Abstract
Macrocyclic compounds are central to discovery of new drugs but their preparation is often challenging because of the energy barrier required for bringing together and fusing the two ends of an acyclic precursor1. Ring-closing metathesis (RCM) 2,3,4 is a catalytic process that has allowed access to countless biologically active macrocyclic organic molecules even on large scale (up to 200 kilograms)5. The potency of a macrocyclic compound can depend on the stereochemistry of its alkene, or one isomer might be needed for subsequent stereoselective modification (e.g., dihydroxylation6). Still, while kinetically controlled Z-selective RCM reactions have been reported7,8,9,10, the only available olefin metathesis approach for accessing macrocyclic E olefins entails selective removal of the Z component of a stereoisomeric mixture by ethenolysis10, sacrificing substantial quantities of material if E/Z ratios are near unity. Use of ethylene can also cause adventitious olefin isomerization, a particularly serious problem when the E alkene is energetically less favored. Here, we show that dienes containing an E-alkenyl–B(pinacolato) group, widely used in catalytic cross-coupling11, possess the requisite electronic and steric attributes to allow them to be converted stereoselectively to E macrocyclic alkenes. Reactions are promoted by a molybdenum monoaryloxide pyrrolide complex and afford products in up to 73 percent yield and >98:2 E:Z ratio. Utility is highlighted by application to preparation of the twelve-membered ring antibiotic recifeiolide12,13 and the eighteen-membered ring Janus kinase 2/Fms-like tyrosine kinase-3 (JAK2/FLT3) inhibitor pacritinib14,15 the Z isomer of which has lower potency than the E16. The eighteen-membered ring moiety of pacritinib, a potent in vivo anti-cancer agent in advanced clinical trials for treatment of lymphoma and myelofibrosis, was prepared by an RCM carried out at 20 times higher concentration than when a ruthenium carbene was employed (0.02 vs. 0.001 M; 73% yield, 92% E).
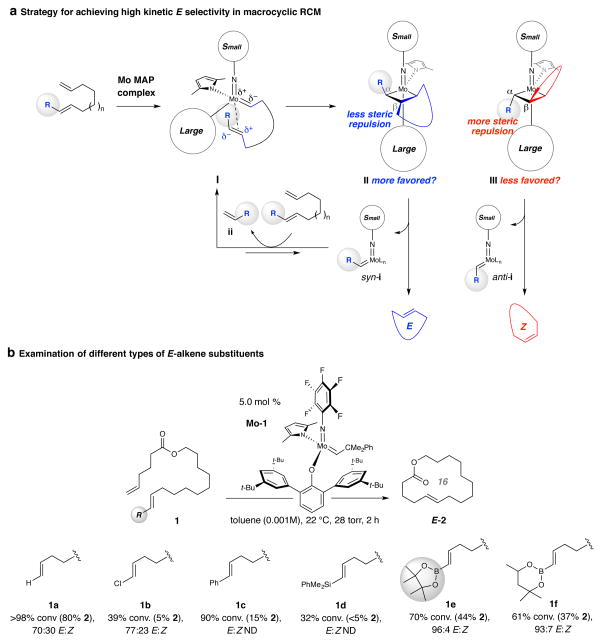
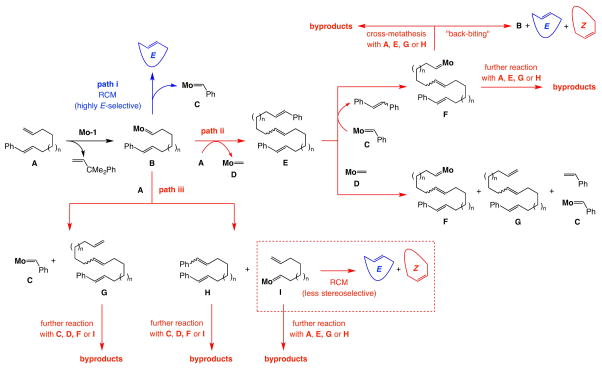
We recently showed that kinetically E-selective cross-metathesis (CM) may be effected between α-olefins and E-dihaloethene compounds with Mo monoaryloxide pyrrolide (MAP) complexes17. However, kinetically E-selective ring-closing metathesis (RCM) poses several distinct challenges. Larger amounts of an alkene reactant cannot be used to maximize efficiency. Moreover, linear products generated by CM or RCM of terminal alkenes are more hindered and cannot as easily re-associate with an active complex to cause loss of kinetic selectivity; in an RCM reaction where one of the substrate olefins is 1,2-disubstituted this distinction no longer applies. In considering a strategy for preparation of biologically active molecules such as recifeiolide13 and pacritinib14, we envisioned that a diene precursor could contain an E-1,2-disubstituted olefin and a suitable substituent (R; Fig. 1a) that can induce facile and selective generation of metallacyclobutane II (via I), leading to an E-macrocyclic alkene. As with the E-selective CM of alkenyl halides, II should be preferred (vs. III); the steric pressure induced by the metallacycle’s Cβ-substituent and the larger aryloxide (vs. imido) ligand in II should be less costly than that involving the group at the more proximal Cα in III.
Figure 1. The strategy and the optimal substituent.
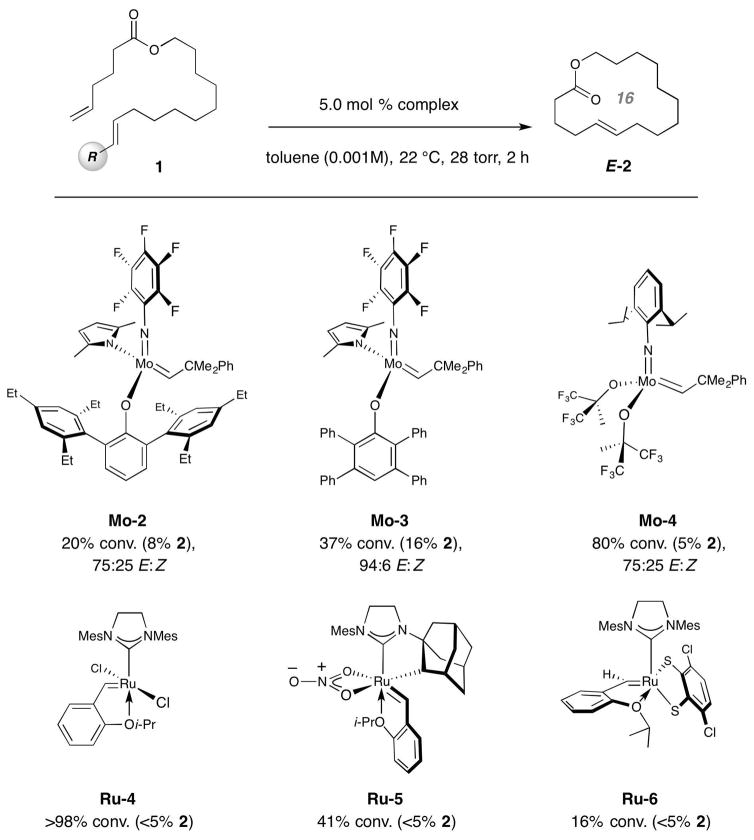
a, One way to achieve high kinetic E selectivity in macrocyclic RCM would be to utilize a substrate where one of the reacting alkene sites is E-1,2-disubstituted, such that preferential transformation through mode of association I and via metallacyclobutane II (vs. III) affords the desired stereochemical preference. The starting acyclic E-alkene must meet several key criteria: it must be readily accessible as the pure E isomer, sufficiently electron withdrawing to facilitate metallacyclobutane formation (cf. I→II), resistant to post-metathesis isomerization, not too large to diminish reaction rates and generate an alkylidene intermediate (syn-i) that is not detrimental to the reaction outcome. b, A model RCM process performed with 5.0 mol % Mo-1 and affording sixteen-membered ring lactone 2 indicated that the (pin)B-substituted E-alkene (cf. 1e) represents the most effective option. In most cases, the major side product is derived from homocoupling of the two terminal alkenes. Abbreviations: R, functional group; Ln, ligands; ND, not determined.
Reactions were performed under N2 atm. Conversion values and E:Z ratios were determined by analysis of 1H NMR spectra of unpurified product mixtures (±2%); stereoselectivity for reaction with 1c could not be determined due to formation of a comparatively complex mixture. See the Supplementary Information for all experimental and analytical details.
A suitable starting E alkene must satisfy the following six requirements: 1) It must be accessible in stereoisomerically pure form (i.e., ≥ 98% E) through reliable, efficient and inexpensive transformations. 2) It must possess the appropriate steric and/or electronic attributes so that it is immune to stereoisomerization. 3) And yet, the substituent (R) must not be so large so that the 1,2-disubstituted olefin is reluctant to undergo RCM at a reasonable rate (i.e., I → II, Fig. 1a). 4) It must bear a substituent that can stabilize the accumulated electron density at the adjacent Mo–C bond (cf. I, Fig. 1a). 5) The corresponding Mo alkylidene generated after metallacyclobutane cycloreversion (cf. syn-i, Fig. 1a) must be sufficiently long living and robust to promote catalyst turnover but not too reactive to engender post-metathesis isomerization and/or facile decomposition. 6) The aforementioned stabilization of alkylidene complex syn-i caused by the substituent (R) must be balanced in order for the RCM to occur at a reasonable rate.
We first investigated several model substrates as precursors to sixteen-membered unsaturated ring lactone 2; representative data involving the use of MAP complex Mo-1 for reactions carried out at ambient temperature and under 28 torr of pressure are shown in Fig. 1b. Bis(α-olefin) 1a was consumed completely after two hours and 80% of the product mixture was macrocycle 2, which was generated with inferior selectivity (70:30 E:Z). The RCM of chloro-substituted alkene 1b was evaluated next in part because we had previously found chloro-olefins to be resistant to isomerization17; the issue was whether the electronegative halogen atom would provide the necessary charge stabilization (cf. II, Fig. 1a). Attempts at cyclization of 1b resulted in only 5% 2 (77:23 E:Z); this might be due to low stability of the chloro-substituted Mo alkylidene, which can be more detrimental in a macrocyclic RCM than a concentrated solution of a CM where its encounter with an α-olefin prior to decomposition is more likely. We then explored the possibility of using an E-β-substituted styrene, another structural motif successfully adopted in CM leading to E-chloro-substituted olefins17. Here too the results were disappointing: attempted RCM with 1c gave a mixture of compounds (90% conv.) within which we could detect ~15% 2. Excessive side products (see Extended Data 1 for details) preempted accurate determination of stereoselectivity; among several possibilities, the derived Mo benzylidene might react with the substrate to generate stilbene (detected in the unpurified mixture) along with bis(α-olefin) 1a. Again, in CM excess dichloroethene may react with a benzylidene to yield β-chlorostyrene and chloro-substituted alkylidene, which can re-enter a productive catalytic cycle; this advantage does not pertain to RCM. Ring formation with the sizeable dimethylphenyl silyl group (cf. 1d), capable of stabilizing the electronic distribution in II by hyperconjugative delocalization of electron density into the low lying Si–C σ* orbital, was similarly inefficient.
With the idea that further attenuation of Mo alkylidene reactivity might result in appropriate activity and chemoselectivity (reaction at the α-olefin vs. the E-disubstituted alkene), we turned to alkenylboronate compounds. Former investigations indicate that pinacolatoboryl [B(pin)] MAP alkylidenes are less reactive compared to their carbon-substituted variants but can deliver higher activity than those with a trimethylsilyl group18. Further, CM between vinyl–B(pin) and terminal alkenes, including those with relatively hindered moieties, are efficient19,20. In the event, RCM with alkenyl–B(pin) compound 1e proceeded to 70% conversion of which 44% was macrocycle 2 formed in 96:4 E:Z selectivity (Fig. 1b). At ambient pressure (vs. 28 torr), there was 47% conversion (35% 2) and stereoselectivity was diminished (77:23 E:Z) probably because of larger amounts of methylidene complex (by reaction with ethylene generated from homocoupling), which is adept at promoting E-to-Z isomerization and especially prone to decomposition21,22. Catalytic RCM with (hexylene glycolato)B-substituted alkene 1f was E-selective as well (93:7 E:Z) but efficiency was lower (37% conv. to 2); this boronate group is less robust and not as practical to use.
Follow-up studies revealed that the alkylidenes derived from Mo-1 do offer the desired balance between catalyst stability (efficiency) and high E selectivity (see Extended Data 2). RCM with a MAP complex containing substituents at the ortho positions of its aryloxide phenyl moieties was much less efficient and E-selective; we attribute this to the steric repulsion within the corresponding metallacycle II. On the other hand, RCM with a species wherein the aforementioned aryl units are smaller was stereoselective but inefficient presumably because of rapid catalyst decomposition. Commonly used chiral Mo22 and Ru complexes7 did not generate appreciable amounts of the macrocyclic alkene (homocoupling was dominant), as was the case with the recently developed stereogenic-at-Ru systems10,23. Accordingly, RCM with substrates containing an α-olefin and an E-alkenyl–B(pin) unit were explored further.
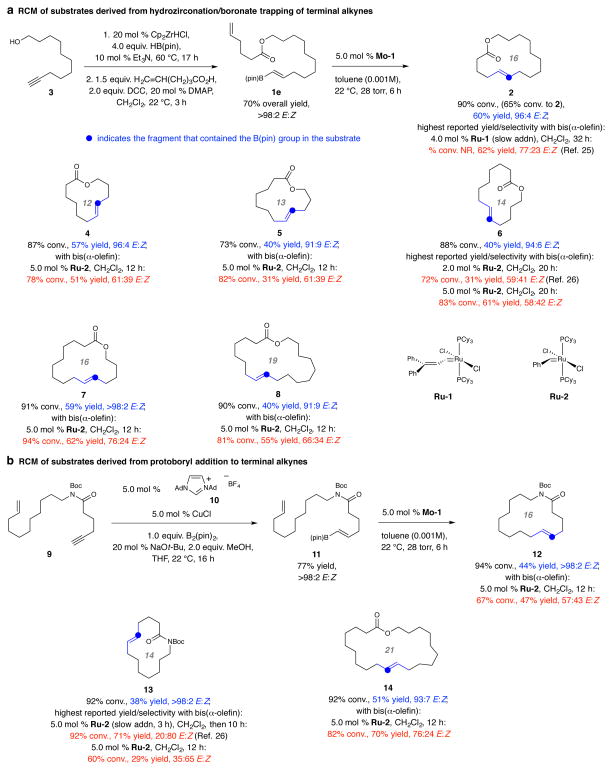
The E-alkenyl–B(pin) moiety is stable to air and moisture and can be accessed by several exceptionally E-selective and broadly applicable protocols catalyzed by readily available and inexpensive complexes and/or reagents (see additional references 31–44). For example, subjection of alkyne 3 (Fig. 2a), bearing an unprotected hydroxy group, to 20 mol % Cp2ZrHCl, 4.0 equivalents of pinacolatoborane and 10 mol % Et3N (60 °C, 17 h; all are commercially available)24 followed by routine ester formation furnished diene 1e in 70% overall yield as a single isomer (>98% E). Treatment of 1e with 5.0 mol % Mo-1 for six hours (vs. 2 h in Fig. 1b) at ambient temperature and 28 torr furnished lactone 2 in 60% yield and 96:4 E:Z selectivity. In comparison (Fig. 1b), the most E-selective RCM reported for bis(α-olefin) 1a was performed with 4.0 mol % Ru-4 and generated 2 in 77:23 E:Z ratio25.
Figure 2. Kinetically E-selective macrocyclic RCM.
Dienes accessed with high E-selectivity by catalytic hydroboration of a terminal alkyne, can be converted to 12- to 21-membered ring macrocyclic alkenes with high E:Z ratios. Transformations carried out with complexes Ru-1 or Ru-2 typically proceed with minimal selectivity or afford the Z isomer preferentially (cf. 13), reactions with Mo-1 are substantially more E-selective (91:9 to >98:2 E:Z). The difference between percent conversion and yield values (of isolated and purified products) is largely due to competitive homocoupling through the α-olefin terminus. Abbreviations: NR, not reported; pin, pinacolato; Boc, tert-butoxycarbonyl; Ad, adamantyl; Cy, cyclohexyl; Cp, cyclopentadienyl; DCC, N,N′-dicyclohexylcarbodiimide; DMAP, 4-dimethylaminopyridine.
Reactions were performed under N2 atm. Conversion (disappearance of the starting diene) and E:Z ratios were determined by analysis of 1H NMR spectra of unpurified product mixtures (±2%). Yields are for isolated and purified products and correspond to the RCM step (±2%). In cases where the transformations have been previously reported, a specific citation is provided. See the Supplementary Information for all experimental and analytical details.
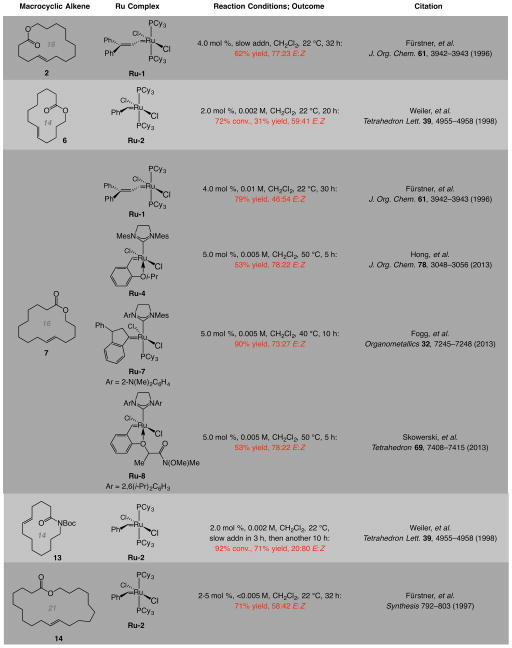
Twelve- to nineteen-membered unsaturated macrocyclic lactones 4–5 and 7–8 are additional examples of the sequence that begins with E-selective zirconocene-catalyzed hydroboration24 of a hydroxy-containing terminal alkyne (Fig. 2a; for synthesis of the precursor to 6 by catalytic protoboryl addition, see below). These macrocyclic compounds were obtained with substantially higher E selectivity (91:9 to >98:2 E:Z) compared to when the related bis(α-olefin) compounds were used along with Ru-1 or Ru-2 (56:44–75:25 E:Z26). Although the latter set of reactions may at times be higher yielding, much of the undesired Z isomer is formed whereas the present protocol delivers products that are substantially more enriched in the E alkene. In our hands, these olefin isomers cannot be separated easily. Use of the more active achiral NHC–Ru-carbene complexes gave typically lower yields and/or E selectivities probably due to post-RCM events (see Extended Data 3 for list of previously reported cases).
The moderate yields in Fig. 3 may be attributed to a lack of a major degree of conformational constraint. Homocoupling reactions thus become more competitive, as manifested by the difference between the values for percent conversion (consumption of the starting material) and the amount of the macrocycle formed. Additionally, although with a diene possessing an α- and a 1,2-disubstituted alkene the possibility of self-metathesis and oligomerization is reduced, RCM may be less facile because of steric hindrance at one of the alkene sites [vs. a bis(α-olefin)]. Also, a homocoupled adduct now contains three disubstituted alkenes [vs. two terminal olefins and a 1,2-disubstituted alkene in a homocoupling product of a bis(α-olefin) starting material] and thus cannot as easily re-enter the catalytic cycle (through “back-biting”)27. The higher yield with which 1a and other bis(α-alkene) substrates are at times converted to 2 and other macrocycles reflects these differences (cf. Fig. 2a and below).
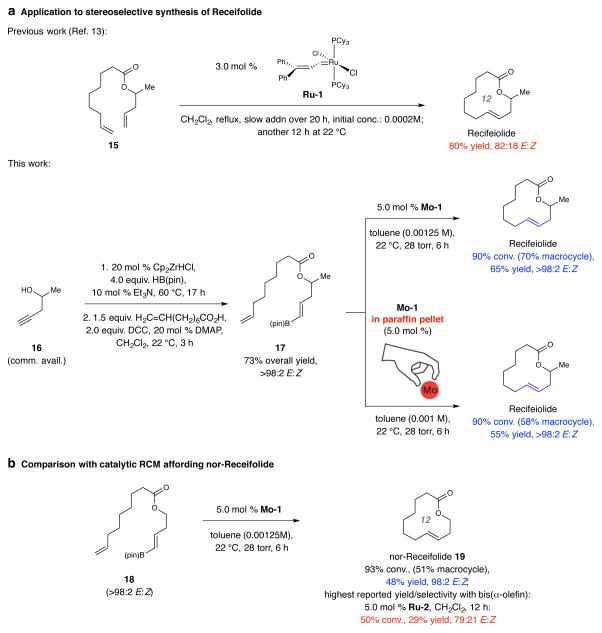
Figure 3. Application to stereoselective synthesis of recifeiolide.
a, Application of the catalytic RCM strategy to the synthesis of antibiotic agent recifeiolide helps underscore the utility of the Mo MAP-catalyzed process. It was previously illustrated that macrocyclic RCM with slow addition of diene 15 to a refluxing dichloromethane solution of carbene complex Ru-1 affords recifeiolide in 82:18 E:Z selectivity after a total of 32 hours of reaction time. The present approach delivers the target molecule with complete E selectivity (>98:2) after only 6 hours at ambient temperature and without the need for slow addition. Further simplifying the E-selective protocol is the possibility of using an air and moisture stable paraffin tablet that contains Mo-1 complex to obtain the desired macrocycle with similar efficiency and stereoselectivity. b, Efficiency of macrocyclic RCM is lower without a methyl substituent, underlining the importance of structural pre-organization to the facility of ring formation. Abbreviations: R, functional group; Cy, cyclohexyl; Cp, cyclopentadienyl; pin, pinacolato; DCC, N,N′-dicyclohexylcarbodiimide; DMAP, 4-dimethylaminopyridine.
Reactions were performed under N2 atm. Conversion values (disappearance of the starting diene) and E:Z ratios were determined by analysis of 1H NMR spectra of unpurified product mixtures (±2%). Yields are for isolated and purified products (±2%). See the Supplementary Information for all experimental and analytical details.
E-Alkenyl–B(pin) fragments may alternatively be synthesized by E-selective protoboryl additions to terminal alkynes catalyzed by N-heterocyclic carbene (NHC) copper catalysts28. Reaction of enyne 9 with B2(pin)2 (Fig. 3b), methanol and the NHC–Cu complex derived from imidazolium salt 10 and CuCl (all are commercially available) afforded 11 in 77% yield, >98% E:Z selectivity and with >98% chemoselectivity (i.e., <2% reaction at α-olefin). The ensuing RCM gave Boc-protected (Boc, tert-butoxycarbonyl) macrolactam 12 in 44% yield as the pure E isomer (<2% Z). Likewise, the catalytic protoboryl addition/RCM route led to the formation of 14-membered macrolactam 13 and 21-membered ring lactone 14 in 38% and 51% yield and >98:2 and 93:7 Z:E selectivity, respectively (Fig. 2b). Lactam 12 was obtained in comparable yield (47% vs. 44% yield) by RCM of the bis(α-olefin) with Ru-2 but as a near equal E and Z isomeric mixture. Again, E:Z ratio was substantially higher with an E-alkenyl–B(pin) substrate (93:7 to >98:2 vs. 20:80–76:24 E:Z). The reaction that affords 14-membered ring lactam 13 (Fig. 2b) is particularly interesting, as with the more commonly used RCM strategy slow addition of the Ru complex is needed and the Z isomer was formed preferentially due to substrate-control (20:80 E:Z); with the present approach only the E macrocyclic alkene was formed (>98% E). Cyclization of the unmasked secondary amide compounds (cf. 12–13) was inefficient (<5%); these transformations are reported to take place more readily with Ru-based carbenes but again with minimal stereocontrol10,26.
There are methods for synthesizing macrocyclic E-alkenes that involve catalytic alkyne metathesis. A two-step procedure entails stereoselective silyl-hydride addition [(EtO)3SiH)] to a macrocyclic alkyne promoted by a cationic ruthenium complex {1.0 mol % [Cp*Ru(MeCN)3]PF6}, Cp* pentamethylcyclopentadienyl] followed by stereoretentive protodesilylation with excess (2.0 equiv.) silver fluoride and methanol29. A more direct approach30 is by E-selective hydrogenation with the aforementioned Ru-based species (5.0 mol %). The RCM of dienes introduced here has significant value because it is strategically distinct. Furthermore, other than the significantly higher cost of Cp*Ru(MeCN)3]PF6 (vs. Cp2ZrHCl or salt 10 and CuCl) alkyne hydrogenations are at times accompanied by over-reduction and/or olefin isomerization30, affording difficult-to-remove byproducts.
We then probed applicability to stereoselective recifeiolide and pacritinib synthesis. A key point was whether increased conformational rigidity of a substrate – however slight it might be – translates into higher yields. Synthesis of recifeiolide’s 12-membered lactone through RCM has been reported previously (Fig. 3a): simultaneous slow addition of a solution of 15 and another of 3.0 mol % Ru-1 to a third (refluxing) solution of dichloromethane over a 20 hour period followed by an additional 12 hours of reaction time (at 22 °C) afforded the natural product in 80% yield and 82:18 mixture of difficult-to-separate E and Z isomers13 (Fig. 3a). In contrast, subjection of commercially available homopropargyl alcohol 16 to the aforementioned zirconocene-catalyzed hydroboration conditions followed by its union with 7-octenoic acid (commercially available) gave diene 17 in 73% overall yield and >98% E selectivity (Fig. 3a). Macrocyclic RCM with Mo-1 after six hours (vs. 32 h needed previously) at ambient temperature and 28 torr of pressure (0.00125 M; ~50 mg scale) delivered recifeiolide in 65% yield and as a single stereoisomer (vs. 82:18 E:Z reported formerly) without the need for manipulation of multiple syringe pumps. What is more, with a paraffin pellet containing ~5.0 mol % of Mo-1 (Fig. 3a) diene 17 was transformed to the natural product in 55% yield and >98% E selectivity (0.001 M in toluene, 22 °C, 28 torr, 6 h). The absence of a methyl group results in lowering of RCM efficiency, as nor-receifolide (19) was isolated in 48% yield and 98:2 E:Z ratio when Mo-1 was used (Fig. 3b); a more significant diminution in yield was observed when the transformation was performed with Ru-2 (29% yield, 79:21 E:Z).
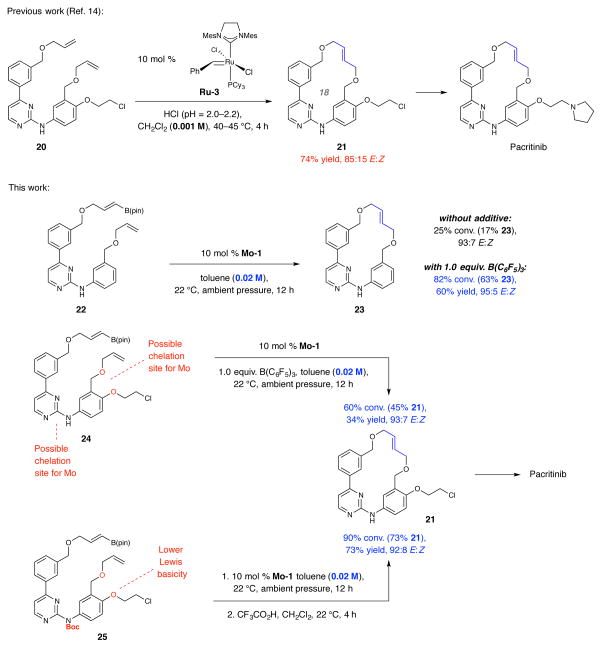
The case of pacritinib (SB1518) is of special interest due to its exceptional therapeutic activity14,15 and because the Lewis basic nitrogen atoms and the involvement of two relatively electron deficient allylic ethers render an RCM approach markedly challenging (Fig. 4). One reported case entails reaction of bis(α-olefin) 20 with 10 mol % Ru-3 at 40–45 °C for four hours, affording 21 in 74% yield and 85:15 E:Z selectivity (inseparable isomers)14. An acidic solution (pH = 2.0–2.2; added HCl) to generate the ammonium derivative of the pyrimidine moiety presumably to counter catalyst inhibition was needed for high efficiency.
Figure 4. Application to synthesis of pacritinib.
Treatment of bis(allyl ether) 20 with 10 mol% Ru-3 under acidic conditions (to counter catalyst deactivation) affords 21 in 85:15 E:Z selectivity. RCM with E-alkenyl–B(pin) derivative 22 with Mo-1 affords higher stereoselectively but less efficiently (17% yield). When the latter transformation was performed in the presence of tris(pentafluorophenyl)borane (to avoid catalyst deactivation), 23 was isolated in 60% yield and 95:5 E:Z selectivity. The same procedure with triether 24 was highly stereoselective but yield was lower (34%). Installation of a Boc unit (cf. 25) to diminish the Lewis basicity of the pyrimidine and the ether moieties furnished the macrocycle with similar E:Z selectivity and in 74% yield (after deprotection). A feature of the RCM with Mo-1 is that a relatively high concentration of the diene substrates (0.02 M vs. 0.001 M for 22, 24 and 25). Abbreviations: Mes, 2,4,6-(Me)3C6H2; pin, pinacolato; Cy, cyclohexyl; Boc, tert-butoxycarbonyl.
Reactions were performed under N2 atm. Conversion values and E:Z ratios were determined by analysis of 1H NMR spectra of unpurified product mixtures (±2%) relate to the disappearance of the starting diene. Yields are for isolated and purified products (±2%). See the Supplementary Information for all experimental and analytical details.
We initially examined the RCM of boryl-diene 22 (Fig. 4), prepared by zirconocene-catalyzed hydroboration of the appropriate propargyl ether. Subjection of 22 to 10 mol % Mo-1 at ambient temperature for 12 hours led to an inefficient reaction [25% conv. (17% 23)] but E selectivity was high (93:7 E:Z). When the RCM was performed with an equivalent of B(C6F5)3 23 could be isolated in 60% yield and 95:5 E:Z selectivity. A notable feature of the transformation with Mo-1 is that it could be carried out at much higher concentration than the previous reactions or when a Ru complex is utilized (i.e., 0.02 M vs. 0.001 M in the transformation involving Ru-3 and the MAP-catalyzed reactions in Fig. 1–3). Namely, 20 times less solvent was needed with a Mo complex, rendering the present approach more practical and cost effective. The reason for this distinction is that homocoupling of an allylic ether by a Mo alkylidene is slower than with a Ru carbene. This feature is manifested by the observation that, while with 2.0 mol % Mo-1 there was 83% homocoupling of 4-phenyl-1-butene within 10 minutes (0.1 M in benzene, 22 °C), <5% of the same byproduct was detected with allyl benzyl ether. In contrast, there was 47% homocoupling with allyl benzyl ether when 2.0 mol % Ru-3 was used. Such advantageous (and largely unappreciated) chemoselectivity is probably because the reactivity of a high oxidation-state Mo alkylidene derived from an allylic ether is diminished by the oxygen substituent (stabilization of electron density at the alkylidene carbon by inductive effect). As a result, the intermolecular reaction of such a species with another electron deficient allylic ether (i.e., homocoupling) is less facile and lower amounts of Mo methylidene are generated. A differently polarized Ru carbene does not offer the same advantage. These considerations imply that RCM involving the Mo alkylidene derived from an α-olefin of an allylic ether and a 1,2-disubstituted alkenyl–B(pin), which is even more electron deficient, is favored because of the intramolecularity of the transformation.
There are two reasons why RCM of the more functionalized triether substrate 24 to afford 21 is compelling (Fig. 4; see the Supplementary Information for synthesis route). 1) Macrocyclic olefin 21 has been previously converted to pacritinib14. 2) This particular cyclization would allow for further examination of the effect of Lewis basic chelating groups on catalyst activity. Compound 24 contains an additional di-allyl ether fragment that might coordinate to the Lewis acidic Mo center to cause reduced catalyst activity; this was a concern since the basicity of the new ether oxygen may be enhanced by the para amino group. Indeed, although E selectivity remained high (93:7 E:Z), conversion of 24 to 21 under the conditions used to prepare 23 was lower (34% vs. 60% yield). The Boc-protected variant (25) was therefore investigated based on the logic that this modification would more firmly diminish the Lewis basicity of the pyrimidine and the bis(ether) moiety. Subjection of 25 to the same reaction conditions [0.02 M (e.g., ~25 mg 24 in ~1.8 mL of toluene), ambient temperature] but without B(C6F5)3 followed by removal of the protecting unit furnished 21 in 73% overall yield and 92:8 E:Z selectivity.
Methods
General Procedure for E-Selective macrocyclic RCM
In a N2-filled glove box, an oven-dried round-bottom flask equipped with a magnetic stir bar is charged with an alkene substrate (1.0 equiv.) and anhydrous toluene. This mixture is then charged with a solution of complex Mo-1 in benzene (5.0 mol %) and the vessel is then connected to a 28 torr vacuum generated from a diaphragm pump. The mixture is allowed to stir for 4 h at 22 °C under vacuum, after which the reaction is quenched by the addition of wet ether (% conversion was determined by 1H NMR analysis of the unpurified mixture). Purification may be performed by silica gel chromatography.
General Procedure for E-Selective RCM with air- and moisture-resistant paraffin tablets
Under N2, an oven-dried round-bottom flask equipped with a magnetic stir bar is charged with an alkene substrate and anhydrous toluene. A paraffin tablet containing Mo-1 is added and the vessel is connected to a 28 torr vacuum generated from a diaphragm pump. The mixture is allowed to stir for 6 h at 22 °C under vacuum. At this time the reaction is quenched by addition of wet ether, and the mixture is concentrated under vacuum. Acetonitrile is added and the mixture is allowed to stir at 22 °C for 10 min. The slurry is filtered through a short plug of silica gel and eluted with acetonitrile (5.0 mL). The filtrate is concentrated in vacuo. Silica gel chromatography may be used to obtain pure product.
The E-alkenyl-B(pin) compounds can be accessed by a variety of reported methods 31–44. Some products can be accessed with low E selectivities through RCM reactions with bis(α-olefin) compounds catalyzed by Ru complexes45–47.
Extended Data
Extended Data 1. Byproducts from RCM with an E-β-substituted styrene.
Mass spectromery analysis of the crude mixture of the reaction of compound 1c confirmed the existence of A, E, G and H and desired ring-closing metathesis product. 1H NMR analysis of the mixture confirmed the existence of stilbene. A: HRMS [M+H]+: Calcd for C23H35O2: 343.26370; found: 343.26277; E: HRMS[M+H]+: Calcd for C44H65O4: 657.48828; found: 657.48808; G: HRMS[M+H]+: Calcd for C38H61O4: 581.45730; found: 581.45698; H: HRMS[M+H]+: Calcd for C29H39O2: 419.29500; found: 419.29383; RCM product: HRMS[M+H]+: Calcd for C15H27O2: 239.20110; found: 239.20019.
Extended Data 2. Performance of other catalyst types.
Examination of alternative Mo MAP complexes (cf. Mo-2-3) shows that the precise identity of the aryloxide ligand is crucial for achieving optimal efficiency and E selectivity. Furthermore, with two widely employed achiral complexes (i.e., Mo-4 and Ru-4), efficiency and E:Z selectivity are low. Two of the more recently introduced Z-selective Ru complexes (Ru-5,6) afford only homocoupling products. Abbreviation: Mes, 2,4,6-(Me)3C6H2; R, functional group; ND, not determined. Reactions were performed under N2 atm. Conversion values and E:Z ratios were determined by analysis of 1H NMR spectra of unpurified product mixtures (±2%). See the Supplementary Information for all experimental and analytical details.
Extended Data 3. Reported RCM reactions with bis(α-olefin) compounds and promoted by Ru complexes.
Supplementary Material
Acknowledgments
This research was supported by a grant from the National Institutes of Health (GM-59426). M. J. K. was supported as a LaMattina Graduate Fellow in Chemical Synthesis. We are grateful to Dr. Sebastian Torker for many helpful discussions and to Dr. Levente Ondi, Dr. Janos Balazs Czirok and Mr. Gergely Mate Nagy for their support and helpful advice on paraffin tablets, which were gifts from XiMo, AG.
Footnotes
Author Contributions. X. S., T. T. N. and M. J. K. developed the catalytic method and analyzed the results regarding various catalysts and substrates. A. W. H. S. first suggested the possibility of controlling RCM stereoselectivity with an appropriate electronically deficient alkene substituent. X. S. carried out the synthesis of recifeiolide and X. S. and D. X. performed the investigations in connection to synthesis of pacritinib. R. R. S. and A. H. H. developed the Mo MAP complexes used in these studies. A. H. H. directed the investigations and composed the manuscript with revisions provided by the other authors.
The authors declare competing financial interests: AHH and RRS are cofounders of a company that has licensed the technology reported in this manuscript.
Data Availability Statement. The authors declare that all the data supporting the findings of this study are available within the paper and its supplementary files.
References
- 1.Martí-Centelles V, Pandey MD, Burguete MI, Luis SV. Macrocyclization reactions: The importance of conformational, configurational, and template-induced preorganization. Chem Rev. 2015;115:8736–8834. doi: 10.1021/acs.chemrev.5b00056. [DOI] [PubMed] [Google Scholar]
- 2.Hanson PR, Maitram S, Chegondi R, Markley JL. In: Handbook of Metathesis. Grubbs RH, O’Leary DJ, editors. Vol. 2. Wiley–VCH; 2014. pp. 1–170. [Google Scholar]
- 3.Mallinson J, Collins I. Macrocycles in new drug discovery. Future Med Chem. 2012;4:1409–1438. doi: 10.4155/fmc.12.93. [DOI] [PubMed] [Google Scholar]
- 4.Gradillas A, Perez-Castells J. In: Metathesis in Natural Product Synthesis. Cossy J, Arseniyades S, Meyer C, editors. Wiley–VCH; 2010. pp. 149–182. [Google Scholar]
- 5.Higman CS, Lummiss JAM, Fogg DE. Olefin metathesis at the dawn of implementation in pharmaceutical and specialty-chemicals manufacturing. Angew Chem Int Ed. 2016;55:3552–3565. doi: 10.1002/anie.201506846. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Wu J, Luo J, Dai WM. A concise total synthesis of amphidinolide T2. Chem Eur J. 2010;16:11530–11534. doi: 10.1002/chem.201001794. [DOI] [PubMed] [Google Scholar]
- 7.Hoveyda AH. Evolution of catalytic stereoselective olefin metathesis: From ancillary transformation to purveyor of stereochemical utility. J Org Chem. 2014;79:4763–4792. doi: 10.1021/jo500467z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu M, Wang C, Kyle AF, Jakubec P, Dixon DJ, Schrock RR, Hoveyda AH. Synthesis of macrocyclic natural products by catalyst-controlled stereoselective ring-closing metathesis. Nature. 2011;479:88–93. doi: 10.1038/nature10563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C, Yu M, Kyle AF, Jakubec P, Dixon DJ, Schrock RR, Hoveyda AH. Efficient and selective formation of macrocyclic disubstituted Z alkenes by ring-closing metathesis (RCM) reactions catalyzed by Mo- or W-based monoaryloxide pyrrolide (MAP) complexes: Applications to total syntheses of epilachnene, yuzu lactone, ambrettolide, epothilone C, and nakadomarin A. Chem Eur J. 2013;19:2726–2740. doi: 10.1002/chem.201204045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marx VM, Herbert MB, Keitz BK, Grubbs RH. Stereoselective access to Z and E macrocycles by ruthenium-catalyzed Z-selective ring-closing metathesis and ethenolysis. J Am Chem Soc. 2013;135:94–97. doi: 10.1021/ja311241q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carboni B, Carreaux F. In: Boronic Acids. Hall DG, editor. Wiley–VCH; 2008. pp. 343–376. [Google Scholar]
- 12.Vesonder RF, Stodola FH, Wickerham LJ, Ellis JJ, Rohwedder WK. 11-Hydroxy-trans-8-dodenoic acid lactone, a 12-membered-ring compound from a fungus. Can J Chem. 1971;49:2029–2032. [Google Scholar]
- 13.Fürstner A, Langemann K. Macrocycles by ring-closing metathesis. Synthesis. 1997:792–803. [Google Scholar]
- 14.William AD, et al. Discovery of macrocycle 11-(2-pyrrolidin-1-yl-ethoxy-14,19-dioxa-5,7,26-triaza-tetracyclo[19.13.1.1(2,6). 1(8,12)]heptacosa-1 (25),2(26),3,5,8,10,12(27),16,21,23-decaene (SB1518), a potent janus kinase 2/Fms-like tyrosine kinase-3 (JAK2/FLT3) inhibitor for the treatment of myelofibrosis and lymphoma. J Med Chem. 2011;54:4638–4658. doi: 10.1021/jm200326p. [DOI] [PubMed] [Google Scholar]
- 15.Verstovsek S, Machida C, Dean JP, Myint H, Bolós J, Castañer R. Pacritinib. Drugs of the Future. 2013;38:375–386. [Google Scholar]
- 16.Poulsen A, William AD, Dymock BW. In: RSC Drug Discovery Series No. 40. Levin J, editor. Wiley–VCH; 2014. pp. 141–205. [Google Scholar]
- 17.Nguyen TT, Koh MJ, Shen X, Romiti F, Schrock RR, Hoveyda AH. Kinetically controlled E-selective catalytic olefin metathesis. Science. 2016;352:569–575. doi: 10.1126/science.aaf4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Townsend EM, Kilyanek SM, Schrock RR, Müller P, Smith SJ, Hoveyda AH. High oxidation state molybdenum imido heteroatom-substituted alkylidene complexes. Organometallics. 2013;32:4612–4617. doi: 10.1021/om400584f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kieswetter ET, O’Brien RV, Yu EC, Meek SJ, Schrock RR, Hoveyda AH. Synthesis of Z-(pinacolato)allylboron and Z-(pinacolato)alkenylboron compounds through selective catalytic cross metathesis. J Am Chem Soc. 2013;135:6026–6029. doi: 10.1021/ja403188t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Speed AWH, Mann TJ, O’Brien RV, Schrock RR, Hoveyda AH. Catalytic Z-selective cross-metathesis in complex molecule synthesis: A convergent stereoselective route to disorazole C1. J Am Chem Soc. 2014;136:16136–16139. doi: 10.1021/ja509973r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robbins J, Bazan GC, Murdzek JS, O’Regan MB, Schrock RR. Reduction of molybdenum imido–alkylidene complexes in the presence of olefins to give molybdenum(IV) complexes. Organometallics. 1991;10:2902–2907. [Google Scholar]
- 22.Schrock RR, Hoveyda AH. Molybdenum and tungsten imido alkylidene complexes as efficient olefin-metathesis catalysts. Angew Chem Int Ed. 2003;42:4592–4633. doi: 10.1002/anie.200300576. [DOI] [PubMed] [Google Scholar]
- 23.Koh MJ, Khan RKM, Torker S, Yu M, Mikus MS, Hoveyda AH. High-value alcohols and higher-oxidation-state compounds by catalytic Z-selective cross-metathesis. Nature. 2015;517:181–186. doi: 10.1038/nature14061. [DOI] [PubMed] [Google Scholar]
- 24.Wang YD, Kimball G, Prashad AS, Wang Y. Zr-Mediated hydroboration: Stereoselective synthesis of vinyl boronic acid. Tetrahedron Lett. 2005;46:8777–8780. [Google Scholar]
- 25.Fürstner A, Langemann K. Conformationally unbiased macrocyclization reactions by using ring closing metathesis. J Org Chem. 1996;61:3942–3943. doi: 10.1021/jo960733v. [DOI] [PubMed] [Google Scholar]
- 26.Goldring WPD, Hodder AS, Weiler L. Synthesis of macrocyclic lactams and lactones via ring-closing olefin metathesis. Tetrahedron Lett. 1998;39:4955–4958. [Google Scholar]
- 27.Conrad JC, Eelman MD, Duarte Silva JA, Monfette S, Parnas HH, Snelgrove JL, Fogg DE. Oligomers as intermediated in ring-closing metathesis. J Am Chem Soc. 2007;129:1024–1025. doi: 10.1021/ja067531t. [DOI] [PubMed] [Google Scholar]
- 28.Jang H, Zhugralin AR, Lee Y, Hoveyda AH. Highly selective methods for synthesis of internal (α-) vinylboronates through efficient NHC–Cu-catalyzed hydroboration of terminal alkynes. Utility in chemical synthesis and mechanistic basis for selectivity. J Am Chem Soc. 2011;133:7859–7871. doi: 10.1021/ja2007643. [DOI] [PubMed] [Google Scholar]
- 29.Radkowski K, Fürstner A. A chemo- and stereoselective reduction of cycloalkynes to (E)-cycloalkenes. Chem Commun. 2002:2182–2183. doi: 10.1039/b207169j. [DOI] [PubMed] [Google Scholar]
- 30.Radkowski K, Sundararaju B, Fürstner A. A functional-group-tolerant catalytic trans hydrogenation of alkynes. Angew Chem Int Ed. 2013;52:355–360. doi: 10.1002/anie.201205946. [DOI] [PubMed] [Google Scholar]
Additional References
- 31.Brown HC, Gupta SK. Hydroboration. XXXIX 1,3,2-Benzodioxaborole (catecholborane) as a new hydroboration reagent for alkenes and alkynes General synthesis of alkane- and alkeneboronic acids and esters via hydroboration Directive effects in the hydroboration of alkenes and alkynes with catecholborane. J Am Chem Soc. 1975;97:5249–5255. [Google Scholar]
- 32.Tucker CE, Davidson J, Knochel P. Mild and stereoselective hydroborations of functionalized alkynes and alkenes using pinacolborane. J Org Chem. 1992;57:3482–3485. [Google Scholar]
- 33.Takai K, Shinomiya N, Kaihara H, Yoshida N, Moriwake T, Utimoto K. Transformation of aldehydes into (E)-1-alkenylboronic esters with a geminal dichromium reagent derived from a dichloromethylboronic ester and CrCl2. Synlett. 1995;9:963–964. [Google Scholar]
- 34.Pereira S, Srebnik M. Hydroboration of alkynes with pinacolborane catalyzed by HZrCp2Cl. Organometallics. 1995;14:3127–3128. [Google Scholar]
- 35.Morrill C, Grubbs RH. Synthesis of functionalized vinyl boronates via ruthenium-catalyzed olefin cross-metathesis and subsequent conversion to vinyl halides. J Org Chem. 2003;68:6031–6034. doi: 10.1021/jo0345345. [DOI] [PubMed] [Google Scholar]
- 36.Takai K, Kunisada Y, Tachibana Y, Yamaji N, Nakatani E. Transformation of aldehydes into (E)-1-alkenylsilanes and (E)-1-alkenylboronic esters with a catalytic amount of a chromium salt. Bull Chem Soc Jpn. 2004;77:1581–1586. [Google Scholar]
- 37.Wang YD, Kimball G, Prashad AS, Wang Y. Zr-mediated hydroboration: stereoselective synthesis of vinyl boronic esters. Tetrahedron Lett. 2005;46:8777–8780. [Google Scholar]
- 38.Jang H, Zhugralin AR, Lee Y, Hoveyda AH. Highly selective methods for synthesis of internal (α-)vinylboronates through efficient NHC-Cu-catalyzed hydroboration of terminal alkynes. Utility in chemical synthesis and mechanistic basis for selectivity. J Am Chem Soc. 2011;133:7859–7871. doi: 10.1021/ja2007643. [DOI] [PubMed] [Google Scholar]
- 39.Takaya J, Kirai N, Iwasawa N. Efficient synthesis of diborylalkenes from alkenes and diboron by a new PSiP-pincer palladium-catalyzed dehydrogenative borylation. J Am Chem Soc. 2011;133:12980–12983. doi: 10.1021/ja205186k. [DOI] [PubMed] [Google Scholar]
- 40.Sun C, Potter B, Morken JP. A catalytic enantiotopic-group-selective Suzuki reaction for the construction of chiral organoboronates. J Am Chem Soc. 2014;136:6534–6537. doi: 10.1021/ja500029w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coombs JR, Zhang L, Morken JP. Synthesis of vinyl boronates from aldehydes by a practical boron–Wittig reaction. Org Lett. 2015;17:1708–1711. doi: 10.1021/acs.orglett.5b00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong S, Zhang W, Liu M, Yao ZJ, Deng W. Transition-metal-free hydroboration of terminal alkynes activated by base. Tetrahedron Lett. 2016;57:1–4. [Google Scholar]
- 43.Reid WB, Spillane JJ, Krause SB, Watson DA. Direct synthesis of alkenyl boronic esters from unfunctionalized alkenes: a boryl-Heck Reaction. J Am Chem Soc. 2016;138:5539–5542. doi: 10.1021/jacs.6b02914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ojha DP, Prabhu KR. Pd-catalyzed hydroborylation of alkynes: a ligand controlled regioselectivity switch for the synthesis of α- or β-vinylboronates. Org Lett. 2016;18:432–435. doi: 10.1021/acs.orglett.5b03416. [DOI] [PubMed] [Google Scholar]
- 45.Jee JE, Cheong JL, Lim J, Chen C, Hong SH, Lee SS. Highly selective macrocycle formations by metathesis catalysts fixated in nanopores. J Org Chem. 2013;78:3048–3056. doi: 10.1021/jo302823w. [DOI] [PubMed] [Google Scholar]
- 46.van Lierop BJ, Fogg DE. On the compatibility of ruthenium metathesis catalysts with secondary phosphines. Organometallics. 2013;32:7245–7248. [Google Scholar]
- 47.Skowerski K, Kasprzycki P, Bieniek M, Olszewski TK. Efficient, durable and reusable olefin metathesis catalysts with high affinity to silica gel. Tetrahedron. 2013;69:7408–7415. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.