The Journal of Comparative Neurology. 1979;183:519–538. doi: 10.1002/cne.901830305. [DOI] [PubMed] [Google Scholar]
- Akeroyd MA, Patterson RD. Discrimination of wideband noises modulated by a temporally asymmetric function. Journal of the Acoustical Society of America. 1995;98:2466–2474. [Google Scholar]
- Anderson S, Parbery-Clark A, White-Schwoch T, Kraus N. Aging Affects Neural Precision of Speech Encoding. The Journal of Neuroscience. 2012;32:14156–14164. doi: 10.1523/JNEUROSCI.2176-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backoff PM, Palombi PS, Caspary DM. γ-Aminobutyric acidergic and glycinergic inputs shape coding of amplitude modulation in the chinchilla cochlear nucleus. Hearing Research. 1999;134:77–88. doi: 10.1016/s0378-5955(99)00071-4. [DOI] [PubMed] [Google Scholar]
- Barsz K, Ison JR, Snell KB, Walton JP. Behavioral and neural measures of auditory temporal acuity in aging humans and mice. Neurobiology of Aging. 2002;23:565–578. doi: 10.1016/s0197-4580(02)00008-8. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Wang X. Long-Lasting Modulation by Stimulus Context in Primate Auditory Cortex. Journal of Neurophysiology. 2005;94:83–104. doi: 10.1152/jn.01124.2004. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Wang X. Neural representations of temporally modulated signals in the auditory thalamus of awake primates. Journal of Neurophysiology. 2007;97:1005–1017. doi: 10.1152/jn.00593.2006. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Wang X. Correlation of neural response properties with auditory thalamus subdivisions in the awake marmoset. Journal of Neurophysiology. 2011;105:2647–2667. doi: 10.1152/jn.00238.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belitski A, Panzeri S, Magri C, Logothetis NK, Kayser C. Sensory information in local field potentials and spikes from visual and auditory cortices: time scales and frequency bands. Journal of Computational Neuroscience. 2010;29:533–545. doi: 10.1007/s10827-010-0230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B. 1995;57:289–300. [Google Scholar]
- Bibikov NG. Coding of amplitude-modulated signals in the cochlear nucleus of a grass frog. Acoustical Physics. 2002;48:388–399. [Google Scholar]
- Bidelman GM, Villafuerte JW, Moreno S, Alain C. Age-related changes in the subcorticalecortical encoding and categorical perception of speech. Neurobiology of Aging. 2014;35:2526–2540. doi: 10.1016/j.neurobiolaging.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Boettcher FA, Madhotra D, Poth EA, Mills JH. The frequency-modulation following response in young and aged human subjects. Hearing Research. 2002;165:10–18. doi: 10.1016/s0378-5955(01)00398-7. [DOI] [PubMed] [Google Scholar]
- Boettcher FA, Poth EA, Mills JH, Dubno JR. The amplitude-modulation following response in young and aged human subjects. Hearing Research. 2001;153:32–42. doi: 10.1016/s0378-5955(00)00255-0. [DOI] [PubMed] [Google Scholar]
- Bullock TH. Signals and signs in the nervous system: The dynamic anatomy of electrical activity is probably information-rich. Proceedings of the National Academy of Sciences. 1997;94:1–6. doi: 10.1073/pnas.94.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burianova J, Ouda L, Profant O, Syka J. Age-related changes in GAD levels in the central auditory system of the rat. Experimental Gerontology. 2009;44:161–169. doi: 10.1016/j.exger.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents — EEG, ECoG, LFP and spikes. Nature Reviews Neuroscience. 2012;13:407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R, Caspary DM. GABAergic inhibition shapes SAM responses in rat auditory thalamus. Neuroscience. 2015;299:146–155. doi: 10.1016/j.neuroscience.2015.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canlon B, Illing RB, Walton JP. Cell Biology and Physiology of the Aging Central Auditory Pathway. In: Gordon-Salant S, Frisina RD, Popper AN, Fay RR, editors. The Aging Auditory System. Springer; New York: 2010. [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. The Journal of Experimental Biology. 2008;211:1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Milbrandt JC, Helfert RH. Central auditory aging: GABA changes in the inferior colliculus. Experimental Gerontology. 1995;30:349–360. doi: 10.1016/0531-5565(94)00052-5. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Palombi PS, Hughes LF. GABAergic inputs shape responses to amplitude modulated stimuli in the inferior colliculus. Hearing Research. 2002;168:163–173. doi: 10.1016/s0378-5955(02)00363-5. [DOI] [PubMed] [Google Scholar]
- Clinard CG, Tremblay KL, Krishnan AR. Aging alters the perception and physiological representation of frequency: Evidence from human frequency-following response recordings. Hearing Research. 2010;264:48–55. doi: 10.1016/j.heares.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey EBJ, Herholz SC, Chepesiuk AMP, Baillet S, Zatorre RJ. Cortical contributions to the auditory frequency-following response revealed by MEG. Nature Communications. 2016;7:11070. doi: 10.1038/ncomms11070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. The Journal of Physiology. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping WJM, Eggermont JJ. Sensitivity of neurons in the auditory midbrain of the grassfrog to temporal characteristics of sound. II. Stimulation with amplitude modulated sound. Hearing Research. 1986;24:55–72. doi: 10.1016/0378-5955(86)90005-5. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Gehlbach G, Caspary DM. On the role of GABA as an inhibitory neurotransmitter in inferior colliculus neurons: iontophoretic studies. Brain Research. 1989;500:302–312. doi: 10.1016/0006-8993(89)90326-0. [DOI] [PubMed] [Google Scholar]
- Furukawa S, Moore BCJ. Effect of the relative phase of amplitude modulation on the detection of modulation on two carriers. Journal of the Acoustical Society of America. 1997;102:3657–3664. doi: 10.1121/1.420152. [DOI] [PubMed] [Google Scholar]
- Gao F, Wang G, Ma W, Ren F, Li M, Dong Y, Liu C, Liu B, Bai X, Zhao B, Edden RAE. Decreased auditory GABA+concentrations in presbycusis demonstrated by edited magnetic resonance spectroscopy. NeuroImage. 2015;106:311–316. doi: 10.1016/j.neuroimage.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Wehr M. A Coding Transformation for Temporally Structured Sounds within Auditory Cortical Neurons. Neuron. 2015;86:1–12. doi: 10.1016/j.neuron.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MJ, Altman DG. Confidence intervals rather than P values: estimation rather than hypothesis testing. Statistics in Medicine. 1986;292:746–750. doi: 10.1136/bmj.292.6522.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gourévitch B, Edeline J-M. Age-related changes in the guinea pig auditory cortex: relationship with brainstem changes and comparison with tone-induced hearing loss. European Journal of Neuroscience. 2011;34:1953–1965. doi: 10.1111/j.1460-9568.2011.07905.x. [DOI] [PubMed] [Google Scholar]
- Grose JH, Mamo SK, Hall III JW. Age effects in temporal envelope processing: speech unmasking and auditory steady state responses. Ear & Hearing. 2009;30:568–575. doi: 10.1097/AUD.0b013e3181ac128f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Nácher V, Luna R, Romo R, Jensen O. α-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proceedings of the National Academy of Sciences. 2011;108:19377–19382. doi: 10.1073/pnas.1117190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil P. First-spike latency of auditory neurons revisited. Current Opinion in Neurobiology. 2004;14:461–647. doi: 10.1016/j.conb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Herdman AT, Lins O, Van Roon P, Stapells DR, Scherg M, Picton TW. Intracerebral Sources of Human Auditory Steady-State Responses. Brain Topography. 2002;15:69–86. doi: 10.1023/a:1021470822922. [DOI] [PubMed] [Google Scholar]
- Herrmann B, Henry MJ, Grigutsch M, Obleser J. Oscillatory Phase Dynamics in Neural Entrainment Underpin Illusory Percepts of Time. The Journal of Neuroscience. 2013a;33:15799–15809. doi: 10.1523/JNEUROSCI.1434-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann B, Henry MJ, Johnsrude IS, Obleser J. Altered temporal dynamics of neural adaptation in the aging human auditory cortex. Neurobiology of Aging. 2016;45:10–22. doi: 10.1016/j.neurobiolaging.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Herrmann B, Henry MJ, Scharinger M, Obleser J. Auditory filter width affects response magnitude but not frequency specificity in auditory cortex. Hearing Research. 2013b;304:128–136. doi: 10.1016/j.heares.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Herrmann B, Parthasarathy A, Han EX, Obleser J, Bartlett EL. Sensitivity of rat inferior colliculus neurons to frequency distributions. Journal of Neurophysiology. 2015;114:2941–2954. doi: 10.1152/jn.00555.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes LF, Turner JG, Parrish JL, Caspary DM. Processing of broadband stimuli across A1 layers in young and aged rats. Hearing Research. 2010;264:79–85. doi: 10.1016/j.heares.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irino T, Patterson RD. Temporal asymmetry in the auditory system. Journal of the Acoustical Society of America. 1996;99:2316–2331. doi: 10.1121/1.415419. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How Inhibition Shapes Cortical Activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris PX, Schreiner CE, Rees A. Neural Processing of Amplitude-Modulated Sounds. Physiological Reviews. 2004;84:541–577. doi: 10.1152/physrev.00029.2003. [DOI] [PubMed] [Google Scholar]
- Joris PX, Yin TC. Responses to amplitude-modulated tones in the auditory nerve of the cat. Journal of the Acoustical Society of America. 1992;91:215–232. doi: 10.1121/1.402757. [DOI] [PubMed] [Google Scholar]
- Kadner A, Berrebi AS. Encoding of temporal features of auditory stimuli in the medial nucleus of the trapezoid body and superior paraolivary nucleus of the rat. Neuroscience. 2008;151:868–887. doi: 10.1016/j.neuroscience.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajikawa Y, Schroeder CE. How Local Is the Local Field Potential? Neuron. 2011;72:847–858. doi: 10.1016/j.neuron.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale S, Heinz MG. Envelope coding in auditory nerve fibers following noise-induced hearing loss. Journal of the Association for Research in Otolaryngology. 2010;11:657–673. doi: 10.1007/s10162-010-0223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser C, Wilson C, Safaai H, Sakata S, Panzeri S. Rhythmic auditory cortex activity at multiple timescales shapes stimulus-response gain and background firing. The Journal of Neuroscience. 2015;35:7750–7762. doi: 10.1523/JNEUROSCI.0268-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH. Hearing Loss Raises Excitability in the Auditory Cortex. The Journal of Neuroscience. 2005;25:3908–3918. doi: 10.1523/JNEUROSCI.5169-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Sanes DH. Deafferentation Weakens Excitatory Synapses in the Developing Central Auditory System. European Journal of Neuroscience. 1997;9:2340–2347. doi: 10.1111/j.1460-9568.1997.tb01651.x. [DOI] [PubMed] [Google Scholar]
- Krishna BS, Semple MN. Auditory temporal processing: responses to sinusoidally amplitude-modulated tones in the inferior colliculus. Journal of Neurophysiology. 2000;84:255–273. doi: 10.1152/jn.2000.84.1.255. [DOI] [PubMed] [Google Scholar]
- Lachaux J-P, Rodriguez E, Martinerie J, Varela FJ. Measuring Phase Synchrony in Brain Signals. Human Brain Mapping. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffont F, Bruneau N, Roux S, Agar N, Minz M, Cathala HP. Effects of age on auditory evoked responses (AER) and augmenting-reducing. Clinical Neurophysiology. 1989;19:15–23. doi: 10.1016/s0987-7053(89)80081-4. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of Neuronal Oscillations as a Mechanism of Attentional Selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- Langner G, Schreiner CE. Periodicity coding in the inferior colliculus of the cat. I. Neuronal mechanisms. Journal of Neurophysiology. 1988;60:1799–1822. doi: 10.1152/jn.1988.60.6.1799. [DOI] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Drivers and modulators in the central auditory pathways. Frontiers in Neuroscience. 2010;4:79–86. doi: 10.3389/neuro.01.014.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano DA, Turner J, Caspary DM. Diminished Cortical Inhibition in an Aging Mouse Model of Chronic Tinnitus. The Journal of Neuroscience. 2012;32:16141–16148. doi: 10.1523/JNEUROSCI.2499-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oettermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annual Review of Physiology. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Lu T, Liang L, Wang X. Temporal and rate representations of time-varying signals in the auditory cortex of awake primates. Nature Neuroscience. 2001;4:1131–1138. doi: 10.1038/nn737. [DOI] [PubMed] [Google Scholar]
- Malone BJ, Scott BH, Semple MN. Dynamic Amplitude Coding in the Auditory Cortex of Awake Rhesus Macaques. Journal of Neurophysiology. 2007;98:1451–1474. doi: 10.1152/jn.01203.2006. [DOI] [PubMed] [Google Scholar]
- Manzoor NF, Licari FG, Klapchar M, Elkin RL, Gao Y, Chen G, Kaltenbach JA. Noise-induced hyperactivity in the inferior colliculus: its relationship with hyperactivity in the dorsal cochlear nucleus. Journal of Neurophysiology. 2012;108:976–988. doi: 10.1152/jn.00833.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt JC, Albin RL, Caspary DM. Age-related decrease in GABAB receptor binding in the Fischer 344 rat inferior colliculus. Neurobiology of Aging. 1994;15:699–703. doi: 10.1016/0197-4580(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Skrodzka E. Detection of frequency modulation by hearing-impaired listeners: Effects of carrier frequency, modulation rate, and added amplitude modulation. The Journal of the Acoustical Society of America. 2002;111:327–335. doi: 10.1121/1.1424871. [DOI] [PubMed] [Google Scholar]
- Neuert V, Pressnitzer D, Patterson RD, Winter IM. The responses of single units in the inferior colliculus of the guinea pig to damped and ramped sinusoids. Hearing Research. 2001;159:36–52. doi: 10.1016/s0378-5955(01)00318-5. [DOI] [PubMed] [Google Scholar]
- Palombi PS, Backoff PM, Caspary DM. Responses of young and aged rat inferior colliculus neurons to sinusoidally amplitude modulated stimuli. Hearing Research. 2001;153:174–180. doi: 10.1016/s0378-5955(00)00264-1. [DOI] [PubMed] [Google Scholar]
- Parthasarathy A, Bartlett EL. Age-related auditory deficits in temporal processing in F-344 rats. Neuroscience. 2011;192:619–630. doi: 10.1016/j.neuroscience.2011.06.042. [DOI] [PubMed] [Google Scholar]
- Parthasarathy A, Bartlett EL. Two-channel recording of auditory-evoked potentials to detect age-related deficits in temporal processing. Hearing Research. 2012;289:52–62. doi: 10.1016/j.heares.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Datta J, Torres JAL, Hopkins C, Bartlett EL. Age-Related Changes in the Relationship Between Auditory Brainstem Responses and Envelope-Following Responses. Journal of the Association for Research in Otolaryngology. 2014;15:649–661. doi: 10.1007/s10162-014-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson RD. The sound of a sinusoid: Spectral models. Journal of the Acoustical Society of America. 1994;96:1409–1418. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; 2006. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK. Processing speed and timing in aging adults: psychoacoustics, speech perception, and comprehension. International Journal of Audiology. 2003;42:S59–S67. doi: 10.3109/14992020309074625. [DOI] [PubMed] [Google Scholar]
- Picton TW, John SM, Dimitrijevic A, Purcell DW. Human auditory steady-state responses. International Journal of Audiology. 2003;42:177–219. doi: 10.3109/14992020309101316. [DOI] [PubMed] [Google Scholar]
- Pollak GD, Burger RM, Klug A. Dissecting the circuitry of the auditory system. Trends in Neurosciences. 2003;26:33–39. doi: 10.1016/s0166-2236(02)00009-7. [DOI] [PubMed] [Google Scholar]
- Pollak GD, Park TJ. The effects of GABAergic inhibition on monaural response properties of neurons in the mustache bat's inferior colliculus. Hearing Research. 1993;65:99–117. doi: 10.1016/0378-5955(93)90205-f. [DOI] [PubMed] [Google Scholar]
- Popelár J, Syka J, Berndt H. Effect of noise on auditory evoked responses in awake guinea pigs. Hearing Research. 1987;26:239–247. doi: 10.1016/0378-5955(87)90060-8. [DOI] [PubMed] [Google Scholar]
- Pressnitzer D, Winter IM, Patterson RD. The responses of single units in the ventral cochlear nucleus of the guinea pig to damped and ramped sinusoids. Hearing Research. 2000;149:155–166. doi: 10.1016/s0378-5955(00)00175-1. [DOI] [PubMed] [Google Scholar]
- Preuß A, Müller-Preuss P. Processing of amplitude modulated sounds in the medial geniculate body of squirrel monkeys. Experimental Brain Research. 1990;79:207–211. doi: 10.1007/BF00228890. [DOI] [PubMed] [Google Scholar]
- Purcell DW, John SM, Schneider BA, Picton TW. Human temporal auditory acuity as assessed by envelope following responses. The Journal of the Acoustical Society of America. 2004;116:3581–3593. doi: 10.1121/1.1798354. [DOI] [PubMed] [Google Scholar]
- Rabang CF, Bartlett EL. A computational model of cellular mechanisms of temporal coding in the medial geniculate body (MGB). PLoS ONE. 2011;6:e29375. doi: 10.1371/journal.pone.0029375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabang CF, Parthasarathy A, Venkataraman Y, Fisher ZL, Gardner SM, Bartlett EL. A computational model of inferior colliculus responses to amplitude modulated sounds in young and aged rats. Frontiers in Neural Circuits. 2012;6:77. doi: 10.3389/fncir.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza A, Milbrandt JC, Arneric SP, Caspary DM. Age-related changes in brainstem auditory neurotransmitters: measures of GABA and acetylcholine function. Hearing Research. 1994;77:221–230. doi: 10.1016/0378-5955(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Rubin DB. requivalent: A Simple Effect Size Indicator. Psychological Methods. 2003;8:492–496. doi: 10.1037/1082-989X.8.4.492. [DOI] [PubMed] [Google Scholar]
- Schatteman TA, Hughes LF, Caspary DM. Age-related loss of temporal processing: Altered responses to amplitude modulated tones in rat dorsal cochlear nucleus. Neuroscience. 2008;154:329–337. doi: 10.1016/j.neuroscience.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BA, K. P-FM, Kowalchuk D, Lamb M. Gap detection and the precedence effect in young and old adults. Journal of the Acoustical Society of America. 1994;95:980–991. doi: 10.1121/1.408403. [DOI] [PubMed] [Google Scholar]
- Schvartz KC, Chatterjee M. Gender identification in younger and older adults: use of spectral and temporal cues in noise-vocoded speech. Ear & Hearing. 2012;33:411–420. doi: 10.1097/AUD.0b013e31823d78dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzberg D, Chrostowski M, Salvi RJ, Allman B. Intracortical circuits amplify sound-evoked activity in primary auditory cortex following systemic injection of salicylate in the rat. Journal of Neurophysiology. 2012;108:200–214. doi: 10.1152/jn.00946.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syka J. The Fischer 344 rat as a model of presbycusis. Hearing Research. 2010;264:70–78. doi: 10.1016/j.heares.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Syka J, Rybalko N, Popelár J. Enhancement of the auditory cortex evoked responses in awake guinea pigs after noise exposure. Hearing Research. 1994;78:158–168. doi: 10.1016/0378-5955(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sanes DH. Developmental hearing loss disrupts synaptic inhibition: implications for auditory processing. Future Neurology. 2009;4:331–349. doi: 10.2217/FNL.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sanes DH. Age-dependent effect of hearing loss on cortical inhibitory synapse function. Journal of Neurophysiology. 2012;107:937–947. doi: 10.1152/jn.00515.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter-Mikaelian M, Sanes DH, Semple MN. Transformation of Temporal Properties between Auditory Midbrain and Cortex in the Awake Mongolian Gerbil. The Journal of Neuroscience. 2007;27:6091–6102. doi: 10.1523/JNEUROSCI.4848-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale C, Juiz JM, Moore DR, Sanes DH. Unilateral cochlear ablation produces greater loss of inhibition in the contralateral inferior colliculus. European Journal of Neuroscience. 2004;20:2133–2140. doi: 10.1111/j.1460-9568.2004.03679.x. [DOI] [PubMed] [Google Scholar]
- Vale C, Sanes DH. The effect of bilateral deafness on excitatory and inhibitory synaptic strength in the inferior colliculus. European Journal of Neuroscience. 2002;16:2394–2404. doi: 10.1046/j.1460-9568.2002.02302.x. [DOI] [PubMed] [Google Scholar]
- Vernier VG, Galambos R. Response of Single Medial Geniculate Units to Repetitive Click Stimuli. American Journal of Physiology. 1957;188:233–237. doi: 10.1152/ajplegacy.1957.188.2.233. [DOI] [PubMed] [Google Scholar]
- Walton JP. Timing is everything: Temporal processing deficits in the aged auditory brainstem. Hearing Research. 2010;264:63–69. doi: 10.1016/j.heares.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JP, Simon H, Frisina RD. Age-Related Alterations in the Neural Coding of Envelope Periodicities. Journal of Neurophysiology. 2002;88:565–578. doi: 10.1152/jn.2002.88.2.565. [DOI] [PubMed] [Google Scholar]
- Wayne RV, Johnsrude IS. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Research Reviews. 2015;23:154–166. doi: 10.1016/j.arr.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- Whittingstall K, Logothetis NK. Frequency-Band Coupling in Surface EEG Reflects Spiking Activity in Monkey Visual Cortex. Neuron. 2009;64:281–289. doi: 10.1016/j.neuron.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Yang L, Pollak GD. Differential Response Properties to Amplitude Modulated Signals in the Dorsal Nucleus of the Lateral Lemniscus of the Mustache Bat and the Roles of GABAergic Inhibition. Journal of Neurophysiology. 1997;77:324–340. doi: 10.1152/jn.1997.77.1.324. [DOI] [PubMed] [Google Scholar]
- Zhang H, Kelly JB. Glutamatergic and GABAergic Regulation of Neural Responses in Inferior Colliculus to Amplitude-Modulated Sounds. Journal of Neurophysiology. 2003;90:477–490. doi: 10.1152/jn.01084.2002. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Escabi MA. Proportional spike-timing precision and firing reliability underlie efficient temporal processing of periodicity and envelope shape cues. Journal of Neurophysiology. 2013;110:587–606. doi: 10.1152/jn.01080.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Escabí MA. Distinct Roles for Onset and Sustained Activity in the Neuronal Code for Temporal Periodicity and Acoustic Envelope Shape. The Journal of Neuroscience. 2008;28:14230–14244. doi: 10.1523/JNEUROSCI.2882-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.