Abstract
Purpose
bone lesions on prostate MRI often raise concern about metastases. This study aimed to evaluate the prevalence of bone metastases on staging prostate MRI and evaluate associations between their MRI features and clinical/pathologic characteristics.
Methods
Retrospective, IRB-approved study of 3765 patients undergoing prostate MRI for newly-diagnosed PCa between 2000-2014. The reference standard to calculate the prevalence of bone metastases was bone biopsy and/or ≥1-year follow-up after MRI. In a subsample of 228 patients, the MRI characteristics of bone lesions were recorded by two radiologists independently. Associations between MRI and clinical/pathologic findings, including National Comprehensive Cancer Network (NCCN) risk categories, were calculated.
Results
57/3765 patients (1.5%, 95%CI:1.2-2.0%) had bone metastases. No patient with NCCN low-risk PCa (Gleason <7, PSA<10 ng/mL, cT1-2a) had bone metastases. In the subsample, ≥1 bone lesion was present on MRI in 74% (95%CI:0.67-0.79) and 72% (95%CI:0.66-0.78) of patients (R1 and R2). Larger lesion diameter (OR:1.33/1.19; p<0.001 for both readers) and absence of intralesional fat (OR:0.07/0.11; p=0.004/0.002 for R1/R2) were significantly associated with bone metastases.
Conclusion
Bone lesions are common in prostate MRI, but only rarely represent metastases. MRI should be interpreted in the context of clinical features that influence the likelihood of metastatic disease.
Keywords: MRI, prostate cancer, bone metastases, staging, detection, imaging
Introduction
Prostate cancer (PCa) is the most commonly diagnosed solid organ malignancy among men in the United States, and the second leading male malignancy worldwide (1). The current management paradigm for patients with newly-diagnosed disease is based on a risk stratification strategy using a combination of clinical, biochemical and pathologic features to triage patients according to the likelihood of cancer-related morbidity and mortality (2). It is now widely accepted that many patients with “low-risk” PCa should be offered active surveillance, while patients with clinically significant disease benefit from definitive therapy (2, 3). However, it is also accepted that the accuracy of these risk-stratification tools is often suboptimal, and in an attempt to improve their performance, imaging is increasingly being incorporated into the workup of newly-diagnosed PCa patients (2, 4-6). The usefulness of prostate magnetic resonance imaging (MRI) has been extensively documented for localizing and staging PCa, evaluating tumor aggressiveness and guiding treatment approaches (7-13). Efforts for standardization of prostate MRI acquisition and reporting, including the Prostate Imaging Reporting and Data System (PI-RADS) have further contributed to its increasing use (14, 15). An unintended consequence of the incorporation of prostate MRI into standard clinical care has been the detection of “nonspecific” bone lesions of unknown clinical importance. Furthermore, unlike other imaging modalities such as bone scintigraphy, which are only indicated in high-risk patients, prostate MRI is increasingly performed in potentially low- and intermediate-risk scenarios such as determination of active surveillance eligibility and assessment for targeted prostate biopsies. In such cases, there is an even higher potential detrimental effect of patients being exposed to additional workup exams or intervention (e.g. bone biopsy) for ultimately benign incidental skeletal findings. On the other hand, as the skeleton is the most common site of distant spread in PCa, these “incidental” bone lesions often raise concern about the possibility of metastatic disease. The lack of evidence-based data on the etiology of these skeletal bone findings to guide MRI reporting and further clinical decision-making is challenging in daily practice. This study aimed to evaluate the prevalence of bone metastases on staging prostate MRI and evaluate associations between their MRI features and clinical/pathologic characteristics.
Materials and Methods
The Institutional Review Board approved this retrospective study and waived the informed consent requirement. The study was complaint with the Health Insurance Portability and Accountability Act.
Eligibility Criteria and Patient Characteristics
We identified consecutive PCa patients who underwent prostate MRI before treatment at our institution between January 2000-June 2014 (n=6771). Patients were excluded if they had recurrent prostate cancer, pre-existing cancer diagnosis in another organ, <1 year follow up, or incomplete data (n=3006), resulting in a final population of 3765 patients.
Nested within this cohort, we used a stratified sampling design to define a subset of patients. Stratifying on the year in which the prostate MRI was done, we selected three patients without bone metastases for every patient with bone metastases. Two independent readers evaluated the prostate MRI exams for MRI features in this subsample. This sampling design was used to facilitate the image review for the purpose of this study without the need for re-interpreting each of the ∼3700 MRI examinations in the entire cohort.
MRI acquisition
Images were acquired on 1.5-Tesla or 3-Tesla whole-body MRI systems (GE Healthcare). A body coil was used for excitation and a multi-channel phased-array coil (with or without endorectal coil) was used for signal reception. During the study period, the MRI parameters varied slightly as per the standard departmental protocol, but all scans included T1-weighted and T2-weighted sequences. The transverse T1-weighted sequence were used for analysis in this study (repetition time/echo time 400-750/10-14 ms, section thickness 5 mm, intersection gap 1mm, field of view 28-36 cm, matrix 256×192).
MRI interpretation
Images were reviewed on a picture archiving and communication system (Centricity GE) by two independent radiologists with 5 and 7 years of experience. Readers were blinded to the clinical features (Gleason grade, PSA, T stage, metastatic status). Readers evaluated the images for the presence of bone lesions on T1-weighted sequences and recorded the size, signal characteristics (hypo-, iso- or hyperintense compared to skeletal muscle) and presence of intralesional fat (punctate areas of hyperintense signal compared to skeletal muscle, similar to subcutaneous fat) of the three largest lesions (>5mm), along with their subjective level of suspicion for the likelihood of bone metastases on MRI using a 5-point scale (1=unlikely metastasis; 5=consistent with metastasis) (Figures 1-3).
Figure 1.
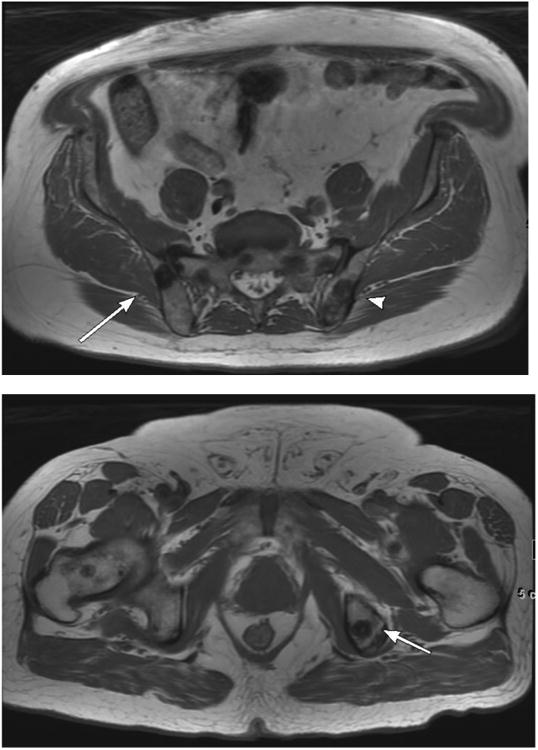
68-year-old male with EAU high-risk (Gleason Score 4+4, PSA 53 ng/mL) prostate carcinoma and osseous metastases. 1a. Large field-of-view axial T1-weighted image of the pelvis shows hypointense osseous metastases in the right (arrow) and left (arrowhead) iliac bones. 1b. Large field-of-view axial T1 weighted image of the pelvis shows a hypointense metastasis in the left ischium (arrow).
Figure 3.
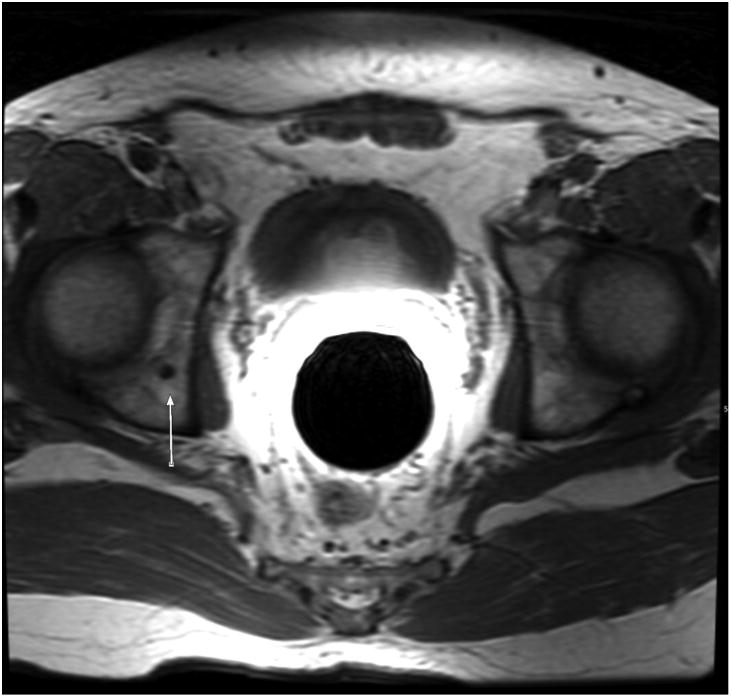
52-year-old male with EAU low-risk (Gleason Score 3+3, PSA 6 ng/mL) prostate carcinoma and no osseous metastases. Large field-of-view axial T1-weighted image of the pelvis shows a benign 0.4 cm hypointense lesion in the right acetabulum (arrow).
Reference standard
For patients who had bone biopsies, the histopathologic biopsy findings were used as the reference standard. As not all patients can be expected to have biopsies, the imaging findings in combination with at least 1-year follow-up were also used to define the presence of bone metastases. Patients were considered negative for bone metastases at the time of staging prostate MRI if there was a negative bone biopsy or if after at least 1 year of imaging/clinical follow-up from the time of the staging MRI there was no evidence of metastatic disease. Patients were considered positive for bone metastasis if metastatic disease was shown in a pelvic bone biopsy (n=18) or if at any point after the staging prostate MRI there was an increase in size of bone lesions and development of overt malignant features (development of soft-tissue component) in a clinical setting supportive of metastatic disease (e.g. rising PSA); or patients with imaging and clinical features consistent with bone metastasis who receive treatment for stage 4 disease (16).
Statistical analysis
Inter-reader agreement was assessed using Cohen's kappa statistic and percent agreement. To evaluate whether the presence of bone lesions differentiated patients with and without metastases, we estimated the non-parametric receiver operating characteristic curve and the corresponding area under the curve (AUC). Sensitivities and specificities were estimated by grouping patients with MRI suspicion scores of 1-3 as negative and patients with scores of 4-5 as positive.
The association of each MRI feature and the presence of bone metastases was evaluated using exact logistic regression for categorical variables and logistic regression for continuous variables, adjusted for year of MRI. Odds ratios and 95% Wald confidence limits (CI) were provided.
P-values<0.05 were considered statistically significant. All analyses were performed using SAS 9.4 (The SAS Institute, Cary, NC).
Results
Patient Characteristics
Of the 3765 patients with newly-diagnosed PCa undergoing staging prostate MRI, 57 (1.5%, 95% CI: 1.2-2.0) had bone metastases (18 biopsy-proven and 39 based on imaging features consistent with bone metastases and treatment for stage 4 disease). All patients with bone metastases had NCCN intermediate (10/57, 18%) or high-risk (47/57, 82%) PCa. None of the patients with NCCN low-risk PCa had bone metastases.
The 228 patients included in the subsample had a median age of 63 years (range: 36-83 years), median PSA 6.3 ng/mL (range: 0.4-222.0) and the majority of patients had Gleason 3+3 (89/228, 39%), stage T1c (121/228, 53%), and NCCN intermediate risk PCa (79/228, 35 %). Within the subsample, 0% (0/76) of NCCN low risk, 10/79 (12%) of intermediate, and 47/73 (64%) of high risk patients had metastatic disease. Demographic, clinical and pathological data are shown in Table 1.
Table 1. Patient Characteristics.
| N (%) | ||
|---|---|---|
| Age at Diagnosis (years) | Median (range) | 62.6 (36.3-83) |
| PreTx PSA (ng/ml) | Median (range) | 6.3 (0.4-222) |
| Gleason Score | ||
| 3+3 | 89 (39) | |
| 3+4 | 61 (26.8) | |
| 4+3 | 27 (11.8) | |
| ≥4+4 | 50 (21.9) | |
| Unknown | 1 (0.4) | |
| T Stage | T1C | 121 (53.1) |
| T2 | 48 (21.1) | |
| T3 | 47 (20.6) | |
| T4 | 12 (5.3) | |
| M Stage | M0 | 175 (76.8) |
| M1 | 53 (23.2) | |
| NCCN Category | Low | 76 (33.3) |
| Medium | 79 (34.6) | |
| High | 73 (32) | |
MRI Findings
At least 1 bone lesion (including benign etiologies) was seen in the majority of patients (168/228 for R1 (74%, 95%CI: 0.67-0.79) and 164/228 patients for R2 (72%, 95%CI: 0.66-0.78). R1 identified 110 lesions >5 mm. Of these, 78 were considered T1 hypointense and 32 T1 hypointense compared to muscle, and 9/110 contained intra-lesional fat on T1. R2 identified 100 lesions >5 mm. Of these, 78 were considered T1 hypointense and 22 T1 hypointense compared to muscle, and 16/100 contained intra-lesional fat on T1.
For detecting bone metastases, sensitivities were 0.89 (95%CI: 0.78-0.96) for R1 and 0.67 (95%CI: 0.53-0.79) for R2, specificities were 0.98 (95%CI: 0.94-0.99) and 0.99 (95%CI: 0.96-1.00) for R1 and R2 and AUCs were 0.97 (95%CI: 0.94-1.00) for R1 and 0.90 (95%CI: 0.84-0.95) for R2. (Table 2). Inter-reader agreement is summarized in Table 3.
Table 2. Diagnostic performance for the detection of bone metastases on prostate MRI.
| Variable | Reader | Sensitivity [95% CI; n] | Specificity [95% CI; n] | AUC [95% CI] |
|---|---|---|---|---|
| Mets Likelihood | 1 | 0.89 [0.78-0.96;51/57] | 0.98 [0.94-0.99;167/171] | 0.97 [0.94-1] |
| 2 | 0.67 [0.53-0.79;38/57] | 0.99 [0.96-1;169/171] | 0.9 [0.84-0.95] |
Table 3. Inter-reader agreement for the MRI features evaluated.
| % Agreement | K | 95% CI | Interpretation | |
|---|---|---|---|---|
| T1 Intralesional Fat | 88.1% | 0.44 | [0.16 - 0.72] | Moderate |
| T1 Signal Intensity | 76.2% | 0.31 | [0.08 - 0.53] | Fair |
| Mets Likelihood | 89.9% | 0.70 | [0.58 - 0.81] | Substantial |
| Any Lesions <5mm | 62.3% | 0.23 | [0.10 - 0.35] | Fair |
| # Lesions > 5mm | 69.3% | 0.50 | [0.41 - 0.59] | Moderate |
K= Cohen kappa statistic
Correlation between MRI features, clinical features and bone metastases
Regarding the MRI features of bone lesions >5mm (110 for R1 and 100 for R2), lack of intralesional fat on T1-weighted images and maximum lesion diameter were significantly associated with bone metastases for both readers. The odds of having bone metastases in patients with lesions containing intralesional fat were lower than for patients without (OR: 0.07, 95% CI:0.00-0.38, p=0.004 for R1 and OR:0.11, 95% CI: 0.01-0.53, p=0.002 for R2), and the odds of having bone metastases were higher in patients with larger lesion size (OR: 1.33, 95% CI: 1.19-1.50, p<0.001 for R1 and OR: 1.19, 95% CI: 1.10-1.30, p<0.001 for R2). T1 signal hypointensity was significantly associated with bone metastases for R2 (OR: 4.54, 95% CI: 1.42-17.42, p=0.007), but not for R1 (OR: 1.72, 95% CI: 0.69-4.37, p=0.282).
Higher pre-treatment PSA and Gleason score were also associated with bone metastases. The median PSA was 5.75 ng/mL (range: 0.40-57.68) in patients without bone metastases, and 11.71 ng/mL (range 1.36-222.01) in patients with bone metastases (OR: 1.07, 95 CI: 1.04-1.10, p<0.001). The odds of having bone metastases were higher in patients with higher Gleason Scores (OR: 6.18, 95% CI: 3.92-9.76, p<0.001). Age at diagnosis did not demonstrate a significant association with bone metastases (OR: 1.02, 95% CI: 0.98-1.06, p=0.331).
Discussion
In this study, we found that 1.5% of patients undergoing prostate MRI for staging newly diagnosed PCa had bone metastases. This supports the notion that the vast majority of bone lesions detected on prostate MRI in newly diagnosed PCa patients are benign. Given the low prevalence of bone metastases in this patient population, we sought to identify clinical features that are associated with their presence. An important finding we report is that out of over 3000 patients who underwent prostate MRI during our study period, none of the patients fulfilling NCCN low-risk criteria (Gleason <7, PSA<10 ng/mL, cT1-2a) were found to have bone metastases. As expected, higher PSA and higher Gleason score were both significantly associated with the presence of bone metastases. These findings emphasize the importance of interpreting MRI in the context of clinical features which increase the likelihood of metastatic disease, especially in the current era where most PCa's are diagnosed at early stages. The clinic-pathologic findings we describe are consistent with previously reported epidemiologic data from the USA. Ryan et al found a reduced incidence of bone metastases over time among newly diagnosed PCa patients in the CaPSURE registry. In the period 1990-1997, 4.2% of 4020 patients had bone metastasis at diagnosis; the percentage decreased to 1.6% of 6093 patients diagnosed in the period between 1998-2003 (17). They also found, using multivariate analysis, that only higher PSA level and higher Gleason score were associated with presence of bone metastases at diagnosis (17-19). An important caveat is the well known difference in sensitivity for bone metastasis detection between the different imaging modalities, as it is unknown whether the increasing use of MRI for prostate cancer staging in recent years would have lead to different results in the CaPSURE registry.
One of the challenges for bone lesion assessment on prostate MRI is that the standard image acquisition protocols for prostate MRI and for dedicated musculoskeletal MRI are different. State-of-the-art prostate MRI includes anatomic T1 and T2-weighted images combined with diffusion-weighted (DW) and/or dynamic contrast-enhanced (DCE) MRI (14). Large field-of-view T1-weighted images of the entire pelvis are acquired from the aortic bifurcation to the perineum, and are used for evaluation of lymph nodes, bone lesions, and postbiopsy changes in the prostate. The T2-weighted, DW and DCE sequences are the primary tool for the primary prostate tumor assessment, and to obtain the best possible anatomical detail, a small field-of-view is used covering only the prostate and seminal vesicles. As such, bone lesions in prostate MRI are often only covered in the T1-weighted images and not in other sequences. Acquisition protocols for bone MRI lesion characterization typically use a small field-of-view focused on the lesion, include some form of fat suppression (e.g. fat-suppression pulse or STIR sequence), and include the lesion on all sequences performed. Given the low prevalence of bone metastases identified, our findings do not support the incorporation of additional MRI sequences to the standard prostate MRI acquisition protocol, as this would unnecessarily prolong examination times and potentially impact patient comfort with a relatively low yield for detection of bone metastasis based on the low prevalence. The MRI features we found to be associated with bone metastasis were larger lesion size and hypointense signal intensity compared to muscle on the T1-weighted sequence, while as previously reported, intralesional fat is associated with benignity(20).
Based on our data we postulate that if bone lesions are present in around 70% of patients undergoing staging prostate MRI, but only 1.5% of patients have bone metastases, the prevalence of bone lesions of doubtful clinical importance may be somewhere around 68.5%.In the absence of previous reports on the prevalence of benign and metastatic bone lesions on prostate MRI, the closest approximation is from the nuclear medicine literature, where bone scan is used for evaluation of PCa. It is important to emphasize that comparison between MRI and bone scan is limited as these two modalities differ in their mechanisms for lesion detection. While bone scans detect osteoblastic activity, MRI detects bone metastases through the differences in signal intensity between tumor tissue and surrounding normal bone marrow. MRI therefore visualizes metastatic tumors directly, and can detect intra-medullary lesions before any cortical destruction occurs and before any osteoblastic reaction is detected on bone scan or radiographic studies (including CT). Furthermore, the typical PCa patient populations undergoing prostate MRI and bone scan can be distinctly different. While bone scintigraphy is only indicated in the context of high risk disease (2), prostate MRI is increasingly performed for potentially low-risk conditions such as rising PSA with negative prostate biopsy and assessment of active surveillance eligibility and follow-up. Notwithstanding this, several studies evaluating the diagnostic value of bone scan in PCa have shown that a substantial portion of bone lesions detected on bone scan do not represent metastatic disease, including clearly benign findings, and equivocal findings that are later proven to be benign (18, 19, 21-23). Jacobson et al evaluated the association between PSA levels and patterns of benign and malignant uptake on bone scintigraphy of newly diagnosed PCa patients, and found benign bone lesions in 281/432 (65%) patients (mostly degenerative disease and post traumatic findings). An additional 100/432 patients (23%) patients with equivocal or suspicious findings did not have metastatic disease on further work-up, increasing the percentage of benign findings even higher (18).
Our study has several limitations. It is a retrospective, single-institution study with the associated limitations of such study design, including selection bias and uncertain generalizability to other practice settings. We evaluated the imaging findings only in a selected sample of the 3765 patients; this could potentially add selection bias and inaccurate results. Although efforts were made within the sampling design to obtain a representative cohort (for example by stratifying on the year in which the prostate MRI was done so the patients in the subset were evenly distributed over the years the entire population was scanned), there is the theoretical possibility the subset of patients evaluated may not be representative of the entire population. Furthermore we had no histopathology to prove the benignity of most of the bone lesions detected on MRI. Although an ideal scenario would include pathologic evaluation of all imaging-detected lesions, this is not in accordance with standard clinical practice where bone lesions are only biopsied if there is sufficient clinical concern for metastatic disease and the imaging findings are indeterminate, or if there is need for tissue confirmation of diagnosis or to perform additional investigational analysis (e.g. genomic testing). Our working assumption, however, is that any bone lesion present at the time of staging prostate MRI, which after ≥1 year of follow-up did not manifest as clear metastatic disease, is highly unlikely to represent metastasis. Another limitation was the inclusion of both 1.5T and 3T MRI examinations performed during a period of 14 years. This allowed us to maximize the number of eligible patients but also introduced potential diagnostic heterogeneity. However, there is no study suggesting a difference in diagnostic performance between 1.5T and 3 T MRI for the detection and characterization of bone lesions.
In Conclusion, bone lesions are present in the majority of patients undergoing initial PCa staging, but only rarely represent metastatic disease. Interpretation of MRI findings should be made in the context of the patient's PSA, Gleason score and clinical stage, in order to increase the likelihood of detection of metastatic disease and avoid unnecessary workup of benign bone lesions.
Supplementary Material
Figure 2.
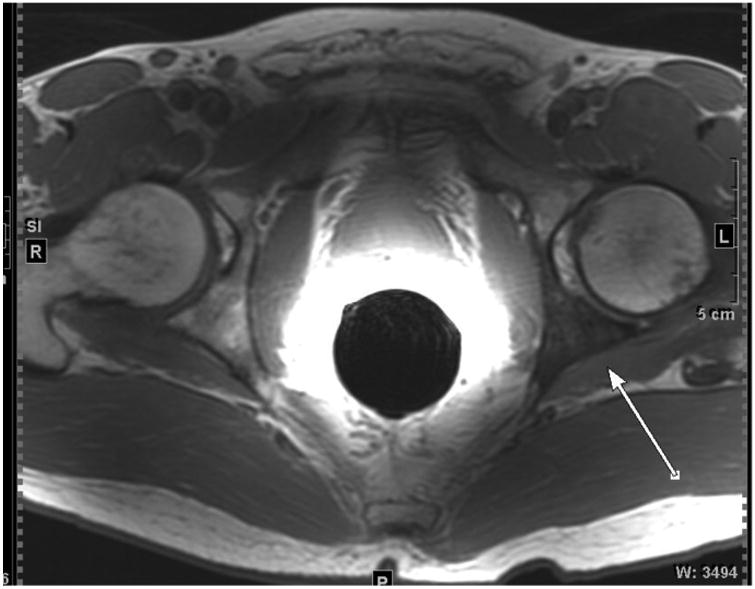
54-year-old male with EAU high-risk (Gleason Score 4+5, PSA 40 ng/mL) prostate carcinoma and osseous metastases. Large field-of-view axial T1-weighted image of the pelvis shows a hypointense metastasis within the left posterior acetabulum (arrow).
Key points.
- Bone lesions are common in prostate MRI, but rarely represent metastases.
- MRI should be interpreted in the context of clinical features influencing the likelihood of metastases.
- None of the patients with NCCN low-risk disease had bone metastases.
Acknowledgments
Funding: This study was partially funded by NIH grant number P30 CA008748
Abbreviations and acronyms
- AUC
area under the curve
- CaPSURE
Cancer of the Prostate Strategic Urologic Research Endeavor
- CI
confidence interval
- MRI
magnetic resonance imaging
- NCCN
National Comprehensive Cancer Network
- OR
odds ratio
- PCa
prostate cancer
- PI-RADS
Prostate Imaging Reporting and Data System
- PSA
prostate-specific antigen
Footnotes
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):252–71. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65(1):124–37. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 3.Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367(3):203–13. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gondo T, Hricak H, Sala E, Zheng J, Moskowitz CS, Bernstein M, et al. Multiparametric 3T MRI for the prediction of pathological downgrading after radical prostatectomy in patients with biopsy-proven Gleason score 3 + 4 prostate cancer. Eur Radiol. 2014;24(12):3161–70. doi: 10.1007/s00330-014-3367-7. [DOI] [PubMed] [Google Scholar]
- 5.Fascelli M, George AK, Frye T, Turkbey B, Choyke PL, Pinto PA. The role of MRI in active surveillance for prostate cancer. Curr Urol Rep. 2015;16(6):507. doi: 10.1007/s11934-015-0507-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eberhardt SC, Carter S, Casalino DD, Merrick G, Frank SJ, Gottschalk AR, et al. ACR Appropriateness Criteria prostate cancer--pretreatment detection, staging, and surveillance. J Am Coll Radiol. 2013;10(2):83–92. doi: 10.1016/j.jacr.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Mullerad M, Chen HN, Eberhardt SC, Kattan MW, Scardino PT, et al. Prostate cancer: incremental value of endorectal MR imaging findings for prediction of extracapsular extension. Radiology. 2004;232(1):133–9. doi: 10.1148/radiol.2321031086. [DOI] [PubMed] [Google Scholar]
- 8.Donati OF, Afaq A, Vargas HA, Mazaheri Y, Zheng J, Moskowitz CS, et al. Prostate MRI: evaluating tumor volume and apparent diffusion coefficient as surrogate biomarkers for predicting tumor Gleason score. Clin Cancer Res. 2014;20(14):3705–11. doi: 10.1158/1078-0432.CCR-14-0044. [DOI] [PubMed] [Google Scholar]
- 9.Sartor AO, Hricak H, Wheeler TM, Coleman J, Penson DF, Carroll PR, et al. Evaluating localized prostate cancer and identifying candidates for focal therapy. Urology. 2008;72(6 Suppl):S12–24. doi: 10.1016/j.urology.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Vargas HA, Akin O, Afaq A, Goldman D, Zheng J, Moskowitz CS, et al. Magnetic resonance imaging for predicting prostate biopsy findings in patients considered for active surveillance of clinically low risk prostate cancer. J Urol. 2012;188(5):1732–8. doi: 10.1016/j.juro.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sciarra A, Barentsz J, Bjartell A, Eastham J, Hricak H, Panebianco V, et al. Advances in magnetic resonance imaging: how they are changing the management of prostate cancer. Eur Urol. 2011;59(6):962–77. doi: 10.1016/j.eururo.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 12.Turkbey B, Brown AM, Sankineni S, Wood BJ, Pinto PA, Choyke PL. Multiparametric prostate magnetic resonance imaging in the evaluation of prostate cancer. CA Cancer J Clin. 2015 doi: 10.3322/caac.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson JE, van Leeuwen PJ, Moses D, Shnier R, Brenner P, Delprado W, et al. The Diagnostic Performance of Multiparametric Magnetic Resonance Imaging to Detect Significant Prostate Cancer. J Urol. 2015 doi: 10.1016/j.juro.2015.10.140. [DOI] [PubMed] [Google Scholar]
- 14.Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol. 2016;69(1):16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vargas HA, Hotker AM, Goldman DA, Moskowitz CS, Gondo T, Matsumoto K, et al. Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. Eur Radiol. 2015 doi: 10.1007/s00330-015-4015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.S LH, G MK, W C. TNM Classification of Malignant Tumors. 7th. UICC International Union Against Cancer; 2009. [Google Scholar]
- 17.Ryan CJ, Elkin EP, Small EJ, Duchane J, Carroll P. Reduced incidence of bony metastasis at initial prostate cancer diagnosis: data from CaPSURE. Urol Oncol. 2006;24(5):396–402. doi: 10.1016/j.urolonc.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson AF. Association of prostate-specific antigen levels and patterns of benign and malignant uptake detected. on bone scintigraphy in patients with newly diagnosed prostate carcinoma. Nucl Med Commun. 2000;21(7):617–22. doi: 10.1097/00006231-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Wan F, Xu L, Zhao N, Xu Z, Wang H, et al. Is it safe to omit baseline bone scan for newly diagnosed prostate cancer patients? Urol Int. 2015;94(3):342–6. doi: 10.1159/000368912. [DOI] [PubMed] [Google Scholar]
- 20.Simpfendorfer CS, Ilaslan H, Davies AM, James SL, Obuchowski NA, Sundaram M. Does the presence of focal normal marrow fat signal within a tumor on MRI exclude malignancy? An analysis of 184 histologically proven tumors of the pelvic and appendicular skeleton. Skeletal radiology. 2008;37(9):797–804. doi: 10.1007/s00256-008-0523-7. [DOI] [PubMed] [Google Scholar]
- 21.Zacho HD, Barsi T, Mortensen JC, Mogensen MK, Bertelsen H, Josephsen N, et al. Prospective multicenter study of bone scintigraphy in consecutive patients with newly diagnosed prostate cancer. Clin Nucl Med. 2014;39(1):26–31. doi: 10.1097/RLU.0000000000000291. [DOI] [PubMed] [Google Scholar]
- 22.Ozgur BC, Gultekin S, Ekici M, Yilmazer D, Alper M. A narrowing range of bone scan in newly diagnosed prostate cancer patients: A retrospective comparative study. Urol Ann. 2015;7(2):193–8. doi: 10.4103/0974-7796.150479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavery HJ, Brajtbord JS, Levinson AW, Nabizada-Pace F, Pollard ME, Samadi DB. Unnecessary imaging for the staging of low-risk prostate cancer is common. Urology. 2011;77(2):274–8. doi: 10.1016/j.urology.2010.07.491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


