Abstract
Diarrhea is a feature of several chronic intestinal disorders that are associated with increased delivery of bile acids into the colon. Although the prevalence of bile acid diarrhea is high, affecting approximately 1% of the adult population, current therapies often are unsatisfactory. By virtue of its capacity to inhibit colonic epithelial fluid secretion and to down-regulate hepatic bile acid synthesis through induction of the ileal fibroblast growth factor 19 release, the nuclear bile acid receptor, farnesoid X receptor, represents a promising target for the development of new therapeutic approaches. Here, we review our current understanding of the pathophysiology of bile acid diarrhea and the current evidence supporting a role for farnesoid X receptor agonists in treatment of the disease.
Keywords: Bile Acid Diarrhea, Enterohepatic Circulation, FGF-19, Chloride Secretion, Epithelium
Abbreviations used in this paper: ASBT, apical sodium-linked bile acid transporter; BAD, bile acid diarrhea; C4, 7α-hydroxy-4-cholesten-3-one; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; EHC, enterohepatic circulation; FGF19, fibroblast growth factor 19; FXR, farnesoid X receptor; LCA, lithocholic acid; OCA, obeticholic acid
Summary.
Although bile acid diarrhea affects ∼1% of the adult population, diagnosis and therapeutic approaches are still unsatisfactory. Herein, we review current concepts of bile acid diarrhea pathogenesis and the potential role for the farnesoid X receptor in its treatment.
The farnesoid X receptor (FXR) is the nuclear hormone receptor that binds bile acids and regulates transcription of multiple genes that are key to many aspects of metabolism, especially those involving bile acids themselves. This review describes the involvement of FXR in the condition known as bile acid diarrhea (BAD).
BAD occurs when excess bile acids are present in the colon. The condition was first recognized in 1967,1 as a result of bile acid malabsorption in patients with ileal resection or disease, particularly those with Crohn’s disease. These patients had high concentrations of fecal bile acids and their symptoms of frequent, unformed bowel motions improved after treatment with bile acid–sequestering resins, such as cholestyramine.2 Later, it became apparent that there was an idiopathic, primary form of BAD in patients who had no obvious ileal disease but who also responded to bile acid sequestrants.3 BAD also can be found to be associated with other gastrointestinal disorders, such as radiation enteropathy or postcholecystectomy. Taking into account these differing etiologies, BAD now is classified as being type 1 (secondary to ileal disease), type 2 (primary, idiopathic), or type 3 (miscellaneous).4
Although primary BAD is common, it still often is unrecognized.5 Several systematic reviews have confirmed that its incidence is approximately 25%–32% of patients suffering from functional bowel disorders with diarrhea.6, 7 This means that the population prevalence is approximately 1% (ie, it is more prevalent than Crohn’s disease or ulcerative colitis and has a similar prevalence to celiac disease). These patients commonly are labeled with diarrhea-predominant irritable bowel syndrome or functional diarrhea, and often will have experienced symptoms for many years before the correct diagnosis is made. Diagnostic methods that have been used include quantification of fecal bile acids, estimates of the retention (and loss) of the γ-emitter 75SeHCAT (a modified bile acid), or measurements of markers of new bile acid synthesis such as 7α-hydroxy-4-cholesten-3-one (C4) or fibroblast growth factor 19 (FGF19),7 however, for a variety of reasons, these tests are not widely available in the United States and Europe.
Consequently, there is a large unmet need in patients with BAD for a better diagnosis and this is coupled with the need for better therapies. Bile acid sequestrants bind bile acids in the colon and prevent them from exerting their adverse effects on water and electrolyte transport, but these drugs can be poorly tolerated, causing abdominal bloating or pain, and have significant potential to interact with other drugs and vitamins.8 Importantly, current therapies do not act on the molecular pathways underlying the pathogenesis of BAD, but studies emerging from our own and other laboratories suggest that drugs that activate the FXR may have future roles to play in this regard.
Physiology of the Enterohepatic Circulation
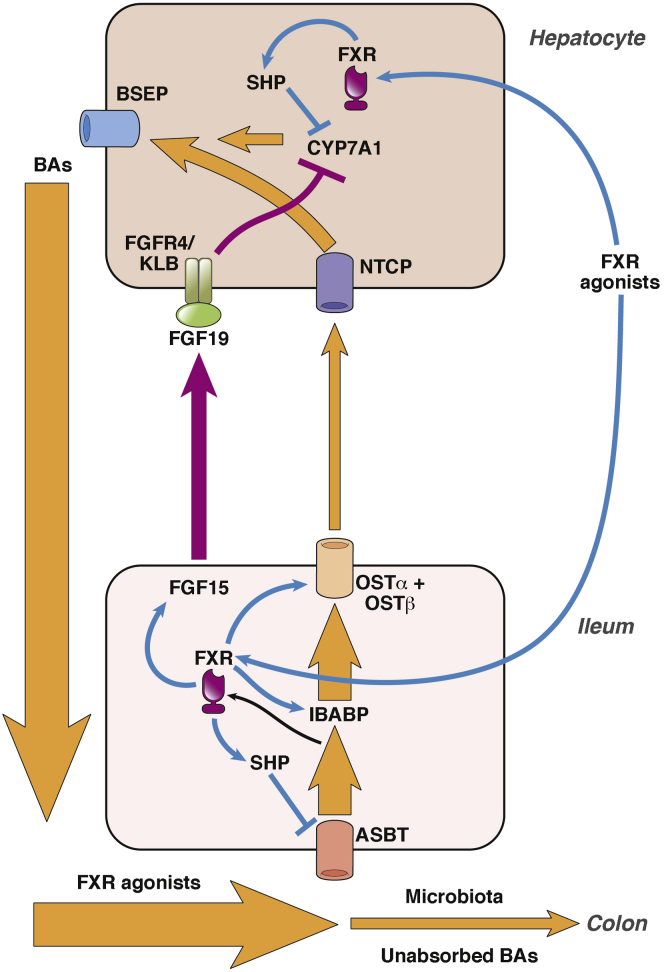
The FXR plays key roles in regulating bile acid metabolism (Figure 1). The primary bile acids in human beings, chenodeoxycholic acid (CDCA) and cholic acid (CA), are synthesized in the liver by way of a multistep process, starting from cholesterol.9 The cytochrome enzyme CYP7A1 is the key regulatory step; C4 is a marker of new bile acid synthesis measurable in serum. CA and CDCA are conjugated with either glycine or taurine, and these more hydrophilic bile acids are secreted from the hepatocyte through the bile salt export protein and are stored as a component of bile in the gallbladder.
Figure 1.
The FXR in regulation of the enterohepatic circulation. Bile acids (BAs) are synthesized in the hepatocyte by CYP7A1 and secreted into the enterohepatic circulation (orange arrows) by the bile salt export pump (BSEP). In ileal enterocytes, BAs are absorbed through the ASBT, bind to the ileal bile acid binding protein (IBABP), and are pumped into the portal circulation by the basolateral organic solute transporter (OST)α-OSTβ dimer. BAs are taken back up into hepatocytes by the Na+-taurocholate co-transporting polypeptide (NTCP). Treatment with FXR agonists (blue lines) induces expression of short heterodimeric partner (SHP) in the liver and of FGF19, IBABP, OSTα-OSTβ, and SHP in ileal enterocytes. FGF19 enters the portal circulation and acts on FGF receptor 4–Klothoβ receptors in hepatocytes (purple arrows) to inhibit CYP7A1 expression. SHP also down-regulates CYP7A1 expression in hepatocytes, further dampening BA synthesis. With each cycle of the EHC, a small proportion of the bile acid pool enters the colon where it is metabolized by the resident microbiota into deconjugated secondary bile acids. In primary BAD, impaired FGF19 production results in increased CYP7A1 expression and enhanced hepatic synthesis of bile acids. In turn, this results in a larger bile acid pool with increased colonic delivery.
Upon ingestion of a meal, gallbladder contraction is induced by cholecystokinin and bile acids are released into the small intestine where they are critical for lipid digestion and absorption. In the ileum, they are reabsorbed, with approximately 95% being recovered. The molecular pathway for bile acid uptake in ileal enterocytes comprises the apical sodium-linked bile transporter (ASBT, also called ileal bile acid transporter, gene symbol SLC10A2), the ileal bile acid binding protein (gene symbol FABP6), and the basolateral membrane organic solute transporters α and β.10 Bile acids enter the portal blood, are taken back up in the liver by the Na+-taurocholate co-transporting polypeptide (SLC10A1), and are recycled; a process known as enterohepatic circulation (EHC). Recycling of bile acids is very efficient and the daily amount of bile acids entering the intestine is 4–6 times that of the total bile acid pool size, with new bile acid synthesis contributing to only a small proportion of this.
With each cycle of the EHC a small proportion (∼5%) of circulating bile acids enter the colon. Here, conjugated primary bile acids are metabolized by enzymes in the microbiota through deconjugation, dehydroxylation, and epimerization to form the secondary bile acids, most notably in human beings, deoxycholic acid (DCA) from CA, and ursodeoxycholic acid and lithocholic acid (LCA) from CDCA. Metabolism of bile acids by the microbiota in this way makes them more lipophilic, enabling their passive reabsorption in the colon and recirculation to the liver where they are reconjugated and re-used in the bile. In human beings, the predominant colonic bile acid is normally DCA, and is present in the range of 50–200 μmol/L in the cecal water.11 However, it should be noted that considerable differences in the nature of the colonic bile acid pool exist between different species, a fact that must be taken into account when translating experimental observations from animal models into human beings. For example, in mice, muricholic acid is a major primary bile acid, and is present in the colon at concentrations similar to those of DCA, but has opposing effects on FXR activation.12
At the molecular level, regulation of the EHC is very complex and is still not fully understood. However, it is now clear that the FXR has key roles to play (Figure 1). The involvement of FXR as a bile acid receptor was first recognized in 1999.13, 14, 15 FXR dimerizes with the retinoid X receptor, another zinc-finger DNA-binding nuclear hormone receptor, and the heterodimer binds to specific bile acid response elements and regulates the transcription of many genes. One important FXR response gene is short heterodimeric partner. This has been shown to down-regulate CYP7A1 transcription in the liver and also ASBT expression in the ileum. Organic solute transporter α and ileal bile acid binding protein in the ileum both are transcriptionally up-regulated by FXR.
The most FXR-responsive gene in human ileum is FGF19,16 and the rodent orthologue is FGF15. FGF19 is a 24-kilodalton protein, produced mainly in enterocytes, and enters the portal circulation and acts as a hormone to provide feedback inhibition in the liver to regulate new bile acid synthesis. FGF15/19 binds the FGF receptor 4 in the liver, which interacts with β-Klotho (KLB) as a co-receptor. Through a kinase signaling pathway, FGF15/19 inhibits the action of CYP7A1,17 resulting in reduced new bile acid synthesis. This provides a feedback mechanism to ensure that if sufficient bile acids are being absorbed in the ileum, hepatic synthesis of new bile acids is inhibited.
Pathophysiology of Bile Acid Diarrhea
Malabsorption of bile acids occurs when the normal ileal bile acid transport systems described earlier are impaired. In human beings, this is most likely to occur as a result of surgical resection of the small intestine or as a consequence of inflammation, such as that associated with Crohn’s disease, leading to reduced expression of ASBT.18 Rarely, children may be born with gene defects in ASBT.10 Inhibition of ASBT function also can be induced pharmacologically by ileal bile acid transporter inhibitors, such as elobixibat, which currently are undergoing development for the treatment of patients with constipation.19
In patients with the primary idiopathic form of BAD, the mechanism for increased colonic bile acid concentrations does not appear to be caused by impaired bile acid absorption.20, 21 Instead, ileal production and reduced serum levels of FGF19 have been shown.20, 22 This defect results in reduced feedback inhibition of hepatic synthesis, and, consequently, overproduction of bile acids. Previous human studies showing unimpaired bile acid uptake in the ileum and a larger pool size support this mechanism of disease pathogenesis. Similarly, studies in mouse models, where the genes for Fxr,23 Fgf15, Fgfr4, and Klb were knocked out, also showed excessive bile acid synthesis and fecal bile acid loss (reviewed by Walters24), whereas monkeys treated with antibodies to FGF1925 developed severe watery diarrhea. These findings all suggest a central role for FGF19 and FXR in the pathogenesis of BAD.
Further evidence comes from studies of human ileal biopsy specimens in short-term culture where FGF19 messenger RNA expression was shown to be stimulated potently by physiological concentrations of natural bile acids. In keeping with their known potency as FXR agonists, CDCA was the most potent natural FGF19 inducer, followed by CA, DCA, and then LCA.26 The semisynthetic bile acid, obeticholic acid (OCA), was effective at even lower concentrations, reflecting findings from other experimental models.27 In a prospective study, serum levels of FGF19 were confirmed to be low in patients with BAD and were a reliable predictor of responses in patients with chronic diarrhea.28 Patients with BAD also were shown to have reduced fasting and bile acid–stimulated levels of ileal FGF19 transcripts.22 Together, these observations have led to the establishment of a model in which dysregulation of the FXR/FGF19 axis, leading to increased hepatic synthesis of bile acids, is the critical step in the pathogenesis of primary BAD.
Bile Acids and Colonic Fluid and Electrolyte Transport
When the EHC is functioning normally, relatively low levels of bile acids enter into the colon. Although bile acid concentrations in the duodenum can be as high as 10 mmol/L, the median concentration in the cecum is reported to be approximately 0.4 mmol/L.11 DCA is the most prominent colonic bile acid, and is present in the colonic fluid at concentrations in the range of 0.1–0.2 mmol/L.11 However, in conditions of bile acid malabsorption, levels of luminal bile acids may be higher. A study from the Mayo Clinic showed that 24-hour fecal bile acid loss was 363 μmol/24 h (interquartile range, 194–762 μmol/24 h) in healthy controls (n = 23), compared with 864 μmol/24 h (interquartile range, 453–1213 μmol/24 h) in a mixed group of patients (n = 21) with undefined diarrhea-predominant IBS.29 Because bile acids in high concentrations are toxic molecules, the body must respond appropriately to such abnormally high levels in the lumen if it is to prevent mucosal damage, ulceration, and inflammation. One of the primary mechanisms by which it does so is through the induction of diarrhea.
Diarrheal Actions of Bile Acids
The cellular and molecular mechanisms by which increased colonic delivery of bile acids promotes fluid accumulation in the colon have been studied extensively in animal models and cultured epithelial cells. Early studies in animal models have shown that the effects of bile acids are mediated by both inhibition of fluid absorption across surface enterocytes and stimulation of secretion from the crypts.30, 31, 32, 33 Although there is some variation in the concentration dependence by which bile acids exert their actions across different species, it is clear that in human beings such effects only occur at high pathophysiological levels.29, 34 It also is apparent that there is a strict structure–activity relationship for bile acids in inducing their cathartic effects, with only dihydroxy bile acids, such as DCA and CDCA, being effective.33, 35, 36 Sulfation of bile acids abolishes their effects on fluid transport, whereas conjugated bile acids are effective only from the basolateral side of the epithelium.36, 37, 38 Thus, the metabolizing activity of the colonic microbiota, which alters bile acids by dehydroxylation, epimerization, deconjugation, and sulfation, has important consequences for their capacity to cause diarrhea.
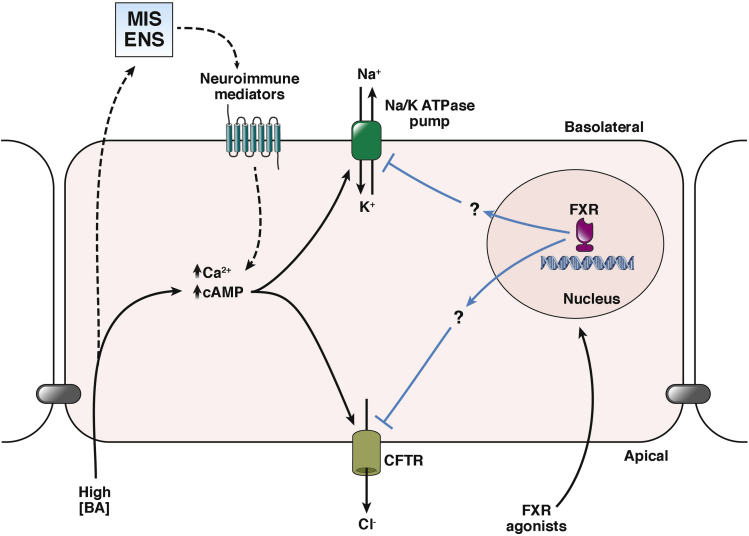
Bile acids exert their effects on colonic fluid transport through both direct and indirect actions on the epithelium. Direct effects have been investigated in cultured epithelial cell lines and primary isolated colonic crypts. When cultured monolayers of crypt epithelial cells are exposed to high concentrations of bile acids, rapid IP3-mediated increases in intracellular Ca2+ result. In turn, this leads to activation of the transport proteins that drive epithelial Cl- secretion, the primary osmotic driving force for intestinal fluid secretion.39, 40, 41 Furthermore, studies on isolated human colonic epithelial cells have shown that bile acids also inhibit Na+ absorption, the main osmotic driving force for colonic fluid absorption. This effect appears to be mediated, at least partly, by Ca2+-dependent inhibition of Na+/H+ and Cl-/HCO3- exchangers in the apical membrane, transport proteins that constitute the pathway for electroneutral Na+ absorption.42, 43
In addition to their direct actions on epithelial cells, bile acids also can regulate colonic fluid transport indirectly through recruitment of cells within the underlying lamina propria. For example, studies in animal models show that luminal bile acids can trigger local neural reflexes that result in activation of secretomotor neurons that synapse directly with the epithelium. Such effector nerves release transmitters, such as acetylcholine, into the neuroepithelial junction to promote Cl- secretion and enhance epithelial permeability.44, 45, 46 Recruitment of these reflex arcs is initiated by activation of sensory neuroendocrine cells interspersed among the transporting enterocytes. Upon activation by bile acids these cells release 5-hydroxytryptamine to stimulate afferent neurons.47 Bile acid activation of intrinsic neural pathways also contributes to the onset of diarrhea by enhancing colonic motility, thereby increasing the speed at which its contents are expelled from the body.48, 49, 50 Recent studies have shown that the G-protein–coupled bile acid receptor TGR5 is expressed on enteroendocrine cells and the ganglia of the myenteric and submucosal plexuses where it appears to mediate bile acid–induced peristalsis in the colon.47
Bile acids also have been shown to regulate epithelial transport function indirectly through recruitment of mucosal immune cells. In particular, mast cells degranulate in response to increased levels of bile acids, resulting in the release of histamine, which then acts directly at epithelial H1 receptors to promote secretion.51 Other mast cell mediators with well-established actions on epithelial transport, such as prostaglandins, leukotrienes, and adenosine, also are likely to be involved, although this has yet to be shown directly. It also should be noted that enteric nerves and mucosal mast cells are located in close proximity to each other within the mucosa, enabling bidirectional communication to occur between the 2 cell types in the regulation of epithelial function.52 This capacity for high concentrations of luminal bile acids to recruit neuroimmune pathways in the colonic mucosa serves to amplify their direct actions on epithelial cells, rapidly leading to induction of diarrhea.
Antidiarrheal Effects of Bile Acids
Although high levels of colonic bile acids have long been associated with induction of diarrhea, only recently have studies from our laboratory begun to address what their role may be in regulating epithelial transport under more normal circumstances. In these studies, we found that, in contrast to their acute prosecretory actions at high concentrations, more physiologically relevant levels of bile acids exert antisecretory actions on colonic epithelial cells. Thus, exposure of cultured monolayers of colonic epithelia to relatively low concentrations of either DCA or CDCA (50–200 μmol/L) led to a slow-onset (>6 h) decrease in their capacity to subsequently evoke secretory responses.53 We have proposed that such down-regulation of epithelial Cl- secretion by low levels of bile acids may serve a physiological role in dampening fluid secretion into the lumen, thereby promoting normal colonic absorptive function.
Subsequent studies have shown that the antisecretory actions of DCA and CDCA are mimicked by the FXR agonists GW4064 and OCA.54 Thus, activation of the FXR with either of these agonists led to a dose-dependent inhibition of subsequent Ca2+ and adenosine 3′,5′-cyclic monophosphate–dependent Cl- secretory responses, both in cell culture and in ex vivo colonic tissues from mice. Further studies have shown that the antisecretory actions of FXR activation are mediated by direct inhibition of key components of the Cl- secretory pathway. In particular, FXR activation inhibits the activity of the cystic fibrosis transmembrane conductance regulator Cl- channels, which are the primary exit pathway for Cl- across the apical membrane of the colonic epithelium. FXR activation also inhibits the activity of basolateral Na+/K+ adenosine triphosphatase pumps, but in this case reduced expression of either the catalytic α or regulatory β subunits of the pump does not appear to be involved. Such effects of FXR agonists on the activity and expression of the transport proteins that comprise the epithelial secretory pathway suggest that these agonists have the potential for development into a new class of directly acting drugs to treat secretory diarrhea. This idea is supported by our findings that administration of GW4064 was effective in preventing luminal fluid accumulation and severity of diarrhea in 2 different models of secretory diarrhea in mice.54 A schematic representation summarizing how increased colonic delivery of bile acids causes BAD and how this can be prevented by FXR agonists is shown in Figure 2.
Figure 2.
The FXR in regulation of bile acid (BA)-induced colonic fluid secretion. Increased colonic delivery of dihydroxy BAs, such as DCA and CDCA, increases epithelial levels of the intracellular second messengers, Ca2+ and adenosine 3′,5′-cyclic monophosphate (cAMP), either by direct (solid black lines) or indirect actions, involving the enteric nervous system (ENS) and the mucosal immune system (MIS) (dashed black lines). These second messengers interact with specific transport proteins to promote transepithelial Cl- secretion, thereby creating an osmotic driving force for fluid secretion, ultimately leading to the onset of diarrhea. Treatment with FXR agonists (blue lines) dampens epithelial fluid secretion by down-regulating the activity and expression of basolateral Na+/K+ adenosine triphosphatase (ATPase) pumps and apical cystic fibrosis transmembrane conductance regulator (CFTR) channels, key transport proteins of the Cl- secretory pathway.
FXR Agonists in the Treatment of BAD
As described earlier, the pathogenic basis of BAD has been assumed to result from a dysregulated EHC, with increased delivery of bile acids to the colon resulting from malabsorption in the ileum.1 This does appear to be the predominant defect occurring in Crohn’s disease or after ileal resection. However, excess bile acid synthesis, resulting from impaired feedback inhibition by FGF19, is an additional pathway that also may be involved in the diarrhea associated with Crohn’s disease,55 and which is likely the predominant pathophysiology underlying primary BAD.20 Increased colonic delivery of bile acids, leading to increased fluid secretion into the lumen, is considered to be a protective mechanism by which toxic secondary bile acids, such as DCA or LCA, are flushed from the colon before they can cause damage to the mucosa. This hypothesis is supported by the observation that, despite the severe toxicity of bile acids, primary BAD is not associated with dramatic changes in mucosal histology.56, 57 However, as discussed earlier, in patients with an underlying pathology that causes prolonged increases in colonic bile acids, the associated symptoms of chronic diarrhea, urgency, and incontinence can impact quality of life severely.8 Given that currently available therapies only address symptoms and often are tolerated poorly, new approaches to the treatment of BAD are required urgently. Based on their ability to prevent hepatic overproduction of bile acids, together with their antisecretory actions in the colon, FXR agonists have excellent therapeutic potential in this regard.
Currently, there are several FXR agonists at differing stages of therapeutic development for various hepatic, intestinal, and metabolic disorders (Table 1). In 2016, OCA became the first member of this class of drugs to receive Food and Drug Administration approval for clinical use in the treatment of primary biliary cholangitis. However, results from a recent phase II clinical trial of OCA in patients with BAD support the idea that FXR agonists also have potential for the treatment of this disease. In a population of patients with primary BAD, daily oral administration of OCA (25 mg/kg) significantly increased serum levels of FGF19, decreased bile acid synthesis (as measured by serum C4 levels), and improved stool form and symptoms of diarrhea.24 OCA also was effective in some patients with secondary bile acid diarrhea caused by Crohn’s disease, but only in patients with relatively short ileal resections (<45 cm). Importantly, symptomatic improvement was seen in 1 week and OCA was well tolerated, with only minor adverse effects including a predicted change in lipids, mild headache in 11% of patients, and no reports of pruritus.
Table 1.
Natural and Synthetic FXR Agonists
| FXR agonists | Source | References | |
|---|---|---|---|
| Natural FXR agonists | Bile acids (CDCA > CA > DCA >> LCA) | Hepatic synthesis | 13, 14, 15, 26 |
| Farnesol | Metabolic compounds | 62 | |
| Cafestol | Food component | 63 | |
| 64 | |||
| Semisynthetic bile acids | Obeticholic acid, Ocalivaa, b, c (6α-ethyl-CDCA, INT-747) | Intercept Pharmaceuticals, Inc/Sumitomo Danippon Pharma | 26, 65 |
| INT-767 | Intercept Pharmaceuticals | 66 | |
| Synthetic molecules | GW4064 | GlaxoSmithKline | 67 |
| Fexaramine | Metacrine | 61 | |
| LJN452b, c | Novartis AG | 68 | |
| PX20606, GS-9674b | Gilead Sciences | 69 | |
| Px-102 | Phenex Pharmaceuticals | 70 |
Licensed by the Food and Drug Administration for use in primary biliary cirrhosis.
Undergoing clinical trials for liver diseases including primary biliary cirrhosis and nonalcoholic fatty liver disease.
Undergoing clinical trials for BAD.
These proof-of-concept studies support a future role for FXR agonists in the treatment of BAD. Further trials using a double-blind, placebo-controlled design with larger numbers of patients clearly are required for a more definitive assessment of long-term efficacy, both in patients with primary and secondary BAD. Further studies into the molecular mechanisms underlying the effects of FXR agonists also are required in both clinical and preclinical models. For example, studies of the expression and activity of epithelial transporters in patients treated with FXR agonists are necessary to define the contribution of alterations in fluid and electrolyte transport to their therapeutic actions, while at the molecular level, the mechanisms by which FXR agonists regulate transport protein function remain to be defined. Also critical to our understanding of BAD pathogenesis and how we can better diagnose and treat the disease is developing our understanding of changes that occur in the colonic microbiota and how these affect the make-up of the colonic bile acid pool. For example, changes in microbial populations that alter the expression of bile salt hydrolases will influence the hydrophobicity of luminal bile acids, in turn, altering their capacity to permeate the epithelium and activate FXR.58 Conversely, activation of FXR can influence antibacterial defense mechanisms in the small intestine and colon, and drugs that act in this way would be expected to alter the make-up of microbiota significantly.59, 60 Although many of these complex studies can be addressed using animal models, it is important to bear in mind that significant interspecies differences exist, not only in how bile acids are synthesized and metabolized, but also in how they evoke intestinal responses. As research moves forward into the era of the microbiome and metabolome, there is a need for new models that will facilitate rapid translation of findings from in vitro and preclinical models into human beings.
It also is likely that differences will be apparent in how patients with BAD respond to different FXR agonists. It is possible that some of these drugs also may have effects at the cell surface bile acid receptor, TGR5, and bioavailability will affect their tissue distribution and site of action. For example, similar to the naturally occurring bile acids, OCA undergoes enterohepatic circulation, and therefore primarily will act in the ileum and liver, with unabsorbed drug potentially having effects in the colon. Non–bile acid agonists would not be accumulated into the EHC and therefore may have greater effects on the colon. For example, fexaramine, an FXR agonist that has not yet been studied in human beings, appears to have only intestinal actions and does not act systemically.61
In conclusion, FXR agonists are an exciting new class of drug that, in addition to the several therapeutic benefits already identified in liver diseases, have significant potential for development in treating patients with diarrheal diseases. Further studies, particularly clinical trials in patients diagnosed with BAD, are likely to help understand the pathogenesis of this disorder and how to better treat it, thereby benefitting a large neglected patient population.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by a Principal Investigator Award from Science Foundation Ireland and a Senior Researcher Award from the Crohn’s and Colitis Foundation of America (S.J.K.), and by research funding from the Bardhan Research and Education Trust and Intercept Pharmaceuticals (J.R.F.W.).
References
- 1.Hofmann A.F. The syndrome of ileal disease and the broken enterohepatic circulation: cholerheic enteropathy. Gastroenterology. 1967;52:752–757. [PubMed] [Google Scholar]
- 2.Hofmann A.F., Poley J.R. Cholestyramine treatment of diarrhea associated with ileal resection. N Engl J Med. 1969;281:397–402. doi: 10.1056/NEJM196908212810801. [DOI] [PubMed] [Google Scholar]
- 3.Thaysen E.H., Pedersen L. Idiopathic bile acid catharsis. Gut. 1976;17:965–970. doi: 10.1136/gut.17.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fromm H., Malavolti M. Bile acid-induced diarrhoea. Clin Gastroenterol. 1986;15:567–582. [PubMed] [Google Scholar]
- 5.Walters J.R. Defining primary bile acid diarrhea: making the diagnosis and recognizing the disorder. Expert Rev Gastroenterol Hepatol. 2010;4:561–567. doi: 10.1586/egh.10.54. [DOI] [PubMed] [Google Scholar]
- 6.Wedlake L., A'Hern R., Thomas K. Systematic review: the prevalence of idiopathic bile acid malabsorption (I-BAM) as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome (IBS) Aliment Pharmacol Ther. 2009;30:707–717. doi: 10.1111/j.1365-2036.2009.04081.x. [DOI] [PubMed] [Google Scholar]
- 7.Valentin N., Camilleri M., Altayar O. Biomarkers for bile acid diarrhoea in functional bowel disorder with diarrhoea: a systematic review and meta-analysis. Gut. 2015 doi: 10.1136/gutjnl-2015-309889. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Walters J.R.F., Pattni S.S. Managing bile acid diarrhoea. Ther Adv Gastroenterol. 2010;3:349–357. doi: 10.1177/1756283X10377126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kh Song, Li T., Owsley E. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology. 2009;49:297–305. doi: 10.1002/hep.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawson P.A., Lan T., Rao A. Bile acid transporters. J Lipid Res. 2009;50:2340–2357. doi: 10.1194/jlr.R900012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton J.P., Xie G., Raufman J.P. Human cecal bile acids: concentration and spectrum. Am J Physiol Gastrointest Liver Physiol. 2007;293:G256–G263. doi: 10.1152/ajpgi.00027.2007. [DOI] [PubMed] [Google Scholar]
- 12.Sayin S.I., Wahlstrom A., Felin J. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Makishima M., Okamoto A.Y., Repa J.J. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 14.Parks D.J., Blanchard S.G., Bledsoe R.K. Bile acids: natural ligands for an orphan receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 15.Wang H., Chen J., Hollister K. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 16.Inagaki T., Choi M., Moschetta A. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Jones S.A. Physiology of FGF15/19. Adv Exp Med Biol. 2012;728:171–182. doi: 10.1007/978-1-4614-0887-1_11. [DOI] [PubMed] [Google Scholar]
- 18.Sauter G.H., Moussavian A.C., Meyer G. Bowel habits and bile acid malabsorption in the months after cholecystectomy. Am J Gastroenterol. 2002;97:1732–1735. doi: 10.1111/j.1572-0241.2002.05779.x. [DOI] [PubMed] [Google Scholar]
- 19.Camilleri M., Busciglio I., Acosta A. Effect of increased bile acid synthesis or fecal excretion in irritable bowel syndrome-diarrhea. Am J Gastroenterol. 2014;109:1621–1630. doi: 10.1038/ajg.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walters J.R.F., Tasleem A.M., Omer O.S. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol. 2009;7:1189–1194. doi: 10.1016/j.cgh.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann A.F., Mangelsdorf D.J., Kliewer S.A. Chronic diarrhea due to excessive bile acid synthesis and not defective ileal transport: a new syndrome of defective fibroblast growth factor 19 release. Clin Gastroenterol Hepatol. 2009;7:1151–1154. doi: 10.1016/j.cgh.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnston I.M., Nolan J.D., Pattni S.S. Characterizing factors associated with differences in FGF19 blood levels and synthesis in patients with primary bile acid diarrhea. Am J Gastroenterol. 2016;111:423–432. doi: 10.1038/ajg.2015.424. [DOI] [PubMed] [Google Scholar]
- 23.Kok T., Hulzebos C.V., Wolters H. Enterohepatic circulation of bile salts in farnesoid X receptor-deficient mice: efficient intestinal bile salt absorption in the absence of ileal bile acid-binding protein. J Biol Chem. 2003;278:41930–41937. doi: 10.1074/jbc.M306309200. [DOI] [PubMed] [Google Scholar]
- 24.Walters J.R. Bile acid diarrhoea and FGF19: new views on diagnosis, pathogenesis and therapy. Nat Rev Gastroenterol Hepatol. 2014;11:426–434. doi: 10.1038/nrgastro.2014.32. [DOI] [PubMed] [Google Scholar]
- 25.Pai R., French D., Ma N. Antibody-mediated inhibition of fibroblast growth factor-19 results in increased bile acids synthesis and ileal malabsorption of bile acids in cynomolgus monkeys. Toxicol Sci. 2012;126:446–456. doi: 10.1093/toxsci/kfs011. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J.H., Nolan J.D., Kennie S.L. Potent stimulation of fibroblast growth factor 19 expression in the human ileum by bile acids. Am J Physiol Gastrointest Liver Physiol. 2013;304:G940–G948. doi: 10.1152/ajpgi.00398.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roda A., Pellicciari R., Gioiello A. Semisynthetic bile acid FXR and TGR5 agonists: physicochemical properties, pharmacokinetics, and metabolism in the rat. J Pharmacol Exp Ther. 2014;350:56–68. doi: 10.1124/jpet.114.214650. [DOI] [PubMed] [Google Scholar]
- 28.Pattni S.S., Brydon W.G., Dew T. Fibroblast growth factor 19 in patients with bile acid diarrhoea: a prospective comparison of FGF19 serum assay and SeHCAT retention. Aliment Pharmacol Ther. 2013;38:967–976. doi: 10.1111/apt.12466. [DOI] [PubMed] [Google Scholar]
- 29.Wong B.S., Camilleri M., Carlson P. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. 2012;10:1009–1015. doi: 10.1016/j.cgh.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Binder H.J., Rawlins C.L. Effect of conjugated dihydroxy bile salts on electrolyte transport in rat colon. J Clin Invest. 1973;52:1460–1466. doi: 10.1172/JCI107320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mekhjian H.S., Phillips S.F. Perfusion of the canine colon with unconjugated bile acids. Effect on water and electrolyte transport, morphology, and bile acid absorption. Gastroenterology. 1970;59:120–129. [PubMed] [Google Scholar]
- 32.Saunders D.R., Hedges J.R., Sillery J. Morphological and functional effects of bile salts on rat colon. Gastroenterology. 1975;68:1236–1245. [PubMed] [Google Scholar]
- 33.Gordon S.J., Kinsey M.D., Magen J.S. Structure of bile acids associated with secretion in the rat cecum. Gastroenterology. 1979;77:38–44. [PubMed] [Google Scholar]
- 34.Mekjian H.S., Phillips S.F., Hofmann A.F. Colonic secretion of water and electrolytes induced by bile acids: perfusion studies in man. J Clin Invest. 1971;50:1569–1577. doi: 10.1172/JCI106644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teem M.V., Phillips S.F. Perfusion of the hamster jejunum with conjugated and unconjugated bile acids: inhibition of water absorption and effects on morphology. Gastroenterology. 1972;62:261–267. [PubMed] [Google Scholar]
- 36.Keely S.J., Scharl M.M., Bertelsen L.S. Bile acid-induced secretion in polarized monolayers of T84 colonic epithelial cells: structure-activity relationships. Am J Physiol Gastrointest Liver Physiol. 2007;292:G290–G297. doi: 10.1152/ajpgi.00076.2006. [DOI] [PubMed] [Google Scholar]
- 37.Hofmann A.F., Loening-Baucke V., Lavine J.E. Altered bile acid metabolism in childhood functional constipation: inactivation of secretory bile acids by sulfation in a subset of patients. J Pediatr Gastroenterol Nutr. 2008;47:598–606. doi: 10.1097/MPG.0b013e31816920a6. [DOI] [PubMed] [Google Scholar]
- 38.Breuer N.F., Rampton D.S., Tammar A. Effect of colonic perfusion with sulfated and nonsulfated bile acids on mucosal structure and function in the rat. Gastroenterology. 1983;84:969–977. [PubMed] [Google Scholar]
- 39.Dharmsathaphorn K., Huott P.A., Vongkovit P. Cl- secretion induced by bile salts. A study of the mechanism of action based on a cultured colonic epithelial cell line. J Clin Invest. 1989;84:945–953. doi: 10.1172/JCI114257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moschetta A., Portincasa P., Debellis L. Basolateral Ca2+-dependent K+-channels play a key role in Cl- secretion induced by taurodeoxycholate from colon mucosa. Biol Cell. 2003;95:115–122. doi: 10.1016/s0248-4900(03)00011-x. [DOI] [PubMed] [Google Scholar]
- 41.Devor D.C., Sekar M.C., Frizzell R.A. Taurodeoxycholate activates potassium and chloride conductances via an IP3-mediated release of calcium from intracellular stores in a colonic cell line (T84) J Clin Invest. 1993;92:2173–2181. doi: 10.1172/JCI116819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alrefai W.A., Saksena S., Tyagi S. Taurodeoxycholate modulates apical Cl-/OH- exchange activity in Caco2 cells. Dig Dis Sci. 2007;52:1270–1278. doi: 10.1007/s10620-006-9090-8. [DOI] [PubMed] [Google Scholar]
- 43.Pallagi-Kunstar E., Farkas K., Maleth J. Bile acids inhibit Na(+)/H(+) exchanger and Cl(-)/HCO(3)(-) exchanger activities via cellular energy breakdown and Ca(2)(+) overload in human colonic crypts. Pflugers Arch. 2015;467:1277–1290. doi: 10.1007/s00424-014-1560-9. [DOI] [PubMed] [Google Scholar]
- 44.Camilleri M., Murphy R., Chadwick V.S. Pharmacological inhibition of chenodeoxycholate-induced fluid and mucus secretion and mucosal injury in the rabbit colon. Dig Dis Sci. 1982;27:865–869. doi: 10.1007/BF01316567. [DOI] [PubMed] [Google Scholar]
- 45.Guyton K.Z., Kensler T.W., Posner G.H. Vitamin D and vitamin D analogs as cancer chemopreventive agents. Nutr Rev. 2003;61:227–238. doi: 10.1301/nr.2003.jul.227-238. [DOI] [PubMed] [Google Scholar]
- 46.Sun Y., Fihn B.M., Sjovall H. Enteric neurones modulate the colonic permeability response to luminal bile acids in rat colon in vivo. Gut. 2004;53:362–367. doi: 10.1136/gut.2003.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alemi F., Poole D.P., Chiu J. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. 2013;144:145–154. doi: 10.1053/j.gastro.2012.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Falconer J.D., Smith A.N., Eastwood M.A. The effects of bile acids on colonic motility in the rabbit. Q J Exp Physiol Cogn Med Sci. 1980;65:135–144. doi: 10.1113/expphysiol.1980.sp002497. [DOI] [PubMed] [Google Scholar]
- 49.Kirwan W.O., Smith A.N., Mitchell W.D. Bile acids and colonic motility in the rabbit and the human. Gut. 1975;16:894–902. doi: 10.1136/gut.16.11.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong B.S., Camilleri M., McKinzie S. Effects of A3309, an ileal bile acid transporter inhibitor, on colonic transit and symptoms in females with functional constipation. Am J Gastroenterol. 2011;106:2154–2164. doi: 10.1038/ajg.2011.285. [DOI] [PubMed] [Google Scholar]
- 51.Gelbmann C.M., Schteingart C.D., Thompson S.M. Mast cells and histamine contribute to bile acid-stimulated secretion in the mouse colon. J Clin Invest. 1995;95:2831–2839. doi: 10.1172/JCI117988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dvorak A.M., McLeod R.S., Onderdonk A.B. Human gut mucosal mast cells: ultrastructural observations and anatomic variation in mast cell-nerve associations in vivo. Int Arch Allergy Immunol. 1992;98:158–168. doi: 10.1159/000236180. [DOI] [PubMed] [Google Scholar]
- 53.Keating N., Mroz M.S., Scharl M.M. Physiological concentrations of bile acids down-regulate agonist induced secretion in colonic epithelial cells. J Cell Mol Med. 2009;13:2293–2303. doi: 10.1111/j.1582-4934.2009.00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mroz M.S., Keating N., Ward J.B. Farnesoid X receptor agonists attenuate colonic epithelial secretory function and prevent experimental diarrhoea in vivo. Gut. 2014;63:808–817. doi: 10.1136/gutjnl-2013-305088. [DOI] [PubMed] [Google Scholar]
- 55.Nolan J.D., Johnston I.M., Pattni S.S. Diarrhea in Crohn's disease: investigating the role of the ileal hormone fibroblast growth factor 19. J Crohns Colitis. 2015;9:125–131. doi: 10.1093/ecco-jcc/jju022. [DOI] [PubMed] [Google Scholar]
- 56.Sciarretta G., Furno A., Morrone B. Absence of histopathological changes of ileum and colon in functional chronic diarrhea associated with bile acid malabsorption, assessed by SeHCAT test: a prospective study. Am J Gastroenterol. 1994;89:1058–1061. [PubMed] [Google Scholar]
- 57.Orekoya O., McLaughlin J., Leitao E. Quantifying bile acid malabsorption helps predict response and tailor sequestrant therapy. Clin Med (Lond) 2015;15:371. doi: 10.7861/clinmedicine.15-4-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joyce S.A., Gahan C.G. The gut microbiota and the metabolic health of the host. Curr Opin Gastroenterol. 2014;30:120–127. doi: 10.1097/MOG.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 59.Inagaki T., Moschetta A., Lee Y.K. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A. 2006;103:3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gadaleta R.M., van Erpecum K.J., Oldenburg B. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60:463–472. doi: 10.1136/gut.2010.212159. [DOI] [PubMed] [Google Scholar]
- 61.Fang S., Suh J.M., Reilly S.M. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21:159–165. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forman B.M., Goode E., Chen J. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 63.Ricketts M.L., Boekschoten M.V., Kreeft A.J. The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors. Mol Endocrinol. 2007;21:1603–1616. doi: 10.1210/me.2007-0133. [DOI] [PubMed] [Google Scholar]
- 64.Jameie-Oskooei S. Cafestol but not resveratrol is a partial agonist of farnesoid X receptor and stimulates FGF19 in human ileal explants. J Hepatol. 2016 [Google Scholar]
- 65.Pellicciari R., Fiorucci S., Camaioni E. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002;45:3569–3572. doi: 10.1021/jm025529g. [DOI] [PubMed] [Google Scholar]
- 66.Rizzo G., Passeri D., De Franco F. Functional characterization of the semisynthetic bile acid derivative INT-767, a dual farnesoid X receptor and TGR5 agonist. Mol Pharmacol. 2010;78:617–630. doi: 10.1124/mol.110.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Willson T.M., Jones S.A., Moore J.T. Chemical genomics: functional analysis of orphan nuclear receptors in the regulation of bile acid metabolism. Med Res Rev. 2001;21:513–522. doi: 10.1002/med.1023. [DOI] [PubMed] [Google Scholar]
- 68.Hegade V.S., Speight R.A., Etherington R.E. Novel bile acid therapeutics for the treatment of chronic liver diseases. Ther Adv Gastroenterol. 2016;9:376–391. doi: 10.1177/1756283X16630712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reiberger T. The non-steroidal FXR agonists PX20606 and GS-9674 improve liver fibrosis and portal hypertension in rodent models of cholestatic, metabolic and toxic liver cirrhosis. Z Gastroenterol. 2016;54:V02. [Google Scholar]
- 70.Gege C., Kinzel O., Steeneck C. Knocking on FXR's door: the “hammerhead”-structure series of FXR agonists - amphiphilic isoxazoles with potent in vitro and in vivo activities. Curr Top Med Chem. 2014;14:2143–2158. doi: 10.2174/1568026614666141112094430. [DOI] [PubMed] [Google Scholar]