Abstract
It is well accepted that parent-child interactions are bidirectional by nature, yet not much is known about the psychophysiological activity underlying these interactions. We examined, during a parent-child interaction, how a child’s negativity statistically predicted maternal frontal EEG asymmetry and how a mother’s negativity statistically predicted child frontal EEG asymmetry. Thirty-four mother-child dyads participated in the study. Maternal and child behavior and physiology were measured during a puzzle task. Results indicated that mothers whose children exhibited more challenging behaviors during the dyadic interaction displayed more right (relative to left) asymmetry, as did children whose mothers were high in negativity during the interaction. These findings suggest that mothers and children react to each other’s signals, not only behaviorally but also physiologically.
One of the most frequently studied areas in child psychology is the link between parental behavior and child developmental outcomes (Belsky & Haan, 2011), yet little is known about the dyadic physiological processes that may connect parenting behavior and child behavior. The study of physiological systems is particularly interesting because children and parents generally do not have conscious control over physiological processes, yet physiological processes are influenced by social context and experience (Ruttle, Serbin, Stack, Schwartzman, & Shirtcliff, 2011; Sethre-Hofstad, & Stansbury, Rice, 2002). Thus, this was a good way to measure how each partner’s behavior may be related to the other partner’s behavior. In the current study, we examined how child and maternal behaviors during an interaction covaried with child and maternal brain electrical activity; we used electroencephalograph (EEG) during those interactions to measure this activity. Specifically, we focused on mother-child similarity in frontal EEG asymmetry. The use of EEG asymmetry is of importance because it refers to the balance of brain activation in left and right frontal areas and is considered to be a reliable indicator of affect regulation (Forbes et al., 2008). Because child behavioral problems are known to elicit negative feelings in parents (Calkins, 2002; Nicholson, Fox, & Johnson, 2005) in situations when the child displays behaviors such as negative affect and noncompliance, the parent is more likely to experience negative emotions arising from the child’s behaviors. Parental negative emotional reactivity and poor emotional regulation can be major components of harsh and abusive parenting behaviors (Deater-Deckard, Wang, Chen, & Bell, 2012; Lorber, O’Leary, & Kendziora, 2003; Mills & McCarroll, 2012). Therefore, it is important to understand the ways in which child behaviors relate to maternal emotional reactivity and regulation at behavioral and psychophysiological levels of measurement and analysis. To the best of our knowledge, there have not been any studies examining dyadic child and maternal EEG asymmetry during mother-child interactions while considering the negative behaviors of either mother or child. The assessment of EEG asymmetry and behavior of both mother and child, during an interaction, may provide unique information on any links between behavior and affect regulation within the mother-child interactions.
Mother-Child Interaction and the Role of Emotional Regulation
It is widely accepted that an infant’s development of self-regulation depends heavily on parents’ responsiveness to the infant’s emotional expression and needs (Graziano, Keane, & Calkins, 2010). Infants learn about regulating their emotions in the context of social interactions (Kopp, 1992). However, these processes are not unidirectional, but rather, involve mutual bidirectional influences (Sameroff, 2010; Tronick, 2007). Because emotions can be influenced and regulated by others during an interaction, children can regulate or disrupt others’ (including their parents’) emotional states and thus affect their parents’ behaviors (Cole, Martin, & Dennis, 2004; Eisenberg & Spinard, 2004; Forbes et al., 2008) via a process of parent-child mutual regulation (Gianino & Tronick, 1988; Tronick, 2007). This literature has relied almost exclusively on behavioral indicators of coreactivity and coregulation.
However, there are studies that have assessed the association between child and parent physiological activity: One body of research investigated the coregulation of cortisol levels of children and parents during an interaction and revealed that maternal behavior and cortisol levels were associated with child’s adrenocortical activity (Granger, 1998; Hibel, Granger, Blair, & & Finegood, 2015). Additionally, Ruttle and colleagues (2011) provided evidence that mother-child dyads showed, overall, some systematic synchronization (“attunement”) of the hypothalamic-pituitary-adrenal (HPA) axis, which was particularly prominent during challenging tasks. But, to the best of our knowledge, there is only one prior study that has examined the mother-child relationship involving child frontal EEG asymmetry (Forbes et al., 2008): Using cross-lagged regression equations, Forbes and colleagues (2008) found that maternal depressive symptoms were predictive of child frontal asymmetry and affect expression and also that child frontal asymmetry predicted maternal depressive symptoms.
Frontal EEG Asymmetry
Research on EEG asymmetry indicates that the frontal region plays a unique and significant role in the regulation of emotional behaviors (Fox, 1994). Frontal asymmetry in the alpha frequency band (8–13 Hz for adults; 6–9 Hz for infants and young children) measures stable individual differences in the functional association between the frontal lobe and the amygdala, a brain network that is involved in processing emotionally salient and relevant information (Davidson, Jackson, & Kalin, 2000). Left and right frontal lobes differentially specialize for approach versus avoidance tendencies, affecting the way individuals engage when behaviorally or emotionally aroused (Davidson & Fox, 1982; Davidson, 1992, Henderson, Fox, & Rubin, 2001).
Here and Now: Assessing EEG Asymmetry in Real-Time Interactions
Frontal EEG asymmetry reflects the balance of brain activation in left and right frontal areas and is widely viewed to be a biomarker of emotional reactivity and regulation in regard to emotional valence and motivational stance (Fox, 1991, 1994; Fox, Bell, & Jones, 1992; Fox, Calkins, & Bell, 1994). In the last two decades, two streams of research have examined the associations between frontal EEG asymmetry and patterns of emotional responses: those examining asymmetry at rest in the laboratory (i.e., baseline; Sutton & Davidson, 1997; Tomarken, Davidson, Wheeler, & Doss, 1992) and asymmetry in response to emotional stimuli during laboratory procedures (Coan & Allen, 2003, 2004; Killeen & Teti, 2012). Studies of asymmetry at rest indicate that left (relative to right) frontal asymmetry is indicative of approach behaviors and expression of positive emotions, and right (relative to left) asymmetry is associated with avoidance behaviors and expression of negative emotions (e.g., Calkins, Fox, & Marshall, 1996; Fox & Davidson, 1984; Fox, Henderson, Rubin, Calkins, & Schmidt, 2001; Smith & Bell, 2010). Studies of asymmetry in response to stimuli have shown similar results, whereby individuals who are exposed to negative avoidance or withdrawal related emotional stimuli – such as those that induce feelings of sadness – respond with greater right (relative to left) asymmetry, but those exposed to approach and positive emotion stimuli show greater left (relative to right) asymmetry (e.g., Coan & Allen, 2003, 2004; Diaz & Bell, 2012; Killeen & Teti, 2012).
However, how would an adult respond if the negative or positive emotional stimuli were being expressed by one’s own child during an interaction with her or him? In a recent study, Dix, Moed, and Anderson (2014) indicated that mothers reacted with either increased (i.e., approached) or decreased (i.e., withdrawn) negative reactivity to their children’s aversive behaviors. That is, both type of behaviors were possible, stressing the need to better understand approach/avoidance reactions to child’s negative behaviors. However, to the best of our knowledge, the only study that has examined parental asymmetry in response to emotion-inducing stimuli is Killeen and Teti’s 2012 study. Killeen and Teti (2012) examined the associations between a mother’s frontal asymmetry at rest and while watching films of her 5–8 month old infant expressing joy, anger, and neutral interest. Maternal frontal asymmetry changed when mothers observed films of their own infants displaying the three emotions. Furthermore, maternal frontal asymmetry at rest was not related to parent-child interaction behaviors (Killeen & Teti, 2012), implying that maternal experienced emotions are more dependent on context and may not reflect individual differences in affective tendencies. Thus, the authors stressed the need to study maternal frontal EEG asymmetry in real time – “here and now” – during the mother-child interaction.
As for children’s asymmetry, prior studies have investigated children’s frontal asymmetry and its links with the children’s developmental outcomes. For example, research has suggested that infants with resting right asymmetry cry more during maternal separation (Davidson & Fox, 1989; Fox et al., 1992), and children who are more dispositionally shy or fearful show more right asymmetry at rest (Fox et al., 1995; Fox et al., 2001). Furthermore, relative right frontal EEG asymmetry was related to elevated levels of child depressive symptoms when children watched happy or sad films (Feng et al., 2012) and to less empathic responses to a crying infant, as well as their own mothers’ simulated distress (Jones, Field, & Davalos, 2000).
The Current Study
Extensive research indicates that both intrinsic and extrinsic factors (i.e., within the individual and outside of the individual, respectively) account for the variance seen in children’s and adults’ emotional reactivity and regulation (Calkins, Howse, & Philippot, 2004; Cicchetti & Rogosch, 1996). Furthermore, based on studies of asymmetry in response to stimuli that have shown that maternal EEG asymmetry correlated with children’s behavior, we examined how the child’s behavior during parent-child interactions in the lab statistically predicted mothers’ frontal EEG asymmetry and how the mother’s behavior statistically predicted the child’s frontal EEG asymmetry, while controlling for within-person associations between one’s own behavior and one’s own frontal asymmetry during interaction. Consistent with theory and empirical literature on parent-child relationships, we hypothesized that each partner’s behavior would statistically predict the other partner’s frontal asymmetry. Specifically, we anticipated that interacting with a partner who was showing more aversive negative behaviors would be linked with greater right frontal asymmetry. We expected that the effects would be comparable for parent and child.
Method
Participants
The sample included 50 biological mother-child dyads. Children and mothers are part of an ongoing longitudinal investigation focused on early cognitive and emotional development. Four to eight weeks after the children’s third-year visit for the longitudinal study (Age = 38 mo; Range = 36–40 mo; SD = 0.97 mo), mothers and children returned to the research lab to participate in parent-child interactions, during which we recorded dyadic EEG and coded their interaction behaviors. Children (17 boys, 33 girls) were 38.5 months old during the second visit (Range = 37–42 mo; SD = 1.38 mo) and mothers were 21–43 years of age (M = 34 yr, SD = 5 yr); 94% of the mothers returning for the visit were Caucasian (one Asian, one Black, one Hispanic). All of the mothers were high school graduates (18% technical degree; 39% bachelor’s degree; 31% graduate degree).
Complete EEG and interaction data were available for 34 mother-child dyads (23 girls, 11 boys); 97% of the mothers were Caucasian (one Asian). All mothers were high school graduates (38% bachelor’s degree; 35% graduate degree). Of the 16 dyads with missing data, five were the result of EEG equipment failure during the dyadic recording, such that either the mother’s or the child’s EEG data were not recorded; seven children refused to wear the EEG cap; three children had too much EEG artifact during the interaction task such that there was less than 10% data available for analysis; and one dyad had no video recording for coding. Mothers received an honorarium for the laboratory visit.
Procedure
Signed consent from the mothers and verbal assent from the children were provided upon arrival to the research lab. The dyad was seated at a small table and EEG electrodes applied to each. Age appropriate resting baseline EEG was recorded for each member of the dyad. For children, 1 minute of EEG was recorded while watching a quiet video (e.g., Finding Nemo; sea turtles riding the East Australian Current). For mothers, 1 minute with eyes opened and 1 minute with eyes closed were recorded, but only the eyes opened data were used in the analyses so that maternal baseline data would be comparable to child baseline data. Mother-child interaction tasks were completed while EEG was recorded. After the interaction tasks, the child left the room and participated in memory games with a research assistant while mothers participated in a battery of computerized executive function and episodic memory tasks. Those results are reported elsewhere (authors’ citation). Mothers received an honorarium, and the university institutional review board approved study procedures.
Interaction tasks
Mothers and children participated in a puzzle interaction task, during which mothers were instructed to assist children in completing a puzzle that was rated as age-appropriate for older children. Mother was instructed to work with her child on the puzzle as she would if they were doing a puzzle at home. The puzzle task averaged 305.98 seconds (Range = 192.02 – 344.48 sec; SD = 28.02 sec).
EEG acquisition, processing, and analysis
Dyadic EEG recordings were made from 28 left and right scalp sites: frontal pole (Fp1, Fp2), frontal (F3, F4, Fz, F7, F8), fronto-central (FC1, FC2, FC5, FC6), central (C3, C4), centro-parietal (CP1, CP2, CP5, CP6), temporal (T7, T8), parietal (P3, P4, Pz, P7, P8), and occipital (O1, O2)], as well as left and right mastoids. All electrodes were referenced to Cz during the recordings. The recordings were obtained using a stretch cap (Electro-Cap, Inc.; Eaton, OH; E1-series cap) with 10 electrodes in the 10/20 system pattern. After the cap was placed on the mother’s head and another placed on the child’s head, a small amount of abrasive gel was placed on each recording site and the scalp was gently rubbed. Conductive gel was then added to the recording sites. Electrode impedances were measured and accepted if they were below 20 kΩ. The electrical activity from each lead was amplified using separate James Long Company Bioamps (James Long Company; Caroga Lake, NY). The 64-channel bioamp system had half of the channels dedicated to the mother and the other half to the child. Thus, mother EEG and child EEG were recorded using the same set of bioamps. An event mark was inserted into the EEG record to note the beginning and ending of the baseline and puzzle interaction task.
During data collection, the high pass filter on the bioamps was a single pole RC filter with a 0.1 Hz cut-off (3 dB or half-power point) and 6 dB per octave roll-off. The low pass filter was a two-pole Butterworth type with a 100 Hz cut-off (3 dB or half-power point) and 12 dB octave roll-off. Activity for each EEG lead for mother and for child was displayed on the monitor of the acquisition computer. The EEG was digitized online at 512 samples per second for each channel to eliminate the effects of aliasing. The acquisition software used was Snapshot-Snapstream (HEM Data Corp., Southfield, MI), and the raw data was stored for later analyses. Prior to the recording of each dyad, a 10 Hz, 50 uV peak-to-peak sine wave was input through each amplifier. This calibration signal was digitized for 30 seconds and stored for subsequent analysis.
Spectral analysis of the calibration signal and computation of power at the 9–11 Hz frequency band was accomplished. The power figures were used to calibrate the power derived from the subsequent spectral analysis of the EEG. Then, EEG data for the mother and for the child were separately examined and analyzed using EEG analysis software developed by the James Long Company. Data were re-referenced via software to an average reference configuration (mastoids were not included in the re-referencing). Use of the average reference configuration requires that a sufficient number of electrodes be sampled and that these electrodes be evenly distributed across the scalp. Currently, there is no agreement concerning the appropriate number of electrodes (Hagemann, Naumann, & Thayer, 2001; Luck, 2005); however our 10/20 configuration satisfies the requirement of having an even scalp distribution. Artifact was scored separately – for eye movements, using a peak-to-peak criterion of 100μVm or greater – for mothers and for children. Gross motor movements over 200μV peak-to-peak were also scored. These artifact scored epochs were eliminated from all subsequent analyses. No artifact correction procedures were used. The data were then analyzed separately for mothers and children with a discrete Fourier transform (DFT) using a Hanning window of 1 second width and 50% overlap. Power values were computed at each site during the baseline and puzzle interaction task. EEG power for mothers and children was expressed as mean square microvolts, and data were transformed using the natural log (ln) to normalize the distribution.
For the mothers, 66% of the baseline EEG was without artifact, whereas 36% of the puzzle task data was artifact free. For the children, the artifact-free values were 69% for baseline and 55% for puzzle task. We did not do eye blink correction and this is the main reason for artifact in the baseline EEG. Mothers had greater artifact during the puzzle task because they were instructed to assist their children. This resulted in mothers doing more talking and reaching for puzzle pieces during the task than did their children. Finally, we calculated the number of usable epochs of data, revealing that for the mothers, there were 82 epochs of non-artifact EEG data during baseline and 224 epochs of non-artifact EEG data during interaction. For children, there were 124 and 367 epochs of non-artifact EEG data during baseline and interaction, respectively.
Measures
Maternal and child negativity
Maternal and child negativity were measured using observers’ ratings. Trained coders used the PARCHISY global ratings system (Deater-Deckard, Pylas, & Petrill, 1997) to rate mother’s behavior during the puzzle task with the child, using the instrument’s 7-point Likert-type scales (1 = no occurrence; 7 = continual occurrence of the behavior). During training, two raters observed the same video independently. For items with a difference in rating scores larger than 1 on the 7-point scale, the two raters would discuss the item and resolve the discrepancy. To calculate the reliability of coding, we randomly selected 20% of the interactions. Each coder’s rating scores were treated as an item and used to calculate the reliability for each item across raters, using a generalizability theory to estimate the coefficient alpha for each item and permitting 1-point discrepancies on the 7-point scale. In this context, alpha represents the overall covariance between raters while accounting for within-rater variance, such that the higher the alpha coefficient the more reliable the ratings of that item (Bakeman & Gottman, 1986, pp. 92–96).
Maternal negativity
In the current study, we examined observed maternal negative affect (e.g., rejecting, frowning, cold/harsh tone; inter-rater reliability of .96), and observed negative control (e.g. use of criticism, physical control of the dials, physical control of the child’s hand/arm/body; inter-rater reliability = .83). Negative control and negative affect were substantially inter-correlated, r = .62. Because our goal was to derive a parenting behavior composite variable that was as reliable as possible, we standardized and averaged the control and affect scores to yield a single maternal negativity score.
Child negativity
We also examined observed child negativity as a composite, including child negative affect, noncompliance, and responsiveness (reversed). Inter-rater reliability was high, ranging between αs = .83–.90. The principal component of these three scales explained 81% of the variance, with loadings ranging from .87 to .93. Using the same rationale and procedure previously described, we standardized and averaged the three indicators of child negativity to yield a child negativity composite. This score was positively skewed.
Frontal EEG asymmetry
Power was computed for the alpha frequency bands, 8–13 Hz for mothers (Coan & Allen, 2004) and 6–9 Hz for children (Bell & Fox, 1994; Marshall, Bar-Haim, & Fox, 2002). We were particularly interested in the asymmetry of brain activity at the F3/F4 scalp locations because past studies have shown that the alpha asymmetry between these two sites index a general response tendency of approach versus inhibition (e.g., Coan & Allen, 2004; Fox et al, 2001) and positive versus negative emotionality (e.g., Jones & Fox, 1992). The asymmetry score was computed by subtracting the natural log of alpha power at the left hemisphere F3 site from the natural log of alpha power at the right hemisphere F4 site. In the EEG literature, brain activation is indicated by lower EEG power values in the alpha frequency band (Coan & Allen, 2004). Thus, greater relative left than right frontal activation is indicated by a positive asymmetry score, and greater relative right than left frontal activation is indicated by a negative asymmetry score.
Results
Descriptive Statistics and Correlations
We first examined the means and standard deviations for all study variables. Maternal asymmetry (M= .02, SD = .26; range: −.80 to .48) and child asymmetry (M=.07, SD = .26, range: −.41 to .52) at rest did not differ on average; (t (32) = −.91, ns). Maternal and child asymmetry during interaction differed significantly (t (34) = − 3.40, p < .01), with the child asymmetry mean being below zero (M= −.06, SD = .23, range: −.43 to .47), reflecting greater relative right frontal asymmetry; the maternal asymmetry mean was above zero (M = .10, SD = .18, range: −.40 to .49), reflecting greater relative left frontal asymmetry. Maternal negativity (M= −.17, SD = .42, range: −.38– to 1.26) and child negativity (M= −.04, SD= .79, range: −.80 to 3.33) during interaction were not significantly different (t (44) = .00, ns).
Bivariate Pearson correlations were estimated and are shown in Table 1. Child frontal asymmetry during baseline covaried substantially with asymmetry during interaction. In contrast, asymmetry at rest and during interaction was only modestly associated (and not significantly) for mothers. Child asymmetry during interaction was associated with maternal negativity, and maternal asymmetry at baseline and during interaction was associated with child negativity. Child asymmetry at baseline and during interaction was not significantly linked with maternal asymmetry at rest and during interaction (r = −.09 and r = .25, accordingly). Finally, maternal and child negativity scores were substantially correlated.
Table 1.
Bivariate Correlations
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| 1.Child EEG Asymmetry (B) | 1.00 | |||||
| 2. Maternal EEG Asymmetry (B) | −.09 | 1.00 | ||||
| 3. Child EEG Asymmetry (Int) | .77*** | −.10 | 1.00 | |||
| 4. Maternal EEG Asymmetry (Int) | .13 | .19 | .25 | 1.00 | ||
| 5. Child Negativity | −.15 | −.44** | −.18 | −.37* | 1.00 | |
| 6. Maternal Negativity | −.16 | −.20 | −.36* | −.29 | .77** | 1.00 |
Note. B = Baseline; Int = mother-child interaction;
p < .05;
p < .01;
p < .001.
Regression Models
To test the hypothesis that each partner’s negativity would statistically predict the other partner’s frontal asymmetry during the interaction task, we used hierarchical regression models. In the first model, which predicted maternal frontal asymmetry during interaction, child asymmetry during interaction and maternal negativity were entered in the first step. In the second step, child negativity was entered. In the second model, which predicted child frontal asymmetry during the interaction task, maternal frontal asymmetry during interaction and child negativity were entered in the first step, and maternal negativity was entered in the second step. In this way, we were able to isolate the variance in each partner’s frontal asymmetry during interaction that was attributable to the covariance with the other partner’s negativity during dyadic interaction – above and beyond any statistical effects – from within-person covariance between asymmetry and negativity and between-person covariance between frontal asymmetry.
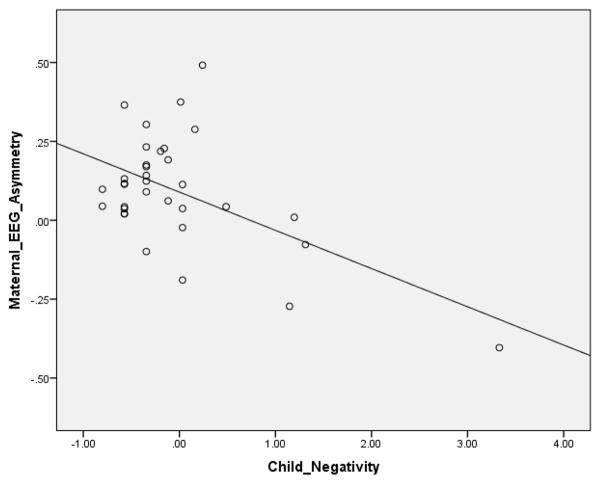
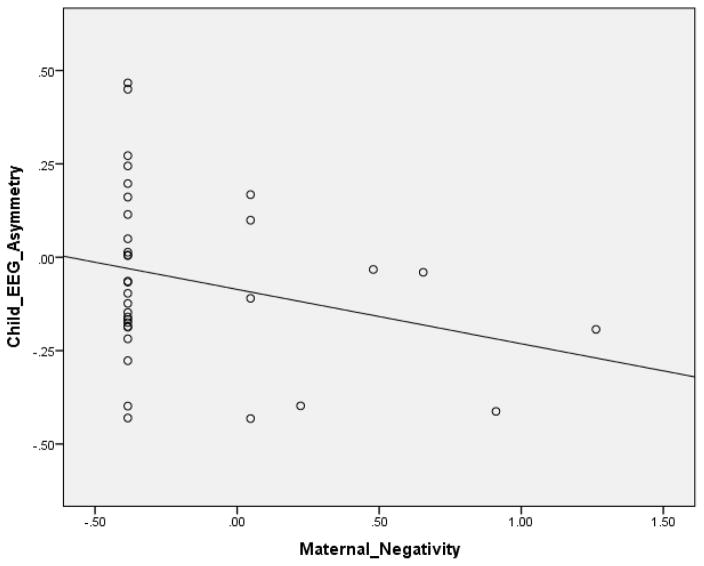
Results are shown in Tables 2 and 3. In both models, we used one-tailed p-values. Scatterplots depicting primary association in Table 2 (i.e., between child negativity and maternal EEG frontal asymmetry) and in Table 3 (i.e., maternal negativity and child EEG frontal asymmetry) are presented in Figure 1 and 2. In the first model, in which we predicted variance in maternal asymmetry during interaction, the overall model was significant, accounting for one third of the variance. As hypothesized, higher levels of child negativity during interaction were associated with greater right asymmetry in the mother. Consistent with this were the results from the second model, in which we predicted variance in child asymmetry during interaction. The overall model was marginally significant, accounting for nearly one fifth of the variance. Higher levels of maternal negativity were associated with greater child right asymmetry.
Table 2.
Hierarchical Regression Results for the Prediction of Maternal Frontal EEG Asymmetry
| β | t-value, sig | Change R2 | |
|---|---|---|---|
| Step 1 | .20 | ||
| Child EEG Asymmetry | .03 | t (31) = .14, ns | |
| Maternal Negativity | −.44 | t (31) = −2.55, p<.05 | |
| Step 2 | .12 | ||
| Child EEG Asymmetry | .07 | t (31) = .41, ns | |
| Maternal Negativity | −.09 | t (31) = −.41, ns | |
| Child Negativity | −.48 | t (31) = −2.29, p<.05 | |
| F(30) = 4.72, p < .001 (one-tailed); R2 = .32 | |||
Note. Results remain the same when controlling for mother’s age and child’s age.
Table 3.
Hierarchical Regression Results for the Prediction of Child Frontal EEG Asymmetry
| B | t-value, sig | Change R2 | |
|---|---|---|---|
| Step 1 | .04 | ||
| Maternal EEG Asymmetry | .13 | t (31) = .60, ns | |
| Child Negativity | −.10 | t (31) = −.49, ns | |
| Step 2 | .15 | ||
| Maternal EEG Asymmetry | .08 | t (31) = .41, ns | |
| Child Negativity | .19 | t (31) = .75, ns | |
| Maternal Negativity | −.46 | t (31) = −1.94, p<.05 | |
| F(30) = 1.75, p = .09 (one-tailed); R2 = .19 | |||
Note. Results remain the same when controlling for mother’s age and child’s age.
Figure 1.
Scatterplots depicting primary association between child negativity and maternal EEG frontal asymmetry during the interaction task.
Figure 2.
Scatterplots depicting primary association between maternal negativity and child EEG frontal asymmetry during the interaction task.
Finally, we repeated the same analyses using one person’s asymmetry during baseline, instead of during interaction, in the first step. These analyses revealed that, similar to the results presented above, one partner’s asymmetry during baseline did not predict other partner’s asymmetry during interaction. These findings further support our understanding that it is the negative behavior performed by one partner and, not their baseline asymmetry that statistically predicts the other partner’s asymmetry during interaction.
Discussion
The current study takes the notion of a bidirectional effect one step forward by adding a physiological aspect that supports the idea that the parent-child relationship is a dynamic process involving both the physiological and the behavioral systems (Kileen & Teti, 2012; Ruttle et al.,2011). The main goal of our study was to move beyond the associations in observed and self-reported parent and child behaviors to an exploration of the dyadic physiological processes that may connect parenting and children’s behavior. We examined how child-observed and mother-observed behaviors covary with their brain electrical activity. Because we were interested in measuring behavior and physiological activity in real time (here and now), data on EEG activity and observed behaviors were recorded during a mother-child play interaction. In fact, this is one of the first studies (cf. author’s citation) to capture both child and maternal EEG asymmetry during joint interactions. Discussion of these results, their implications, limitations, and future directions follow.
Bidirectional Effect at the Physiological Level
Maternal asymmetry
Our hypothesis, which proposed that interaction with a partner who was showing more aversive negative behaviors would be linked with greater right (relative to left) frontal asymmetry, was supported. Results indicated that mothers whose children exhibited more challenging behaviors (e.g., higher in noncompliance and in negative affect and lower in responsiveness) during the dyadic interaction displayed more right (relative to left) asymmetry, as did children whose mothers were high in negativity (e.g., rejecting, frowning, criticizing, and physically controlling the child) during the interaction. Frontal EEG asymmetry is theorized to provide a noninvasive way to gain insight about an individual’s emotional expression, experience, and regulation (Davidson et al., 2000). Our results indicate that mothers and children seem to react to each other’s signals, not only behaviorally but also physiologically. These findings support Ruttle and colleagues’ (2011) findings indicating that mother-child dyads showed some attunement of the HPA axis and expand them to another physiological mechanism, the brain activation. Specifically, it seems that children’s negative behaviors act as natural stimuli, eliciting mothers’ right asymmetry, which may, in turn, be related to more maternal harsh discipline (Chen, Bell, & Deater-Deckard, 2015). The strong correlations between child’s negativity and maternal right asymmetry support previous research indicating that mothers who shift toward greater right (relative to left) frontal activation, in response to brief videos of their infants showing angry distress, were associated with more self-reported sadness and irritability as well as elevated levels of maternal psychopathology, in particular, anxiety and depression (Killeen & Teti, 2011). The current study expands previous studies by showing that maternal asymmetry is related not only to general mood induction elicited during video observation (Coan & Allen, 2003, 2004) or to laboratory procedures where the mood induction stimuli are videos of their own child expressing various moods (Killeen & Teti, 2011), but also to concurrent – here and now – child behavior.
Furthermore, in accordance with previous research highlighting the need to incorporate ecologically valid methods by which a mother could take action (Killeen & Teti, 2012) – natural mother-child interaction versus passive observation of a video of her child – the current study examined maternal and child frontal asymmetry during a common mother-child play interaction (assembling a puzzle). The results are important because maternal harsh discipline, mostly known as a result of dysregulation (Chen et al., 2015), is a major causal factor in the development of child psychopathology (Teti & Cole, 2011). Thus, understanding brain activation during interaction may enhance the understanding of the way mothers and children regulate or deregulate each other. It is worth noting that child negativity was related to both maternal EEG asymmetry during interaction as well as during baseline. A possible explanation is that maternal frontal asymmetry is contextual as previously claimed (Killen & Teti, 2012), but based on a history with the child and not solely on the “here and now” interaction. This supports recent findings related to cortisol, which proposed that adrenocortical attunement is a result of a combination of diurnal and momentary synchronicity (Hibel et al., 2015). Thus, children’s behavior is related to both baseline and task maternal frontal asymmetry. Furthermore, our results indicated that child EEG asymmetry decreased significantly from baseline to task, moving from positive to negative asymmetry, proposing that children react with some negative affect to the challenging puzzle task. However, maternal asymmetry remained stable and positive, suggesting that mothers managed to regulate their emotions during their task.
Moreover, the significant correlations between child’s behavior to both baseline and task asymmetry suggests that mothers were able to stay relatively regulated and connected to their children’s behaviors even under more challenging conditions. A parent maintaining stable and positive frontal asymmetry while remaining connected to his/her child’s changes in mood and regulation seems to be a foundation of good parenting (Killen & Teti, 2012).
Child asymmetry
Guided by a bidirectional model (Bell, 1968), we demonstrated that an equivalent process occurs for the child. In other words, children’s emotional regulation systems are linked to their mothers’ negative behaviors. This finding is novel and expands upon previous research showing the links between child frontal asymmetry and maternal behavior/psychopathology. For example, research suggests that infants of depressed mothers exhibit right frontal asymmetry (Dawson et al., 1999) and that right frontal asymmetry is related to early child internalizing and externalizing problems (Fox, Schmidt, Calkins, Rubin, & Coplan, 1996). However, no study to date has assessed concurrent online child frontal asymmetry and maternal behaviors.
The theory on coregulation proposes that the parent-child dyad is critical for the development of a child’s ability to self-regulate (Tronick, 2007). Children develop self-regulation through dynamic interaction with their caregivers (Calkins, 1994). A sensitive mother who recognizes her child’s signals and responds to them in an appropriate manner has a child who develops the ability to regulate the dyadic exchange and eventually him/herself (Tronick, 2007). Our results support these findings and expand on them by showing that during mother-child interactions, not only does child emotional regulation develop, but so does maternal emotional regulation, progressively achieving a mutual regulation (i.e. reciprocity). Although most studies focus on one direction (e.g., Killeen & Teti, 2012) or the other (e.g., Forbes et al., 2008), we highlight the need to include both concurrent pathways of influence. Finally, it is worth noting, with some caution, that child’s behavior had a larger contribution to maternal EEG asymmetry than the mothers’ behavior had to their children’s EEG asymmetry. It seems that mothers who are in charge of their children’s socialization, including teaching appropriate behavior and how to behave outside the home, were more affected by their children’s behavior when the children displayed more negative behavior. Because mothers may feel responsible for their children’s behavior, they may be more influenced in this context. However, it is important to interpret these results with caution because we have yet to test these hypotheses.
Most studies have examined EEG asymmetry with high risk/pathological populations (e.g., Calkins & Dedmon, 2000; Forbes et al., 2008); however, not much is known about the typically developing population. A low risk sample was recruited for this study, enabling the examination of normative mechanisms that operate on everyday activities (e.g., assembling a puzzle).
The current study has several limitations. The physiological and behavioral data for mothers and for children were coded separately, and a time-lagged model was not used. Thus, we cannot identify the specific transactions or sequences of influence (Sameroff, 2010). However, our findings indicated that both the behavioral and physiological systems were involved during the mother-child interaction. Furthermore, because mothers were instructed to help their children during the task, a large percentage of the EEG data had artifacts. Future studies that collect more data, or alternatively, that use a task requiring less movement, will increase the amount of artifact-free data. Because this is the first study to assess same-time maternal and child behavior and frontal asymmetry – a first step in the area – there are still other avenues for future research. Further, sample characteristics were too narrow, for example, families participating in the study were middle-class, European-American families. Replicating these results with a more diverse population (e.g., ethnicity, SES, risk level for maternal harsh discipline, and child problem behavior) is necessary before these results can be generalized. Finally, only mothers were included in the study. There is a need to replicate these findings with fathers before we can generalize these results to both parents.
To conclude, the results of this study suggest that the behavioral and physiological systems are only modestly related and that during an interaction both mothers’ and children’s frontal asymmetry – a measure of response to emotional events – are more related to each other’s behaviors than to their own behaviors. Killeen and Teti (2012) claimed that, “New perspectives on parenting at risk call for increased study of parents’ in the moment, or online, emotional experiences and emotion regulation capacities during parenting events” (p. 16). The current study attended to this call and examined online EEG asymmetry while mother and child interacted with each other, rather than observing static images or videos of each other.
This study also has several implications. Results may assist in the identification of risk and resilience factors within the parent-child relationship, which may predict adaptive or pathological child and parental development. For example, intervention enhancing both the mother’s and the child’s ability to be reflective – to understand their own as well each other’s behaviors and feelings (Slade, 2005) – may help them interpret each other’s negative behaviors differently and thus reduce their emotional reaction at the physiological level.
Acknowledgments
This research was supported by grant HD057319 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) awarded to Martha Ann Bell. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health. We are grateful to the families for their participation in our research. The assistance of Leslie Patton, Morgan Hubble, and Laurel Marburg with data collection and coding is greatly appreciated.
References
- Bakeman R, Gottman JM. Observing interaction: An introduction to sequential analysis. New York, NY: Cambridge University Press; 1986. [Google Scholar]
- Bell MA, Fox NA. Brain development over the first year of life. In: Dawson G, Fischer KW, editors. Human behavior and the developing brain. New York, NY: Guilford; 1994. pp. 314–345. [Google Scholar]
- Bell RQ. A reinterpretation of the direction of effects in studies of socialization. Psychological Review. 1968;75:81–95. doi: 10.1037/h0025583. [DOI] [PubMed] [Google Scholar]
- Belsky J, de Haan M. Annual research review: Parenting and children’s brain development: The end of the beginning. Journal of Child Psychology and Psychiatry. 2011;52:409–428. doi: 10.1111/j.1469-7610.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- Calkins SD. Origins and outcomes of individual differences in emotion regulation. Monographs of the Society for Research in Child Development. 1994;59:53–72. [PubMed] [Google Scholar]
- Calkins SD. Does aversive behavior during toddlerhood matter? The effects of difficult temperament on maternal perceptions and behavior. Infant Mental Health Journal. 2002;23:381–402. [Google Scholar]
- Calkins SD, Dedmon SE. Physiological and behavioral regulation in two-year-old children with aggressive/destructive behavior problems. Journal of Abnormal Child Psychology. 2000;28:103–118. doi: 10.1023/a:1005112912906. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Development. 1996;67:523–540. [PubMed] [Google Scholar]
- Calkins SD, Howse RB, Philippot P. Individual differences in self-regulation: Implications for childhood adjustment. The Regulation of Emotion. 2004:307–332. [Google Scholar]
- Chen N, Bell MA, Deater-Deckard K. Maternal Frontal EEG Asymmetry and chronic stressors moderate the link between child conduct problems and maternal negativity. Social Development. 2015;24:323–340. doi: 10.1111/sode.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Equifinality and multifinality in developmental psychopathology. Development and Psychopathology. 1996;8:597–600. [Google Scholar]
- Coan JA, Allen JJ. Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology. 2003;40:106–114. doi: 10.1111/1469-8986.00011. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJ. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67:7–50. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJ, Harmon-Jones E. Voluntary facial expression and hemispheric asymmetry over the frontal cortex. Psychophysiology. 2001;38:912–925. doi: 10.1111/1469-8986.3860912. [DOI] [PubMed] [Google Scholar]
- Cole PM, Martin SE, Dennis TA. Emotion regulation as a scientific construct: Methodological challenges and directions for child development research. Child Development. 2004;75:317–333. doi: 10.1111/j.1467-8624.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain and Cognition. 1992;20:125–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Emotion and affective style: Physiological substrates. Electroencephalography and Clinical Neurophysiology. 1997;102:1. [Google Scholar]
- Davidson RJ, Fox NA. Asymmetrical brain activity discriminates between positive and negative affective stimuli in human infants. Science. 1982;218:1235–1237. doi: 10.1126/science.7146906. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Fox NA. Frontal brain asymmetry predicts infants’response to maternal separation. Journal of Abnormal Psychology. 1989;98:127. doi: 10.1037//0021-843x.98.2.127. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: Perspectives from affective neuroscience. Psychological Bulletin. 2000;126:890. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Dawson G, Frey K, Panagiotides H, Yamada E, Hessl D, Osterling J. Infants of depressed mothers exhibit atypical frontal electrical brain activity during interactions with mother and with a familiar, nondepressed adult. Child Development. 1999;70:1058–1066. doi: 10.1111/1467-8624.00078. [DOI] [PubMed] [Google Scholar]
- Deater-Deckard K, Pylas M, Petrill SA. Parent-child interaction system. London, UK: Institute of Psychiatry; 1997. [Google Scholar]
- Deater-Deckard K, Wang Z, Chen N, Bell MA. Maternal executive function, harsh parenting, and child conduct problems. Journal of Child Psychology and Psychiatry. 2012;53:1084–1091. doi: 10.1111/j.1469-7610.2012.02582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz A, Bell MA. Frontal EEG asymmetry and fear reactivity in different contexts at 10 months. Developmental Psychobiology. 2012;54:536–545. doi: 10.1002/dev.20612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix T, Moed A, Anderson ER. Mothers’ Depressive Symptoms Predict Both Increased and Reduced Negative Reactivity Aversion Sensitivity and the Regulation of Emotion. Psychological Science. 2014;25:1353–1361. doi: 10.1177/0956797614531025. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Spinrad TL. Emotion-related regulation: Sharpening the definition. Child Development. 2004;75:334–339. doi: 10.1111/j.1467-8624.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- Feng X, Forbes EE, Kovacs M, George CJ, Lopez-Duran NL, Fox NA, Cohn JF. Children’s depressive symptoms in relation to EEG frontal asymmetry and maternal depression. Journal of Abnormal Child Psychology. 2012;40:265–276. doi: 10.1007/s10802-011-9564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Shaw DS, Silk JS, Feng X, Cohn JF, Fox NA, Kovacs M. Children’s affect expression and frontal EEG asymmetry: Transactional associations with mothers’ depressive symptoms. Journal of Abnormal Child Psychology. 2008;36:207–221. doi: 10.1007/s10802-007-9171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA. If it’s not left, it’s right: Electroencephalograph asymmetry and the development of emotion. American Psychologist. 1991;46:863. doi: 10.1037//0003-066x.46.8.863. [DOI] [PubMed] [Google Scholar]
- Fox NA. Dynamic cerebral processes underlying emotion regulation. Monographs of the Society for Research in Child Development. 1994;59:152–166. [PubMed] [Google Scholar]
- Fox NA, Bell MA, Jones NA. Individual differences in response to stress and cerebral asymmetry. Developmental Neuropsychology. 1992;8:161–184. [Google Scholar]
- Fox NA, Calkins SD, Bell MA. Neural plasticity and development in the first two years of life: Evidence from cognitive and socioemotional domains of research. Development and Psychopathology. 1994;6:677–696. [Google Scholar]
- Fox NA, Davidson RJ, editors. The psychobiology of affective development. Hillsdale, NJ: Lawrence Erlbaum Associates; 1984. [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Rubin KH, Calkins SD, Marshall TR, Coplan RJ, Porges SW, Stewart S. Frontal activation asymmetry and social competence at four years of age. Child Development. 1995;66:1770–1784. [PubMed] [Google Scholar]
- Fox NA, Schmidt LA, Calkins SD, Rubin KH, Coplan RJ. The role of frontal activation in the regulation and dysregulation of social behavior during the preschool years. Development and Psychopathology. 1996;8:89–102. [Google Scholar]
- Gianino A, Tronick EZ. The mutual regulation model: The infant’s self and interactive regulation and coping and defensive capacities. In: Field TM, McCabe PM, Schneiderman N, editors. Stress and coping across development. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1988. pp. 47–68. [Google Scholar]
- Granger DA. Children’s salivary cortisol, internalising behaviour problems, and family environment: Results from the Concordia Longitudinal Risk Project. International Journal of Behavioral Development. 1998;22:707–728. [Google Scholar]
- Graziano PA, Keane SP, Calkins SD. Maternal behaviour and children’s early emotion regulation skills differentially predict development of children’s reactive control and later effortful control. Infant and Child Development. 2010;19:333–353. doi: 10.1002/icd.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann D, Naumann E, Thayer JF. The quest for the EEG reference revisited: A glance from brain asymmetry research. Psychophysiology. 2001;38:847–857. [PubMed] [Google Scholar]
- Henderson HA, Fox NA, Rubin KH. Temperamental contributions to social behavior: The moderating roles of frontal EEG asymmetry and gender. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40:68–74. doi: 10.1097/00004583-200101000-00018. [DOI] [PubMed] [Google Scholar]
- Hibel LC, Granger DA, Blair C, Finegood ED. Maternal-child adrenocortical attunement in early childhood: Continuity and change. Developmental Psychobiology. 2015;57:83–95. doi: 10.1002/dev.21266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NA, Field T, Davalos M. Right frontal EEG asymmetry and lack of empathy in preschool children of depressed mothers. Child Psychiatry and Human Development. 2000;30:189–204. doi: 10.1023/a:1021399605526. [DOI] [PubMed] [Google Scholar]
- Jones NA, Fox NA. Electroencephalogram asymmetry during emotionally evocative films and its relation to positive and negative affectivity. Brain and Cognition. 1992;20:280–299. doi: 10.1016/0278-2626(92)90021-d. [DOI] [PubMed] [Google Scholar]
- Killeen LA, Teti DM. Mothers’ frontal EEG asymmetry in response to infant emotion states and mother-infant emotional availability, emotional experience, and internalizing symptoms. Development and Psychopathology. 2012;24:9–21. doi: 10.1017/S0954579411000629. [DOI] [PubMed] [Google Scholar]
- Kopp CB. Emotional distress and control in young children. New Directions for Child and Adolescent Development. 1992;1992:41–56. doi: 10.1002/cd.23219925505. [DOI] [PubMed] [Google Scholar]
- Lorber MF, O’Leary SG, Kendziora KT. Mothers’ over-reactive discipline and their encoding and appraisals of toddler behavior. Journal of Abnormal Child Psychology. 2003;31:485–494. doi: 10.1023/a:1025496914522. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An Introduction to the Event-Related Potential Technique. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology. 2002;113:1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Mills H, McCarroll E. Ramifications associated with child abuse. Advances in Applied Sociology. 2012;2:274. [Google Scholar]
- Nicholson BC, Fox RA, Johnson SD. Parenting young children with challenging behaviour. Infant and Child Development. 2005;14:425–428. [Google Scholar]
- Ruttle PL, Serbin LA, Stack DM, Schwartzman AE, Shirtcliff EA. Adrenocortical attunement in mother–child dyads: Importance of situational and behavioral characteristics. Biological Psychology. 2011;88:104–111. doi: 10.1016/j.biopsycho.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Sameroff A. A unified theory of development: A dialectic integration of nature and nurture. Child Development. 2010;81:6–22. doi: 10.1111/j.1467-8624.2009.01378.x. [DOI] [PubMed] [Google Scholar]
- Sethre-Hofstad L, Stansbury K, Rice MA. Attunement of maternal and child adrenocortical response to child challenge. Psychoneuroendocrinology. 2002;27:731–747. doi: 10.1016/s0306-4530(01)00077-4. [DOI] [PubMed] [Google Scholar]
- Slade A. Parental reflective functioning: An introduction. Attachment & Human Development. 2005;7:269–281. doi: 10.1080/14616730500245906. [DOI] [PubMed] [Google Scholar]
- Smith CL, Bell MA. Stability in infant frontal asymmetry as a predictor of toddlerhood internalizing and externalizing behaviors. Developmental Psychobiology. 2010;52:158–167. doi: 10.1002/dev.20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science. 1997;8:204–210. [Google Scholar]
- Teti DM, Cole PM. Parenting at risk: New perspectives, new approaches. Journal of Family Psychology. 2011;25:625. doi: 10.1037/a0025287. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, Doss RC. Individual differences in anterior brain asymmetry and fundamental dimensions of emotion. Journal of Personality and Social Psychology. 1992;62:676. doi: 10.1037//0022-3514.62.4.676. [DOI] [PubMed] [Google Scholar]
- Tronick E. The neurobehavioral and social-emotional development of infants and children. New York, NY: WW Norton & Company; 2007. [Google Scholar]