Abstract
With the combined purpose of facilitating useful vision over a lifetime, a number of ocular cells have evolved specialized features not found elsewhere in the body. The trabecular meshwork (TM) cell at the irido-corneal angle, which is a key regulator of intraocular pressure, is no exception. Examination of cells in culture isolated from the human TM has shown that they are unique in many ways, displaying characteristic features of several different cell types. Thus, these neural crest derived cells display expression patterns and behaviors typical of endothelia, fibroblasts, smooth muscle and macrophages, owing to the multiple roles and two distinct environments where they operate to maintain intraocular pressure homeostasis. In most individuals, TM cells function normally over a lifetime in the face of persistent stressors, including phagocytic, oxidative, mechanical and metabolic stresses. Study of TM cells isolated from ocular hypertensive eyes has shown a compromised ability to perform their daily duties. This review highlights the many responsibilities of the TM cell and its challenges, progress in our understanding of TM biology over the past 30 years, as well as discusses unanswered questions about TM dysfunction that results in IOP dysregulation and glaucoma.
Keywords: trabecular meshwork, glaucoma, cell culture, cellular phenotype
I. Trabecular Meshwork Structure and Function
Trabecular meshwork (TM) cells are the primary cell type that occupy and form the proximal portion of the conventional outflow pathway, the primary egress route for aqueous humor from the eye. In this passageway resistance to unobstructed outflow is generated, regulated and responsible for homeostatic intraocular pressure (IOP) control. In coordination with the inner wall of Schlemm’s canal (SC), IOP is rigorously maintained within a couple of millimeters of mercury by the TM and SC for about 90% of people over a lifetime (David et al., 1987, Klein et al., 1992). Unfortunately, cellular dysfunction in the conventional outflow pathway, including the TM, results in the generation of “extra” resistance that causes elevated IOP (ocular hypertension) characteristic of most types of primary open-angle glaucoma (POAG) (Grant, 1951, Grant, 1963). Thus, in POAG, the most common type of glaucoma, the irido-corneo angle is open and there are no gross abnormalities or clinically visible accumulation of material in the TM.
The TM is an avascular, architecturally complex connective tissue bridging Schwalbe’s line to the scleral spur/ciliary muscle; and spanning the entire length of SC. The TM can be anatomically divided into three regions (from inner to outermost): (i) the uveal meshwork, which is closest to the anterior chamber and consists of a network of collagen and elastin lamellae covered by TM cells with large “open spaces” in between individual lamellae; (ii) the corneoscleral meshwork is the “middle layer”, which is composed of a series of perforated collagen and elastin plates covered by TM cells; and (iii) the juxtacanalicular (JCT) TM is a loose connective tissue containing TM cells surrounded by extracellular matrix between the outermost corneoscleral plates and the inner wall of SC. The ciliary muscle is anatomically and functionally linked to the SC inner wall via a network of elastin fibers that extend from the tips of the longitudinal fibers through the corneoscleral and JCT TM, anchoring onto the inner wall (reviewed by (Tamm, 2009)). These three divisions are considered part of the “filtering” TM since they lay directly over SC. A fourth division called the “insert” region of the TM is considered “non-filtering” since it resides just below Schwalbe’s line, adjacent to, but not in front of SC. Data suggest that this region contains a population of TM stem cells (reviewed by (Kelley et al., 2009)).
The TM tissue has two primary responsibilities to assure effective outflow resistance regulation over nearly a century of life. As aqueous humor leaves the eye, it first encounters the uveal and corneoscleral TM, which function as a self-cleaning biological filter; intercepting cellular debris and reactive oxygen species before reaching the resistance generating region of the JCT. The JCT region of the TM is populated and maintained by JCT-TM and inner wall of SC cells. The precise manner by which JCT-TM cells and SC cells function to generate and regulate outflow resistance (and thus IOP) is not completely known. However, it appears that the two cell types work together physically and functionally to generate outflow resistance, to restrict flow of aqueous humor into the lumen of SC and onto the systemic venous system (Reviewed by (Overby et al., 2009)).
While TM cells that populate the conventional outflow tissues have two different morphologies, both have a common embryological origin, the neural crest (Tripathi and Tripathi, 1989). Uniquely, these mesenchymal cells display characteristics of four different cell types, supporting the two primary responsibilities of the TM (Table 1). Thus, requisite for an occupation as a biological filter, cells of the inner TM have macrophage-like activity. Acting as professional phagocytes, TM cells clear cellular debris derived from shed pigmented epithelia propelled forward toward the irido-corneo angle by the flow of aqueous humor (Samuelson et al., 1984, Grierson and Lee, 1973, Rohen and van der Zypen, 1968). Importantly, debris is rapidly cleared by the inner TM before reaching deep into the TM where it might accumulate and interfere with resistance generation and regulation. Cultured TM cells as well as TM cells in vivo are actively phagocytic (Johnson et al., 1989, Epstein et al., 1986, Tripathi and Tripathi, 1982), which appears to be an essential part of keeping the “outflow filter” clean. In addition to engulfing pigment granules and debris, TM cells also experimentally phagocytize latex beads and other labeled particles (Johnson et al., 1989, Yue et al., 1987, Grierson et al., 1986). Similar to macrophages, TM cells express scavenger receptors, thought to be used for uptake of foreign and waste materials. In fact, early identification of TM cells was partially dependent upon their unique expression of receptors that mediate acetylated low density lipoprotein uptake (Chang et al., 1991, Stamer et al., 1995b). Interestingly, factors other than defects in phagocytosis are thought responsible for secondary forms of glaucoma that display accumulation of pigment or exfoliation material (Matsumoto and Johnson, 1997, Epstein et al., 1986).
Table 1.
TM phenotypes, tissue location, cell behavior and associated responsibilities
| Phenotype | Location | Cell Behavior | Responsibilities |
|---|---|---|---|
| Endothelial | Uveal/corneoscleral | Endothelia | Maintain passageway patency |
| Neutralize reactive oxygen species | |||
| Macrophage | Biological filter/phagocytosis | ||
| Immune mediation | |||
| Fibroblastic | Juxtacanalicular tissue | Fibroblast | ECM turnover/tissue repair |
| Smooth muscle | Contractile tone | ||
| Mechanotrasduction |
To maintain a clear outflow pathway, the cells of the inner TM completely cover the elaborate collagen-elastin lamellae and plates as thin continuous monolayers. Functioning as endothelia, TM cells produce large quantities of antithrombogenic substances, like heparin sulfate and tissue-plasminogen activator (tPA). Incredibly, TM cells produce one hundred times more tPA than vascular endothelia, emphasizing the importance of passageway patency (Snyder et al., 1993, Shuman et al., 1988). Also like endothelia, cells from the inner TM participate in antigen presentation and inflammation mediation, producing major histocompatibility proteins and a wide array of inflammatory cytokines, respectively (Shifera et al., 2010, Tripathi et al., 1990a, Latina et al., 1988, Lynch et al., 1987). In fact, laser trabeculoplasty takes advantage of the TM’s role in local inflammatory mediation and resolution to decrease outflow resistance. Hence, delivery of laser energy to the TM results in the rapid secretion of IL-1α and TNFα in response to the injury, stimulating extracellular matrix turnover and debris phagocytosis (Bradley et al., 2000, Latina et al., 1998).
In line with their second responsibility, resistance generation, TM cells in the JCT region have both fibroblastic and smooth muscle-like qualities. For example, TM cells secrete a number of extracellular matrix proteins and their cognate degradation enzymes to support the continual remodeling of extracellular matrix (reviewed by (Keller et al., 2009). Indeed, the activity of TM cells resembles fibroblasts at a wound site, turning over matrix proteins as rapidly as every 48 hours (Acott et al., 1988). In keeping with the interplay between extracellular matrix turnover and cytoskeletal tension, TM cells in the JCT and corneoscleral region are contractile, expressing smooth muscle actin and myosin (reviewed by (Tian et al., 2009)). Cellular contractile force generation by the TM cells counter tension that is imposed by the ciliary muscle, which extends elastic tendons into the TM, connecting with its elastic network that extends to the inner wall of SC (Overby et al., 2014, Rohen et al., 1981). Thus, contraction of the ciliary muscle pulls on the TM elastic network, opening spaces between lamellae and cribiform plates plus preventing collapse of the SC lumen (Li et al., 2014, Lutjen-Drecoll, 1973). Together, the relationship between the ciliary muscle, TM and SC modifies/maintains flow pathways to increase outflow facility. Paradoxically, treatment of TM tissues with drugs that decrease contractility, such as actin depolymerizing agents or rho kinase inhibitors, also increases outflow facility (Rao et al., 2001, Kaufman and Erickson, 1982). While the precise mechanism is unknown, rho kinase inhibitors increase the separation distance between the outermost cribiform plate and the SC inner wall (Yang et al., 2013), emphasizing the importance of the relationship between JCT TM and SC inner wall cells in outflow resistance generation and dampening of IOP spikes.
II. TM cells reside in a biologically demanding environment
Owing to their location, pressure-sensitivity, lack of a blood supply and continual exposure to byproducts of UV radiation and cellular metabolism, the cells of the TM weather a combination of stresses not experienced by most other cells in the body. For instance, TM cells on a daily basis experience mechanical stress due to routine daily activities such as eye rubbing, squinting, blinking, ocular pulse and saccades that range from a 20–105% increase of their original dimensions (Coleman and Trokel, 1969, Johnstone and Grant, 1973). In fact, recent studies by Downs show that about 15% of all energy in the eye is due to pressure spikes (on top of steady state IOP)(Downs, 2015). To survive such insults, TM cells possess adaptations such as a prominent cytoskeleton, complex cell-cell and cell-matrix attachments and expression of water channels to facilitate rapid changes in cell volume following stretch (Baetz et al., 2009, Grierson and Lee, 1975, Bhatt et al., 1995, Tumminia et al., 1998).
TM cells, particularly in the inner meshwork, continually endure oxidative stress in the form of byproducts from UV energy interacting with aqueous humor, cornea and lens epithelium. Cells of the TM express a number of oxidative reducing agents, including super oxide dismutase, glutathione reductase and peroxidase to neutralize reactive species (Russell and Johnson, 1996, De La Paz and Epstein, 1996, Nguyen et al., 1985). An example of the extraordinary oxidative capacity of TM cells was demonstrated when 1 mM hydrogen peroxide was injected intracamerally into calf eyes and hydrogen peroxide was not detectable in effluent exiting the aqueous veins (Nguyen et al., 1988).
Because the conventional outflow tract is the final destination for aqueous humor before it leaves the eye, TM cells experience constant metabolic and phagocytic stress. Metabolic activity of other avascular ocular cells upstream of the TM, such as crystalline lens and cornea deplete the aqueous humor of nutrients and deposit waste products. Aqueous humor also carries cell debris from epithelial turnover and apoptosis events upstream plus pigment granules from the iris rubbing on the crystalline lens that increases with age. The inner TM cells intercept these materials, protecting the functionality of the outer TM for proper resistance generation. Thus, filtering of aqueous humor of cell debris, in addition to the normal remodeling of the tissue, impose a continual phagocytic stress on the TM. Like a macrophage, TM cells that have phagocytosed large cellular debris detach from their matrix and leave the TM (Sherwood and Richardson, 1988). It is unclear in healthy eyes whether detached TM cells are replaced by TM insert (stem) cells; however, trabecular beams in healthy eyes remain cellularized, suggesting that lost cells are replaced.
III. Original isolation and characterization of cultured TM cells
Significant advances in our understanding of the normal function of the TM and TM cells have come from pioneering studies on cultured of TM cells from several species. The first report of TM cell culture was published by Rohen and colleagues, who cultured TM cells from dissected primate TM explants (Rohen et al., 1975). The cultured TM cells appeared metabolically active with high amounts of endoplasmic reticulum, ribosomes, and mitochondria. These cells were also capable of phagocytosis of pigment granules. This same group subsequently showed that these primate TM cells synthesized the glycosaminoglycans hyaluronic acid, dermatan sulfate, and chondroitin-4-sulfate and suggested that these GAGs might function as an outflow filter for the aqueous humor (Schachtschabel et al., 1982). Soon after, Jon Polansky, Jorge Alvarado and colleagues cultured and characterized human TM cells from explants dissected from postmortem eyes (Alvarado et al., 1982, Polansky et al., 1979). They morphologically and ultrastructurally compared these cells to human corneal keratinocytes, scleral fibroblasts, and corneal endothelial cells, which could be potential contaminating cells upon dissection of the TM explants. TM cells formed confluent monolayers with long overlapping processes, whereas the keratinocytes and scleral fibroblasts grew much faster, in multiple layers, and had the characteristic elongated fibroblast morphology and cellular foci. They reported that the cultured human TM cells had many features of TM cells in vivo, including the expression of fibronectin, gap junctions, apical villous processes (including cilia), and numerous coated vesicles at the basal surface. About the same time, Ramesh and Brenda Tripathi cultured human TM cells and morphologically and ultrastructurally compared these cells to corneal endothelial cells, keratocytes, and scleral fibroblasts, with similar findings (Tripathi and Tripathi, 1982). They also reported that these human TM cells had greater phagocytic activity compared to the other cell types, which could be blocked with the microfilament disrupting agent cytochalasin B. Ian Grierson and colleagues cultured bovine TM cells and found that confluent, contact inhibited monolayers better reflected the behavior and activities of TM cells in vivo (Grierson et al., 1985). They also showed that the cultured bovine TM cells were phagocytically active (Grierson et al., 1986). Therefore, early studies of cultured TM cells from 3 different species displayed a number of common morphological and functional characteristics of TM cells in vivo.
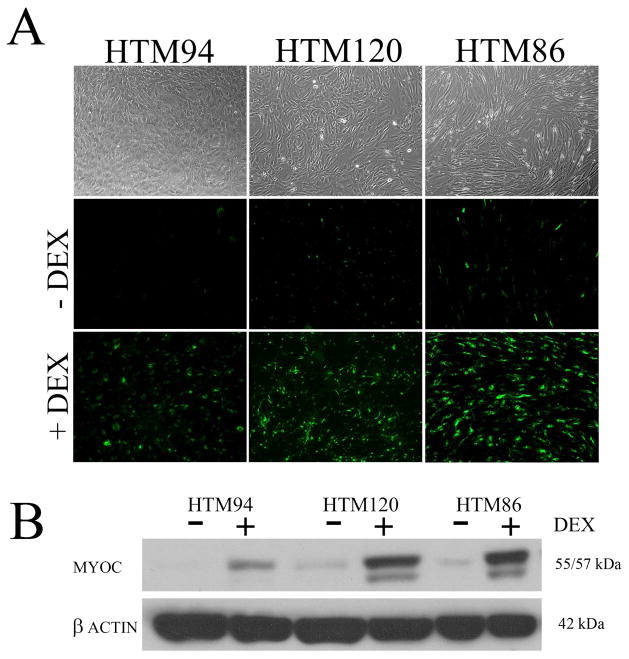
Cell isolates from the TM have one of two general morphologies: Cells obtained from the inner TM that cover collagen/elastic beams/plates of the uveal and corneoscleral TM have a round to oval shape, a large cell body with some overlapping processes. These TM cells appear “endothelial-like” upon reaching confluence in culture, with cobblestone morphology (Figure 1). In contrast, cells isolated from the deeper JCT region of the TM have a more elongated, spindle-shaped morphology with many overlapping processes. Upon reaching confluence in culture, JCT cells appear to have a smooth muscle-like or fibroblastic appearing morphology (Figure 1). Whether TM and JCT cells are the same cell “type” in different biological environments or two different cell types has been the subject of debate for decades (Flugel et al., 1991, Coroneo et al., 1991) (Ge et al., 2016). Regardless of their phenotype, TM cells are contact inhibited with primary isolates and low passage cells having a doubling time in culture of less than two days (Polansky et al., 1979). The proportion of endothelial-like cells versus fibroblastic/smooth muscle-like cells obtained during isolations depends upon two factors: the depth of dissection into the TM and the age of the donor eyes. For example, cells obtained from eyes of younger donors with well-populated lamellae tend to give preferentially endothelial-like cultures, whereas eyes from older donors with less populated lamellae generally give cultures dominated by fibroblastic/smooth muscle-like TM cells. Of course, the fresher the donor tissue, the more cells are obtained in either case. Not surprising, TM cells isolated from eyes of younger donors are smaller, more abundant, have faster doubling times and can be passaged more times before reaching senescence.
Figure 1.
Morphology and response to corticosteroid of human trabecular meshwork cell monolayers in culture. Panel A shows phase contrast and immunofluorescence microscopic images of three human trabecular meshwork cell monolayers, each isolated from different human donor eyes and displaying different morphologies. HTM 94 shows predominant “endothelial-like” morphology, while HTM120 and HTM86 are mixed cultures with primarily “myofibroblast” morphologies. Cell monolayers were probed with antibodies raised against myocilin in the presence or absence of 100 nM dexamethasone (DEX) treatment for 5 days. To assess magnitude of myocilin induction, corresponding western blots of treated and untreated TM cell monolayers are shown in panel B.
IV. Types of TM Cell Cultures
TM cells have a very limited replicative potential in situ (Kimpel and Johnson, 1992, Dueker et al., 1990). However, unlike their corneal endothelial neighbors, isolated primary TM cells proliferate until they form a contact inhibited monolayer in culture. As mentioned above, primary TM cell cultures are often started from TM tissue explants (Steely et al., 1992, Grierson et al., 1985, Tripathi and Tripathi, 1982, Polansky et al., 1979, Rohen et al., 1975). However, some use proteases to partially digest the dissected TM tissue prior to culture (Stamer et al., 1995b). Although primary TM cell cultures are normally passaged by trypsin, the cells become more senescent with each passage. Therefore some laboratories routinely passage cells using gelatin-coated Cytodex beads (Steely et al., 1992), where the TM cells are not routinely exposed to trypsin and appear to maintain their initial phenotypes for longer periods in culture. Using these methods, primary TM cell cultures have been derived from multiple species including human (Polansky et al., 1979, Snyder et al., 1993, Steely et al., 1992, Tripathi and Tripathi, 1982), nonhuman primates (Rohen et al., 1975)], cows (Mao et al., 2012a, Grierson et al., 1985), pigs (Alexander et al., 1998, O’Brien et al., 1996, Tripathi et al., 1991), and mice (Mao et al., 2013). All these TM cells have consistent phenotypes; however, TM cell cultures from cows and pigs tend to be dominated by “endothelial-like” cells due to their anatomy; having prominent inner TM and angular aqueous plexi instead of SC.
TM cells are routinely grown on plastic culture plates or flasks in defined culture medium containing fetal bovine serum (FBS). Most investigators use high amounts of serum (10–20%) to promote cell division while expanding cultures. Some, use a differentiation step once cells are confluent whereby supplementary growth factors are withdrawn (such as FGF) and/or fetal bovine serum levels are reduced (to 1–10%) (Wax et al., 1989, Ge et al., 2016). Freddo and colleagues have reported in vivo leakage of serum proteins at the iris root adjacent to TM tissue, so it is likely that the TM in vivo is also exposed to serum components (Freddo, 1993). Estimates place serum concentrations in the TM at about 1% v/v of aqueous humor. Alternatively, Fautsch and colleagues have cultured human TM cells in medium mixed with aqueous humor rather than FBS (Resch et al., 2010) because TM cells in situ are bathed in AH. For example, myocilin expression is significantly diminished in cultured TM cells, but expression is greatly increased in the presence of AH. While TM cells effectively and rapidly lay down ECM on plastic, there was concern that plastic is not ideal for studying TM cell biology. Paul Russell has measured the normal elasticity (Young’s modulus) of human TM tissue (Last et al., 2011) and has cultured human TM cells on substrates of varying stiffness, demonstrating that TM cell behavior and phenotypes are quite different compared to cells grown on plastic (Wood et al., 2011, McKee et al., 2011). Others have cultured TM cells on porous filters and exposed the cells in attempt to more closely mimic aqueous flow through the TM (Ramos et al., 2009, Perkins et al., 1988). Confluent TM cells make their own ECM substrate, and depending on the culture conditions, the ECM content and stiffness can change. For example, NTM cells treated with the glucocorticoid, dexamethasone, deposit a stiffer substrate compared to control TM cells (Raghunathan et al., 2015).
Because some primary TM cells grow rather slowly in culture, particularly from older donor eyes, and have a finite passage lifetime, several labs have generated transformed or spontaneously immortalized TM cells. Transformed human TM cell lines, including GTM3 and NTM5 cells (Pang et al., 1994), grow faster and proliferate indefinitely, and these cell lines are widely used (Figure 2). However, these cell lines have a very different phenotype compared to primary TM cells: (1) they are not contact inhibited and continue to grow past the monolayer stage, (2) the cells are smaller and have less cytoplasm, (3) the cells do not form an extensive cytoskeleton, and (4) they do not make as much ECM as primary cultures. One major advantage of these transformed and immortalized cells is that they are much easier to transfect compared to primary TM cell cultures. Note that many eye research journals (including Experimental Eye Research, IOVS, and Molecular Vision) require that any work done with transformed cell lines be replicated in primary cell cultures, ex vivo or in vivo models. Unlike the other transformed human TM cell lines, the spontaneous immortalized bovine BTM28T cell line is diploid with 60 pairs of chromosomes and is contact inhibited growing as a monolayer (Mao et al., 2012b). This cell line has the same phenotype as primary bovine TM cells except that it has been propagated greater than 50 passages.
Figure 2.
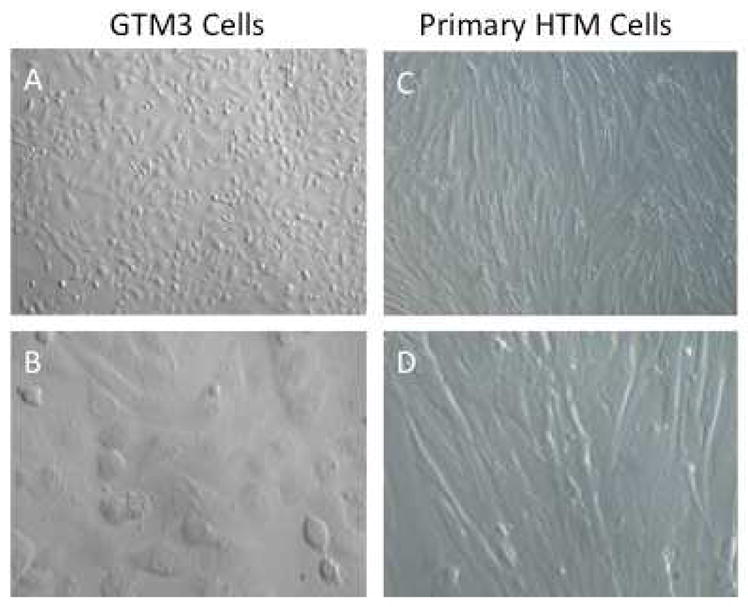
Morphological comparison between transformed GTM3 cells and primary cultures of human TM cells. GTM3 cells (A-10X and B-40X) are smaller cells with less cytoplasm and continue cell division (rounded cells) despite being confluent. Primary human TM cells (NTM176-04)(C-10X and D-40X) are larger and more elongated with overlapping processes. These cells are contact inhibited and stop dividing when they become a confluent monolayer.
In attempt to provide a more natural environment for cultured TM cells, perfusion “organ” cultured anterior segments have been developed for human (Johnson and Tschumper, 1987), nonhuman primate (Hu et al., 2006), bovine (Mao et al., 2011, Erickson-Lamy et al., 1988), and porcine (Keller et al., 2008) eyes. The lens, iris, and ciliary body are removed from these anterior segments prior to being mounted on specially designed Plexiglass dishes, sealed with an O-ring, and perfusion cultured with culture medium (generally lacking FBS). The perfusion medium enters the bottom of the dish and is perfused through the trabecular outflow pathway and into the episcleral veins. These ex vivo cultures can be run under conditions of constant flow (using a perfusion pump) and variable pressure (continuously recording pressure with a pressure transducer) or as constant pressure (medium reservoir set at specific height) and variable flow (flow measured by loss of fluid from medium reservoir). In many labs, these ex vivo models can be cultured for weeks with very healthy TM tissue. These ex vivo models have been extremely useful to study and validate glaucoma related pathways (discussed below), drug effects on the trabecular outflow system (Pang et al., 2001, Pang et al., 2000, Gottanka et al., 2004, Wan et al., 2007), and circumferential differences in outflow resistance and biochemistry (Keller et al., 2011).
V. TM cell markers
The search for marker proteins that specifically identify TM cells in culture began over 30 years ago, when TM cells were first isolated. Unfortunately, decades later a specific marker is still not available. In addition to positive identification of TM cells in culture, specific marker proteins will enable selective genetic targeting and modification of TM cells in organ culture and transgenic mouse models. Moreover, the future of gene therapy and genome editing in the TM, a tissue ideally suited for such modifications, is likely dependent upon tissue-specific promoters. There are two main reasons that have made TM marker protein discovery difficult. Firstly, TM cell responsibilities overlap with neighboring cells and secondly, TM cells reside in two distinct environments within the conventional pathway, demanding different aptitudes. Thus, when TM cells are isolated, cultures are generally mixed, comprised of both cell “phenotypes” (Figure 1). In the future, it will be useful to expression profile cells from each region, isolated from the same donor eye. Alternatively, profiling of TM versus neighboring cells using RNAseq in inbred mice will speed up the identification of unique promotors/proteins in the TM.
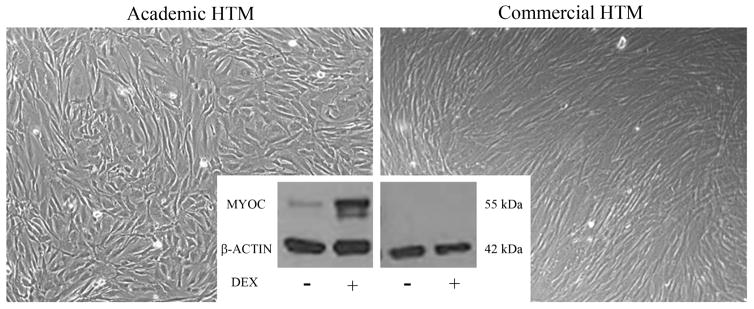
Since the detection of a single TM marker has been elusive, researchers rely on a panel of proteins (positive selection markers) and behaviors that typify TM cells in culture (Table 2). Studies have revealed a few proteins preferentially expressed by TM cells including aquaporin-1, alpha 2A adrenergic receptors, collagen IV, alpha-B crystallin and myocilin (Stamer et al., 1996, Stamer et al., 1998, Stamer et al., 1995a, Nguyen et al., 1998a, Polansky et al., 2000, Siegner et al., 1996). These proteins are differentially expressed or induced by TM cells compared to their cell neighbors that include ciliary muscle cells, scleral spur cells, scleral fibroblasts, Schlemm’s canal endothelia and corneal endothelia (Welge-Lussen et al., 1999, Hernandez et al., 1987). For example, myocilin is a protein whose expression is inordinately high in the TM compared to cell neighbors, but decreases with time in culture (Fautsch et al., 2005). Interestingly, myocilin is dramatically upregulated when TM cells are treated with glucocoticoids, a feature that is not shared by neighboring cells (Figures 1 and 3) (Polansky et al., 2000). Thus, glucocorticoid induction of myocilin is the most common and reliable way used today to identify TM cells. It is important to monitor changes in myocilin expression, both by immunocytochemistry and western blot. Immunocytochemistry shows the percentage of isolated cells responsive to glucocorticoid (70–80% is typical) and whether myocilin immunoreactivity displays a peri-Golgi and vesicular phenotype (O’Brien et al., 1999, Stamer et al., 1998). Western blot will validate specificity of anti-myocilin antibody being used, appearing as a 55–57 kDa doublet band. Some anti-myocilin antibodies have reported a larger 65 kDa band that appears to be non-specific staining, likely cross-reacting with albumin (molecular mass of 66.5 kDa). On a cautionary note, cells that are purchased commercially (Sciencell, Innoprot, etc) must be characterized before use, since methods used by companies to characterize commercial cells often are not standard practice for the field. For example, Figure 3 shows that while a commercial “TM” cell strain appears similar morphologically to human TM cells, this particular strain did not upregulate myocilin in response to glucocorticoid treatment and thus is likely not a TM cell.
Table 2.
Identification of TM cells in culture; differentially expressed proteins and responses to stimuli compared to potential contaminating cells
| Positive Markers | Negative Markers | Cellular responses |
|---|---|---|
| Myocilin | VE-cadherin | Acetylated LDL uptake |
| Matrix GLA protein | Fibulin-2 | αvb5/β3-mediated phagocytosis |
| Chitinase-3 like-1 | Integrin α6 | CS induction of myocilin, FN, CLANs |
| Aquaporin-1 | Keratin | CS down regulation of tPA, phagocytosis |
| Smooth muscle actin | Desmin | PMA, TNFα and IL-1α stimulation of MMP-3/-9 |
| Smooth muscle myosin | VEGFR3 | TGFβ induction of alpha-B crystallin, PAI-I, MMP2, CLANs |
| Alpha-B crystallin | Prox-1 | Stretch induction of MMP-2/-3 |
Abbreviations: VE=vascular endothelial; LDL=low density lipoprotein; CS= corticosteroid; FN=fibronectin; CLANs=cross-linked actin networks; tPA= tissue-type plasminogen activator; PMA=phorbol ester; TNF= tumor necrosis factor; IL= interleukin; MMP= matrix metalloproteinase; PAI= plasminogen activator inhibitor; VEGFR3: vascular endothelial growth factor receptor type 3.
Figure 3.
Morphology and corticosteroid response of a commercial human “trabecular meshwork” cell (HTM) culture compared to a human trabecular meshwork cell strain isolated in an academic setting. The phase contrast images show subtle phenotypic differences; however, western blot analysis of myocilin show clear differences in both basal expression and induction by dexamethasone (DEX).
Other examples of TM behavior are TGFβ induction of PAI-I, MMP2 and myocilin (Tamm et al., 1999). Moreover, TM cells consistently upregulate MMP3 and MMP9 when treated with IL-1α, TNFα or phorbol ester (Kelley et al., 2007a, Kelley et al., 2007b, Hosseini et al., 2006, Fleenor et al., 2003, Alexander and Acott, 2003, Shearer and Crosson, 2001) (Table 2). As mentioned above, TM cells readily take up acetylated low density lipoprotein (Chang et al., 1991).
Array and proteomic expression studies have also revealed a number of proteins that are preferentially expressed by TM cells, including, matrix GLA protein and chitinase-3 like-1 (Gonzalez et al., 2004, Liton et al., 2005). Unfortunately, these proteins are not expressed by all cells in the TM, but only subpopulations. Similarly, only a portion of cells of the TM express smooth muscle actin and myosin (de Kater et al., 1992, de Kater et al., 1990). There are a few marker proteins that TM cells do not express that are used as negative selection markers. For example the endothelial proteins PECAM-1, VE-cadherin, integrin α6, VEGFR3, Prox-1 and fibulin-2 are expressed by SC cells, but not by TM (Heimark et al., 2002, Kizhatil et al., 2014, Perkumas and Stamer, 2012, VanderWyst et al., 2011). Likewise, the epithelial proteins keratin and desmin are not expressed by TM cells (Welge-Lussen et al., 1998, Weinreb and Ryder, 1990, Tripathi and Tripathi, 1982).
VI. TM Cell responses to stress hormones and cytokines
Cultured TM cells respond to a wide variety of glaucoma-like insults. Glucocorticoid (GC) treatment of TM cells in culture led to the identification of the first POAG gene Myocilin (MYOC) (Nguyen et al., 1998b, Stone et al., 1997, Polansky et al., 1997). Since then, GC induction of myocilin has been described in bovine (Mao et al., 2012a) and mouse TM cells (Mao et al., 2013), making this a very reproducible phenotype to characterize and validate TM cells as mentioned above. GCs also induce a major actin cytoskeletal rearrangement to form cross-linked actin networks (CLANs) in confluent cultured human TM cells (Clark et al., 1994, Wilson et al., 1993), which has also been shown in cultured bovine (Wade et al., 2009) and mouse (Mao et al., 2013) TM cells. Significantly, these CLANs are observed in the TM tissues of perfusion cultured human anterior segments treated with dexamethasone (Clark et al., 2005). Moreover, CLANs are more prevalent in cultured glaucomatous TM cells (Clark and Wordinger, 2009, Clark et al., 1995) and in situ in human glaucoma eyes (Hoare et al., 2009). GCs have also been shown to induce extracellular matrix production in cultured TM cells and tissues, including fibronectin (Steely et al., 1992), laminin (Dickerson et al., 1998), collagens (Zhou et al., 1998, Hernandez et al., 1985), and glycosaminoglycans (Engelbrecht-Schnur et al., 1997, Johnson et al., 1990). GCs inhibit TM cell proliferation and migration (Clark et al., 1994) as well as inhibit TM cell phagocytosis (Zhang et al., 2007). Therefore, GC treatment of TM cells has been used extensively to better understand GC-induced ocular hypertension. Importantly, GC-induced changes in TM cells are similar in many ways to glaucomatous changes in the TM (Wordinger and Clark, 1999).
The anti-inflammatory profibrotic cytokine, TGFβ2, has been implicated in the pathogenesis of POAG because: (1) TGFβ2 levels are elevated in the aqueous humor (Lutjen-Drecoll, 2005, Tripathi et al., 1994, Agarwal et al., 2015, Trivedi et al., 2011, Min et al., 2006, Yamamoto et al., 2005, Ozcan et al., 2004, Ochiai and Ochiai, 2002, Picht et al., 2001, Inatani et al., 2001) and TM (Tovar-Vidales et al., 2008) of POAG patients, and (2) activated TGFβ2 elevates IOP in perfusion cultured human (Fleenor et al., 2006, Gottanka et al., 2004) and porcine (Bachmann et al., 2006) anterior segments as well as in mouse eyes in vivo where the TM was transduced with an Ad5.TGFβ2 expression vector (Shepard et al., 2010). The time course of IOP induction occurred over days, consistent with observed effects on ECM accumulation in the TM. TGFβ serves as a profibrotic signal for cultured TM cells due to induced expression of α-smooth muscle actin (Tamm et al., 1996), a variety of extracellular matrix proteins, including fibronectin (Medina-Ortiz et al., 2013, Fleenor et al., 2006), collagens (Fuchshofer et al., 2007), plasminogen activator inhibitor-1 (PAI-1) (Fuchshofer et al., 2003), and extracellular matrix crosslinking enzymes transglutaminase-2 (TGM2) (Tovar-Vidales et al., 2011, Welge-Lussen et al., 2000), lysyl oxidase (LOX), and LOXL1–4 (Sethi et al., 2011b). TGFβ2 also inhibits TM cell proliferation (Wordinger et al., 1998), which may at least partially be responsible for the decreased cell density in the inner TM tissues of POAG eyes (Alvarado et al., 1984).
Expression of connective tissue growth factor (CTGF) is induced by TGFβ2 and has been implicated in a number of fibrotic diseases (Moussad and Brigstock, 2000, Franklin, 1997). CTGF also is induced in human TM cells by TGFβ1, mechanical stretch, and increased IOP (Chudgar et al., 2006). CTGF directly increases the expression of a wide variety of ECM proteins, including fibronectin, collagens I, III, IV, and VI as well as self-induction of CTGF, thereby generating feed-forward signaling. Blocking CTGF expression by RNA interference inhibited the TGFβ2 induction of CTGF and fibronectin (Junglas et al., 2009). Anti-CTGF antibody treatment also decreased ECM production in cultured TM cells (Wallace et al., 2013). In addition, a recent study showed that nanoparticles coated with cyclic RGD inhibited CTGF signaling in TM cells (Hennig et al., 2016). These studies suggest that at least part of the profibrotic effects of TGFβ2 are mediated by CTGF. The question of whether CTGF plays a role in regulating IOP was nicely answered in mice with Ad5.CTGF transduction of TM tissue and a transgenic mouse model where CTGF expression was driven by a βB1-crystallin promoter (Junglas et al., 2012). Junglas and colleagues showed increased expression of CTGF in the TM in both the inducible and transgenic models, which led to significantly elevated IOP and optic nerve damage. This was associated increased actin stress fiber formation and increased expression of α-smooth muscle actin.
As implicated with TGFβ2/CTGF treatments, aqueous humor outflow resistance is mediated in part by the TM ECM. Inhibition of ECM degrading matrix metalloproteinases (MMPs) leads to increased outflow resistance in perfusion cultured human anterior segments (Bradley et al., 1998), and TM cells express a number of MMPs, including MMP1–3, MMP9, and MMP14 (Conley et al., 2004, Fuchshofer et al., 2003, Pang et al., 2003, Alexander and Acott, 2001). The therapeutic success of argon laser trabeculectomy is mediated by activation and release of proinflammatory cytokines, especially TNFα and IL-1α from TM cells (Bradley et al., 2000), which “remodel” the TM tissue via activation of MMP3 (Fleenor et al., 2003, Alexander and Acott, 2003, Parshley et al., 1995). Urokinase and tissue plasminogen activators, which are also expressed in TM cells (Tripathi et al., 1990b, Park et al., 1987), help proteolytically convert the zymogen proMMPs to MMPs.
VII. Glaucomatous TM Cells
There are a number of pathological changes that have been reported in the glaucomatous TM. There is a progressive decrease of TM cellularity with age that is exacerbated in POAG eyes (Grierson and Howes, 1987, Alvarado et al., 1984). There also is the accumulation of plaque-like material, extracellular material in the JCT (Lutjen-Drecoll et al., 1986, Rohen, 1983, Lutjen-Drecoll et al., 1981), and increased deposition of fibronectin (Medina-Ortiz et al., 2013, Babizhayev and Brodskaya, 1989) in the TM tissues of POAG eyes. There is increased endoplasmic reticulum and protein misfolding stress in glaucomatous TM tissue and cells, as seen by significantly increased expression of GRP78, GRP94 and CHOP, suggesting that the GTM cells undergo chronic protein stress in glaucoma (Peters et al., 2015). Using atomic force microscopy, Last and colleagues showed that TM tissue at the level of the JCT is stiffer (decreased elasticity) and regionally more variable in POAG eyes compared to aged-matched normal eyes (Last et al., 2011). However, the circumferential elasticity of POAG TM (including uveal, corneoscleral and JCT) tissue appears to be greater (Camras et al., 2014) and may contribute to enhanced SC collapse and irreversible herniations into collector channels found in glaucoma (personal communication, Haiyan Gong).
Knepper and colleagues have shown the aqueous humor of POAG eyes has higher concentrations of soluble CD44 (sCD44) compared to control eyes (Knepper et al., 2002) and that this sCD44 isoform was hypophosphorylated (Knepper et al., 2005). Moreover, aqueous humor concentrations of sCD44 correlate with progressive visual field loss in POAG patients (Nolan et al., 2007). Consistent with this observation, transduction of the TM in mouse eyes with either Ad5.sCD44 or Ad5.CD44 significantly elevates IOP (Giovingo et al., 2013). These data suggest that sCD44, a multifunctional glycoprotein protein involved in cell-cell interactions and adhesion, may play a pathogenic role in POAG.
Normal TM cells have been cultured with a wide variety of glaucoma-like insults and display some features of glaucomatous TM cells. For example, treatment of normal TM cells with exogenous TGFβ2 enhances the production of some ECM molecules (including the profibrotic fibronectin isoform EDA) and increases PAI1 expression, thereby inhibiting plasminogen activator activation of MMPs. TGFβ2 also increases the expression of ECM cross-linking enzymes such as TGM2 and LOXs as well as induces CLAN cytoskeletal rearrangement (O’Reilly et al., 2011) in cultured TM cells. There is increased expression of the EDA fibronectin isoform (Medina-Ortiz et al., 2013), TGM2 (Tovar-Vidales et al., 2008), and CLANs (Hoare et al., 2009) in POAG TM tissues.
Some laboratories have been able to culture TM cells from OAG donor eyes that display detectible differences compared to cells isolated from donor eyes without a history of OAG (Table 3). For example, primary cultures of GTM cells demonstrate decreased migratory ability from explants and defects in autophagy (Porter et al., 2015, Stamer et al., 2000). Moreover, the majority of POAG patients are considered GC responders (Clark and Wordinger, 2009, Wordinger and Clark, 1999), and cultured GTM cells are more responsive to GCs compared to NTM cells. For example, these GTM cells have a greater basal level of CLANs and are more responsive to GC-induced CLAN formation (Uchida et al., 2008, Clark et al., 1995). GTM cells have a greater induction of a GRE-luciferase reporter gene compared to NTM cells (Zhang et al., 2005). An alternatively spliced form of the glucocorticoid receptor (GRβ) acts as a dominant regulator of GC activity (Schaaf and Cidlowski, 2002), and GRβ expression is lower in GTM cells, making these more sensitive to GCs (Zhang et al., 2005), possibly due to altered expression of splicesome protein SFRS5.1, which increases splicing of GRβ in cultured TM cells (Jain et al., 2012).
Table 3.
Summary of Differences Between NTM and GTM Cells
| Feature | Difference in GTM | |
|---|---|---|
| TM Tissue (in vivo) | TM cellularity | Decrease |
| Deposition of ECM in outflow pathway | Increase | |
| Fibronectin expression | Increase total Increase EDA isoform | |
| Elasticity | Decrease at JCT Increase circumferentially | |
| TGFβ2 expression | Increase | |
| TGM2 expression | Increase | |
| sCD44 expression | Increase | |
| ELAM1 expression | Increase | |
| Cochlin expression | Increase | |
| ER/protein misfolding stress | Increase | |
| TM Cells (in vitro) | Migratory ability | Decrease |
| Autophagy | Decrease | |
| GC responsiveness | Increase | |
| GRβ expression | Decrease | |
| CLANs | Increase | |
| TGFβ2 expression | Increase | |
| Gremlin expression | Increase | |
| SFRP1 expression | Increase | |
| Cochlin expression | Increase | |
| SAA2 expression | Increase |
[See Sections VI and VII for details and references]
Gene and protein expression comparing normal TM cells and tissues to GTM cells and tissues have lead to the discovery of a number of new pathogenic pathways. Cultured GTM cells express higher levels of TGFβ2 (Tovar-Vidales et al., 2008), possibly due to epigenetic regulation of histone acetylation in TM cells [Bermudez et al., submitted for publication]. Treatment with a potent HDAC inhibitor elevates TGFβ2 expression in TM cells and tissues, with a corresponding increase in IOP in perfusion cultured anterior segments. TM cells express bone morphogentic proteins (BMPs) and BMP receptors (Wordinger et al., 2002), which normally antagonize TGFβ2 signaling (Wordinger et al., 2007, Fuchshofer et al., 2007). However, expression of the BMP antagonist gremlin is elevated in GTM cells, and over-expression of gremlin elevates IOP in perfusion cultured human anterior segments (Wordinger et al., 2007) and in mouse eyes (McDowell et al., 2015). TGFβ2 increases gremlin expression and gremlin increases TGFβ2 expression in cultured TM cells (Sethi et al., 2011a), creating a profibrotic environment. In addition to TGFβ2/BMP signaling, TM cells also have a functional canonical β-catenin Wnt pathway that regulates aqueous outflow and IOP (Mao et al., 2012b, Wang et al., 2008b). GTM cells and tissues have higher mRNA and protein expression of SFRP1, an inhibitor of Wnt signaling (Wang et al., 2008b), and overexpression of SFRP1 increases aqueous humor outflow resistance and elevates IOP in perfusion cultured human anterior segments and in mouse eyes (Mao et al., 2012a, Wang et al., 2008b). mRNA and protein expression of the acute phase gene SAA2 is elevated in cultured GTM cells, and treatment of perfusion cultured human anterior segments with recombinant SAA2 elevated IOP (Wang et al., 2008a). SAA2 treated TM cells increased IL-8 secretion and did not cause obvious amyloid deposits in the TM. A proteomic analysis of trabeculectomy tissue from POAG patients compared to TM tissue dissected from normal donor eyes identified increased expression of cochlin, and expression of this protein was also elevated in the TM region of the glaucomatous DBA/2J mouse (Bhattacharya et al., 2005a, Bhattacharya et al., 2005b). Perfusion culture of monkey anterior segments with recombinant cochlin increased aqueous outflow resistance (Lee et al., 2010). Expression of ELAM1 in POAG TM tissue has also been reported to be a distinct marker of POAG, and ELAM1 expression activated by IL-1 in an autocrine feedback loop in GTM cells (Wang et al., 2001). Global changes in gene expression of normal and POAG TM tissues have recently been reported (Liu et al., 2013), which may further increase our understanding of glaucomatous damage to the TM.
VIII. Unresolved questions and future directions
Given the handful of laboratories working in the area of TM cell biology, progress has been exemplary over the past 30 years. For example, despite only 20–25 laboratories/year funded over the past decade by NIH to study TM biology (NIH Reporter, using trabecular meshwork as searchable term in abstracts) these research teams have been instrumental in helping bring 5 drugs to late stage clinical trials that target the trabecular meshwork (Kopczynski and Epstein, 2014).
Drugs in trials are thought to function by relaxing the TM, improving access of aqueous humor to SC and opening new flow pathways plus enhancing turnover of ECM. For example, basic research on TM cell biology have resulted in the development of drugs that target rho kinase (Rao et al., 2001), adenosine A1 receptors (Shearer and Crosson, 2002) and cyclic GMP (Wiederholt et al., 1994). In addition to these, there will likely be multiple other drug classes that target the conventional outflow pathway to restore functionality. Consequently, there is a clinical need for (i) drugs that transiently “clean out” accumulated debris, particularly after some cataract removals and in exfoliation glaucoma; (ii) drugs that assist in phagocytic processing of materials; (iii) drugs that stimulate resident stem cell division (Bradley et al., 2000), (v) specific disease modifying therapies based on our understanding of molecular pathogenesis, and (vi) gene therapeutics. Several drugs that target the diseased conventional outflow tract will be needed because “glaucoma” is a group of diseases, most of which have in common elevated IOP and defects in conventional outflow. Depending upon the type of glaucoma (e.g.: myocilin versus psuedoexfoliation versus steroid-induced), the diseased pathway in the conventional tract will likely be different.
Even with such progress, there remain many questions about this complicated tissue that when answered will uncover new drug targets and specific treatments for TM pathologies.
Which TM cell functions and/or interactions with the inner wall of SC altered in glaucoma are responsible for increased outflow resistance and elevated IOP?
What strategies are needed to identify specific TM cell marker(s)?
Are JCT cells really TM cells in a different environment or are they a separate cell type or epigenetically modified?
What is the relationship between JCT cells and SC inner wall cells and how do they determine outflow resistance in normal and glaucoma eyes?
Does TM cell and tissue stiffness affect outflow resistance?
Are CLANs involved in outflow resistance, and why are they increased in POAG?
Does enhanced TM ECM crosslinking affect outflow resistance?
Which of the plethora of GC and TGFβ1/2 effects on the TM are responsible for IOP elevation?
Is there cross-talk between the pathogenic signaling pathways identified to date?
What awakens stem cell population in TM insert region- are these cells unresponsive in some types of glaucoma?
What happens to TM cells in psuedoexfoliation/pigment dispersion syndrome vs. glaucoma that makes them unresponsive to shed cellular materials?
How can mouse models help us better understand the TM?
Highlights.
Trabecular meshwork cells are responsible for regulating aqueous humor outflow resistance
Defective trabecular meshwork function can cause elevated IOP and the development of glaucoma
Trabecular meshwork cells are a hybrid cells, containing properties of endothelia, myofibroblasts, and monocytes
The study of TM cells in culture has led to the development of new glaucoma therapies
Acknowledgments
The authors would like to acknowledge the following grant support: EY022356 and Prevent Blindness Foundation (for WDS) as well as EY016242, EY022363 and EY024259 (for AFC). We would also like to thank Iris Navarro, Mike Dismuke, and Tara Tovar-Vidales for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ACOTT TS, KINGSLEY PD, SAMPLES JR, VAN BUSKIRK EM. Human trabecular meshwork organ culture: morphology and glycosaminoglycan synthesis. Invest Ophthalmol Vis Sci. 1988;29:90–100. [PubMed] [Google Scholar]
- AGARWAL P, DAHER AM, AGARWAL R. Aqueous humor TGF-beta2 levels in patients with open-angle glaucoma: A meta-analysis. Mol Vis. 2015;21:612–20. [PMC free article] [PubMed] [Google Scholar]
- ALEXANDER JP, ACOTT TS. Involvement of protein kinase C in TNFalpha regulation of trabecular matrix metalloproteinases and TIMPs. Invest Ophthalmol Vis Sci. 2001;42:2831–8. [PubMed] [Google Scholar]
- ALEXANDER JP, ACOTT TS. Involvement of the Erk-MAP kinase pathway in TNFalpha regulation of trabecular matrix metalloproteinases and TIMPs. Invest Ophthalmol Vis Sci. 2003;44:164–9. doi: 10.1167/iovs.01-1201. [DOI] [PubMed] [Google Scholar]
- ALEXANDER JP, SAMPLES JR, ACOTT TS. Growth factor and cytokine modulation of trabecular meshwork matrix metalloproteinase and TIMP expression. Curr Eye Res. 1998;17:276–85. doi: 10.1076/ceyr.17.3.276.5219. [DOI] [PubMed] [Google Scholar]
- ALVARADO J, MURPHY C, JUSTER R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984;91:564–79. doi: 10.1016/s0161-6420(84)34248-8. [DOI] [PubMed] [Google Scholar]
- ALVARADO JA, WOOD I, POLANSKY JR. Human trabecular cells. II. Growth pattern and ultrastructural characteristics. Invest Ophthalmol Vis Sci. 1982;23:464–78. [PubMed] [Google Scholar]
- BABIZHAYEV MA, BRODSKAYA MW. Fibronectin detection in drainage outflow system of human eyes in ageing and progression of open-angle glaucoma. Mech Ageing Dev. 1989;47:145–57. doi: 10.1016/0047-6374(89)90017-1. [DOI] [PubMed] [Google Scholar]
- BACHMANN B, BIRKE M, KOOK D, EICHHORN M, LUTJEN-DRECOLL E. Ultrastructural and biochemical evaluation of the porcine anterior chamber perfusion model. Invest Ophthalmol Vis Sci. 2006;47:2011–20. doi: 10.1167/iovs.05-1393. [DOI] [PubMed] [Google Scholar]
- BAETZ NW, HOFFMAN EA, YOOL AJ, STAMER WD. Role of aquaporin-1 in trabecular meshwork cell homeostasis during mechanical strain. Exp Eye Res. 2009;89:95–100. doi: 10.1016/j.exer.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHATT K, GONG H, FREDDO TF. Freeze-fracture studies of interendothelial junctions in the angle of the human eye. Invest Ophthalmol Vis Sci. 1995;36:1379–89. [PubMed] [Google Scholar]
- BHATTACHARYA SK, ANNANGUDI SP, SALOMON RG, KUCHTEY RW, PEACHEY NS, CRABB JW. Cochlin deposits in the trabecular meshwork of the glaucomatous DBA/2J mouse. Exp Eye Res. 2005a;80:741–4. doi: 10.1016/j.exer.2005.01.028. [DOI] [PubMed] [Google Scholar]
- BHATTACHARYA SK, ROCKWOOD EJ, SMITH SD, BONILHA VL, CRABB JS, KUCHTEY RW, ROBERTSON NG, PEACHEY NS, MORTON CC, CRABB JW. Proteomics reveal Cochlin deposits associated with glaucomatous trabecular meshwork. J Biol Chem. 2005b;280:6080–4. doi: 10.1074/jbc.M411233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADLEY JM, ANDERSSOHN AM, COLVIS CM, PARSHLEY DE, ZHU XH, RUDDAT MS, SAMPLES JR, ACOTT TS. Mediation of laser trabeculoplasty-induced matrix metalloproteinase expression by IL-1beta and TNFalpha. Invest Ophthalmol Vis Sci. 2000;41:422–30. [PubMed] [Google Scholar]
- BRADLEY JM, VRANKA J, COLVIS CM, CONGER DM, ALEXANDER JP, FISK AS, SAMPLES JR, ACOTT TS. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest Ophthalmol Vis Sci. 1998;39:2649–58. [PubMed] [Google Scholar]
- CAMRAS LJ, STAMER WD, EPSTEIN D, GONZALEZ P, YUAN F. Circumferential tensile stiffness of glaucomatous trabecular meshwork. Invest Ophthalmol Vis Sci. 2014;55:814–23. doi: 10.1167/iovs.13-13091. [DOI] [PubMed] [Google Scholar]
- CHANG IL, ELNER G, YUE YJT, CORNICELLI A, KAWA JE, ELNER VM. Expression of modified low-density lipoprotein by trabecular meshwork cells. Curr Eye Res. 1991;10:1101–1112. doi: 10.3109/02713689109024127. [DOI] [PubMed] [Google Scholar]
- CHUDGAR SM, DENG P, MADDALA R, EPSTEIN DL, RAO PV. Regulation of connective tissue growth factor expression in the aqueous humor outflow pathway. Mol Vis. 2006;12:1117–26. [PubMed] [Google Scholar]
- CLARK AF, BROTCHIE D, READ AT, HELLBERG P, ENGLISH-WRIGHT S, PANG IH, ETHIER CR, GRIERSON I. Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil Cytoskeleton. 2005;60:83–95. doi: 10.1002/cm.20049. [DOI] [PubMed] [Google Scholar]
- CLARK AF, MIGGANS ST, WILSON K, BROWDER S, MCCARTNEY MD. Cytoskeletal changes in cultured human glaucoma trabecular meshwork cells. J Glaucoma. 1995;4:183–8. [PubMed] [Google Scholar]
- CLARK AF, WILSON K, MCCARTNEY MD, MIGGANS ST, KUNKLE M, HOWE W. Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1994;35:281–94. [PubMed] [Google Scholar]
- CLARK AF, WORDINGER RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009;88:752–9. doi: 10.1016/j.exer.2008.10.004. [DOI] [PubMed] [Google Scholar]
- COLEMAN DJ, TROKEL S. Direct-recorded intraocular pressure variations in a human subject. Archives of Ophthalmology. 1969;82:637–40. doi: 10.1001/archopht.1969.00990020633011. [DOI] [PubMed] [Google Scholar]
- CONLEY SM, BRUHN RL, MORGAN PV, STAMER WD. Selenium’s effects on MMP-2 and TIMP-1 secretion by human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2004;45:473–9. doi: 10.1167/iovs.03-0767. [DOI] [PubMed] [Google Scholar]
- CORONEO MT, KORBMACHER C, FLUGEL C, STIEMER B, LUTJEN-DRECOLL E, WIEDERHOLT M. Electrical and morphological evidence for heterogeneous populations of cultured bovine trabecular meshwork cells. Exp Eye Res. 1991;52:375–88. doi: 10.1016/0014-4835(91)90032-a. [DOI] [PubMed] [Google Scholar]
- DAVID R, ZANGWILL L, STONE D, YASSUR Y. Epidemiology of intraocular pressure in a population screened for glaucoma. Br J Ophthalmol. 1987;71:766–71. doi: 10.1136/bjo.71.10.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE KATER A, SHAHSAFAEI A, EPSTEIN D. Localization of smooth muscle and nonmuscle actin isoforms in the human aqueous outflow pathway. Invest Ophthalmol and Vis Sci. 1992;33:424–429. [PubMed] [Google Scholar]
- DE KATER A, SPURR-MICHAUD S, GIPSON I. Localization of smooth muscle myosin-containing cells in the aqueous outflow pathway. Invest Ophthalmol and Vis Sci. 1990;31:347–353. [PubMed] [Google Scholar]
- DE LA PAZ MA, EPSTEIN DL. Effect of age on superoxide dismutase activity of human trabecular meshwork. Invest Ophthalmol Vis Sci. 1996;37:1849–53. [PubMed] [Google Scholar]
- DICKERSON JE, JR, STEELY HT, JR, ENGLISH-WRIGHT SL, CLARK AF. The effect of dexamethasone on integrin and laminin expression in cultured human trabecular meshwork cells. Exp Eye Res. 1998;66:731–8. doi: 10.1006/exer.1997.0470. [DOI] [PubMed] [Google Scholar]
- DOWNS JC. IOP telemetry in the nonhuman primate. Exp Eye Res. 2015;141:91–8. doi: 10.1016/j.exer.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUEKER DK, NORBERG M, JOHNSON DH, TSCHUMPER RC, FEENEY-BURNS L. Stimulation of cell division by argon and Nd:YAG laser trabeculoplasty in cynomolgus monkeys. Invest Ophthalmol Vis Sci. 1990;31:115–24. [PubMed] [Google Scholar]
- ENGELBRECHT-SCHNUR S, SIEGNER A, PREHM P, LUTJEN-DRECOLL E. Dexamethasone treatment decreases hyaluronan-formation by primate trabecular meshwork cells in vitro. Exp Eye Res. 1997;64:539–43. doi: 10.1006/exer.1996.0232. [DOI] [PubMed] [Google Scholar]
- EPSTEIN DL, FREDDO TF, ANDERSON PJ, PATTERSON MM, BASSETT-CHU S. Experimental obstruction to aqueous outflow by pigment particles in living monkeys. Invest Ophthalmol Vis Sci. 1986;27:387–95. [PubMed] [Google Scholar]
- ERICKSON-LAMY K, ROHEN JW, GRANT WM. Outflow facility studies in the perfused bovine aqueous outflow pathways. Curr Eye Res. 1988;7:799–807. doi: 10.3109/02713688809033211. [DOI] [PubMed] [Google Scholar]
- FAUTSCH MP, HOWELL KG, VRABEL AM, CHARLESWORTH MC, MUDDIMAN DC, JOHNSON DH. Primary trabecular meshwork cells incubated in human aqueous humor differ from cells incubated in serum supplements. Invest Ophthalmol Vis Sci. 2005;46:2848–56. doi: 10.1167/iovs.05-0101. [DOI] [PubMed] [Google Scholar]
- FLEENOR DL, PANG IH, CLARK AF. Involvement of AP-1 in interleukin-1alpha-stimulated MMP-3 expression in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2003;44:3494–501. doi: 10.1167/iovs.02-0757. [DOI] [PubMed] [Google Scholar]
- FLEENOR DL, SHEPARD AR, HELLBERG PE, JACOBSON N, PANG IH, CLARK AF. TGFbeta2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci. 2006;47:226–34. doi: 10.1167/iovs.05-1060. [DOI] [PubMed] [Google Scholar]
- FLUGEL C, TAMM E, LUTJEN-DRECOLL E. Different cell populations in bovine trabecular meshwork: an ultrastructural and immunocytochemical study. Exp Eye Res. 1991;52:681–90. doi: 10.1016/0014-4835(91)90020-f. [DOI] [PubMed] [Google Scholar]
- FRANKLIN TJ. Therapeutic approaches to organ fibrosis. Int J Biochem Cell Biol. 1997;29:79–89. doi: 10.1016/s1357-2725(96)00121-5. [DOI] [PubMed] [Google Scholar]
- FREDDO TF. The Glenn A. Fry Award Lecture 1992: aqueous humor proteins: a key for unlocking glaucoma? Optom Vis Sci. 1993;70:263–70. doi: 10.1097/00006324-199304000-00003. [DOI] [PubMed] [Google Scholar]
- FUCHSHOFER R, WELGE-LUSSEN U, LUTJEN-DRECOLL E. The effect of TGF-beta2 on human trabecular meshwork extracellular proteolytic system. Exp Eye Res. 2003;77:757–65. doi: 10.1016/s0014-4835(03)00220-3. [DOI] [PubMed] [Google Scholar]
- FUCHSHOFER R, YU AH, WELGE-LUSSEN U, TAMM ER. Bone morphogenetic protein-7 is an antagonist of transforming growth factor-beta2 in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007;48:715–26. doi: 10.1167/iovs.06-0226. [DOI] [PubMed] [Google Scholar]
- GE P, NAVARRO ID, KESSLER MM, BERNIER SG, PERL NR, SARNO R, MASFERRER J, HANNIG G, STAMER WD. The Soluble Guanylate Cyclase Stimulator IWP-953 Increases Conventional Outflow Facility in Mouse Eyes. Invest Ophthalmol Vis Sci. 2016;57:1317–26. doi: 10.1167/iovs.15-18958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIOVINGO M, NOLAN M, MCCARTY R, PANG IH, CLARK AF, BEVERLEY RM, SCHWARTZ S, STAMER WD, WALKER L, GRYBAUSKAS A, SKURAN K, KUPRYS PV, YUE BY, KNEPPER PA. sCD44 overexpression increases intraocular pressure and aqueous outflow resistance. Mol Vis. 2013;19:2151–64. [PMC free article] [PubMed] [Google Scholar]
- GONZALEZ P, CABALLERO M, LITON PB, STAMER WD, EPSTEIN DL. Expression analysis of the matrix GLA protein and VE-cadherin gene promoters in the outflow pathway. Invest Ophthalmol Vis Sci. 2004;45:1389–95. doi: 10.1167/iovs.03-0537. [DOI] [PubMed] [Google Scholar]
- GOTTANKA J, CHAN D, EICHHORN M, LUTJEN-DRECOLL E, ETHIER CR. Effects of TGF-beta2 in perfused human eyes. Invest Ophthalmol Vis Sci. 2004;45:153–8. doi: 10.1167/iovs.03-0796. [DOI] [PubMed] [Google Scholar]
- GRANT WM. Clinical tonography. Trans Am Acad Ophthalmol Otolaryngol. 1951;55:774–81. [PubMed] [Google Scholar]
- GRANT WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963;69:783–801. doi: 10.1001/archopht.1963.00960040789022. [DOI] [PubMed] [Google Scholar]
- GRIERSON I, DAY J, UNGER WG, AHMED A. Phagocytosis of latex microspheres by bovine meshwork cells in culture. Graefes Arch Clin Exp Ophthalmol. 1986;224:536–44. doi: 10.1007/BF02154742. [DOI] [PubMed] [Google Scholar]
- GRIERSON I, HOWES RC. Age-related depletion of the cell population in the human trabecular meshwork. Eye (Lond) 1987;1( Pt 2):204–10. doi: 10.1038/eye.1987.38. [DOI] [PubMed] [Google Scholar]
- GRIERSON I, LEE WR. Erythrocyte phagocytosis in the human trabecular meshwork. Br J Ophthalmol. 1973;57:400–15. doi: 10.1136/bjo.57.6.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIERSON I, LEE WR. The fine structure of the trabecular meshwork at graded levels of intraocular pressure. (1) Pressure effects within the near-physiological range (8–30 mmHg) Exp Eye Res. 1975;20:505–21. doi: 10.1016/0014-4835(75)90218-3. [DOI] [PubMed] [Google Scholar]
- GRIERSON I, ROBINS E, UNGER W, MILLAR L, AHMED A. The cells of the bovine outflow system in tissue culture. Exp Eye Res. 1985;40:35–46. doi: 10.1016/0014-4835(85)90106-x. [DOI] [PubMed] [Google Scholar]
- HEIMARK RL, KAOCHAR S, STAMER WD. Human Schlemm’s canal cells express the endothelial adherens proteins, VE-cadherin and PECAM-1. Curr Eye Res. 2002;25:299–308. doi: 10.1076/ceyr.25.5.299.13495. [DOI] [PubMed] [Google Scholar]
- HENNIG R, KUESPERT S, HAUNBERGER A, GOEPFERICH A, FUCHSHOFER R. Cyclic RGD peptides target human trabecular meshwork cells while ameliorating connective tissue growth factor-induced fibrosis. J Drug Target. 2016:1–8. doi: 10.3109/1061186X.2016.1163709. [DOI] [PubMed] [Google Scholar]
- HERNANDEZ MR, WEINSTEIN BI, DUNN MW, GORDON GG, SOUTHREN AL. The effect of dexamethasone on the synthesis of collagen in normal human trabecular meshwork explants. Invest Ophthalmol Vis Sci. 1985;26:1784–8. [PubMed] [Google Scholar]
- HERNANDEZ MR, WEINSTEIN BI, SCHWARTZ J, RITCH R, GORDON GG, SOUTHREN AL. Human trabecular meshwork cells in culture: morphology and extracellular matrix components. Invest Ophthalmol Vis Sci. 1987;28:1655–60. [PubMed] [Google Scholar]
- HOARE MJ, GRIERSON I, BROTCHIE D, POLLOCK N, CRACKNELL K, CLARK AF. Cross-linked actin networks (CLANs) in the trabecular meshwork of the normal and glaucomatous human eye in situ. Invest Ophthalmol Vis Sci. 2009;50:1255–63. doi: 10.1167/iovs.08-2706. [DOI] [PubMed] [Google Scholar]
- HOSSEINI M, ROSE AY, SONG K, BOHAN C, ALEXANDER JP, KELLEY MJ, ACOTT TS. IL-1 and TNF induction of matrix metalloproteinase-3 by c-Jun N-terminal kinase in trabecular meshwork. Invest Ophthalmol Vis Sci. 2006;47:1469–76. doi: 10.1167/iovs.05-0451. [DOI] [PubMed] [Google Scholar]
- HU Y, GABELT BT, KAUFMAN PL. Monkey organ-cultured anterior segments: technique and response to H-7. Exp Eye Res. 2006;82:1100–8. doi: 10.1016/j.exer.2005.12.004. [DOI] [PubMed] [Google Scholar]
- INATANI M, TANIHARA H, KATSUTA H, HONJO M, KIDO N, HONDA Y. Transforming growth factor-beta 2 levels in aqueous humor of glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 2001;239:109–13. doi: 10.1007/s004170000241. [DOI] [PubMed] [Google Scholar]
- JAIN A, WORDINGER RJ, YORIO T, CLARK AF. Spliceosome protein (SRp) regulation of glucocorticoid receptor isoforms and glucocorticoid response in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2012;53:857–66. doi: 10.1167/iovs.11-8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON DH, BRADLEY JM, ACOTT TS. The effect of dexamethasone on glycosaminoglycans of human trabecular meshwork in perfusion organ culture. Invest Ophthalmol Vis Sci. 1990;31:2568–71. [PubMed] [Google Scholar]
- JOHNSON DH, RICHARDSON TM, EPSTEIN DL. Trabecular meshwork recovery after phagocytic challenge. Curr Eye Res. 1989;8:1121–30. doi: 10.3109/02713688909000037. [DOI] [PubMed] [Google Scholar]
- JOHNSON DH, TSCHUMPER RC. Human trabecular meshwork organ culture. A new method. Invest Ophthalmol Vis Sci. 1987;28:945–53. [PubMed] [Google Scholar]
- JOHNSTONE M, GRANT M. Pressure-dependent changes in structures of the aqueous outflow system of human and monkey eyes. Am J Ophthalmol. 1973;75:365–383. doi: 10.1016/0002-9394(73)91145-8. [DOI] [PubMed] [Google Scholar]
- JUNGLAS B, KUESPERT S, SELEEM AA, STRULLER T, ULLMANN S, BOSL M, BOSSERHOFF A, KOSTLER J, WAGNER R, TAMM ER, FUCHSHOFER R. Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am J Pathol. 2012;180:2386–403. doi: 10.1016/j.ajpath.2012.02.030. [DOI] [PubMed] [Google Scholar]
- JUNGLAS B, YU AH, WELGE-LUSSEN U, TAMM ER, FUCHSHOFER R. Connective tissue growth factor induces extracellular matrix deposition in human trabecular meshwork cells. Exp Eye Res. 2009;88:1065–75. doi: 10.1016/j.exer.2009.01.008. [DOI] [PubMed] [Google Scholar]
- KAUFMAN PL, ERICKSON KA. Cytochalasin B and D dose-outflow facility response relationships in the cynomolgus monkey. Invest Ophthalmol Vis Sci. 1982;23:646–50. [PubMed] [Google Scholar]
- KELLER KE, AGA M, BRADLEY JM, KELLEY MJ, ACOTT TS. Extracellular matrix turnover and outflow resistance. Exp Eye Res. 2009;88:676–82. doi: 10.1016/j.exer.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLER KE, BRADLEY JM, KELLEY MJ, ACOTT TS. Effects of modifiers of glycosaminoglycan biosynthesis on outflow facility in perfusion culture. Invest Ophthalmol Vis Sci. 2008;49:2495–505. doi: 10.1167/iovs.07-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLER KE, BRADLEY JM, VRANKA JA, ACOTT TS. Segmental versican expression in the trabecular meshwork and involvement in outflow facility. Invest Ophthalmol Vis Sci. 2011;52:5049–57. doi: 10.1167/iovs.10-6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLEY MJ, ROSE A, SONG K, LYSTRUP B, SAMPLES JW, ACOTT TS. p38 MAP kinase pathway and stromelysin regulation in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007a;48:3126–37. doi: 10.1167/iovs.06-1375. [DOI] [PubMed] [Google Scholar]
- KELLEY MJ, ROSE AY, KELLER KE, HESSLE H, SAMPLES JR, ACOTT TS. Stem cells in the trabecular meshwork: present and future promises. Exp Eye Res. 2009;88:747–51. doi: 10.1016/j.exer.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLEY MJ, ROSE AY, SONG K, CHEN Y, BRADLEY JM, ROOKHUIZEN D, ACOTT TS. Synergism of TNF and IL-1 in the induction of matrix metalloproteinase-3 in trabecular meshwork. Invest Ophthalmol Vis Sci. 2007b;48:2634–43. doi: 10.1167/iovs.06-1445. [DOI] [PubMed] [Google Scholar]
- KIMPEL MW, JOHNSON DH. Factors influencing in vivo trabecular cell replication as determined by 3H-thymidine labelling; an autoradiographic study in cats. Curr Eye Res. 1992;11:297–306. doi: 10.3109/02713689209001783. [DOI] [PubMed] [Google Scholar]
- KIZHATIL K, RYAN M, MARCHANT JK, HENRICH S, JOHN SW. Schlemm’s canal is a unique vessel with a combination of blood vascular and lymphatic phenotypes that forms by a novel developmental process. PLoS Biol. 2014;12:e1001912. doi: 10.1371/journal.pbio.1001912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEIN BE, KLEIN R, LINTON KL. Intraocular pressure in an American community. The Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1992;33:2224–8. [PubMed] [Google Scholar]
- KNEPPER PA, MAYANIL CS, GOOSSENS W, WERTZ RD, HOLGREN C, RITCH R, ALLINGHAM RR. Aqueous humor in primary open-angle glaucoma contains an increased level of CD44S. Invest Ophthalmol Vis Sci. 2002;43:133–9. [PubMed] [Google Scholar]
- KNEPPER PA, MILLER AM, CHOI J, WERTZ RD, NOLAN MJ, GOOSSENS W, WHITMER S, YUE BY, RITCH R, LIEBMANN JM, ALLINGHAM RR, SAMPLES JR. Hypophosphorylation of aqueous humor sCD44 and primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2005;46:2829–37. doi: 10.1167/iovs.04-1472. [DOI] [PubMed] [Google Scholar]
- KOPCZYNSKI CC, EPSTEIN DL. Emerging trabecular outflow drugs. J Ocul Pharmacol Ther. 2014;30:85–7. doi: 10.1089/jop.2013.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAST JA, PAN T, DING Y, REILLY CM, KELLER K, ACOTT TS, FAUTSCH MP, MURPHY CJ, RUSSELL P. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest Ophthalmol Vis Sci. 2011;52:2147–52. doi: 10.1167/iovs.10-6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LATINA M, FLOTTE T, CREAN E, SHERWOOD ME, GRANSTEIN RD. Immunohistochemical staining of the human anterior segment. Evidence that resident cells play a role in immunologic responses. Arch Ophthalmol. 1988;106:95–9. doi: 10.1001/archopht.1988.01060130101037. [DOI] [PubMed] [Google Scholar]
- LATINA MA, SIBAYAN SA, SHIN DH, NOECKER RJ, MARCELLINO G. Q-switched 532-nm Nd:YAG laser trabeculoplasty (selective laser trabeculoplasty): a multicenter, pilot, clinical study. Ophthalmology. 1998;105:2082–8. doi: 10.1016/S0161-6420(98)91129-0. discussion 2089–90. [DOI] [PubMed] [Google Scholar]
- LEE ES, GABELT BT, FARALLI JA, PETERS DM, BRANDT CR, KAUFMAN PL, BHATTACHARYA SK. COCH transgene expression in cultured human trabecular meshwork cells and its effect on outflow facility in monkey organ cultured anterior segments. Invest Ophthalmol Vis Sci. 2010;51:2060–6. doi: 10.1167/iovs.09-4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI G, FARSIU S, CHIU SJ, GONZALEZ P, LUTJEN-DRECOLL E, OVERBY DR, STAMER WD. Pilocarpine-induced dilation of Schlemm’s canal and prevention of lumen collapse at elevated intraocular pressures in living mice visualized by OCT. Invest Ophthalmol Vis Sci. 2014;55:3737–46. doi: 10.1167/iovs.13-13700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITON PB, LIU X, STAMER WD, CHALLA P, EPSTEIN DL, GONZALEZ P. Specific targeting of gene expression to a subset of human trabecular meshwork cells using the chitinase 3-like 1 promoter. Invest Ophthalmol Vis Sci. 2005;46:183–90. doi: 10.1167/iovs.04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Y, ALLINGHAM RR, QIN X, LAYFIELD D, DELLINGER AE, GIBSON J, WHEELER J, ASHLEY-KOCH AE, STAMER WD, HAUSER MA. Gene expression profile in human trabecular meshwork from patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2013;54:6382–9. doi: 10.1167/iovs.13-12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUTJEN-DRECOLL E. Structural factors influencing outflow facility and its changeability under drugs. A study in Macaca arctoides. Invest Ophthalmol. 1973;12:280–94. [PubMed] [Google Scholar]
- LUTJEN-DRECOLL E. Morphological changes in glaucomatous eyes and the role of TGFbeta2 for the pathogenesis of the disease. Exp Eye Res. 2005;81:1–4. doi: 10.1016/j.exer.2005.02.008. [DOI] [PubMed] [Google Scholar]
- LUTJEN-DRECOLL E, FUTA R, ROHEN JW. Ultrahistochemical studies on tangential sections of the trabecular meshwork in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 1981;21:563–73. [PubMed] [Google Scholar]
- LUTJEN-DRECOLL E, SHIMIZU T, ROHRBACH M, ROHEN JW. Quantitative analysis of ‘plaque material’ between ciliary muscle tips in normal- and glaucomatous eyes. Exp Eye Res. 1986;42:457–65. doi: 10.1016/0014-4835(86)90005-9. [DOI] [PubMed] [Google Scholar]
- LYNCH MG, PEELER JS, BROWN RH, NIEDERKORN JY. Expression of HLA class I and II antigens on cells of the human trabecular meshwork. Ophthalmology. 1987;94:851–7. doi: 10.1016/s0161-6420(87)33539-0. [DOI] [PubMed] [Google Scholar]
- MAO W, LIU Y, MODY A, MONTECCHI-PALMER M, WORDINGER RJ, CLARK AF. Characterization of a spontaneously immortalized bovine trabecular meshwork cell line. Exp Eye Res. 2012a;105:53–9. doi: 10.1016/j.exer.2012.10.007. [DOI] [PubMed] [Google Scholar]
- MAO W, LIU Y, WORDINGER RJ, CLARK AF. A magnetic bead-based method for mouse trabecular meshwork cell isolation. Invest Ophthalmol Vis Sci. 2013;54:3600–6. doi: 10.1167/iovs.13-12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAO W, MILLAR JC, WANG WH, SILVERMAN SM, LIU Y, WORDINGER RJ, RUBIN JS, PANG IH, CLARK AF. Existence of the canonical Wnt signaling pathway in the human trabecular meshwork. Invest Ophthalmol Vis Sci. 2012b;53:7043–51. doi: 10.1167/iovs.12-9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAO W, TOVAR-VIDALES T, YORIO T, WORDINGER RJ, CLARK AF. Perfusion-cultured bovine anterior segments as an ex vivo model for studying glucocorticoid-induced ocular hypertension and glaucoma. Invest Ophthalmol Vis Sci. 2011;52:8068–75. doi: 10.1167/iovs.11-8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUMOTO Y, JOHNSON DH. Trabecular meshwork phagocytosis in glaucomatous eyes. Ophthalmologica. 1997;211:147–52. doi: 10.1159/000310782. [DOI] [PubMed] [Google Scholar]
- MCDOWELL CM, HERNANDEZ H, MAO W, CLARK AF. Gremlin Induces Ocular Hypertension in Mice Through Smad3-Dependent Signaling. Invest Ophthalmol Vis Sci. 2015;56:5485–92. doi: 10.1167/iovs.15-16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCKEE CT, WOOD JA, SHAH NM, FISCHER ME, REILLY CM, MURPHY CJ, RUSSELL P. The effect of biophysical attributes of the ocular trabecular meshwork associated with glaucoma on the cell response to therapeutic agents. Biomaterials. 2011;32:2417–23. doi: 10.1016/j.biomaterials.2010.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEDINA-ORTIZ WE, BELMARES R, NEUBAUER S, WORDINGER RJ, CLARK AF. Cellular fibronectin expression in human trabecular meshwork and induction by transforming growth factor-beta2. Invest Ophthalmol Vis Sci. 2013;54:6779–88. doi: 10.1167/iovs.13-12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIN SH, LEE TI, CHUNG YS, KIM HK. Transforming growth factor-beta levels in human aqueous humor of glaucomatous, diabetic and uveitic eyes. Korean J Ophthalmol. 2006;20:162–5. doi: 10.3341/kjo.2006.20.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOUSSAD EE, BRIGSTOCK DR. Connective tissue growth factor: what’s in a name? Mol Genet Metab. 2000;71:276–92. doi: 10.1006/mgme.2000.3059. [DOI] [PubMed] [Google Scholar]
- NGUYEN KP, CHUNG ML, ANDERSON PJ, JOHNSON M, EPSTEIN DL. Hydrogen peroxide removal by the calf aqueous outflow pathway. Invest Ophthalmol Vis Sci. 1988;29:976–81. [PubMed] [Google Scholar]
- NGUYEN KP, WEISS H, KARAGEUZIAN LN, ANDERSON PJ, EPSTEIN DL. Glutathione reductase of calf trabecular meshwork. Invest Ophthalmol Vis Sci. 1985;26:887–90. [PubMed] [Google Scholar]
- NGUYEN T, CHEN P, HUANG W, CHEN H, JOHNSON D, POLANSKY J. Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J Biol Chem. 1998a;273:6341–6350. doi: 10.1074/jbc.273.11.6341. [DOI] [PubMed] [Google Scholar]
- NGUYEN TD, CHEN P, HUANG WD, CHEN H, JOHNSON D, POLANSKY JR. Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J Biol Chem. 1998b;273:6341–50. doi: 10.1074/jbc.273.11.6341. [DOI] [PubMed] [Google Scholar]
- NOLAN MJ, GIOVINGO MC, MILLER AM, WERTZ RD, RITCH R, LIEBMANN JM, ALLINGHAM RR, HERNDON LW, WAX MB, SMOLYAK R, HASAN F, BARNETT EM, SAMPLES JR, KNEPPER PA. Aqueous humor sCD44 concentration and visual field loss in primary open-angle glaucoma. J Glaucoma. 2007;16:419–29. doi: 10.1097/IJG.0b013e318050ab4b. [DOI] [PubMed] [Google Scholar]
- O’BRIEN ET, PERKINS SL, ROBERTS BC, EPSTEIN DL. Dexamethasone inhibits trabecular cell retraction. Exp Eye Res. 1996;62:675–88. doi: 10.1006/exer.1996.0078. [DOI] [PubMed] [Google Scholar]
- O’BRIEN TE, METHENEY CD, POLANSKY JR. Immunofluorescence method for quantifying the trabecular meshwork glucocorticoid response (TIGR) protein in trabecular meshwork and Schlemm’s canal cells. Curr Eye Res. 1999;19:517–24. doi: 10.1076/ceyr.19.6.517.5285. [DOI] [PubMed] [Google Scholar]
- O’REILLY S, POLLOCK N, CURRIE L, PARAOAN L, CLARK AF, GRIERSON I. Inducers of cross-linked actin networks in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52:7316–24. doi: 10.1167/iovs.10-6692. [DOI] [PubMed] [Google Scholar]
- OCHIAI Y, OCHIAI H. Higher concentration of transforming growth factor-beta in aqueous humor of glaucomatous eyes and diabetic eyes. Jpn J Ophthalmol. 2002;46:249–53. doi: 10.1016/s0021-5155(01)00523-8. [DOI] [PubMed] [Google Scholar]
- OVERBY DR, BERTRAND J, SCHICHT M, PAULSEN F, STAMER WD, LUTJEN-DRECOLL E. The structure of the trabecular meshwork, its connections to the ciliary muscle, and the effect of pilocarpine on outflow facility in mice. Invest Ophthalmol Vis Sci. 2014;55:3727–36. doi: 10.1167/iovs.13-13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OVERBY DR, STAMER WD, JOHNSON M. The changing paradigm of outflow resistance generation: towards synergistic models of the JCT and inner wall endothelium. Exp Eye Res. 2009;88:656–70. doi: 10.1016/j.exer.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZCAN AA, OZDEMIR N, CANATAROGLU A. The aqueous levels of TGF-beta2 in patients with glaucoma. Int Ophthalmol. 2004;25:19–22. doi: 10.1023/b:inte.0000018524.48581.79. [DOI] [PubMed] [Google Scholar]
- PANG IH, HELLBERG PE, FLEENOR DL, JACOBSON N, CLARK AF. Expression of matrix metalloproteinases and their inhibitors in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2003;44:3485–93. doi: 10.1167/iovs.02-0756. [DOI] [PubMed] [Google Scholar]
- PANG IH, MCCARTNEY MD, STEELY HT, CLARK AF. Human ocular perfusion organ culture: a versatile ex vivo model for glaucoma research. J Glaucoma. 2000;9:468–79. doi: 10.1097/00061198-200012000-00009. [DOI] [PubMed] [Google Scholar]
- PANG IH, MOLL H, MCLAUGHLIN MA, KNEPPER PA, DE SANTIS L, EPSTEIN DL, CLARK AF. Ocular hypotensive and aqueous outflow-enhancing effects of AL- 3037A (sodium ferri ethylenediaminetetraacetate) Exp Eye Res. 2001;73:815–25. doi: 10.1006/exer.2001.1087. [DOI] [PubMed] [Google Scholar]
- PANG IH, SHADE DL, CLARK AF, STEELY HT, DESANTIS L. Preliminary characterization of a transformed cell strain derived from human trabecular meshwork. Curr Eye Res. 1994;13:51–63. doi: 10.3109/02713689409042398. [DOI] [PubMed] [Google Scholar]
- PARK JK, TRIPATHI RC, TRIPATHI BJ, BARLOW GH. Tissue plasminogen activator in the trabecular endothelium. Invest Ophthalmol Vis Sci. 1987;28:1341–5. [PubMed] [Google Scholar]
- PARSHLEY DE, BRADLEY JM, SAMPLES JR, VAN BUSKIRK EM, ACOTT TS. Early changes in matrix metalloproteinases and inhibitors after in vitro laser treatment to the trabecular meshwork. Curr Eye Res. 1995;14:537–44. doi: 10.3109/02713689508998400. [DOI] [PubMed] [Google Scholar]
- PERKINS TW, ALVARADO JA, POLANSKY JR, STILWELL L, MAGLIO M, JUSTER R. Trabecular meshwork cells grown on filters. Conductivity and cytochalasin effects. Invest Ophthalmol Vis Sci. 1988;29:1836–46. [PubMed] [Google Scholar]
- PERKUMAS KM, STAMER WD. Protein markers and differentiation in culture for Schlemm’s canal endothelial cells. Exp Eye Res. 2012;96:82–7. doi: 10.1016/j.exer.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERS JC, BHATTACHARYA S, CLARK AF, ZODE GS. Increased Endoplasmic Reticulum Stress in Human Glaucomatous Trabecular Meshwork Cells and Tissues. Invest Ophthalmol Vis Sci. 2015;56:3860–8. doi: 10.1167/iovs.14-16220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PICHT G, WELGE-LUESSEN U, GREHN F, LUTJEN-DRECOLL E. Transforming growth factor beta 2 levels in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefes Arch Clin Exp Ophthalmol. 2001;239:199–207. doi: 10.1007/s004170000252. [DOI] [PubMed] [Google Scholar]
- POLANSKY J, FAUSS D, ZIMMERMAN C. Regulation of TIGR/MYOC gene expression in human trabecular meshwork cells. Eye. 2000;14:503–514. doi: 10.1038/eye.2000.137. [DOI] [PubMed] [Google Scholar]
- POLANSKY JR, FAUSS DJ, CHEN P, CHEN H, LUTJEN-DRECOLL E, JOHNSON D, KURTZ RM, MA ZD, BLOOM E, NGUYEN TD. Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica. 1997;211:126–39. doi: 10.1159/000310780. [DOI] [PubMed] [Google Scholar]
- POLANSKY JR, WEINREB RN, BAXTER JD, ALVARADO J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979;18:1043–9. [PubMed] [Google Scholar]
- PORTER K, HIRT J, STAMER WD, LITON PB. Autophagic dysregulation in glaucomatous trabecular meshwork cells. Biochim Biophys Acta. 2015;1852:379–85. doi: 10.1016/j.bbadis.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAGHUNATHAN VK, MORGAN JT, PARK SA, WEBER D, PHINNEY BS, MURPHY CJ, RUSSELL P. Dexamethasone Stiffens Trabecular Meshwork, Trabecular Meshwork Cells, and Matrix. Invest Ophthalmol Vis Sci. 2015;56:4447–59. doi: 10.1167/iovs.15-16739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMOS RF, SUMIDA GM, STAMER WD. Cyclic mechanical stress and trabecular meshwork cell contractility. Invest Ophthalmol Vis Sci. 2009;50:3826–32. doi: 10.1167/iovs.08-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAO PV, DENG PF, KUMAR J, EPSTEIN DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42:1029–37. [PubMed] [Google Scholar]
- RESCH ZT, HANN CR, COOK KA, FAUTSCH MP. Aqueous humor rapidly stimulates myocilin secretion from human trabecular meshwork cells. Exp Eye Res. 2010;91:901–8. doi: 10.1016/j.exer.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROHEN JW. Why is intraocular pressure elevated in chronic simple glaucoma? Anatomical considerations. Ophthalmology. 1983;90:758–65. doi: 10.1016/s0161-6420(83)34492-4. [DOI] [PubMed] [Google Scholar]
- ROHEN JW, FUTA R, LUTJEN-DRECOLL E. The fine structure of the cribriform meshwork in normal and glaucomatous eyes as seen in tangential sections. Invest Ophthalmol Vis Sci. 1981;21:574–85. [PubMed] [Google Scholar]
- ROHEN JW, SCHACHTSCHABEL OO, MATTHIESSEN PF. In vitro studies on the trabecular meshwork of the primate eye. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1975;193:95–107. doi: 10.1007/BF00419354. [DOI] [PubMed] [Google Scholar]
- ROHEN JW, VAN DER ZYPEN E. The phagocytic activity of the trabecularmeshwork endothelium. An electron-microscopic study of the vervet (Cercopithecus aethiops) Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1968;175:143–60. doi: 10.1007/BF02385060. [DOI] [PubMed] [Google Scholar]
- RUSSELL P, JOHNSON DH. Enzymes protective of oxidative damage present in all decades of life in the trabecular meshwork, as detected by two-dimensional gel electrophoresis protein maps. J Glaucoma. 1996;5:317–24. [PubMed] [Google Scholar]
- SAMUELSON DA, GELATT KN, GUM GG. Kinetics of phagocytosis in the normal canine iridocorneal angle. Am J Vet Res. 1984;45:2359–66. [PubMed] [Google Scholar]
- SCHAAF MJ, CIDLOWSKI JA. Molecular mechanisms of glucocorticoid action and resistance. J Steroid Biochem Mol Biol. 2002;83:37–48. doi: 10.1016/s0960-0760(02)00263-7. [DOI] [PubMed] [Google Scholar]
- SCHACHTSCHABEL DO, ROHEN JW, WEVER J, SAMES K. Synthesis and composition of glycosaminoglycans by cultured human trabecular meshwork cells. Graefes Arch Clin Exp Ophthalmol. 1982;218:113–7. doi: 10.1007/BF02215647. [DOI] [PubMed] [Google Scholar]
- SETHI A, JAIN A, ZODE GS, WORDINGER RJ, CLARK AF. Role of TGFbeta/Smad signaling in gremlin induction of human trabecular meshwork extracellular matrix proteins. Invest Ophthalmol Vis Sci. 2011a;52:5251–9. doi: 10.1167/iovs.11-7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SETHI A, MAO W, WORDINGER RJ, CLARK AF. Transforming growth factor-beta induces extracellular matrix protein cross-linking lysyl oxidase (LOX) genes in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011b;52:5240–50. doi: 10.1167/iovs.11-7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEARER T, CROSSON CE. Activation of extracellular signal-regulated kinase in trabecular meshwork cells. Exp Eye Res. 2001;73:25–35. doi: 10.1006/exer.2001.1007. [DOI] [PubMed] [Google Scholar]
- SHEARER TW, CROSSON CE. Adenosine A1 receptor modulation of MMP-2 secretion by trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2002;43:3016–20. [PubMed] [Google Scholar]
- SHEPARD AR, MILLAR JC, PANG IH, JACOBSON N, WANG WH, CLARK AF. Adenoviral gene transfer of active human transforming growth factor-{beta}2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Invest Ophthalmol Vis Sci. 2010;51:2067–76. doi: 10.1167/iovs.09-4567. [DOI] [PubMed] [Google Scholar]
- SHERWOOD ME, RICHARDSON TM. Phagocytosis by trabecular meshwork cells: sequence of events in cats and monkeys. Exp Eye Res. 1988;46:881–95. doi: 10.1016/s0014-4835(88)80040-x. [DOI] [PubMed] [Google Scholar]
- SHIFERA AS, TRIVEDI S, CHAU P, BONNEMAISON LH, IGUCHI R, ALVARADO JA. Constitutive secretion of chemokines by cultured human trabecular meshwork cells. Exp Eye Res. 2010;91:42–7. doi: 10.1016/j.exer.2010.04.001. [DOI] [PubMed] [Google Scholar]
- SHUMAN MA, POLANSKY JR, MERKEL C, ALVARADO JA. Tissue plasminogen activator in cultured human trabecular meshwork cells. Predominance of enzyme over plasminogen activator inhibitor. Invest Ophthalmol Vis Sci. 1988;29:401–5. [PubMed] [Google Scholar]
- SIEGNER A, MAY CA, WELGE-LUSSEN UW, BLOEMENDAL H, LUTJEN-DRECOLL E. alpha B-crystallin in the primate ciliary muscle and trabecular meshwork. Eur J Cell Biol. 1996;71:165–9. [PubMed] [Google Scholar]
- SNYDER RW, STAMER WD, KRAMER TR, SEFTOR RE. Corticosteroid treatment and trabecular meshwork proteases in cell and organ culture supernatants. Exp Eye Res. 1993;57:461–8. doi: 10.1006/exer.1993.1148. [DOI] [PubMed] [Google Scholar]
- STAMER DW, ROBERTS BC, EPSTEIN DL, ALLINGHAM RR. Isolation of primary open-angle glaucomatous trabecular meshwork cells from whole eye tissue. Curr Eye Res. 2000;20:347–50. [PubMed] [Google Scholar]
- STAMER WD, HUANG Y, SEFTOR RE, SVENSSON SS, SNYDER RW, REGAN JW. Cultured human trabecular meshwork cells express functional alpha 2A adrenergic receptors. Invest Ophthalmol Vis Sci. 1996;37:2426–33. [PubMed] [Google Scholar]
- STAMER WD, ROBERTS BC, HOWELL DN, EPSTEIN DL. Isolation, culture, and characterization of endothelial cells from Schlemm’s canal. Invest Ophthalmol Vis Sci. 1998;39:1804–12. [PubMed] [Google Scholar]
- STAMER WD, SEFTOR RE, SNYDER RW, REGAN JW. Cultured human trabecular meshwork cells express aquaporin-1 water channels. Curr Eye Res. 1995a;14:1095–1100. doi: 10.3109/02713689508995815. [DOI] [PubMed] [Google Scholar]
- STAMER WD, SEFTOR RE, WILLIAMS SK, SAMAHA HA, SNYDER RW. Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Curr Eye Res. 1995b;14:611–7. doi: 10.3109/02713689508998409. [DOI] [PubMed] [Google Scholar]
- STEELY HT, BROWDER SL, JULIAN MB, MIGGANS ST, WILSON KL, CLARK AF. The effects of dexamethasone on fibronectin expression in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1992;33:2242–50. [PubMed] [Google Scholar]
- STONE EM, FINGERT JH, ALWARD WL, NGUYEN TD, POLANSKY JR, SUNDEN SL, NISHIMURA D, CLARK AF, NYSTUEN A, NICHOLS BE, MACKEY DA, RITCH R, KALENAK JW, CRAVEN ER, SHEFFIELD VC. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–70. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- TAMM ER. The trabecular meshwork outflow pathways: structural and functional aspects. Exp Eye Res. 2009;88:648–55. doi: 10.1016/j.exer.2009.02.007. [DOI] [PubMed] [Google Scholar]
- TAMM ER, RUSSELL P, EPSTEIN DL, JOHNSON DH, PIATIGORSKY J. Modulation of myocilin/TIGR expression in human trabecular meshwork. Invest Ophthalmol Vis Sci. 1999;40:2577–82. [PubMed] [Google Scholar]
- TAMM ER, SIEGNER A, BAUR A, LUTJEN-DRECOLL E. Transforming growth factor-beta 1 induces alpha-smooth muscle-actin expression in cultured human and monkey trabecular meshwork. Exp Eye Res. 1996;62:389–97. doi: 10.1006/exer.1996.0044. [DOI] [PubMed] [Google Scholar]
- TIAN B, GABELT BT, GEIGER B, KAUFMAN PL. The role of the actomyosin system in regulating trabecular fluid outflow. Exp Eye Res. 2009;88:713–7. doi: 10.1016/j.exer.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOVAR-VIDALES T, CLARK AF, WORDINGER RJ. Transforming growth factor-beta2 utilizes the canonical Smad-signaling pathway to regulate tissue transglutaminase expression in human trabecular meshwork cells. Exp Eye Res. 2011;93:442–51. doi: 10.1016/j.exer.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOVAR-VIDALES T, ROQUE R, CLARK AF, WORDINGER RJ. Tissue transglutaminase expression and activity in normal and glaucomatous human trabecular meshwork cells and tissues. Invest Ophthalmol Vis Sci. 2008;49:622–8. doi: 10.1167/iovs.07-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRIPATHI BJ, TRIPATHI RC. Neural crest origin of human trabecular meshwork and its implications for the pathogenesis of glaucoma. Am J Ophthalmol. 1989;107:583–90. doi: 10.1016/0002-9394(89)90253-5. [DOI] [PubMed] [Google Scholar]
- TRIPATHI BJ, TRIPATHI RC, WONG P, RAJA S. Expression of HLA by the human trabecular meshwork and corneal endothelium. Exp Eye Res. 1990a;51:269–76. doi: 10.1016/0014-4835(90)90023-n. [DOI] [PubMed] [Google Scholar]
- TRIPATHI BJ, TRIPATHI RC, YANG C, MILLARD CB, DIXIT VM. Synthesis of a thrombospondin-like cytoadhesion molecule by cells of the trabecular meshwork. Invest Ophthalmol Vis Sci. 1991;32:181–8. [PubMed] [Google Scholar]
- TRIPATHI RC, CHAN WF, LI J, TRIPATHI BJ. Trabecular cells express the TGF-beta 2 gene and secrete the cytokine. Exp Eye Res. 1994;58:523–8. doi: 10.1006/exer.1994.1046. [DOI] [PubMed] [Google Scholar]
- TRIPATHI RC, TRIPATHI BJ. Human trabecular endothelium, corneal endothelium, keratocytes, and scleral fibroblasts in primary cell culture. A comparative study of growth characteristics, morphology, and phagocytic activity by light and scanning electron microscopy. Exp Eye Res. 1982;35:611–24. doi: 10.1016/s0014-4835(82)80074-2. [DOI] [PubMed] [Google Scholar]
- TRIPATHI RC, TRIPATHI BJ, PARK JK. Localization of urokinase-type plasminogen activator in human eyes: an immunocytochemical study. Exp Eye Res. 1990b;51:545–52. doi: 10.1016/0014-4835(90)90085-9. [DOI] [PubMed] [Google Scholar]
- TRIVEDI RH, NUTAITIS M, VROMAN D, CROSSON CE. Influence of race and age on aqueous humor levels of transforming growth factor-beta 2 in glaucomatous and nonglaucomatous eyes. J Ocul Pharmacol Ther. 2011;27:477–80. doi: 10.1089/jop.2010.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUMMINIA SJ, MITTON KP, ARORA J, ZELENKA P, EPSTEIN DL, RUSSELL P. Mechanical stretch alters the actin cytoskeletal network and signal transduction in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1998;39:1361–71. [PubMed] [Google Scholar]
- UCHIDA K, INOUE M, OTAKE K, MIKI C, KUSUNOKI M. Efficacy of dermabond for closing lymphatic leakage after resection and OK-432 treatment of a lymphangioma. Plast Reconstr Surg. 2008;122:156e–157e. doi: 10.1097/PRS.0b013e31818823fa. [DOI] [PubMed] [Google Scholar]
- VANDERWYST SS, PERKUMAS KM, READ AT, OVERBY DR, STAMER WD. Structural basement membrane components and corresponding integrins in Schlemm’s canal endothelia. Mol Vis. 2011;17:199–209. [PMC free article] [PubMed] [Google Scholar]
- WADE NC, GRIERSON I, O’REILLY S, HOARE MJ, CRACKNELL KP, PARAOAN LI, BROTCHIE D, CLARK AF. Cross-linked actin networks (CLANs) in bovine trabecular meshwork cells. Exp Eye Res. 2009;89:648–59. doi: 10.1016/j.exer.2009.06.006. [DOI] [PubMed] [Google Scholar]
- WALLACE DM, CLARK AF, LIPSON KE, ANDREWS D, CREAN JK, O’BRIEN CJ. Anti-connective tissue growth factor antibody treatment reduces extracellular matrix production in trabecular meshwork and lamina cribrosa cells. Invest Ophthalmol Vis Sci. 2013;54:7836–48. doi: 10.1167/iovs.13-12494. [DOI] [PubMed] [Google Scholar]
- WAN Z, WOODWARD DF, CORNELL CL, FLIRI HG, MARTOS JL, PETTIT SN, WANG JW, KHARLAMB AB, WHEELER LA, GARST ME, LANDSVERK KJ, STRUBLE CS, STAMER WD. Bimatoprost, prostamide activity, and conventional drainage. Invest Ophthalmol Vis Sci. 2007;48:4107–15. doi: 10.1167/iovs.07-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG N, CHINTALA SK, FINI ME, SCHUMAN JS. Activation of a tissue-specific stress response in the aqueous outflow pathway of the eye defines the glaucoma disease phenotype. Nat Med. 2001;7:304–9. doi: 10.1038/85446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG WH, MCNATT LG, PANG IH, HELLBERG PE, FINGERT JH, MCCARTNEY MD, CLARK AF. Increased expression of serum amyloid A in glaucoma and its effect on intraocular pressure. Invest Ophthalmol Vis Sci. 2008a;49:1916–23. doi: 10.1167/iovs.07-1104. [DOI] [PubMed] [Google Scholar]
- WANG WH, MCNATT LG, PANG IH, MILLAR JC, HELLBERG PE, HELLBERG MH, STEELY HT, RUBIN JS, FINGERT JH, SHEFFIELD VC, STONE EM, CLARK AF. Increased expression of the WNT antagonist sFRP-1 in glaucoma elevates intraocular pressure. J Clin Invest. 2008b;118:1056–64. doi: 10.1172/JCI33871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAX MB, MOLINOFF PB, ALVARADO J, POLANSKY J. Characterization of beta-adrenergic receptors in cultured human trabecular cells and in human trabecular meshwork. Invest Ophthalmol Vis Sci. 1989;30:51–7. [PubMed] [Google Scholar]
- WEINREB RN, RYDER MI. In situ localization of cytoskeletal elements in the human trabecular meshwork and cornea. Invest Ophthalmol Vis Sci. 1990;31:1839–47. [PubMed] [Google Scholar]
- WELGE-LUSSEN U, EICHHORN M, BLOEMENDAL H, LUTJEN-DRECOLL E. Classification of human scleral spur cells in monolayer culture. Eur J Cell Biol. 1998;75:78–84. doi: 10.1016/S0171-9335(98)80050-2. [DOI] [PubMed] [Google Scholar]
- WELGE-LUSSEN U, MAY CA, EICHHORN M, BLOEMENDAL H, LUTJEN-DRECOLL E. AlphaB-crystallin in the trabecular meshwork is inducible by transforming growth factor-beta. Invest Ophthalmol Vis Sci. 1999;40:2235–41. [PubMed] [Google Scholar]
- WELGE-LUSSEN U, MAY CA, LUTJEN-DRECOLL E. Induction of tissue transglutaminase in the trabecular meshwork by TGF-beta1 and TGF-beta2. Invest Ophthalmol Vis Sci. 2000;41:2229–38. [PubMed] [Google Scholar]
- WIEDERHOLT M, STURM A, LEPPLE-WIENHUES A. Relaxation of trabecular meshwork and ciliary muscle by release of nitric oxide. Invest Ophthalmol Vis Sci. 1994;35:2515–20. [PubMed] [Google Scholar]
- WILSON K, MCCARTNEY MD, MIGGANS ST, CLARK AF. Dexamethasone induced ultrastructural changes in cultured human trabecular meshwork cells. Curr Eye Res. 1993;12:783–93. doi: 10.3109/02713689309020383. [DOI] [PubMed] [Google Scholar]
- WOOD JA, MCKEE CT, THOMASY SM, FISCHER ME, SHAH NM, MURPHY CJ, RUSSELL P. Substratum compliance regulates human trabecular meshwork cell behaviors and response to latrunculin B. Invest Ophthalmol Vis Sci. 2011;52:9298–303. doi: 10.1167/iovs.11-7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WORDINGER RJ, AGARWAL R, TALATI M, FULLER J, LAMBERT W, CLARK AF. Expression of bone morphogenetic proteins (BMP), BMP receptors, and BMP associated proteins in human trabecular meshwork and optic nerve head cells and tissues. Mol Vis. 2002;8:241–50. [PubMed] [Google Scholar]
- WORDINGER RJ, CLARK AF. Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma. Prog Retin Eye Res. 1999;18:629–67. doi: 10.1016/s1350-9462(98)00035-4. [DOI] [PubMed] [Google Scholar]
- WORDINGER RJ, CLARK AF, AGARWAL R, LAMBERT W, MCNATT L, WILSON SE, QU Z, FUNG BK. Cultured human trabecular meshwork cells express functional growth factor receptors. Invest Ophthalmol Vis Sci. 1998;39:1575–89. [PubMed] [Google Scholar]
- WORDINGER RJ, FLEENOR DL, HELLBERG PE, PANG IH, TOVAR TO, ZODE GS, FULLER JA, CLARK AF. Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest Ophthalmol Vis Sci. 2007;48:1191–200. doi: 10.1167/iovs.06-0296. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO N, ITONAGA K, MARUNOUCHI T, MAJIMA K. Concentration of transforming growth factor beta2 in aqueous humor. Ophthalmic Res. 2005;37:29–33. doi: 10.1159/000083019. [DOI] [PubMed] [Google Scholar]
- YANG CY, LIU Y, LU Z, REN R, GONG H. Effects of Y27632 on aqueous humor outflow facility with changes in hydrodynamic pattern and morphology in human eyes. Invest Ophthalmol Vis Sci. 2013;54:5859–70. doi: 10.1167/iovs.12-10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUE BY, ELNER VM, ELNER SG, DAVIS HR. Lysosomal enzyme activities in cultured trabecular-meshwork cells. Exp Eye Res. 1987;44:891–7. doi: 10.1016/s0014-4835(87)80051-9. [DOI] [PubMed] [Google Scholar]
- ZHANG X, CLARK AF, YORIO T. Regulation of glucocorticoid responsiveness in glaucomatous trabecular meshwork cells by glucocorticoid receptor-beta. Invest Ophthalmol Vis Sci. 2005;46:4607–16. doi: 10.1167/iovs.05-0571. [DOI] [PubMed] [Google Scholar]
- ZHANG X, OGNIBENE CM, CLARK AF, YORIO T. Dexamethasone inhibition of trabecular meshwork cell phagocytosis and its modulation by glucocorticoid receptor beta. Exp Eye Res. 2007;84:275–84. doi: 10.1016/j.exer.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU L, LI Y, YUE BY. Glucocorticoid effects on extracellular matrix proteins and integrins in bovine trabecular meshwork cells in relation to glaucoma. Int J Mol Med. 1998;1:339–46. [PubMed] [Google Scholar]