Abstract
Pathobiont expansion, such as that of adherent-invasive Escherichia coli (AIEC), is an emerging factor associated with inflammatory bowel disease. The intestinal epithelial barrier is the first line of defense against these pathogens. Inflammation plays a critical role in altering the epithelial barrier and is a major factor involved in promoting the expansion and pathogenesis of AIEC. AIEC in turn can exacerbate intestinal epithelial barrier dysfunction by targeting multiple elements of the barrier. One critical element of the epithelial barrier is the tight junction. Increasing evidence suggests that AIEC may selectively target protein components of tight junctions, leading to increased barrier permeability. This may represent one mechanism by which AIEC could contribute to the development of inflammatory bowel disease. This review article discusses potential mechanisms by which AIEC can disrupt epithelial tight junction function and intestinal barrier function.
Keywords: Tight Junctions, Intestinal Permeability, Inflammatory Bowel Disease
Abbreviations used in this paper: AIEC, adherent-invasive Escherichia coli; AJ, adherens junction; AJC, apical junctional complex; BP, bacterial peptidoglycans; CD, Crohn’s disease; CEACAM6, carcinoembryonic antigen–related cell-adhesion molecule; IBD, inflammatory bowel disease; IEC, intestinal epithelial cell; IFN, interferon; IL, interleukin; JAM-A, junctional adhesion molecule-A; LPF, long polar fimbriae; MLC, myosin light chain; MLCK, myosin light chain kinase; NF-κB, nuclear factor-κB; NOD2, nucleotide-binding oligomerization domain 2; PDZ, PSD95-DlgA-zonula occludens-1 homology domain; TJ, tight junction; TNF, tumor necrosis factor; UC, ulcerative colitis; ZO, zonula occludens
Summary.
Expansion of pathobionts such as adherent-invasive Escherichia coli during inflammation is associated with inflammatory bowel disease and contributes to intestinal epithelial barrier dysfunction. This review discusses mechanisms by which these bacteria disrupt epithelial barrier and tight junction function.
Inflammation plays a major role in altering the intestinal microenvironment, including the intestinal microbiome, and can result in the promotion of pathobiont expansion.1 A clinically important example of this involves the expansion of the adherent-invasive Escherichia coli (AIEC) LF82, originally isolated from ileal mucosa of a Crohn’s disease patient.2 Crohn’s disease (CD) and ulcerative colitis (UC) are multifaceted conditions that collectively are referred to as inflammatory bowel disease (IBD).3, 4 Factors contributing to IBD onset and development include dysregulation of gut bacteria and an increase in intestinal epithelial barrier permeability.1 This review article discusses the emergence of AIEC as a potential pathogenic factor in IBD and the molecular mechanisms through which it disrupts intestinal epithelial homeostasis and tight junction (TJ) integrity.
AIEC
Although the etiology of IBD is unknown, many investigators have probed whether a pathogenic cause can be attributed to onset of disease, either in full or in part.5, 6, 7 Although no pathogen has been shown consistently to be associated with IBD, a subset of CD patients do show increased prevalence of a unique enteropathogenic strain of the B2 phylotype E coli termed AIEC.2, 8, 9 Ileal biopsy specimens from CD and UC patients have shown that AIEC are associated primarily with CD; however, more recent studies have shown an equal prevalence of AIEC strains in UC as well as CD, suggesting that this pathobiont may have a greater association with IBD than first thought.10, 11, 12 Although LF82 is the most referenced AIEC, it is only one of many AIEC strains that were found in IBD patients.2, 13
It has been remarkably difficult to identify a specific genetic or molecular marker of AIEC, thus in vitro assays are used to validate AIEC.14 However, there may be some genetic signatures that differ between the B2 AIEC phylotype compared with other AIEC phylotypes.12 One potential AIEC candidate gene is the novel AIEC serine protease Vat-AIEC, which promotes expansion and adherence of AIEC to intestinal epithelial cells (IECs).15 Expression of this gene was approximately 3-fold higher in CD-AIEC isolates compared with non-AIEC isolates from CD patients or healthy subjects.15 It therefore could be considered a selective rather than an exclusive genetic marker for AIEC.
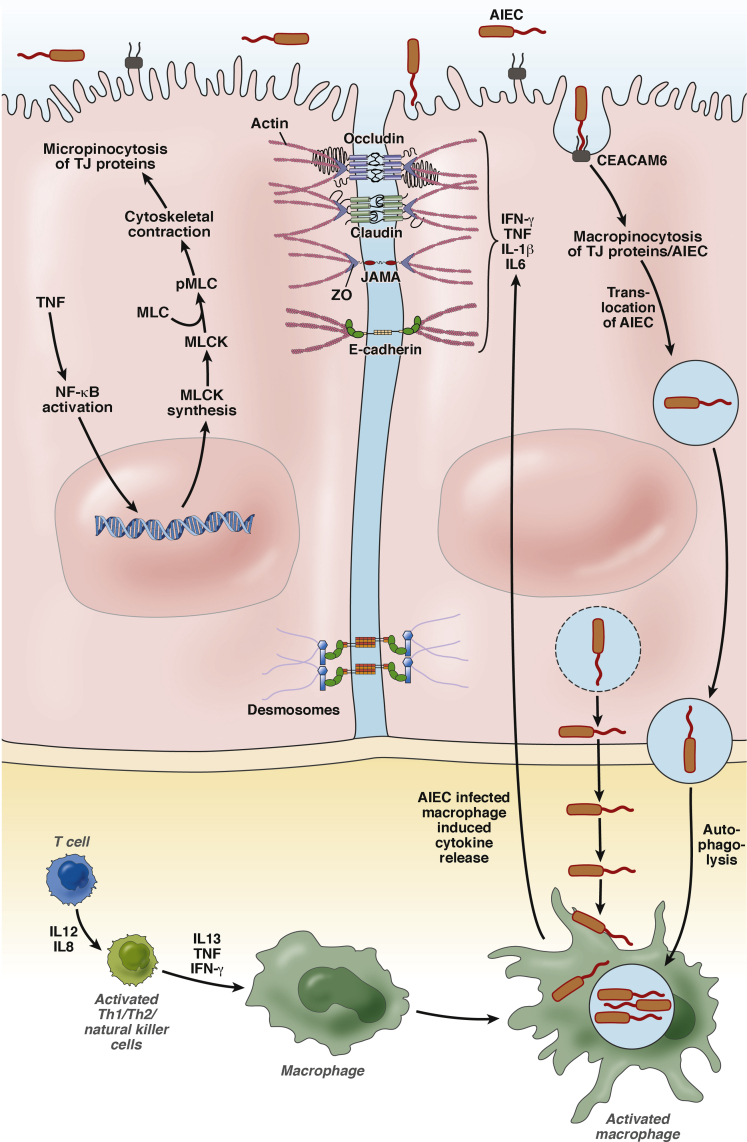
AIEC are distinct from other strains of E coli because they show nonclassic virulence factors of adherence and invasion (ie, lack of a type III secretion system).13 Notably, AIEC pathogenesis involves survival and replication in IECs and macrophages in vivo and in vitro that further exacerbated barrier dysfunction, this topic is discussed later (Figure 1).9, 13 Martinez-Medina and Garcia-Gil16 published an excellent comprehensive review on the definition, characteristics, and molecular basis of AIEC pathogenicity.
Figure 1.
Effect of AIEC on major junctional proteins and regulatory pathways in simplified IECs. Indirect internalization of TJ proteins by a micropinocytosis process can occur as a result of AIEC direct or indirect TNF-α–dependent activation of NF-κB, leading to increased expression of MLCK and cytoskeletal contraction. AIEC interaction with IECs results in direct internalization of TJ proteins and indirectly by activation of proinflammatory cytokines. Intestinal barrier permeability is compromised, permitting the internalization, survival, and replication of AIEC, invasion of macrophages, and further exacerbation of barrier permeability. pMLC, phosphorylated MLC; Th, T-helper cell.
Interaction of AIEC With IECs
The intestine is lined by a single layer of epithelial cells that are interconnected by transmembrane proteins to create physical connections with one another and to form a selectively permeable barrier.17 This leaky barrier serves multiple functions that are essential for intestinal homeostasis: generation of ion solute concentration gradients, absorption of nutrients, antimicrobial peptide secretion (reviewed by McCole and Barrett18), and protection of the host from toxins and pathogens (reviewed by Peterson and Artis19). Disruption of barrier function can lead to loss of microbiome diversity and promotion of AIEC expansion and pathogenesis.1, 6
IECs express molecular sensors that sample the luminal content for bacterial antigens, and in some cases the bacteria itself, and translate this information to host immune cells.19 This process prevents the host immune system from a robust proinflammatory response against commensal bacteria while maintaining the ability to protect against pathogens. The first identified IBD-associated gene, cytoplasmic nucleotide-binding oligomerization domain 2 (NOD2/CARD15), a NOD-like receptor family member, serves as a sensor to bacterial peptidoglycans (BPs) (reviewed by Philpott et al20). Binding of BPs to NOD2 results in the activation of nuclear factor–κB (NF-κB) and autophagolysis,21 and the appearance of NOD2 at the site of bacterial endocytosis in epithelial cells drives autophagolysis.20 Mutations in NOD2 result in an increased immune response to bacteria and bacterial antigens22 that is accompanied by impaired autophagolysis of AIEC and subsequently survival of AIEC in immune cells.23, 24 Conversely, overexpression of NOD2 and other genes involved in autophagy (eg, by activation of the eukaryotic translation initiation factor 2 alpha kinase 4-eukaryotic translation initiation factor 2 alpha-activating transcription factor 4 [EIF2AK4-EIF2A-ATF4] pathway), results in an increased autophagy response to AIEC and a reduction in AIEC survival in macrophages.25, 26, 27 Thus, functional autophagolysis in both intestinal epithelial cells and macrophages is required for AIEC degradation and prevention of AIEC-induced barrier dysfunction.24
It is clear that proinflammatory cytokine signaling plays a major role in altered intestinal permeability28 and this may be associated with AIEC pathogenesis. Loss of negative regulation of proinflammatory cytokines (eg, interferon-γ [IFN-γ] and tumor necrosis factor-α [TNF-α]), and, conversely, overstimulation of the proinflammatory response, plays a role in altered intestinal permeability and this may be associated with AIEC expansion and pathogenesis.22, 29, 30, 31 A key mediator of signaling by many cytokines, NF-κB has been shown to regulate the development and response of the immune system, inflammation, and cancer.32 Defective NF-κB activation can result in a defective immune cell response to BPs (driven by interleukin [IL]8) and bacterial lipopolysaccharides, and defective autophagy.33, 34, 35 Taken together, dysfunctional regulation of proinflammatory cytokines may be associated with an inappropriate response to AIEC and may play a role in the promotion of AIEC expansion and pathogenesis.
AIEC Adherence
With respect to the clinical intersection between altered expression of genes in IBD and genes involved in AIEC expansion, patients who are at risk of developing CD also overexpress carcinoembryonic antigen–related cell-adhesion molecule 6 (CEACAM6), placing them at a higher risk for AIEC adherence and invasion via interaction of bacterial type 1 pili and long polar fimbriae (LPF) to host CEACAM6.36 CEACAM6, normally expressed on the apical membrane of human epithelial cells from the large intestine,37 is regulated by the proinflammatory cytokines IFN-γ and TNF-α.36, 38 Studies using isolated human Peyer’s patches or mouse ileal biopsy specimens containing Peyer’s patches showed that bacterial pili specifically targeted Peyer’s patch M-cells (discussed later) expressing CEACAM6; however, LPF-independent AIEC invasion and adherence also has been shown in other types of intestinal epithelial cells.36, 39 Nevertheless, AIEC can up-regulate CEACAM6 indirectly via activation of proinflammatory cytokines,40 thus leading to increased expression of CEACAM6 and further binding and internalization of AIEC.
Carcinoembryonic antigen human bacterial artifical chromosome 10 (CEABAC10) transgenic mice that overexpress human CEACAMs show no clinical evidence of colitis; however, these same mice infected with AIEC show a barrier defect.41 It would be expected, therefore, that patients with NOD2 mutations and/or increased expression of ileal CEACAM6 are more at risk to develop IBD. In support of this statement, CEACAM6 and the pore-forming TJ protein claudin-2, isolated from ileal biopsy specimens of CD patients, are not only co-localized, but expression levels are both increased compared with control subjects,41 thereby promoting a favorable environment for AIEC adherence and invasion and further exacerbation of barrier dysfunction, ultimately leading to a vicious cycle of proinflammatory cytokine release and increased barrier permeability. Interestingly, although CEACAM6 is overexpressed in ileal mucosa of IBD patients, the protein is not normally expressed in ileal mucosa.41 The mechanism(s) regulating CEACAM6 expression in IBD currently are not known.
AIEC Invasion
The mechanism of AIEC internalization involves macropinocytosis and vacuolization of the bacteria into IECs and macrophages.9, 42, 43, 44 AIEC have been found in late endosomes of intestinal epithelial cells, as evident by co-localization with the lysosomal marker lysosomal-associated membrane protein-1 (LAMP1).24, 44 Similarly, Mycobacterium avium subspecies paratuberculosis has been localized in endosomes of intestinal epithelial cells.45 The process of internalization into endosomes also occurs during reorganization of TJs and is discussed later. AIEC-containing endosomes mature into phagolysosomes, which then can exit IECs, as discussed later. Alternatively, AIEC replication is permitted in autophagolysis-deficient, AIEC-containing phagosome/endosome/lysosomes, leading to loss of organelle membrane integrity, release of the bacteria into the cytoplasm, and basolateral exit of the bacteria from the IEC (Figure 1).46, 47 AIEC translocation through the epithelial barrier is dependent on type 1 pili and binding of LPF to glycoprotein 2 (GP2) to AIEC Disruption of Epithelial Junctions microfold cells (M-cells).39, 43, 48 M-cells are required for the development of host immunity,49 are involved in bacterial sampling,50, 51, 52, 53 and can serve as an alternative gateway for AIEC39, 54 into and through the epithelium (reviewed by Pravda55). The AIEC-containing endosome can mature into a phagosome and exit basolaterally from the IEC, where it can be processed by macrophages (Figure 1).42, 44
After basolateral exit from epithelial cells, AIEC exposure to lamina propria immune cells induces a proinflammatory response and a TNF-α–dependent increase in M-cell development,56 thereby further permitting the adherence and invasion of AIEC and exacerbation of barrier permeability. AIEC invasion elicits a proinflammatory response by triggering secretion of proinflammatory cytokines from IECs, resulting in recruitment of immune cells,13, 57 and, subsequently, NF-κB signaling,13 which allows survival of AIEC in macrophages.58 In summary, cytokines play a key role in the pathogenesis of AIEC and AIEC-induced epithelial barrier dysfunction.
AIEC-Dependent Disruption of the Apical Junctional Complex
In addition to the unknown etiology of IBD, it is uncertain whether inflammation precedes epithelial barrier dysfunction or if barrier dysfunction results in chronic inflammation such as that observed in IBD. However, some evidence points to an underlying barrier defect that renders individuals susceptible to disease. First-degree relatives of CD patients show asymptomatic barrier dysfunction associated with subclinical immune activation, indicating that a barrier defect may precede or is at least an early event in the disease.59, 60 In addition, altered permeability across the intestinal epithelium is a predictor of relapse in human CD61; whereas increased permeability precedes inflammation in rodent models,62 canine models,63 and human disease,59, 64, 65, 66 and has been shown to be the underlying mechanism of IBD in these patients. Thus, host health is dependent on the integrity of the epithelial barrier. Pathologic stimuli, such as AIEC, in part influence epithelial barrier integrity by altering the network of proteins that regulate barrier permeability known as the intercellular apical junctional complex (AJC).67
The AJC, comprised of several different proteins with distinct responsibilities, directs the paracellular movement of ions and solutes and thus is responsible to maintain appropriate epithelial barrier permeability. The structural units of the AJC responsible for regulating barrier integrity and permeability are adherens junctions (AJ) and apical TJs.17 AJs and TJs are associated with scaffolding proteins that cooperate with the cytoskeleton of the cell providing the AJC with a dynamic means of regulation.67 Dysregulation of TJ proteins is observed in IBD patients (Table 1).68, 69, 70, 71 That the AJC is required for barrier integrity has led to the hypothesis that defects in the AJC may play a role in IBD pathogenesis. Alteration of the AJC can occur in response to exposure to AIEC and other enteropathogenic E coli.71, 72, 73, 74 AIEC have been shown to decrease transepithelial electrical resistance of IECs owing to disruption of the AJC.47, 67 The molecular interaction of AIEC with IECs induces an inflammatory response leading to the overproduction of proinflammatory cytokines,75 with IFN-γ and TNF-α as the major effectors, and this is a key step in the pathogenesis of IBD. IFN-γ and TNF-α both have been shown to increase gut permeability by acting on TJ proteins and TJ strand complexity,76, 77 which then further exacerbates barrier dysfunction. AIEC-dependent, cytokine-induced alterations in AJ and TJ proteins is discussed later.
Table 1.
Major AIEC-Affected Host Junctional Proteins and Mutations Associated With IBD
| Protein | Gene | Function | Impact of AIEC | Mechanisms | IBD References |
|---|---|---|---|---|---|
| CEACAM6 | CEACAM6/CD66c/CEAL/NCA | Bacterial pili receptor recognizing AIEC | Increase in expression | IFN-γ and TNF-α indirect induction | 36, 40 |
| Claudin-2 | CLDN2 | Paracellular transport of Na+ and H2O; regulates leak pathway; major integral protein of TJs | Increase in expression | IFN-γ, TNF-α, and MLCK-dependent internalization | 92 |
| E-cadherin | CDH1 | Major adherens junction protein; cell–cell adhesion and communication | Displace E-cadherin in vitro | Presumed TNF-α, IFN-γ, and MLCK-induced internalization; cleavage? | 47, 91, 93 |
| JAM-A | F11R | Regulator of TJ assembly; leukocyte transmigration | Decrease in expression | Presumably macrophage release of proinflammatory cytokines | 69, 94 |
| MLCK | MYLK | Phosphorylates myosin regulatory light chains to facilitate myosin interaction with actin filaments to produce contractile activity; membrane distribution (and redistribution) of TJ proteins | Down-regulation of ZO-1, redistribution of TJ proteins | TNF-α–dependent activation of NF-κB and activation of MLCK | 67, 95 |
| NOD2 | CARD15 | Recognition of pathogen-associated molecular patterns; bacterial sensing; drive autophagolysis of bacteria containing phagosomes | Decrease in expression; indirect: LPS binding with NOD2 results in autophagolysis of AIEC |
Endocytosis activation of NF-κB leading to dysregulated proinflammatory response and promotes invasion of AIEC |
20, 21, 35 |
| Occludin | OCLN | Integral TJ protein involved in stability and cytokine-induced regulation of TJs | Redistribution/down-regulation of occludin | Induction of TNF-α, activation of MLCK | 69, 96 |
| Partitioning defective protein-3 (PAR-3) | PARD3 | Asymmetric cell growth; cell polarization; targeting of TJ proteins to membrane | Unknown | Presumably disruption of adherens junctions resulting in increased Cldn-2 and decreased membrane localization of ZO-1 | 91, 97 |
| Zonula occludens-1 | TJP1 | Plaque protein; scaffolding of TJ proteins to the cytoskeleton | Disruption of ZO-1 resulting in increased appearance of gaps between cells | Presumably TNF-α, IFN-γ, and MLCK- induced internalization | 47, 92, 93 |
CEACAM6
As previously discussed, an increase in expression of both CEACAM6 and claudin-2 is associated with increased barrier permeability. Interestingly, targeting of adherens junction proteins, occludin, and claudin 3/4 and other tight junction proteins by Helicobacter pylori, Vibrio cholera, and enteropathogenic E coli, respectively, has been shown previously,78, 79 supporting the possibility that AIEC directly bind to TJ proteins. It has been shown that AIEC results in increased claudin-2 expression in mice and human beings,41 and because AIEC pathogenesis is not dependent solely on interaction of CEACAM6 with AIEC, it is possible that claudin-2 could serve as a gateway for AIEC entry into IECs while co-internalization of TJ proteins and AIEC may promote AIEC invasion and increased barrier permeability. Whether AIEC are able to bind directly to TJ proteins has yet to be shown experimentally.
Claudin-2
Distinct from the functions of adherens junctions, TJs serve as a paracellular gateway for passive fluid movement and solute flux and limit the passive movement of proteins and lipids, thereby generating a concentration gradient as well as playing a role in polarization of epithelia.71 The conglomeration of proteins that make up the TJs include the claudin family of transmembrane proteins, junctional adhesion molecule-A (JAM-A), occludin, and zonula occludens members 1–3 (others not included in this article are reviewed elsewhere67) (Figure 1).
Claudin-2 is a prominent member of the claudin family because it forms a cation-selective pore that permits paracellular sodium and water flux.80, 81 Overexpression of claudin-2 results in an increase in epithelial barrier permeability both in vitro and in vivo.82, 83, 84, 85 IL13 and TNF-α induce a barrier defect in UC by up-regulating claudin-2 to facilitate the pore pathway, although IL6 also has been shown to increase claudin-2 expression in intestinal epithelial cells.86, 87 That epithelial barrier leakiness is related inversely to TJ strand complexity67 supports the association of increased levels of claudin-2 with increased barrier permeability in IBD.70, 88 Conversely, a decrease in claudin-2 expression results in a tighter epithelial barrier.89
E-cadherin
Adherens junctions are primarily responsible for cell–cell recognition as well as initiating and maintaining cell–cell contact and polarization.90 The major AJ protein is E-cadherin, a transmembrane protein associated with the catenin family of cytoplasmic proteins (Figure 1).90 E-cadherin plays a critical role in adhesion of cells and is required for the formation of TJs.91 E-cadherin single-nucleotide polymorphisms and dysregulation of E-cadherin are associated with IBD (reviewed by McCole91), supporting a role for AJ defects in IBD-associated barrier dysfunction.67 Furthermore, AIEC, in addition to other intestinal pathogens,91 are able to displace E-cadherin in vitro,47, 93 potentially providing another mechanism of invasion and barrier disruption.
Junctional Adhesion Molecule
JAM-A is another PSD95-DlgA-zonula occludens-1 homology domain (PDZ)-domain–containing integral protein localized to TJs.98 JAM-A shows PDZ-domain–dependent interactions with the scaffolding protein zonula occludens 1 (ZO-1) and cell polarity partitioning defective protein-3.99 Expression of JAM-A was shown to be decreased69 together with ZO-1100 in IBD, and loss of JAM-A results in increased epithelial barrier permeability.101, 102 It is unknown whether AIEC directly results in the down-regulation of JAM-A or if it is a consequence of down-regulation of ZO-1; however, AIEC may regulate JAM-A indirectly via the release of inflammatory cytokines from infected macrophages.94 Nevertheless, it is possible that AIEC, in addition to altering ZO-1, may alter JAM-A and partitioning defective protein-3–dependent targeting of TJ proteins to the membrane,97 resulting in disrupted TJ complex integrity and altered cell polarity, further promoting IBD pathogenesis.
Occludin
Occludin, a 65-kilodalton integral transmembrane protein, is involved in the stability and regulation of TJs.103 Although down-regulation of occludin is observed in CD and UC patients,69 the UC-associated proinflammatory cytokine IL13 alone was not able to abolish occludin protein levels in a colorectal cancer cell line (HT-29).86 This discrepancy was attributed to a dilution of the number of cells isolated from the inflamed tissue of UC patients. Nevertheless, it has been shown from in vitro work that occludin plays a key role in promoting barrier function and is redistributed away from tight junctions after enterohemorrhagic E coli infection of human colonoid monolayers.17, 74 Further work to elucidate the in vivo role of occludin is needed because mice lacking occludin do not show defects in TJ morphology despite histologic abnormalities in some tissues.104 Proinflammatory cytokine-dependent phosphorylation of myosin light chain (MLC) by myosin light chain kinase (MLCK) or Rho-associated kinase results in rapid reorganization of the AJC.67, 83, 105 Although it remains to be confirmed, AIEC could affect occludin distribution indirectly through bacteria-dependent induction of proinflammatory cytokines from immune cells, activation of MLCK or Rho-associated kinase, and subsequent redistribution of occludin.
Zonula Occludens
ZO is a PDZ homology domain containing plaque protein involved in scaffolding of TJ proteins to the cytoskeleton.106 ZO-1, -2, and -3 contain PDZ domains that facilitate anchorage of TJ proteins such as claudins,107 occludin,108 and JAM-A100 to the cytoplasm and thereby play a key role in TJ formation and regulation. TNF-α induces an increase in TJ permeability via NF-κB–dependent down-regulation of ZO-1 protein and MLCK-dependent95, 96, 109, 110, 111 redistribution of TJ proteins76 in an apoptosis-independent manner (reviewed by Bruewer et al67). Similarly, AIEC disrupt TJ composition by altering ZO-1, resulting in increased appearance of gaps between cells.47, 93 Conversely, probiotic and commensal bacteria promote up-regulation of ZO proteins and strengthening of the epithelial barrier (reviewed by Ulluwishewa et al112), potentially supporting the notion that when probiotic bacteria outcompete pathogens, this not only will return the balance of the microbiota, but also enable restitution of the TJ complex.
In the absence of bacteria, regulation of the AJC is mediated by the interaction of the cytoskeleton with the proteins of the AJC or by cytokines. IFN-γ induces macropinocytosis92 of occludin, JAM-A, and claudin-1 via myosin 2–dependent vacuolarization of the apical membrane105 into early endosomes, resulting in an increase in barrier permeability.113 That AIEC also are internalized by macropinocytosis illustrates a direct disruption of the AJC by the bacteria and establishes an additional virulence factor for the invasion of AIEC.
Conclusions
Data to date clearly support the notion that IBD pathogenesis is dependent on dysregulation of multiple factors including disruption of the intestinal barrier and immune dysregulation, and is associated with profound alterations in gut microbial communities. Altered intestinal homeostasis drives expansion of AIEC and results in compromised barrier function. AIEC are able to invade, survive, and replicate within host cells, leading to exacerbation of barrier dysfunction by disruption of TJ proteins, and ultimately contributing to IBD pathogenesis. Recent clinical findings have indicated that AIEC is not associated exclusively with ileal CD. If AIEC does indeed play an important role in IBD pathogenesis it will be vital to better understand unresolved questions regarding its appearance in disease. Specifically: (1) what are the host genetic and environmental factors that promote AIEC expansion in some patients but not all; (2) how is regional variation in AIEC expansion determined; and (3) can therapeutic interventions (probiotics, prebiotics, small molecules) specifically neutralize or modify AIEC pathogenic behavior? AIEC are capable of compromising intestinal epithelial barrier properties both directly and indirectly to enhance their pathogenic impact. Therefore, in addition to the earlier-described approaches, it will be of key interest to determine if strategies designed to enhance the epithelial barrier can minimize the pathogenic impact of AIEC and its putative contribution to IBD.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by the National Institute of Diabetes and Digestive and Kidney DiseasesR01DK091281 and Crohn's and Colitis Foundation of America Senior Research Award (D.F.M.).
References
- 1.Craven M., Egan C.E., Dowd S.E. Inflammation drives dysbiosis and bacterial invasion in murine models of ileal Crohn's disease. PLoS One. 2012;7:e41594. doi: 10.1371/journal.pone.0041594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Darfeuille-Michaud A., Neut C., Barnich N. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology. 1998;115:1405–1413. doi: 10.1016/s0016-5085(98)70019-8. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y.Z., Li Y.Y. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91–99. doi: 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaser A., Zeissig S., Blumberg R.S. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nataro J.P., Kaper J.B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stecher B. The roles of inflammation, nutrient availability and the commensal microbiota in enteric pathogen infection. In: Conway T, Cohen PS, eds. Metabolism and bacterial pathogenesis. District of Columbia: ASM Press, 2015:297–320. [DOI] [PubMed]
- 7.Chow J., Mazmanian S.K. A pathobiont of the microbiota balances host colonization and intestinal inflammation. Cell Host Microbe. 2010;7:265–276. doi: 10.1016/j.chom.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnich N., Darfeuille-Michaud A. Role of bacteria in the etiopathogenesis of inflammatory bowel disease. World J Gastroenterol. 2007;13:5571–5576. doi: 10.3748/wjg.v13.i42.5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boudeau J., Glasser A.L., Masseret E. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease. Infect Immun. 1999;67:4499–4509. doi: 10.1128/iai.67.9.4499-4509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bibiloni R., Mangold M., Madsen K.L. The bacteriology of biopsies differs between newly diagnosed, untreated, Crohn's disease and ulcerative colitis patients. J Med Microbiol. 2006;55:1141–1149. doi: 10.1099/jmm.0.46498-0. [DOI] [PubMed] [Google Scholar]
- 11.Darfeuille-Michaud A., Boudeau J., Bulois P. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology. 2004;127:412–421. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 12.Desilets M., Deng X., Rao C. Genome-based definition of an inflammatory bowel disease-associated adherent-invasive Escherichia coli pathovar. Inflamm Bowel Dis. 2016;22:1–12. doi: 10.1097/MIB.0000000000000574. [DOI] [PubMed] [Google Scholar]
- 13.Eaves-Pyles T., Allen C.A., Taormina J. Escherichia coli isolated from a Crohn's disease patient adheres, invades, and induces inflammatory responses in polarized intestinal epithelial cells. Int J Med Microbiol. 2008;298:397–409. doi: 10.1016/j.ijmm.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien C.L., Bringer M.A., Holt K.E. Comparative genomics of Crohn's disease-associated adherent-invasive Escherichia coli. Gut. 2016 doi: 10.1136/gutjnl-2015-311059. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Gibold L., Garenaux E., Dalmasso G. The Vat-AIEC protease promotes crossing of the intestinal mucus layer by Crohn's disease-associated Escherichia coli. Cell Microbiol. 2016;18:617–631. doi: 10.1111/cmi.12539. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Medina M., Garcia-Gil L.J. Escherichia coli in chronic inflammatory bowel diseases: an update on adherent invasive Escherichia coli pathogenicity. World J Gastrointest Pathophysiol. 2014;5:213–227. doi: 10.4291/wjgp.v5.i3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner J.R. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 18.McCole D.F., Barrett K.E. Varied role of the gut epithelium in mucosal homeostasis. Curr Opin Gastroenterol. 2007;23:647–654. doi: 10.1097/MOG.0b013e3282f0153b. [DOI] [PubMed] [Google Scholar]
- 19.Peterson L.W., Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 20.Philpott D.J., Sorbara M.T., Robertson S.J. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol. 2014;14:9–23. doi: 10.1038/nri3565. [DOI] [PubMed] [Google Scholar]
- 21.Correa R.G., Milutinovic S., Reed J.C. Roles of NOD1 (NLRC1) and NOD2 (NLRC2) in innate immunity and inflammatory diseases. Biosci Rep. 2012;32:597–608. doi: 10.1042/BSR20120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogler G. The effects of NOD2/CARD15 mutations on the function of the intestinal barrier. J Crohns Colitis. 2007;1:53–60. doi: 10.1016/j.crohns.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Cooney R., Baker J., Brain O. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16:90–97. doi: 10.1038/nm.2069. [DOI] [PubMed] [Google Scholar]
- 24.Lapaquette P., Bringer M.A., Darfeuille-Michaud A. Defects in autophagy favour adherent-invasive Escherichia coli persistence within macrophages leading to increased pro-inflammatory response. Cell Microbiol. 2012;14:791–807. doi: 10.1111/j.1462-5822.2012.01768.x. [DOI] [PubMed] [Google Scholar]
- 25.Negroni A., Colantoni E., Vitali R. NOD2 induces autophagy to control AIEC bacteria infectiveness in intestinal epithelial cells. Inflamm Res. 2016;65:803–813. doi: 10.1007/s00011-016-0964-8. [DOI] [PubMed] [Google Scholar]
- 26.Vazeille E., Chassaing B., Buisson A. GipA factor supports colonization of Peyer's patches by Crohn's disease-associated Escherichia coli. Inflamm Bowel Dis. 2016;22:68–81. doi: 10.1097/MIB.0000000000000609. [DOI] [PubMed] [Google Scholar]
- 27.Bretin A., Carriere J., Dalmasso G. Activation of the EIF2AK4-EIF2A/eIF2alpha-ATF4 pathway triggers autophagy response to Crohn disease-associated adherent-invasive Escherichia coli infection. Autophagy. 2016;12:770–783. doi: 10.1080/15548627.2016.1156823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Sadi R., Boivin M., Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci (Landmark Ed) 2009;14:2765–2778. doi: 10.2741/3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hugot J.P., Chamaillard M., Zouali H. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 30.Warhurst A.C., Hopkins S.J., Warhurst G. Interferon gamma induces differential upregulation of alpha and beta chemokine secretion in colonic epithelial cell lines. Gut. 1998;42:208–213. doi: 10.1136/gut.42.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishima Y., Liu B., Hansen J.J. Resident bacteria-stimulated IL-10-secreting B cells ameliorate T cell-mediated colitis by inducing Tr-1 cells that require IL-27-signaling. Cell Mol Gastroenterol Hepatol. 2015;1:295–310. doi: 10.1016/j.jcmgh.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell S., Vargas J., Hoffmann A. Signaling via the NFkappaB system. Wiley Interdiscip Rev Syst Biol Med. 2016;8:227–241. doi: 10.1002/wsbm.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Travassos L.H., Carneiro L.A.M., Ramjeet M. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 2010;11:55–62. doi: 10.1038/ni.1823. [DOI] [PubMed] [Google Scholar]
- 34.Kim Y.G., Shaw M.H., Warner N. Cutting edge: Crohn's disease-associated Nod2 mutation limits production of proinflammatory cytokines to protect the host from Enterococcus faecalis-induced lethality. J Immunol. 2011;187:2849–2852. doi: 10.4049/jimmunol.1001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inohara N., Ogura Y., Chen F.F. Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J Biol Chem. 2001;276:2551–2554. doi: 10.1074/jbc.M009728200. [DOI] [PubMed] [Google Scholar]
- 36.Barnich N., Carvalho F.A., Glasser A.L. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest. 2007;117:1566–1574. doi: 10.1172/JCI30504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scholzel S., Zimmermann W., Schwarzkopf G. Carcinoembryonic antigen family members CEACAM6 and CEACAM7 are differentially expressed in normal tissues and oppositely deregulated in hyperplastic colorectal polyps and early adenomas. Am J Pathol. 2000;156:595–605. doi: 10.1016/S0002-9440(10)64764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fahlgren A., Baranov V., Frangsmyr L. Interferon-gamma tempers the expression of carcinoembryonic antigen family molecules in human colon cells: a possible role in innate mucosal defence. Scand J Immunol. 2003;58:628–641. doi: 10.1111/j.1365-3083.2003.01342.x. [DOI] [PubMed] [Google Scholar]
- 39.Chassaing B., Rolhion N., de Vallee A. Crohn disease–associated adherent-invasive E. coli bacteria target mouse and human Peyer's patches via long polar fimbriae. J Clin Invest. 2011;121:966–975. doi: 10.1172/JCI44632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cieza R.J., Cao A.T., Cong Y. Immunomodulation for gastrointestinal infections. Expert Rev Anti Infect Ther. 2012;10:391–400. doi: 10.1586/eri.11.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Denizot J., Sivignon A., Barreau F. Adherent-invasive Escherichia coli induce claudin-2 expression and barrier defect in CEABAC10 mice and Crohn's disease patients. Inflamm Bowel Dis. 2012;18:294–304. doi: 10.1002/ibd.21787. [DOI] [PubMed] [Google Scholar]
- 42.Glasser A.L., Boudeau J., Barnich N. Adherent invasive Escherichia coli strains from patients with Crohn's disease survive and replicate within macrophages without inducing host cell death. Infect Immun. 2001;69:5529–5537. doi: 10.1128/IAI.69.9.5529-5537.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boudeau J., Barnich N., Darfeuille-Michaud A. Type 1 pili-mediated adherence of Escherichia coli strain LF82 isolated from Crohn's disease is involved in bacterial invasion of intestinal epithelial cells. Mol Microbiol. 2001;39:1272–1284. doi: 10.1111/j.1365-2958.2001.02315.x. [DOI] [PubMed] [Google Scholar]
- 44.Bringer M.A., Glasser A.L., Tung C.H. The Crohn's disease-associated adherent-invasive Escherichia coli strain LF82 replicates in mature phagolysosomes within J774 macrophages. Cell Microbiol. 2006;8:471–484. doi: 10.1111/j.1462-5822.2005.00639.x. [DOI] [PubMed] [Google Scholar]
- 45.Pott J., Basler T., Duerr C.U. Internalization-dependent recognition of Mycobacterium avium ssp. paratuberculosis by intestinal epithelial cells. Cell Microbiol. 2009;11:1802–1815. doi: 10.1111/j.1462-5822.2009.01372.x. [DOI] [PubMed] [Google Scholar]
- 46.Lapaquette P., Glasser A.L., Huett A. Crohn's disease-associated adherent-invasive E. coli are selectively favoured by impaired autophagy to replicate intracellularly. Cell Microbiol. 2010;12:99–113. doi: 10.1111/j.1462-5822.2009.01381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wine E., Ossa J.C., Gray-Owen S.D. Adherent-invasive Escherichia coli, strain LF82 disrupts apical junctional complexes in polarized epithelia. BMC Microbiol. 2009;9:180. doi: 10.1186/1471-2180-9-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strober W. Adherent-invasive E. coli in Crohn disease: bacterial “agent provocateur”. J Clin Invest. 2011;121:841–844. doi: 10.1172/JCI46333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neutra M.R. M cells in antigen sampling in mucosal tissues. Curr Top Microbiol Immunol. 1999;236:17–32. doi: 10.1007/978-3-642-59951-4_2. [DOI] [PubMed] [Google Scholar]
- 50.Secott T.E., Lin T.L., Wu C.C. Mycobacterium avium subsp. paratuberculosis fibronectin attachment protein facilitates M-cell targeting and invasion through a fibronectin bridge with host integrins. Infect Immun. 2004;72:3724–3732. doi: 10.1128/IAI.72.7.3724-3732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jensen V.B., Harty J.T., Jones B.D. Interactions of the invasive pathogens Salmonella typhimurium, Listeria monocytogenes, and Shigella flexneri with M cells and murine Peyer's patches. Infect Immun. 1998;66:3758–3766. doi: 10.1128/iai.66.8.3758-3766.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Owen R.L., Pierce N.F., Apple R.T. M cell transport of Vibrio cholerae from the intestinal lumen into Peyer's patches: a mechanism for antigen sampling and for microbial transepithelial migration. J Infect Dis. 1986;153:1108–1118. doi: 10.1093/infdis/153.6.1108. [DOI] [PubMed] [Google Scholar]
- 53.Blanco L.P., DiRita V.J. Bacterial-associated cholera toxin and GM1 binding are required for transcytosis of classical biotype Vibrio cholerae through an in vitro M cell model system. Cell Microbiol. 2006;8:982–998. doi: 10.1111/j.1462-5822.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- 54.Roberts C.L., Keita A.V., Duncan S.H. Translocation of Crohn's disease Escherichia coli across M-cells: contrasting effects of soluble plant fibres and emulsifiers. Gut. 2010;59:1331–1339. doi: 10.1136/gut.2009.195370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pravda J. Crohn's disease: evidence for involvement of unregulated transcytosis in disease etio-pathogenesis. World J Gastroenterol. 2011;17:1416–1426. doi: 10.3748/wjg.v17.i11.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bennett K.M., Parnell E.A., Sanscartier C. Induction of colonic M cells during Intestinal Inflammation. Am J Pathol. 2016;186:1166–1179. doi: 10.1016/j.ajpath.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Subramanian S., Rhodes J.M., Hart C.A. Characterization of epithelial IL-8 response to inflammatory bowel disease mucosal E. coli and its inhibition by mesalamine. Inflamm Bowel Dis. 2008;14:162–175. doi: 10.1002/ibd.20296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dunne K.A., Allam A., McIntosh A. Increased S-nitrosylation and proteasomal degradation of caspase-3 during infection contribute to the persistence of adherent invasive Escherichia coli (AIEC) in immune cells. PLoS One. 2013;8:e68386. doi: 10.1371/journal.pone.0068386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hollander D. Permeability in Crohn's disease: altered barrier functions in healthy relatives? Gastroenterology. 1993;104:1848–1851. doi: 10.1016/0016-5085(93)90668-3. [DOI] [PubMed] [Google Scholar]
- 60.Peeters M., Geypens B., Claus D. Clustering of increased small intestinal permeability in families with Crohn's disease. Gastroenterology. 1997;113:802–807. doi: 10.1016/s0016-5085(97)70174-4. [DOI] [PubMed] [Google Scholar]
- 61.Wyatt J., Vogelsang H., Hubl W. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet. 1993;341:1437–1439. doi: 10.1016/0140-6736(93)90882-h. [DOI] [PubMed] [Google Scholar]
- 62.Olson T.S., Reuter B.K., Scott K.G.E. The primary defect in experimental ileitis originates from a nonhematopoietic source. J Exp Med. 2006;203:541–552. doi: 10.1084/jem.20050407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hall E.J., Carter S.D., Barnes A. Immune responses to dietary antigens in gluten-sensitive enteropathy of Irish setters. Res Vet Sci. 1992;53:293–299. doi: 10.1016/0034-5288(92)90129-p. [DOI] [PubMed] [Google Scholar]
- 64.Hollander D., Vadheim C.M., Brettholz E. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- 65.Katz K.D., Hollander D., Vadheim C.M. Intestinal permeability in patients with Crohn's disease and their healthy relatives. Gastroenterology. 1989;97:927–931. doi: 10.1016/0016-5085(89)91499-6. [DOI] [PubMed] [Google Scholar]
- 66.Teahon K., Smethurst P., Levi A.J. Intestinal permeability in patients with Crohn's disease and their first degree relatives. Gut. 1992;33:320–323. doi: 10.1136/gut.33.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bruewer M., Samarin S., Nusrat A. Inflammatory bowel disease and the apical junctional complex. Ann N Y Acad Sci. 2006;1072:242–252. doi: 10.1196/annals.1326.017. [DOI] [PubMed] [Google Scholar]
- 68.Oshitani N., Watanabe K., Nakamura S. Dislocation of tight junction proteins without F-actin disruption in inactive Crohn's disease. Int J Mol Med. 2005;15:407–410. [PubMed] [Google Scholar]
- 69.Kucharzik T., Walsh S.V., Chen J. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol. 2001;159:2001–2009. doi: 10.1016/S0002-9440(10)63051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gassler N., Rohr C., Schneider A. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol. 2001;281:G216–G228. doi: 10.1152/ajpgi.2001.281.1.G216. [DOI] [PubMed] [Google Scholar]
- 71.Laukoetter M.G., Bruewer M., Nusrat A. Regulation of the intestinal epithelial barrier by the apical junctional complex. Curr Opin Gastroenterol. 2006;22:85–89. doi: 10.1097/01.mog.0000203864.48255.4f. [DOI] [PubMed] [Google Scholar]
- 72.Muza-Moons M.M., Schneeberger E.E., Hecht G.A. Enteropathogenic Escherichia coli infection leads to appearance of aberrant tight junctions strands in the lateral membrane of intestinal epithelial cells. Cell Microbiol. 2004;6:783–793. doi: 10.1111/j.1462-5822.2004.00404.x. [DOI] [PubMed] [Google Scholar]
- 73.Shifflett D.E., Clayburgh D.R., Koutsouris A. Enteropathogenic E. coli disrupts tight junction barrier function and structure in vivo. Lab Invest. 2005;85:1308–1324. doi: 10.1038/labinvest.3700330. [DOI] [PubMed] [Google Scholar]
- 74.In J., Foulke-Abel J., Zachos N.C. Enterohemorrhagic Escherichia coli reduce mucus and intermicrovillar bridges in human stem cell-derived colonoids. Cell Mol Gastroenterol Hepatol. 2016;2:48–62. doi: 10.1016/j.jcmgh.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raddatz D., Bockemuhl M., Ramadori G. Quantitative measurement of cytokine mRNA in inflammatory bowel disease: relation to clinical and endoscopic activity and outcome. Eur J Gastroenterol Hepatol. 2005;17:547–557. doi: 10.1097/00042737-200505000-00012. [DOI] [PubMed] [Google Scholar]
- 76.Ma T.Y., Iwamoto G.K., Hoa N.T. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367–G376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- 77.Söderholm J.D., Streutker C., Yang P.C. Increased epithelial uptake of protein antigens in the ileum of Crohn's disease mediated by tumour necrosis factor alpha. Gut. 2004;53:1817–1824. doi: 10.1136/gut.2004.041426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ribet D., Cossart P. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect. 2015;17:173–183. doi: 10.1016/j.micinf.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 79.Guttman J.A., Finlay B.B. Tight junctions as targets of infectious agents. Biochim Biophys Acta. 2009;1788:832–841. doi: 10.1016/j.bbamem.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 80.Rosenthal R., Milatz S., Krug S.M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci. 2010;123:1913–1921. doi: 10.1242/jcs.060665. [DOI] [PubMed] [Google Scholar]
- 81.Rosenthal R., Günzel D., Schulzke J. Claudin-2 mediated cation and water transport share a common pore. Acta Physiologica. 2016 doi: 10.1111/apha.12742. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amasheh S., Meiri N., Gitter A.H. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 83.Weber C.R., Nalle S.C., Tretiakova M. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest. 2008;88:1110–1120. doi: 10.1038/labinvest.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ridyard A.E., Brown J.K., Rhind S.M. Apical junction complex protein expression in the canine colon: differential expression of claudin-2 in the colonic mucosa in dogs with idiopathic colitis. J Histochem Cytochem. 2007;55:1049–1058. doi: 10.1369/jhc.7A7211.2007. [DOI] [PubMed] [Google Scholar]
- 85.Zeissig S., Burgel N., Gunzel D. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heller F., Florian P., Bojarski C. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 87.Al-Sadi R., Ye D., Boivin M. Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. PLoS One. 2014;9:e85345. doi: 10.1371/journal.pone.0085345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schmitz H., Barmeyer C., Fromm M. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301–309. doi: 10.1016/s0016-5085(99)70126-5. [DOI] [PubMed] [Google Scholar]
- 89.Luettig J., Rosenthal R., Barmeyer C. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers. 2015;3:e977176. doi: 10.4161/21688370.2014.977176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yap A.S., Brieher W.M., Gumbiner B.M. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- 91.McCole D.F. IBD candidate genes and intestinal barrier regulation. Inflamm Bowel Dis. 2014;20:1829–1849. doi: 10.1097/MIB.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bruewer M., Utech M., Ivanov A.I. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005;19:923–933. doi: 10.1096/fj.04-3260com. [DOI] [PubMed] [Google Scholar]
- 93.Sasaki M., Sitaraman S.V., Babbin B.A. Invasive Escherichia coli are a feature of Crohn's disease. Lab Invest. 2007;87:1042–1054. doi: 10.1038/labinvest.3700661. [DOI] [PubMed] [Google Scholar]
- 94.Bruewer M., Luegering A., Kucharzik T. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- 95.Clayburgh D.R., Musch M.W., Leitges M. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest. 2006;116:2682–2694. doi: 10.1172/JCI29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shen L., Black E.D., Witkowski E.D. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci. 2006;119:2095–2106. doi: 10.1242/jcs.02915. [DOI] [PubMed] [Google Scholar]
- 97.Schumann M., Gunzel D., Buergel N. Cell polarity-determining proteins Par-3 and PP-1 are involved in epithelial tight junction defects in coeliac disease. Gut. 2012;61:220–228. doi: 10.1136/gutjnl-2011-300123. [DOI] [PubMed] [Google Scholar]
- 98.Martin-Padura I., Lostaglio S., Schneemann M. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ebnet K., Suzuki A., Ohno S. Junctional adhesion molecules (JAMs): more molecules with dual functions? J Cell Sci. 2004;117:19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- 100.Itoh M., Sasaki H., Furuse M. Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J Cell Biol. 2001;154:491–497. doi: 10.1083/jcb.200103047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Laukoetter M.G., Nava P., Lee W.Y. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med. 2007;204:3067–3076. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vetrano S., Rescigno M., Cera M.R. Unique role of junctional adhesion molecule-a in maintaining mucosal homeostasis in inflammatory bowel disease. Gastroenterology. 2008;135:173–184. doi: 10.1053/j.gastro.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 103.Furuse M., Hirase T., Itoh M. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saitou M., Furuse M., Sasaki H. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Utech M., Ivanov A.I., Samarin S.N. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell. 2005;16:5040–5052. doi: 10.1091/mbc.E05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fanning A.S., Anderson J.M. PDZ domains: fundamental building blocks in the organization of protein complexes at the plasma membrane. J Clin Invest. 1999;103:767–772. doi: 10.1172/JCI6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Itoh M., Furuse M., Morita K. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karczewski J., Troost F.J., Konings I. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol. 2010;298:G851–G859. doi: 10.1152/ajpgi.00327.2009. [DOI] [PubMed] [Google Scholar]
- 109.Turner J.R., Rill B.K., Carlson S.L. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997;273:C1378–C1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- 110.Clayburgh D.R., Barrett T.A., Tang Y. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115:2702–2715. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang F., Graham W.V., Wang Y. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ulluwishewa D., Anderson R.C., McNabb W.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- 113.Watson C.J., Hoare C.J., Garrod D.R. Interferon-gamma selectively increases epithelial permeability to large molecules by activating different populations of paracellular pores. J Cell Sci. 2005;118:5221–5230. doi: 10.1242/jcs.02630. [DOI] [PubMed] [Google Scholar]