Abstract
Background
The targeting of the prostate-specific membrane antigen (PSMA) is of particular interest for radiotheragnostic purposes of prostate cancer. Radiolabeled PSMA-617, a 1,4,7,10-tetraazacyclododecane-N,N′,N′′,N′′′-tetraacetic acid (DOTA)-functionalized PSMA ligand, revealed favorable kinetics with high tumor uptake, enabling its successful application for PET imaging (68Ga) and radionuclide therapy (177Lu) in the clinics. In this study, PSMA-617 was labeled with cyclotron-produced 44Sc (T 1/2 = 4.04 h) and investigated preclinically for its use as a diagnostic match to 177Lu-PSMA-617.
Results
44Sc was produced at the research cyclotron at PSI by irradiation of enriched 44Ca targets, followed by chromatographic separation. 44Sc-PSMA-617 was prepared under standard labeling conditions at elevated temperature resulting in a radiochemical purity of >97% at a specific activity of up to 10 MBq/nmol. 44Sc-PSMA-617 was evaluated in vitro and compared to the 177Lu- and 68Ga-labeled match, as well as 68Ga-PSMA-11 using PSMA-positive PC-3 PIP and PSMA-negative PC-3 flu prostate cancer cells. In these experiments it revealed similar in vitro properties to that of 177Lu- and 68Ga-labeled PSMA-617. Moreover, 44Sc-PSMA-617 bound specifically to PSMA-expressing PC-3 PIP tumor cells, while unspecific binding to PC-3 flu cells was not observed. The radioligands were investigated with regard to their in vivo properties in PC-3 PIP/flu tumor-bearing mice. 44Sc-PSMA-617 showed high tumor uptake and a fast renal excretion. The overall tissue distribution of 44Sc-PSMA-617 resembled that of 177Lu-PSMA-617 most closely, while the 68Ga-labeled ligands, in particular 68Ga-PSMA-11, showed different distribution kinetics. 44Sc-PSMA-617 enabled distinct visualization of PC-3 PIP tumor xenografts shortly after injection, with increasing tumor-to-background contrast over time while unspecific uptake in the PC-3 flu tumors was not observed.
Conclusions
The in vitro characteristics and in vivo kinetics of 44Sc-PSMA-617 were more similar to 177Lu-PSMA-617 than to 68Ga-PSMA-617 and 68Ga-PSMA-11. Due to the almost four-fold longer half-life of 44Sc as compared to 68Ga, a centralized production of 44Sc-PSMA-617 and transport to satellite PET centers would be feasible. These features make 44Sc-PSMA-617 particularly appealing for clinical application.
Electronic supplementary material
The online version of this article (doi:10.1186/s13550-017-0257-4) contains supplementary material, which is available to authorized users.
Keywords: 44Sc, 68Ga, 177Lu, Prostate cancer, PSMA, PET imaging, Theragnostics, Cyclotron
Background
Prostate cancer is the second most frequently diagnosed cancer type in men in the US [1]. Patients with localized disease have a good prognosis for survival; however, those diagnosed with metastatic castration-resistant prostate cancer, have much less chance of successful treatment [1, 2]. Sensitive and specific imaging tools are needed in order to enable tumor localization and staging of the disease, as well as monitoring therapy [3]. Currently, nuclear imaging of prostate cancer and related metastases is performed using 11C- and 18F-choline for positron emission tomography (PET) and 99mTc-methanediphosphonic acid for single photon emission computed tomography (SPECT), respectively [4, 5]. However, the clinical value of these radiotracers has been controversially discussed due to the low specificity and sensitivity [6, 7]. More promising may be a class of radioligands for targeting prostate-specific membrane antigen (PSMA) that has been widely investigated over the last two decades [8–10]. PSMA is a transmembrane protein, upregulated in poorly differentiated, metastatic, and hormone-refractory prostate carcinomas, while physiological expression is restricted to only a few sites, including the kidneys [11]. In recent years, a number of PSMA-targeted nuclear imaging agents were developed [12–14], among those PSMA-11, which comprises an acyclic N,N′-bis-[2-hydroxy-5-(carboxyethyl)benzyl]ethylenediamine-N,N′-diacetic acid (HBED-CC)-chelator suitable for coordination of 68Ga (T 1/2 = 68 min, Eβ+ av = 830 keV; Fig. 1, Table 1) [15, 16]. This PSMA radioligand has been used successfully in clinics for PET imaging of prostate cancer [17–19]. More recently, a structurally modified PSMA ligand, referred to as PSMA-617, has been designed with a 1,4,7,10-tetraazacyclododecane-N,N′,N′′,N′′′-tetraacetic acid (DOTA) chelator (Fig.1, Table 1). This derivative allows coordination of diagnostic and therapeutic radionuclides and, hence, it paved the way towards a theragnostic approach [20, 21]. Clinical studies performed so far demonstrated the promising potential of 68Ga- and 177Lu-labeled PSMA-617 to be used for the management of prostate cancer (Table 1) [21, 22]. 68Ga-PSMA-11 and 68Ga-PSMA-617 showed similar accumulation in most organs, albeit the renal clearance of 68Ga-PSMA-617 was significantly faster than for 68Ga-PSMA-11 [20, 21]. In patients, application of 68Ga-PSMA-11 resulted in an increased contrast on delayed images; however, all lesions were already visible on the 1 h post injection (p.i.) scans [21, 23]. On the other hand, it was suggested to scan patients 2–3 h after injection of 68Ga-PSMA-617 due to the improving image quality over time [21]. For the same reason, the scanning at even later time points (>3 h p.i.) was supposed to enable discovering additional lesions [21]; however, longer-lived radionuclides would be required for this purpose. Several 18F-based PSMA-ligands are currently under development, and the first clinical applications with 18F-DCFPyl and 18F-PSMA-1007 revealed promising results [24, 25]. The concept of radiotheragnostics, as proposed herein, is based on the use of a diagnostic and a therapeutic radionuclide with the same PSMA-targeting ligand.
Fig. 1.
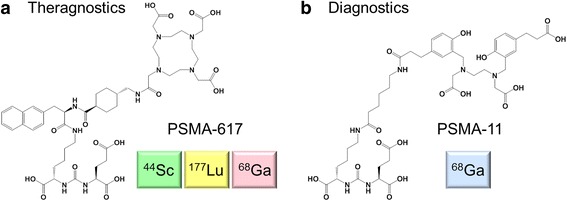
Chemical structures of a PSMA-617 and b PSMA-11 suitable for labeling with the corresponding radionuclides for theragnostic or diagnostic intervention
Table 1.
Overview of radionuclides and corresponding ligands used in this study
| Nuclide | Half-life | Radiation energy | Ligand | Chelator | Application | Refs. |
|---|---|---|---|---|---|---|
| 44Sc | 4.04 h | Eβ+
av = 632 keV Eγ = 1157 keV* |
PSMA-617 | DOTA | PET (β+-radiation) |
Evaluated in this study |
| 177Lu | 6.65 days | Eβ−
av = 134 keV Eγ = 113 keV, 208 keV |
PSMA-617 | DOTA | Therapy/SPECT (β−/γ-radiation) |
[22, 37–39] |
| 68Ga | 68 min | Eβ+
av = 830 keV Eγ = 1077 keV** |
PSMA-617 PSMA-11 |
DOTA HBED-CC |
PET (β+-radiation) |
[21] [17, 18, 40] |
*I = 99.9%; **I = 3.2%
In this regard, we proposed 44Sc (T 1/2 = 4.04 h, Table 1 [26]) as an alternative radionuclide to 68Ga for PET imaging allowing to use 44Sc-PSMA-617 as a diagnostic match to 177Lu-PSMA-617. The emitted positrons have a lower energy (Eβ+ av = 632 keV) as compared to 68Ga (Eβ+ av = 830 keV), enabling PET imaging with a potentially favorable spatial resolution [27, 28]. Recently, the production of 44Sc via the 44Ca(p,n)44Sc nuclear reaction has been implemented at the research cyclotron at Paul Scherrer Institut, providing this radionuclide with high radionuclidic purity (>99%) and at high activities (>2 GBq) [29]. There is great potential of using 44Sc clinically, as it can be produced at medical cyclotrons typically installed in PET centers worldwide. Another means of producing 44Sc is via the 44Ti/44Sc generator [30]; however, the quantity of eluted 44Sc activity is very limited (<200 MBq) in this case. In contrast, the proposed production route of 44Sc at a cyclotron would address the clinical needs and, thus, be a favorable option for application of this radionuclide in patients.
The aim of this study was to investigate PSMA-617, labeled with cyclotron-produced 44Sc, in preclinical experiments. 44Sc-PSMA-617 was prepared and evaluated for direct comparison with 177Lu- and 68Ga-PSMA-617, as well as with 68Ga-PSMA-11. PSMA-positive (PC-3 PIP) and PSMA-negative (PC-3 flu) prostate cancer cells were used for the performance of in vitro studies and, as xenografts in mice, for preclinical in vivo experiments including PET imaging.
Methods
PSMA-ligands and radionuclides
The PSMA ligands PSMA-617 and PSMA-11 were obtained from Advanced Biochemical Compounds (ABX GmbH, Radeberg, Germany). 44Sc was prepared by proton irradiation of enriched 44Ca targets at the Injector 2 cyclotron at PSI, as previously reported [29]. The irradiation of targets with ~11 MeV protons at a beam current of 50 μA lasted for 90 min. The separation of the produced 44Sc from the target material was carried out by chromatographic methods using DGA resin [29]. 44Sc was provided in an acidic solution (~0.1 M HCl, pH ~1 in ~700 μL) and was used directly for labeling reactions. No-carrier added 177Lu in HCl 0.5 M was provided by Isotope Technologies Garching (ITG GmbH, Germany). 68Ga was obtained from a 68Ge/68Ga generator (Eckert & Ziegler, Berlin, Germany) using 0.1 M HCl as an eluent (radionuclidic purity >99%).
Radiolabeling of PSMA-617 and PSMA-11
PSMA-617 was labeled with 44Sc, 68Ga, and 177Lu in a mixture of sodium acetate (0.5 M, pH 8) and HCl (0.05–0.1 M, pH ~1) at a pH of 3.5–4.5. The reaction mixture was incubated for 10 min at 95 °C. PSMA-11 was labeled with 68Ga under the same reaction conditions. Quality control of the radiolabeled PSMA ligands was performed using high-performance liquid chromatography (HPLC) with a C-18 reversed-phase column (XterraTM MS, C18, 5 μm, 150 × 4.6 mm; waters) (Additional file 1).
Determination of n-octanol/PBS distribution coefficients
The distribution coefficients (logD values) of the radioligands were determined by a shake-flask method using liquid-liquid extraction followed by phase separation, as previously reported (Additional file 1) [31]. In brief, the PSMA ligands were labeled at a specific activity of 5 MBq/nmol. Samples containing 1.25 MBq (250 pmol) of the radioligand in a volume of 25 μL were added to each vial containing 1475 μL of PBS (pH 7.4) and 1500 μL of n-octanol. The vials were vortexed vigorously for 1 min and then centrifuged for 6 min for phase separation. The concentration of radioactivity in a defined volume of each layer was measured in a γ-counter (Perkin Elmer, Wallac Wizard 1480). The distribution coefficients were expressed as the logarithm of the ratio of counts per minute (cpm) measured in the n-octanol phase to the cpm measure in the PBS phase. The values are reported as the average of at least three independent measurements (± standard deviation, SD), each performed with five replicates. Data were analyzed for significance using a one-way ANOVA test (GraphPad Prism software, version 7). A p value of <0.05 was considered as statistically significant.
Cell culture
The PC-3 PIP (PSMApos) and PC-3 flu (PSMAneg) tumor cells were kindly provided by Prof. Dr. Martin Pomper (John Hopkins Institutions, Baltimore, USA) [32]. The cells were grown in RPMI cell culture medium supplemented with 10% fetal calf serum, L-glutamine, antibiotics, and puromycin (2 μg/mL) to maintain PSMA expression (Additional file 1) [33].
Cell experiments
Determination of the PSMA affinity was performed by saturation binding assays using PC-3 PIP cells and different concentrations of nat/44Sc-, nat/177Lu-, nat/68Ga-PSMA-617, or nat/68Ga-PSMA-11, respectively (Additional file 1). The relative affinities were defined as the average of at least three independent experiments and expressed as inverse molar ratio of compound needed for half-maximal binding to PSMA and the relative affinity of 177Lu-PSMA-617 was set to 1.
Cell uptake and internalization experiments were performed with 44Sc-, 177Lu-, and 68Ga-PSMA-617 as well as 68Ga-PSMA-11 using PSMApos PC-3 PIP and PSMAneg PC-3 flu cells in order to investigate whether they behaved equally and whether the uptake was PSMA-specific. For this purpose, cells were seeded in 12-well plates (~5 × 105 cells in 2 mL RPMI medium/well) allowing adhesion and growth overnight at 37 °C. After removal of the supernatant, cells were washed once with PBS prior to the addition of RPMI medium without supplements (975 μL/well), followed by the addition of the radiolabeled PSMA ligands (MBq/nmol) to each well (25 μL, 7.5 pmol). Some of the cell samples were co-incubated with excess 2-(phosphonomethyl)pentane-1,5-dioic acid (2-PMPA; 100 μM) to block PSMA on the surface of PC-3 PIP cells. The well plates were incubated at 37 °C for 2 and 4 h, respectively. The cells were washed three times with ice-cold PBS to determine the total uptake of the radioligands (PSMA-bound fraction on the surface and internalized fraction). The internalized fraction of the radioligands was determined in cells which were washed with ice-cold PBS, then incubated for 10 min with acidic stripping buffer (0.05 M glycine stripping buffer in 100 mM NaCl, pH 2.8) followed by an additional washing step with ice-cold PBS. Cell samples were lysed by addition of NaOH (1 M, 1 mL) to each well. The samples of the cell suspensions were measured in a γ-counter (Perkin Elmer, Wallac Wizard 1480). After homogenization of the cell suspensions, the protein concentration was determined for each sample using a Micro BCA Protein Assay kit (Pierce, Therma Scientific). The results were expressed as percentage of total added radioactivity per 300 μg/mL protein.
Tumor mouse model
In vivo experiments were approved by the local veterinarian department and conducted in accordance with the Swiss law of animal protection. All mice were obtained from Charles River Laboratories (Sulzfeld, Germany), at the age of 5–6 weeks. Female, athymic nude Balb/c mice were subcutaneously inoculated with PC-3 PIP cells (6 × 106 cells in 100 μL Hank’s balanced salt solution (HBSS) with Ca2+/Mg2+) on the right shoulder and with PC-3 flu cells (5 × 106 cells in 100 μL HBSS with Ca2+/Mg2+) on the left shoulder 2 weeks before the performance of the experiments.
Biodistribution studies
44Sc-, 177Lu-, and 68Ga-PSMA-617 as well as 68Ga-PSMA-11 were intravenously injected (5 MBq, 1 nmol, 100–200 μL). Mice were sacrificed at different time points post injection (p.i.) of the radioligands. Selected tissues and organs were collected, weighed and measured using a γ-counter. The results were decay-corrected and listed as a percentage of the injected activity per gram of tissue mass (% IA/g).
Imaging studies
PET/CT scans were performed using a small-animal bench-top PET/CT scanner (G8, Sofie Biosciences, Culver City, California, USA, and Perkin Elmer, Massachusetts, USA) and a small-animal SPECT/CT scanner (NanoSPECT/CTTM, Mediso Medical Imaging Systems, Budapest, Hungary), respectively (Additional file 1). During the scans, the mice were anesthetized with a mixture of isoflurane and oxygen.
Static whole-body PET scans of 20 min duration were performed at 30 min, 2 and 4 h after injection of 44Sc-PSMA-617 (~5 MBq, 1 nmol) and of 10 min duration at 30 min and 2 h after injection of 68Ga-PSMA-617 and 68Ga-PSMA-11 (~5 MBq, 1 nmol), respectively. The PET scans were followed by a CT of 1.5 min. The SPECT scan of 45 min duration was performed 2 h after injection of 177Lu-PSMA-617 (~50 MBq, 1 nmol) followed by a CT of 7.5 min. Reconstruction of the acquired data was performed by using the software of the respective scanner.
All images were prepared using VivoQuant post-processing software (version 2.10, inviCRO Imaging Services and Software, Boston USA). A Gauss post-reconstruction filter (full width at half maximum = 1 mm) was applied to the images, which were presented with the scale adjusted to allow visualization of the most important organs and tissues, usually by cutting 0.5–1% of the lower scale.
Results
Radiolabeling and in vitro evaluation of PSMA-targeted radioligands
The radiochemical yield was always >97% for all radiolabeled compounds at a specific activity of up to 10 MBq/nmol (Additional file 1: Figure S1). The n-octanol/PBS distribution coefficients (logD values) were in the same range for 44Sc-, 177Lu-, and 68Ga-PSMA-617, but somewhat reduced for 68Ga-PSMA-11 (Table 2). The KD values obtained for 44Sc-PSMA-617 and 177Lu-PSMA-617 were in the same range, but somewhat higher values were determined for the 68Ga-labeled PSMA ligands (Additional file 1: Figure S2). The results were converted into relative PSMA-binding affinities, which were similar for 44Sc-PSMA-617 and 177Lu-PSMA-617, but slightly reduced for the 68Ga-labeled PSMA ligands (Table 2).
Table 2.
In vitro characteristics of the radiolabeled PSMA-ligands
| Radioligand | LogD valuesa | Relative PSMA-binding affinityb |
|---|---|---|
| 44Sc-PSMA-617 | −4.21 ± 0.04 | 1.2 |
| 177Lu-PSMA-617 | −4.18 ± 0.06 | 1.0 |
| 68Ga-PSMA-617 | −4.30 ± 0.10 | 0.5 |
| 68Ga-PSMA-11 | −4.82 ± 0.07 | 0.4 |
aLogD values represent the average (±SD) of 3–5 independent experiments performed in triplicate
bBinding affinity is the inverse molar ratio of the average KD values determined in four independent experiments performed in triplicates
Cell internalization studies of PSMA radioligands
Uptake and internalization of all four radioligands was investigated using PC-3 PIP/flu cells (Fig. 2). The uptake of all radioligands into PC-3 PIP cells (PSMApos) was comparable and in the range of 55–70%, whereas the internalized fraction was about 10–15% of total added activity (Fig. 2a). The uptake of all radioligands dropped to <0.5% when PC-3 flu cells (PSMAneg) were used, which proves PSMA-specific uptake/internalization of all four radioligands (Fig. 2b).
Fig. 2.
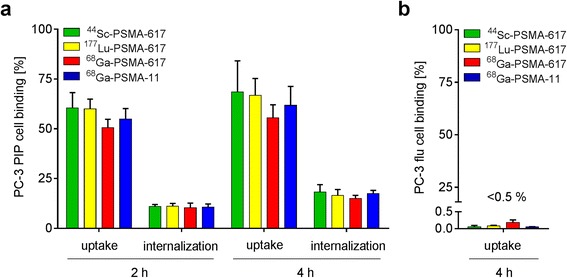
Uptake and internalization of all radioligands using a PSMApos PC-3 PIP and b PSMAneg PC-3 flu tumor cells
Biodistribution studies in tumor-bearing mice
The tissue distribution profiles of 44Sc-PSMA-617 and 177Lu-PSMA-617 were investigated in PC-3 PIP/flu tumor-bearing mice over a period of 6 h (Fig. 3, Additional file 1: Tables S1/S2). Relatively high radioactivity levels were detected in the blood shortly after injection (~7% IA/g, 15 min p.i.); however, blood activity decreased quickly over time to less than 0.5% IA/g at 2 h p.i. The uptake of both radioligands in PC-3 PIP tumors was already high 15 min after injection (36.5 ± 7.44 and 32.3 ± 3.54% IA/g) and increased further to reach a maximum uptake (51.9 ± 4.05 and 56.0 ± 8.0% IA/g) after 4 h (Fig. 3a). In PC-3 flu tumor xenografts, however, the accumulated activity was clearly below the blood level, indicating that unspecific accumulation of the radioligands did not occur (Fig. 3b). The uptake of the radioligands in the kidneys (37.7 ± 0.82 and 30.8 ± 4.52% IA/g, 15 min p.i.) was cleared quickly, resulting in renal retention of ~6% IA/g after 2 h and ~3% IA/g after 6 h (Fig. 3c). Most importantly, the tissue distribution kinetics and excretion pattern of 44Sc-PSMA-617 was comparable to that of 177Lu-PSMA-617 (Fig. 3).
Fig. 3.
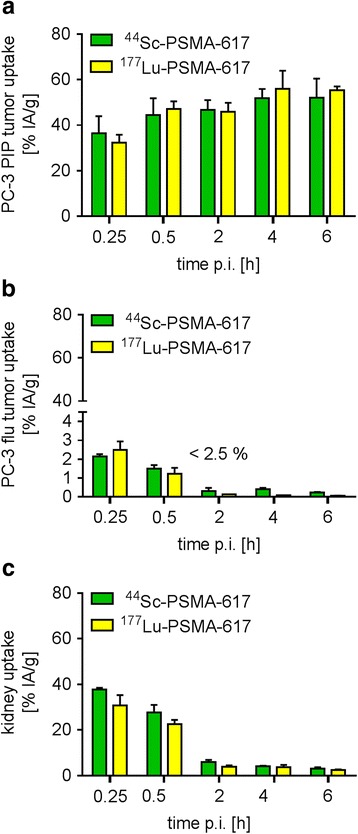
Biodistribution data of 44Sc-PSMA-617 and 177Lu-PSMA-617, respectively, in PC-3 PIP/flu tumor-bearing mice. a Uptake of the radioligands in PSMApos PC-3 PIP tumors, b in PSMAneg PC-3 flu tumors, and c clearance through the kidneys
All four radioligands were investigated under the same in vivo conditions over a period of 2 h (Fig. 4, Table 3, Additional file 1: Tables S1–S5). Clearance of radioactivity from the blood pool was fast (<0.5% IA/g, 2 h p.i) resulting in very high tumor-to-blood ratios (>200) at 2 h p.i. The accumulation of activity in PC-3 PIP tumors was comparable for all radioligands (45–49% IA/g, 2 h p.i.). The tumor-to-background ratios of 44Sc-PSMA-617 were virtually the same as those of 177Lu-PSMA-617. Renal retention of 44Sc-PSMA-617 was comparable to the 68Ga- and 177Lu-labeled versions; however, 68Ga-PSMA-11 showed a much higher uptake in the kidneys (~60% IA/g, 2 h p.i., Fig. 4). This resulted in reduced tumor-to-kidney ratios of 68Ga-PSMA-11 as compared to the other radioligands. Moreover, uptake of 68Ga-PSMA-11 in the spleen was also slightly increased. In comparison to 44Sc-PSMA-617 and 177Lu-PSMA-617, 68Ga-PSMA-617 showed reduced tumor-to-liver ratios which is due to the increased liver uptake of this radioligand.
Fig. 4.
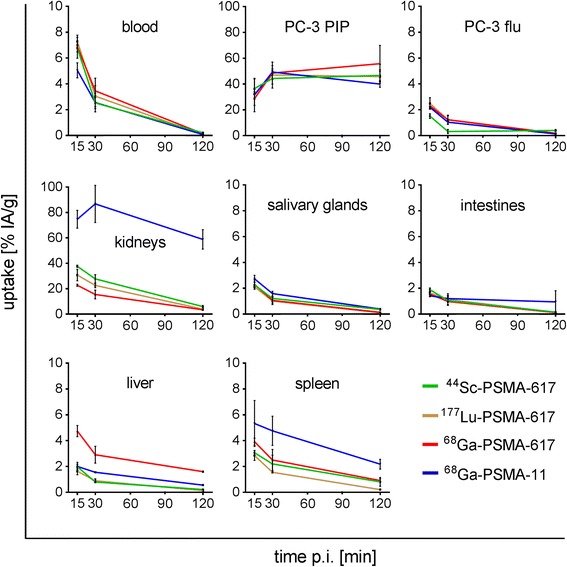
Time-dependent uptake and retention of radioactivity in different organs and tissues over the first 2 h after injection of 44Sc-PSMA-617 (green), 177Lu-PSMA-617 (yellow-brown), 68Ga-PSMA-617 (red) and 68Ga-PSMA-11 (blue)
Table 3.
Tumor-to-background ratios obtained in PC-3 PIP/flu tumor-bearing mice
| 15 min after injection | ||||
| PSMA-ligand | 44Sc-PSMA-617 | 177Lu-PSMA-617 | 68Ga-PSMA-617 | 68Ga-PSMA-11 |
| Tumor-to-blood | 5.38 ± 0.90 | 4.45 ± 0.66 | 4.15 ± 0.24 | 6.10 ± 1.84 |
| Tumor-to-liver | 18.8 ± 5.02 | 20.4 ± 5.48 | 6.02 ± 0.59 | 15.3 ± 3.96 |
| Tumor-to-kidney | 0.97 ± 0.21 | 1.06 ± 0.14 | 1.26 ± 0.25 | 0.41 ± 0.14 |
| 30 min after injection | ||||
| 44Sc-PSMA-617 | 177Lu-PSMA-617 | 68Ga-PSMA-617 | 68Ga-PSMA-11 | |
| Tumor-to-blood | 17.6 ± 1.23 | 16.0 ± 3.52 | 16.9 ± 4.22 | 20.5 ± 6.40 |
| Tumor-to-liver | 55.6 ± 7.36 | 53.1 ± 6.68 | 19.0 ± 2.32 | 31.7 ± 2.48 |
| Tumor-to-kidney | 1.60 ± 0.22 | 2.09 ± 0.24 | 3.62 ± 0.81 | 0.58 ± 0.10 |
| 2 h after injection | ||||
| 44Sc-PSMA-617 | 177Lu-PSMA-617 | 68Ga-PSMA-617 | 68Ga-PSMA-11 | |
| Tumor-to-blood | >200 | >200 | >200 | >200 |
| Tumor-to-liver | >200 | >200 | 35.2 ± 9.94 | 71.3 ± 7.48 |
| Tumor-to-kidney | 7.98 ± 1.71 | 11.6 ± 0.87 | 15.9 ± 3.26 | 0.69 ± 0.12 |
Imaging studies in PC-3 PIP/flu tumor-bearing mice
Nuclear imaging studies were performed with PC-3 PIP/flu tumor-bearing mice 2 h after injection of 44Sc-, 177Lu-, and 68Ga-PSMA-617 as well as 68Ga-PSMA-11 (Fig. 5, Additional file 1: Figure S3). PC-3 PIP tumor xenografts, located on the right shoulder, showed high uptake of activity in all four cases. No activity was detected, however, in PC-3 flu tumors on the left shoulder, demonstrating the PSMA-specific tumor accumulation of the radioligands. The PET images obtained with 44Sc- and 68Ga-PSMA-617 as well as the SPECT image obtained with 177Lu-PSMA-617 showed a similar distribution pattern and confirmed the post mortem data. The tissue distribution of 68Ga-PSMA-11 was, however, different in that it accumulated to a significantly higher extent in the kidneys.
Fig. 5.
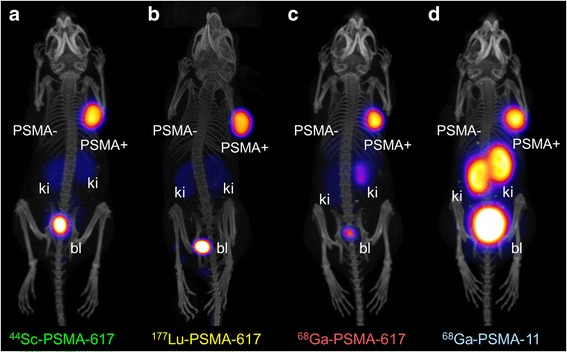
Images (PET/CT and SPECT/CT, respectively) as maximum intensity projections (MIPs) of PC-3 PIP/flu tumor-bearing mice 2 h after injection of a 44Sc-PSMA-617, b 177Lu-PSMA-617, c 68Ga-PSMA-617, and d 68Ga-PSMA-11. PSMA- PC-3 flu tumor, PSMA+ PC-3 PIP tumor, ki kidney, bl urinar bladder
PET/CT scans were performed at 30 min, 2 and 4 h after injection of 44Sc-PSMA-617 to visualize the tissue distribution in the same animal over time (Fig. 6). The PC-3 PIP tumor (PSMApos) was visible already 30 min p.i.; however, at this time point, retention of radioactivity was also seen in the kidneys. At delayed time points, when renal activity was cleared, the tumor became better visible and was finally the only site showing accumulated activity. These results demonstrated increasing tumor-to-background contrast over time and confirmed the advantage of late imaging.
Fig. 6.
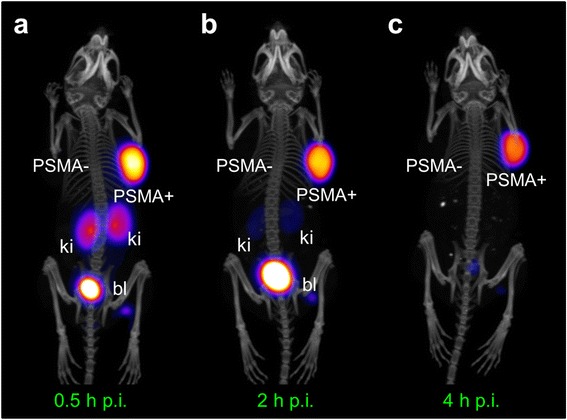
PET/CT images as maximum intensity projections (MIPs) of PC-3 PIP/flu tumor-bearing mice a 0.5, b 2, and c 4 h after injection of 44Sc-PSMA-617. PSMA- PC-3 flu tumor, PSMA+ PC-3 PIP tumor, ki kidney, bl urinary bladder
Discussion
In this study, 44Sc-PSMA-617 was compared to 68Ga-PSMA-617 and 68Ga-PSMA-11 in vitro and in tumor-bearing mice as a potential diagnostic match to the therapeutically employed 177Lu-PSMA-617 (Fig. 1). The PC-3 PIP/flu tumor cells enabled the investigation of PSMA-specific and -unspecific uptake of the radioligands in vitro and in vivo by using a PC-3 PIP and PC-3 flu tumor xenograft in the same animal.
A comparable in vitro behavior of 44Sc-PSMA-617 and 177Lu-PSMA-617, as was determined in this study, was expected due to the chemical similarities of 44Sc and 177Lu with regard to their complexation using DOTA [34]. Our data revealed almost identical characteristics of 44Sc-PSMA-617 and 177Lu-PSMA-617 with regard to their hydrophilic properties and PSMA-binding affinities (Table 2). 44Sc-PSMA-617 showed PSMA-specific cell uptake and internalization, as was the case for all three other radioligands (Fig. 2).
The evaluation of the radioligands in mice revealed a favorable tumor accumulation of 44Sc-PSMA-617 already shortly after injection and a relatively fast clearance of background activity through the kidneys (Fig. 4). These circumstances enabled PET/CT imaging with increasing tumor-to-background contrast over time (Fig. 6). The tissue distribution profile of 44Sc-PSMA-617 was largely identical to that obtained with 177Lu-PSMA-617 over the investigated period of 6 h (Fig. 3). Comparable pharmacokinetic properties of 44Sc- and 177Lu-labeled compounds were expected as previously shown in preclinical experiments with a DOTA-functionalized folate conjugate [35]. The chemical similarities of these nuclides and, as a consequence, comparable pharmacokinetics of ligands labeled with 44Sc and 177Lu, respectively, would thus, allow predicting the exact tissue distribution of 177Lu-PSMA-617 based on the PET imaging results obtained with 44Sc-PSMA-617.
The chemical structure of 68Ga-PSMA-11 is fundamentally different (Fig. 1). Not surprisingly, the tissue distribution profile of 68Ga-PSMA-11 in mice varied significantly from those of the other investigated radioligands, as was observed previously in preclinical experiments [15, 20]. High uptake of activity was found in the kidneys promptly after injection of 68Ga-PSMA-11 (Fig. 4). This observation was in agreement with the fact that in patients 68Ga-PSMA-11 showed an increased retention of activity in the kidneys as compared to 68Ga-PSMA-617 [21, 23].
Even when using the same chelator, the chemical properties of 68Ga- and 177Lu-labeled compounds are not identical due to the different coordination chemistry of these radiometals [34] which may potentially result in different in vivo kinetics [36]. In the case of 68Ga-PSMA-617, the pharmacokinetics were similar to those of the 44Sc- and 177Lu-labeled versions, with the exception of an increased uptake of 68Ga-PSMA-617 in the liver (Fig. 4). Exactly the same phenomenon has been previously observed in mice, when they were injected with 68Ga-labeled DOTA peptides [28]. In patients, increased hepatic uptake of radioactivity was not detected after injection of 68Ga-PSMA-617 [21, 23].
Although 68Ga-labeled PSMA ligands—in particular 68Ga-PSMA-11—are successfully employed in the clinics, it is indisputable that the high cost for a 68Ge/68Ga generator is a disadvantage when considering the limited activity that can be eluted daily. The short half-life of 68Ga is also a limiting factor, making transportation of activity over long distances unattractive. The almost fourfold longer half-life of 44Sc as compared to 68Ga would enable the shipment of 44Sc-based radiopharmaceuticals to PET centers without cyclotron and radiopharmaceutical facilities. Concerns of radiation safety with regard to the high-energy γ-radiation of 44Sc (Eγ = 1157 keV) may be addressed by using tungsten-based containers as are employed for other commercial PET nuclides such as 89Zr, which emits high-energy γ-radiation (Eγ = 909 keV) as well.
The use of 44Sc-PSMA-617 as a diagnostic radioligand would be novel and favorable, since the longer half-life of 44Sc permits a greater flexibility of patient scheduling to accommodate urgent cases in between, thus, allowing optimization of patient management in nuclear medicine departments. Moreover, 44Sc-PSMA-617 would allow PET/CT imaging of patients several hours after injection, potentially allowing dosimetry estimations and enabling the detection of small pathological lesions due to increased tumor-to-background ratios and, consequently, improved image contrast.
A clinical translation of the proposed concept, as is planned for the near future, will allow addressing the diverse aspects of this promising approach. Appropriate acquisition parameters for PET imaging of patients with prostate cancer remain to be determined after collecting practical experiences with 44Sc-PSMA-617 in a clinical setting.
Conclusions
In this study, the potential of 44Sc-PSMA-617 for PET imaging was demonstrated in a preclinical setting. The results indicate more similar characteristics of 44Sc-PSMA-617 to 177Lu-PSMA-617 than is the case for 68Ga-PSMA-11. An important advantage of using 44Sc over 68Ga is the feasibility of transporting 44Sc-based PSMA-ligands to PET centers without cyclotron and radiopharmaceutical facilities due to its longer half-life. The fact that 44Sc-PSMA-617 can enable delayed PET imaging makes it particularly appealing for clinical application as it may allow pretherapeutic dosimetry and an improved image quality at later time points after injection.
Acknowledgements
The authors thank Dr. Christiaan Vermeulen, Walter Hirzel, Alexander Sommerhalder, Katharina A. Domnanich, Nadezda Gracheva, Klaudia Siwowska, and Susan Cohrs for technical assistance at PSI and Dr. Konstantin Zhernosekov (ITG GmbH) for providing the 177Lu.
Funding
The research project was financially supported by the Swiss National Science Foundation (grants CR23I2_156852 and IZLIZ3_156800). The G8 PET/CT scanner was partially funded by the Swiss National Science Foundation (R’Equip grant 316030_157763).
Authors’ contributions
CAU performed and analyzed the in vitro and in vivo studies and contributed to the writing of the manuscript and preparation of the figures. MB contributed to the experiments and writing of the manuscript. RMS assisted the in vitro and in vivo studies and reviewed the manuscript. NvdM was responsible for the 44Sc production/separation and reviewed the manuscript. AT and RS reviewed the manuscript. CM designed the study, supervised the practical experiments, and finalized the manuscript and figures. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Compliance with ethical standards
This article does not contain any studies with human participants performed by any of the authors. All applicable international, national, and/or institutional guidelines for the care and use of test animals were followed.
Abbreviations
- 2-PMPA
2-(Phosphonomethyl)pentane-1,5-dioic acid
- PSMA
prostate-specific membrane antigen
- DOTA
1,4,7,10-Tetraazacyclododecane- N,N′,N′′,N′′′-tetraacetic acid
- HBED-CC
N,N′-bis-[2-hydroxy-5-(carboxyethyl)benzyl]ethylenediamine-N,N′-diacetic acid
- HBSS
Hank’s balanced salt solution
- HPLC
High performance liquid chromatography
- IA
Injected activity
- p.i.
Post injection
- PBS
Phosphate-buffered saline pH 7.4
- PET
Positron emission tomography
- PSMA
Prostate-specific membrane antigen
- SPECT
Single photon emission computed tomography
Additional file
Supporting information. (DOCX 671 kb)
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13550-017-0257-4) contains supplementary material, which is available to authorized users.
Contributor Information
Christoph A. Umbricht, Email: christoph.umbricht@psi.ch
Martina Benešová, Email: martina.benesova@psi.ch.
Raffaella M. Schmid, Email: raffaella.schmid@psi.ch
Andreas Türler, Email: andreas.tuerler@psi.ch.
Roger Schibli, Email: roger.schibli@psi.ch.
Nicholas P. van der Meulen, Email: nick.vandermeulen@psi.ch
Cristina Müller, Phone: +41-56-310 44 54, Email: cristina.mueller@psi.ch.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Chu KC, Tarone RE, Freeman HP. Trends in prostate cancer mortality among black men and white men in the United States. Cancer. 2003;97(6):1507–1516. doi: 10.1002/cncr.11212. [DOI] [PubMed] [Google Scholar]
- 3.Turkbey B, Pinto PA, Choyke PL. Imaging techniques for prostate cancer: implications for focal therapy. Nature Rev Urol. 2009;6:191–203. [DOI] [PMC free article] [PubMed]
- 4.Krause BJ, Souvatzoglou M, Treiber U. Imaging of prostate cancer with PET/CT and radioactively labeled choline derivates. Urol Oncol. 2013;31(4):427–435. doi: 10.1016/j.urolonc.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Xi R, Li C, Wang H, Wu Z, Li S. The value of 99mTc-MDP bone imaging semi-quantitative analysis in differential diagnosis of benign and malignant lesions in the prostate cancer patients. J Nucl Med. 2016;57(no. supplement 2):1768. [Google Scholar]
- 6.Tombal B, Lecouvet F. Modern detection of prostate cancer’s bone metastasis: is the bone scan era over? Adv Urol. 2012;2012:893193. doi: 10.1155/2012/893193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vali R, Loidl W, Pirich C, Langesteger W, Beheshti M. Imaging of prostate cancer with PET/CT using 18F-Fluorocholine. Am J Nucl Med Mol Imaging. 2015;5(2):96–108. [PMC free article] [PubMed] [Google Scholar]
- 8.Bouchelouche K, Choyke PL, Capala J. Prostate specific membrane antigen—a target for imaging and therapy with radionuclides. Discov Med. 2010;9(44):55–61. [PMC free article] [PubMed] [Google Scholar]
- 9.Kiess AP, Banerjee SR, Mease RC, Rowe SP, Rao A, Foss CA, et al. Prostate-specific membrane antigen as a target for cancer imaging and therapy. QJ Nucl Med Imaging. 2015;59(3):241–268. [PMC free article] [PubMed] [Google Scholar]
- 10.Haberkorn U, Eder M, Kopka K, Babich JW, Eisenhut M. New strategies in prostate cancer: prostate-specific membrane antigen (PSMA) ligands for diagnosis and therapy. Clin Cancer Res. 2016;22(1). doi:10.1158/1078-0432.CCR-15-0820. [DOI] [PubMed]
- 11.Silver DA, Pellicer I, Fair WR, Heston WDW, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 12.Foss CA, Mease RC, Fan H, Wang Y, Ravert HT, Dannals RF, et al. Radiolabeled small-molecule ligands for prostate-specific membrane antigen: in vivo imaging in experimental models of prostate cancer. Clin Cancer Res. 2005;11:4022–4028. doi: 10.1158/1078-0432.CCR-04-2690. [DOI] [PubMed] [Google Scholar]
- 13.Hillier SM, Maresca KP, Femia FJ, Marquis JC, Foss CA, Nguyen N, et al. Preclinical evaluation of novel glutamate-urea-lysine analogues that target prostate-specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer. Cancer Res. 2009;69(17):6932–6940. doi: 10.1158/0008-5472.CAN-09-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banerjee SR, Pullambhatla M, Byun Y, Nimmagadda S, Green G, Fox JJ, et al. 68Ga-labeled inhibitors of prostate-specific membrane antigen (PSMA) for imaging prostate cancer. J Med Chem. 2010;53:5333–41. [DOI] [PMC free article] [PubMed]
- 15.Eder M, Schäfer M, Bauder-Wust U, Hull WE, Wängler C, Mier W, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 2012;23(4):688–697. doi: 10.1021/bc200279b. [DOI] [PubMed] [Google Scholar]
- 16.Eder M, Neels O, Müller M, Bauder-Wust U, Remde Y, Schäfer M, et al. Novel preclinical and radiopharmaceutical aspects of [68Ga]Ga-PSMA-HBED-CC: a new PET tracer for imaging of prostate cancer. Pharmaceuticals (Basel) 2014;7(7):779–796. doi: 10.3390/ph7070779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterzing F, Kratochwil C, Fiedler H, Katayama S, Habl G, Kopka K, et al. 68Ga-PSMA-11 PET/CT: a new technique with high potential for the radiotherapeutic management of prostate cancer patients. Eur J Nucl Med Mol Imaging. 2016;43:34–41. doi: 10.1007/s00259-015-3188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the 68Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;42:197–209. doi: 10.1007/s00259-014-2949-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56(5):668–74. [DOI] [PubMed]
- 20.Benesova M, Schäfer M, Bauder-Wust U, Afshar-Oromieh A, Kratochwil C, Mier W, et al. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med. 2015;56(6):914–920. doi: 10.2967/jnumed.114.147413. [DOI] [PubMed] [Google Scholar]
- 21.Afshar-Oromieh A, Hetzheim H, Kratochwil C, Benesova M, Eder M, Neels OC, et al. The theranostic PSMA ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: biodistribution in humans, radiation dosimetry, and first evaluation of tumor lesions. J Nucl Med. 2015;56:1697–1705. doi: 10.2967/jnumed.115.161299. [DOI] [PubMed] [Google Scholar]
- 22.Ahmadzadehfar H, Rahbar K, Kürpig S, Bögemann M, Claesener M, Eppard E, et al. Early side effects and first results of radioligand therapy with 177Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res. 2015;36(5). [DOI] [PMC free article] [PubMed]
- 23.Afshar-Oromieh A, Hetzheim H, Kübler W, Kratochwil C, Giesel FL, Hope TA, et al. Radiation dosimetry of 68Ga-PSMA-11 (HBED-CC) and preliminary evaluation of optimal imaging timing. Eur J Nucl Med Mol Imaging. 2016;43(9):1611–1620. doi: 10.1007/s00259-016-3419-0. [DOI] [PubMed] [Google Scholar]
- 24.Szabo Z, Mena E, Rowe SP, Plyku, D.,, Nidal R, Eisenberger MA, Antonarakis ES, et al. Initial evaluation of [18F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015;17(4):565–74. [DOI] [PMC free article] [PubMed]
- 25.Giesel FL, Cardinale J, Schäfer M, Neels O, Benesova M, Mier W, et al. 18F-Labelled PSMA-1007 shows similarity in structure, biodistribution and tumour uptake to the theragnostic compound PSMA-617. Eur J Nucl Med Mol Imaging. 2016;43(10):1929–1930. doi: 10.1007/s00259-016-3447-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Torano E, Peyres V, Roteta M, Sanchez-Cabezudo AI, Romero E, Martinez Ortega A. Standardisation and precise determination of the half-life of 44Sc. Appl Radiat Isot. 2016;109:314–318. doi: 10.1016/j.apradiso.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Bunka M, Müller C, Vermeulen C, Haller S, Türler A, Schibli R, et al. Imaging quality of 44Sc in comparison with five other PET radionuclides using Derenzo phantoms and preclinical PET. Appl Radiat Isot. 2016;110:129–133. doi: 10.1016/j.apradiso.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Domnanich KA, Müller C, Farkas R, Schmid MR, Ponsard B, Schibli R, et al. 44Sc for labelng of DOTA- and NODAGA-functionalized peptides: preclinical in vitro and in vivo investigations. EJNMMI Radiopharm Chem. 2016;1:8. doi: 10.1186/s41181-016-0013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Meulen NP, Bunka M, Domnanich KA, Müller C, Haller S, Vermeulen CE, et al. Cyclotron production of 44Sc: from bench to bedside. Nucl Med Biol 2015;42:745–51. [DOI] [PubMed]
- 30.Rösch F. Scandium-44: benefits of a long-lived PET radionuclide available from the 44Ti/44Sc generator system. Curr Radiopharm. 2012;5(3):187–201. doi: 10.2174/1874471011205030187. [DOI] [PubMed] [Google Scholar]
- 31.Müller C, Mindt TL, de Jong M, Schibli R. Evaluation of a novel radiofolate in tumour-bearing mice: promising prospects for folate-based radionuclide therapy. Eur J Nucl Med Mol Imaging. 2009;36(6):938–946. doi: 10.1007/s00259-008-1058-9. [DOI] [PubMed] [Google Scholar]
- 32.Banerjee SR, Ngen EJ, Rotz MW, Kakkad S, Lisok A, Pracitto R, et al. Synthesis and evaluation of Gd(III)-based magnetic resonance contrast agents for molecular imaging of prostate-specific membrane antigen. Angew Chem Int Ed Engl. 2015;54(37):10778–10782. doi: 10.1002/anie.201503417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee SR, Pullambhatla M, Foss CA, Nimmagadda S, Ferdani R, Anderson CJ, et al. 64Cu-labeled inhibitors of prostate-specific membrane antigen for PET imaging of prostate cancer. J Med Chem. 2014;57(6):2657–2669. doi: 10.1021/jm401921j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majkowska-Pilip A, Bilewicz A. Macrocyclic complexes of scandium radionuclides as precursors for diagnostic and therapeutic radiopharmaceuticals. J Inorg Biochem. 2011;105(2):313–320. doi: 10.1016/j.jinorgbio.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Müller C, Bunka M, Reber J, Fischer C, Zhernosekov K, Türler A, et al. Promises of cyclotron-produced 44Sc as a diagnostic match for trivalent β−-emitters: in vitro and in vivo study of a 44Sc-DOTA-folate conjugate. J Nucl Med. 2013;54(12):2168–2174. doi: 10.2967/jnumed.113.123810. [DOI] [PubMed] [Google Scholar]
- 36.Sainz-Esteban A, Prasad V, Schuchardt C, Zachert C, Carril JM, Baum RP. Comparison of sequential planar 177Lu-DOTA-TATE dosimetry scans with 68Ga-DOTA-TATE PET/CT images in patients with metastasized neuroendocrine tumours undergoing peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging. 2012;39(3):501–511. doi: 10.1007/s00259-011-2003-x. [DOI] [PubMed] [Google Scholar]
- 37.Das T, Guleria M, Parab A, Kale C, Shah H, Sarma HD, et al. Clinical translation of 177Lu-labeled PSMA-617: initial experience in prostate cancer patients. Nucl Med Biol. 2016;43(5):296–302. doi: 10.1016/j.nucmedbio.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Rahbar K, Bode A, Weckesser M, Avramovic N, Claesener M, Stegger L, et al. Radioligand therapy with 177Lu-PSMA-617 as a novel therapeutic option in patients with metastatic castration resistant prostate cancer. Clin Nucl Med. 2016;41(7):522–528. doi: 10.1097/RLU.0000000000001240. [DOI] [PubMed] [Google Scholar]
- 39.Delker A, Fendler WP, Kratochwil C, Brunegraf A, Gosewisch A, Gildehaus FJ, et al. Dosimetry for 177Lu-DKFZ-PSMA-617: a new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43(1):42–51. doi: 10.1007/s00259-015-3174-7. [DOI] [PubMed] [Google Scholar]
- 40.Dietlein M, Kobe C, Kuhnert G, Stockter S, Fischer T, Schomäcker K, et al. Comparison of [18F]DCFPyL and [68Ga]Ga-PSMA-HBED-CC for PSMA-PET imaging in patients with relapsed prostate cancer. Mol Imaging Biol. 2015;17:575–84. [DOI] [PMC free article] [PubMed]


