Abstract
Intestinal-type gastric adenocarcinoma evolves in a field of pre-existing metaplasia. Over the past 20 years, a number of murine models have been developed to address aspects of the physiology and pathophysiology of metaplasia induction. Although none of these models has achieved true recapitulation of the induction of adenocarcinoma, they have led to important insights into the factors that influence the induction and progression of metaplasia. Here, we review the pathologic definitions relevant to alterations in gastric corpus lineages and classification of metaplasia by specific lineage markers. In addition, we review present murine models of the induction and progression of spasmolytic polypeptide (TFF2)–expressing metaplasia, the predominant metaplastic lineage observed in murine models. These models provide a basis for the development of a broader understanding of the physiological and pathophysiological roles of metaplasia in the stomach.
Keywords: SPEM, Intestinal Metaplasia, Gastric Cancer, TFF2, Chief Cell, Hyperplasia
Abbreviations used in this paper: ATPase, adenosine triphosphatase; BMP, bone morphogenic protein; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; Hip1r, Huntington interacting protein 1 related; IFN, interferon; MUC, mucin; SDF1, stromal-derived factor 1; SPEM, spasmolytic polypeptide–expressing metaplasia; TFF, trefoil factor; Tg, transgene; TGF, transforming growth factor; Th, T-helper
Summary.
While mouse models have been relatively poor at recapitulating true adenocarcinoma in the gastric corpus, a number of models have been successful in modeling pre-neoplastic metaplasias. These models demonstrate key insights into the origins of metaplasia and their modulation by intrinsic mucosal and immune cell-derived factors.
Gastric adenocarcinoma is a leading cause of worldwide cancer-related deaths. Poor clinical outcomes result from the lack of early clinical indicators and late presentation. Insights into the progression of preneoplastic processes that promote gastric cancer therefore are needed to facilitate early intervention in gastric carcinogenesis. The sequence of metaplasia to neoplasia is at the root of the most common type of gastric cancer worldwide, intestinal-type adenocarcinoma, which occurs in the setting of chronic infection with the bacterium Helicobacter pylori. Murine models currently used are helpful in understanding the molecular mechanisms that drive the development of metaplasia in the stomach and its progression toward neoplasia. Unfortunately, no mouse models have reproduced the human later-stage progression to a true intestinal-type cancer with tumor masses that lead to local or distal metastasis. In short, the greatest utility of mouse models lies in the analysis of mechanisms that are responsible for the induction and progression of precancerous lesions, in particular, metaplasia. The following discussion examines models of metaplasia and gastric neoplasia in the corpus of mice and the insights they can provide into the origin and progression of human disease. To aid in interpretation of the various specific models, we first offer a primer on the terms used for the relevant mouse lesions in the stomach.
Definition of Hyperplastic, Metaplastic, and Preneoplastic Lineages in Mouse Stomach Models
The nomenclature used to describe the gastric pathology in the mouse stomach has never been standardized. Inconsistent terminology hinders progress toward developing and interpreting models that can help elucidate the molecular and cellular progression of metaplastic pathology. Establishing terminology that is specific for particular pathologic features is necessary to accurately classify cellular changes at key stages. Later, we attempt to define the most commonly observed lesions in a way that we hope will guide interpretation of future experiments. First, however, we must define some key pathology terms we use to describe the lesions: hyperplasia, metaplasia, and dysplasia. Hyperplasia refers to a pathologic lesion characterized by expansion of a normal cell lineage that resides in the tissue where it normally is found. Metaplasia refers to the presence of an otherwise normal cell lineage (or lineages) in a tissue where such a lineage is not normally found. Dysplasia is the presence of cells with abnormal cellular features and implies that the cells, which could resemble either normal or metaplastic lineages, have acquired mutations or epigenetic alterations that provide increased risk for malignant (eg, invasive) progression.
Oxyntic Atrophy
Atrophy, to a pathologist, means loss of cells. Parietal cells, whose primary job is to secrete acid, also are known as oxyntic cells. Thus, oxyntic atrophy is the pathologic process characterized by loss of parietal cells. In human beings and mouse models, loss of parietal cells usually correlates with the onset of metaplastic lesions, such that oxyntic atrophy has been termed the sine qua non for metaplasia.1, 2 In human beings and mice, chronic Helicobacter infection can lead to loss of parietal cells in the corpus of the stomach.3, 4, 5, 6 Oxyntic atrophy is diagnosed easily on H&E staining because the absence of highly eosinophilic parietal cells is obvious. During oxyntic atrophy, mature chief cells (digestive enzyme–secreting or zymogenic), which are mixed H&E positive, also are absent. Work in mouse models and human beings suggests that the loss of mature chief cells may not simply be because they all die similar to parietal cells, but rather that chief cells, in response to loss of parietal cells, change their differentiation state. Specifically, they reprogram into metaplastic mucous cells.7, 8, 9, 10, 11 Such a reprogramming of cell fate also is known as transdifferentiation. For a more definitive analysis beyond H&E, cell-type and lineage-specific markers can be used with immunofluorescent or immunohistochemical techniques: for example, antibodies against the proton pump, H+/K+–adenosine triphosphatase (ATPase) (α or β subunit) will label only mature parietal cells, whereas antibodies against the basic Helix-Loop-Helix transcription factor, MIST1 (A15), will label only chief cells.2, 7, 12
Foveolar Hyperplasia
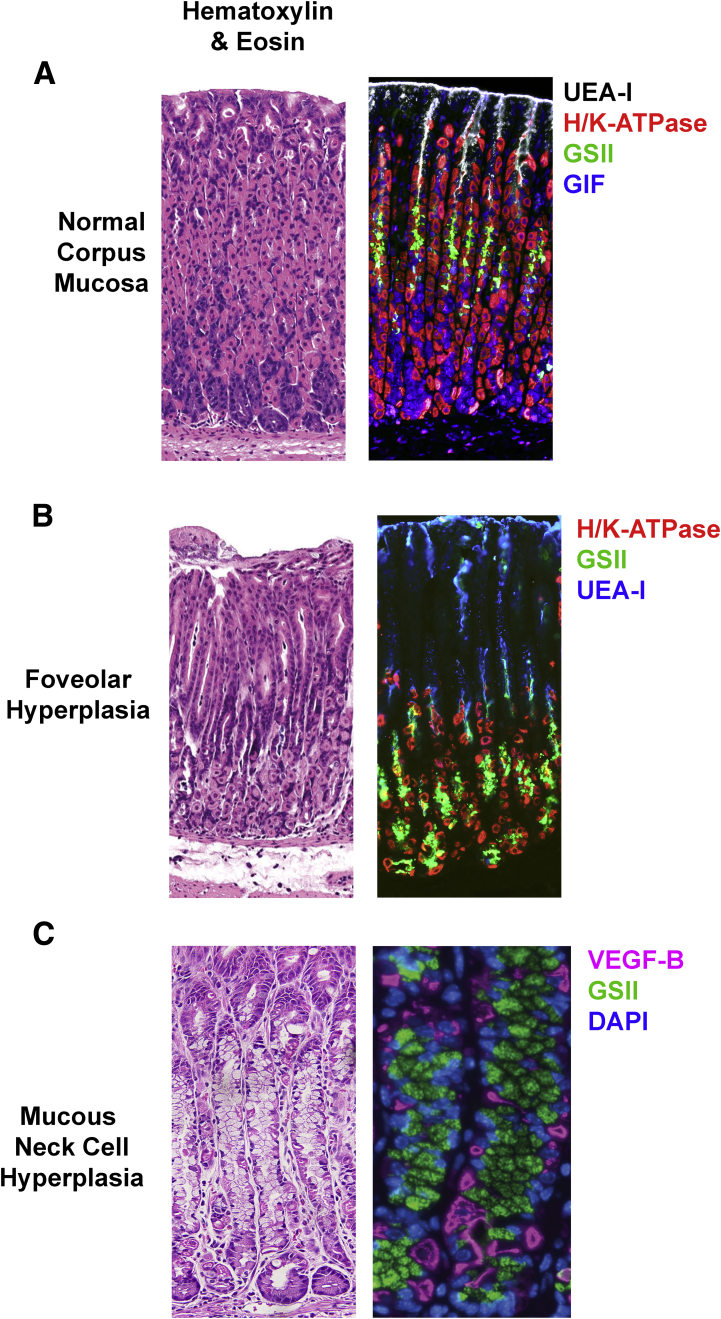
Foveolar cells are the simple columnar mucous cells lining the surface of the stomach and extending downward toward the gastric gland (Figure 1). They face the harshest conditions, being closest to the lumen of the stomach, and turn over the fastest.13, 14 Gastric units are shaped roughly like a funnel, with the glandular portion (the part with the parietal and chief cells) below the neck of the funnel, and the foveolar cells in the wide mouth.15 Thus, the foveolar region also resembles the opening to a pit. Hence, foveolar cells also are known as pit cells in the literature. Hyperplasia, as mentioned, is an expansion of normal cells. Hence, foveolar hyperplasia represents an expansion of these surface or pit mucous cells. Foveolar hyperplasia (Figure 1) usually is associated with an increase in proliferation in the normal progenitor cells in the isthmus of the gastric unit.10 A common cause of foveolar hyperplasia in mice and human beings is an increase of gastrin.16 Increased signaling through the epidermal growth factor (EGF) receptor (eg, by increased abundance of its ligand transforming growth factor α) also causes foveolar hyperplasia; human Ménétrier disease is caused by such overactive signaling.17, 18 Interestingly, oxyntic atrophy and foveolar hyperplasia often are linked. Long-term loss of parietal cells causes decreased stomach acid (hypochlorhydria), which causes gastrin-secreting cells in the antrum of the stomach (G cells) to secrete gastrin in an attempt to stimulate parietal cell function. The increased gastrin has several effects, including inducing foveolar hyperplasia.10 Gastrin-secreting tumors of the gastrointestinal tract (as occurs in Zollinger–Ellison syndrome), also can result in foveolar hyperplasia.19 Thus, in general, foveolar hyperplasia correlates with hypochlorhydria and hypergastrinemia.
Figure 1.
Hyperplastic lesions in the gastric corpus. (A) Normal gastric mucosa stained with Ulex Europaeus Agglutinin I (UEA-I) lectin for surface cells (inverse greyscale), GSII lectin for mucous neck cells (green), antibodies against H/K-ATPase for parietal cells (red), and antibodies against GIF (blue). (B) Mucosal section showing foveolar hyperplasia after 3 days dosing of DMP-777 stained with UEA-I (blue), H/K-ATPase (red), and GSII (green). (C) Mucosa with mucous neck cell hyperplasia stained with antibodies against vascular endothelial growth factor (VEGF)-B for parietal cells (red) and GSII lectin (GSII).
Mucous Neck Cell Hyperplasia/Mucinous Metaplasia
Mucous neck cell hyperplasia connotes expansion of normal mucous neck cells (Figure 1). A related term often used by pathologists, especially veterinary pathologists, is mucous metaplasia, which often may be the same lesion as mucous neck cell hyperplasia. A reason for the possible confusion is that mucous metaplasia typically is diagnosed by conventional histochemical staining (H&E, periodic acid–Schiff, and Alcian blue). It describes a lesion characterized by abnormally increased numbers of mucous-expressing neck cells in the glands of the stomach (ie, not in the foveolar/pit region). In our experience, mucinous metaplasias usually are caused by expansion specifically of normal mucous neck cells. Thus, mucous metaplasia is often a misnomer because no new (metaplastic) cell lineages are found in the stomach. Mucous neck cells may have a metaplastic look when they expand markedly because in the normal stomach they usually are difficult to see, given that their cytoplasms do not stain with H&E, and those nonstaining cytoplasms are localized predominantly in the lumen of the gland because parietal cells occupy the vast majority of the basement membrane.20 When mucous neck cells expand significantly, they do so at the expense of parietal cells, giving a morphology that appears as if a new population of cells has appeared. On immunohistochemical or immunofluorescent staining, however, the cells in these lesions label exclusively with mucous neck cell lineage markers (including Griffonia simplicifolia lectin (GS-II), Trefoil factor 2 (TFF2), mucin 6 (MUC6), and gastrokine 3 (GKN3)).21, 22 Thus, this lesion is best described as mucous neck cell hyperplasia.
Mucous neck cell hyperplasia has been reported in a number of settings with alterations of ion channels (eg, loss of KVLQT1) or endocrine cell influences.22 In our experience, mucous neck cell hyperplasia also arises spontaneously in otherwise healthy mice. The lesion usually is focal and characterized by a neutrophil-predominate inflammatory infiltrate. The expansion of mucous neck cells usually is not associated with markedly increased proliferation within the mucous neck cell population, but rather appears to reflect either an increased production of mucous neck cells from multipotent progenitors or a slowing of differentiation of mucous neck cells into chief cells.7, 23 It is important to note that combinations of mucous neck cell hyperplasia can exist in association with other truly metaplastic lesions. For example, spasmolytic polypeptide (TFF2)-expressing metaplasia (SPEM), which will be discussed later, often is characterized by abundant mucous-containing cells (Figure 1). SPEM may look like mucous neck cell hyperplasia on histochemical stain, and it can combine with mucous neck cell hyperplasia.7
Spasmolytic Polypeptide (TFF2)-Expressing Metaplasia/Pseudopyloric Metaplasia/Antralization
Loss of parietal cells in mice or human beings correlates with the induction of metaplasia in the corpus glands.2, 7, 10, 12, 24, 25, 26 In particular, as mentioned previously, this oxyntic atrophy is characterized by loss of mature chief cells. As mentioned earlier, mouse models and examination of human tissue suggest that many of these chief cells do not die, but actually transdifferentiate or reprogram into a metaplastic mucous-secreting lineage: SPEM. SPEM shares many features with the cells that populate the gland in embryonic and juvenile stomach.7, 11, 12, 27 Namely, SPEM is characterized by expression of lower levels of chief cell proteins (such as the digestive enzyme pepsinogen C and, in mice, gastric intrinsic factor), with markers of mucous neck cells (TFF2, GKN3, and MUC6) (Figure 2). The pattern of corpus units that have no parietal cells and abundant basal cells co-expressing chief and neck cell markers is similar to the organization, at least at the superficial level, of units in the more distal stomach (the antrum or pylorus) (Figure 1). Thus, this lesion also has been termed “pseudopyloric metaplasia,” representing a process of “antralization.”28 SPEM also may represent a reparative lineage equivalent to the ulcer-associated lineages identified in association with healing mucosa in inflammatory bowel disease.29 Indeed, recent studies have noted that SPEM develops at the edges of healing gastric ulcers and contributes to ulcer healing.30, 31
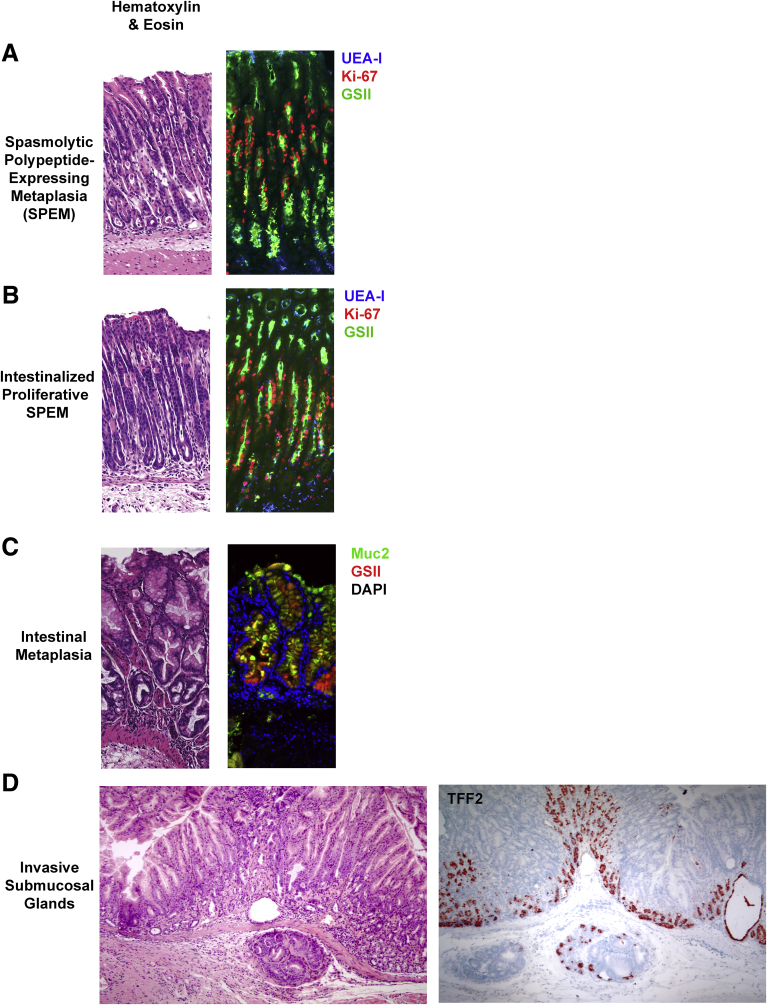
Figure 2.
Metaplastic lesions in the mouse gastric corpus. (A) Corpus section with SPEM stained with GSII lectin (green), Ulex Europaeus Agglutinin I (UEA-I)-I lectin (blue), and Ki-67 (Red). (B) Section with proliferative SPEM with intestinalizing characteristics stained with GSII lectin (green), UEA-I lectin (blue), and Ki-67 (Red). (C) Section with intestinal metaplasia stained with Muc2 (green), GSII lectin (red), and 4′,6-diamidino-2-phenylindole (DAPI) (blue). (D) Section with penetrating submucosal gland stained for TFF2.
As mentioned, it is thought that SPEM cells arise in part via reprogramming/transdifferentiation of chief cells. The key evidence is that lineage mapping studies in mice using the Mist1 reporter of chief cell differentiation have shown that SPEM cells emerging during loss of parietal cells were once MIST1-positive (ie, they were chief cells).7 The chief cells that reprogram after loss of parietal cells down-regulate expression of chief cell differentiation markers (eg, the endogenous Mist1 gene) and begin to express high levels of proteins that were expressed in mucous neck cell lineages, including TFF2 and MUC6.25, 26, 32 Thus, the metaplastic cells can be identified in the base of gastric glands (the normal niche for chief cells) by strong immunolabeling for TFF2, which is the origin for the moniker SPEM.33, 34, 35 This lineage often can be identified by pink staining in diastase-resistant periodic acid–Schiff staining, compared with the purple staining in surface mucous cells.24 Most importantly, SPEM glands usually show, especially at their bases, hybrid cells, which express both chief cell markers (eg, intrinsic factor in mice) and mucous cell markers (eg, TFF2, MUC6, or GS-II lectin).2, 25, 26, 36 SPEM is a true metaplasia because SPEM-type cells are not normal corpus lineages, but resemble deep antral gland cells.27
Markers to positively determine SPEM
SPEM cannot be determined definitively in the antrum, where deep mucous gland cells show many of the same characteristics. It is a lesion specifically of glands from the corpus/body (also known as fundic-type glands) that develop metaplasia. SPEM likely occurs with similar characteristics in all mammals, but has been documented definitively in mice, rats, gerbils, and human beings.10, 33, 34, 35, 37 The organization of SPEM glands, as mentioned, superficially resembles the antrum, with TFF2-positive cells also labeling with a lower abundance of chief cell markers (gastricintrinsic factor in mice, or pepsinogen C), and the center of proliferation moves from near the gastric lumen (at the isthmus, where the funnel of pit/foveolar cells meet the deeper glandular cells) in the normal oxyntic gland, toward the base in SPEM glands. SPEM cells often show proliferation with TFF2/GIF dual-positive cells also labeling with Ki-6737 or nucleotide analogs such as bromodeoxyuridine, especially in the presence of inflammation.20 Investigations have identified some biomarkers of SPEM that do not appear in normal corpus lineages including HE-4 (WFDC2)25 and CD44 variant 9.38 It also is clear in human patients and in animal models that morphologically apparent SPEM lineages often can show varying levels of intestinalizing transcripts including TFF3, MAL2, CFTR, and MUC4.36, 39 This intestinalized SPEM is morphologically identical to other SPEM lineages and can be recognized only by immunostaining with specific intestinal markers.36
Intestinal Metaplasia
Although both SPEM and intestinal metaplasia are present in the stomachs of human beings with atrophic gastritis secondary to H pylori infection,2, 12 few instances of documented intestinal metaplasia exist in mouse models. In human stomach, Alcian blue staining of intestinal-type goblet cells often is used to define intestinal metaplasia. However, murine deep antral mucous cells and SPEM often both are strongly Alcian blue positive.24 Thus, more specific markers are required in mice. MUC2 and TFF3 labeling of cells with intestinal goblet cell–like morphology provides the most specific indication of intestinal metaplasia (Figure 2C).12 Notably, expression of TFF3 and MUC2 has been reported in lineages with immunocytochemical evidence of SPEM, so there may be intermediates of intestinalized SPEM that reflect evolution of metaplastic phenotypes.36, 40 Expression of nuclear CDX1 and CDX2 has been noted in human intestinal metaplasia,41 but has been less apparent in mouse models. MUC2-positive goblet cells have been identified in older amphiregulin knockout mice.42 More recently, CDX1-positive cells and MUC2-positive goblet cells have been observed in Mist1-Kras (G12D) mice, but CDX2 expression was not observed.43 The expression of CDX1 rather than CDX2 may reflect the more prominent expression of CDX1 in the duodenum, consistent with the concept that intestinal metaplasia in the stomach may represent more specifically a “duodenal metaplasia.”44 Indeed, previous studies have suggested that intestinal metaplasia in the human corpus may reflect a duodenal lineage paradigm based on PDX1 expression.44 It is notable that forced expression of the intestinal master regulatory transcription factor Cdx2 in the stomach caused complete intestinalization of the stomach mucosa.45, 46 However, it is difficult to interpret these results within the context of adult-onset metaplasia because the gastric epithelium in these mice was specified during development with forced expression of an intestine-promoting transcription factor in cells that otherwise would have become stomach. Thus, the epithelium in this case simply may be (developmentally) intestine, rather than metaplastic stomach. Only specific marker staining can differentiate between SPEM with intestinalizing characteristics and frank intestinal metaplasia with the presence of goblet cells and villin-expressing absorptive cells.43
Invasive Submucosal Glands
The presence of glands penetrating into the submucosa (sometimes referred to as gastritis cystica profunda) usually is considered a dysplastic lesion in human beings. For example, the presence of gastritis cystica profunda in human beings has been noted decades after gastrojejunostomy for ulcer disease and is considered a preneoplastic lesion.47 Intermediate levels of penetrating or invasive glands also can be observed. Although invasive submucosal glands have been reported in H pylori–infected Mongolian gerbils, some investigations have indicated that invasive glands are reversible after bacterial eradication.48 Recent detailed studies in gerbils have noted that invasive submucosal glands usually are connected to SPEM glands within the mucosa.49 Numerous mouse models have assumed that invasive glands are synonymous with dysplasia or even invasive neoplasia (malignancy).3, 4, 50 However, we are unaware of any models in which investigators have shown truly neoplastic qualities in these lesions. As noted in mouse models of colon cancer,51 in our experience, the mouse stomach has a propensity for extension of metaplastic lesions. In some occasions, tremendous proliferation in the epithelium can result in cross-sectional cuts of the tissue that appear invasive but are not. In other cases, the epithelial cells in the mucosa actually may breach the relatively thin muscularis mucosa of the mouse stomach. Although these glands thus technically might appear to be invasive, the examples we have observed in the literature and in our own studies do not show any additional features of neoplasia. In other words, such invasive glands remain symmetrical and are indistinguishable from surrounding metaplastic glands that are not apparently in the submucosa. Furthermore, the glands in question typically do not show other signs of dysplasia/neoplasia, such as: loss of nuclear polarity, cells piling up with nuclear crowding, irregular gland forms with an invasive-seeming leading edge, nuclear atypia, or surrounding stromal hyperplasia (desmoplasia). In addition, these invasive glands usually are populated by TFF2-positive lineages (Figure 2D) and do not show proliferative rates any greater than associated intramucosal SPEM. Furthermore, we are unaware of models showing metastatic behavior typical of invasive adenocarcinomas of the stomach in human beings. Thus, there is no evidence that such glands actually invade the way human tumors do. Therefore, the neoplastic implications of seemingly invasive submucosal glands, which might be assumed in human beings to be premalignant lesions, are unclear in mouse models.
Dysplasia
Dysplastic phenotypes are defined by cellular and glandular morphologies on H&E staining. No specific markers of dysplasia exist. The interpretation of dysplasia therefore is fraught with controversy even among human pathologists diagnosing lesions in patients. The importance of a dysplasia diagnosis is essentially that it is prognostic for increased progression to cancer. Given that no actual metastatic or truly invasive adenocarcinoma models in the mouse stomach seem to exist, it is difficult to define the term dysplasia in a standard way in mouse models. If used, it must be strictly defined based on which atypical, potentially neoplastic features are present in the lesion in question.
Adenocarcinoma
In human beings, cells with aberrant, invasive, and metastatic characteristics are defined as adenocarcinoma. The aberrant morphologies generally are defined by patterns in H&E staining, including the following: multilayered cells lacking polarity, complex glandular patterns with irregular intervening lumens (back-to-back cribriform glands), altered and inconsistent nuclear morphology and positioning, and irregular chromatin patterns (eg, prominent nucleoli). Proliferative rates are expected to be high, but metaplastic lineages also can show high proliferative rates. No present studies have defined specific mutations that might define a transition to cancer in mice. To our knowledge, there is only 1 purely genetically driven mouse model of epithelial carcinogenesis that results in invasive metastatic tumors that invade to the serosal surface (and do not just form the seemingly invasive submucosal glands discussed earlier) and then metastasize to regional lymph nodes.52, 53 In this model, large T antigen was expressed as a transgene in parietal cell progenitors, which eventually led to an invasive carcinoma that was always neuroendocrine in morphology and molecular phenotype. No specific model of intestinal type cancer in the gastric body that is driven by a controlled genotype of mouse (as opposed to tumors induced by mutagens) has been reported.
Human Disease Inception and Progression
Chronic infection with virulent Helicobacter pylori strains causes the most gastric adenocarcinomas worldwide.54 H pylori is a gram-negative bacteria that inhabits more than 50% of the global population.55 Chronic H pylori infection can trigger changes in the fundic mucosa that correlate with increased risk for a subset of individuals to gastric adenocarcinoma. Most pathologists recognize chronic atrophic gastritis as the first lesion indicating an increased risk for progression of an H pylori infection to gastric cancer. This lesion pairs a chronic immune cell infiltrate (including resident plasma cells, establishment of lymphoid follicles) with oxyntic atrophy (loss of parietal cells). As mentioned earlier, oxyntic atrophy seems to correlate invariably with induction of SPEM.2 Thus, chronic atrophic gastritis and SPEM appear as correlated lesions.
We discuss the literature correlating animal models of SPEM with human lesions. Work over the past several years by our groups and others in both human beings47, 56, 57 and rodents26, 34, 35 has established that SPEM is a common metaplastic phenotype observed in the atrophic human and rodent stomachs. In the setting of a chronic inflammatory infiltrate that does not resolve in human beings, SPEM may progress to intestinal metaplasia.2, 12 Correa58 originally described the association of intestinal metaplasia with the development of intestinal-type gastric cancer. When intestinal-type gastric adenocarcinoma develops, it usually occurs in the setting of both SPEM and intestinal metaplasia lesions.33 Thus, the presence of metaplastic cell lineages in the stomach carries increased risk for progression to neoplasia. The adenocarcinomas caused by H pylori in this setting are exceedingly heterogeneous from patient to patient, but they vary on a spectrum from neoplastic glands with more obviously gastric differentiation to ones with obvious intestinal differentiation.54, 56 In general, these types of gastric cancers originally were referred to as “intestinal-type” because they arise in the background of intestinal metaplasia.59 It is easy to assume that the cancers, which have varying degrees of intestinal morphology, thus arise from the intestinal metaplasia, but that may be too simplistic an interpretation.12, 32 An alternative view, for example, is that inducing postmitotic, differentiated cells to re-enter the cell cycle is an inherently risky process that can induce, unmask, or generate mutations owing to massive changes in the chromatin landscape. Thus, abundant metaplasia simply may reflect a sign of abundant, risky, reprogramming events.11, 60
Murine Models of Gastric Preneoplasia
A review of studies over the past 20 years that show SPEM phenotypes in association with varying degrees of gastric neoplasia is detailed later. In particular, we review the pathologic phenotypes of metaplasia in these models (summarized in Table 1) and how they inform our knowledge of the evolution of preneoplastic lesions.
Table 1.
Pathological Lesions in Mouse Models of Metaplasia
| Mouse model | SPEM | IM | Foveolar hyperplasia | Proliferative SPEM/intestinalized SPEM | Inflammatory infiltrate | Invasive glands |
|---|---|---|---|---|---|---|
| Helicobacter species infection | Yes | No | No | Yes | Yes | Yes |
| DMP-777 treatment | Yes | No | Yes | No | No | No |
| Tamoxifen treatment | Yes | No | Yes | No | Yes? | No |
| L635 treatment | Yes | No | Yes | Yes | Yes, M2 macrophage | No |
| Insulin-gastrin mouse | Yes | No | Yes | Yes | Yes | Yes |
| Gastrin KO mouse | Accelerated after DMP777 | No | No | No | No | No |
| Claudin 18 KO mouse | Yes | No | No | Yes | Yes, neutrophils | No |
| KLF4 KO mouse | Yes | No | Yes | No | No | No |
| Runx3 KO | Yes | No | No | Yes | No | No |
| H/K-cholera toxin mouse | Yes | No | Yes | ND | Yes, lymphocyte follicles | No |
| Mist1-Kras mouse | Yes | Yes | Yes | Yes | Yes, M2 macrophage | No |
| Smad3 KO mouse | Yes, proximal corpus | No | No | Yes | No | Yes |
| H/K-noggin mouse | Yes | No | Yes | No | No | No |
| Amphiregulin KO mouse | Yes | Yes, focal | Yes | Yes | Yes | Yes |
| H/K-IFNγ mouse | Yes | No | Yes | Yes | Yes | No |
| HIP1r KO mouse | Yes | No | Yes | No | Yes | No |
| TxA23 mouse | Yes | No | Yes | Yes | Yes | No |
| H/K-IL1β mouse | Yes | No | No | Yes | Yes | Yes |
| K19-C2mE mouse | Yes | No | Yes | No | Yes, macrophage | No |
| GAN mouse | Yes | No | Yes | Yes | Yes | Yes |
| IL11 treatment | Yes | No | Yes | No | Yes, PMN | No |
| IL33 treatment | Yes | No | No | No | Yes | No |
KLF4, Kruppel-like factor 4; KO, knockout; ND, not determined; PMN, polymorphonuclear leukocyte.
Helicobacter infection
Mice infected with Helicobacter felis recapitulate much of the pathology associated with H pylori infection observed in human beings, including parietal cell loss and metaplasia, and these bacteria have been used for years specifically in the C57BL/6 mouse strain to study metaplasia.4, 5, 6, 61 Mouse-adapted clinical isolates of H pylori from infected human beings also can be used to recapitulate the chronic infection and metaplasia seen in patients.62 The mechanism by which Helicobacter causes parietal cell loss is not known. The host inflammatory response, however, is thought to be critical both in human beings and in mouse models.3, 36, 63, 64, 65 In general, the immune response can be categorized along 2 distinct paradigms: innate and adaptive. Innate immunity includes the epithelial cells themselves (eg, parietal cells produce tremendous microbial killing via acid) along with phagocytic cells such as monocytes and granulocytes, in particular neutrophils, that are recruited rapidly to areas of injury or infection. The slower, adaptive immune response is associated with antibody-producing B cells, antigen-recognizing CD4+ T-cells, and cytotoxic CD8+ T cells. Studies in the early 1990s focused on understanding how the immune system contributed to the development of gastric pathology associated with Helicobacter infection. The inflammatory infiltrate observed in H felis–infected mice is mixed, containing populations of lymphocytes, neutrophils, and macrophages within the metaplastic mucosa. Roth et al66 identified that T cells were necessary for Helicobacter-associated parietal cell loss because T-cell–deficient mice did not develop oxyntic atrophy or metaplasia. Murine models also have aided in the further understanding of metaplastic events occurring after parietal cell loss. Six months after Helicobacter infection, mice developed oxyntic atrophy and TFF2-expressing metaplasia.5, 6 In further studies, the mucous cell lineage expansion was identified as SPEM,33 derived from the transdifferentiation of mature chief cells.7 SPEM is highly proliferative throughout the corpus in H felis–infected mice.5, 6, 37 Although the metaplasia is extensive, intestinal metaplasia as seen in human beings is notably absent from Helicobacter-infected mice. However, there is evidence for intestinalization of SPEM during chronic H felis and H pylori infection.35, 37, 40
Acute Drug-Induced Models of SPEM
A caveat of using Helicobacter infection to study oxyntic atrophy and metaplasia is that alterations, especially to the immune system, may affect the competency of Helicobacter to induce parietal cell loss, a prerequisite for metaplasia development. Infection models also induce large-scale metaplasia slowly (after approximately 6 months) and asynchronously. Acute drug treatments that induce parietal cell death directly have some advantages in that they rapidly and synchronously induce metaplasia, bypassing chronic immune mechanisms usually required for parietal cell loss. This rapid and direct induction of SPEM allows study of specific stages of metaplasia using both molecular and histologic techniques.
Oral administration of DMP-777
Treatment with the parietal cell–specific protonophore drug DMP-777 leads to rapid loss of parietal cells within 3 days, followed by induction of SPEM after 10–14 days of treatment.10, 34 Loss of parietal cells is abrogated when animals are pretreated with omeprazole before DMP-777 administration.67 Although no proliferative chief cells were identified in the normal mucosa, scattered proliferative SPEM cells were identified after DMP-777 treatment. All of the metaplasia is reversible within 7–14 days after cessation of DMP-777 treatment. Interestingly, in both mice and rats, administration of DMP-777 for up to 2 years led to prominent continuous metaplasia, but never resulted in any dysplastic or neoplastic lesions.34 The absence of dysplastic lesions in the face of chronic metaplasia appears to be caused by a lack of inflammation, likely because of the other action of DMP-777 as a cell-permeant elastase inhibitor that could serve to block neutrophil function and innate immunity.10, 34, 37
Intraperitoneal injections of high doses of tamoxifen
Mice given 3 consecutive daily doses of 5 mg of fully emulsified bolus doses of tamoxifen68 had a 90% reduction in parietal cell mass in the stomach by 3 days after the first injection.26, 69 High-dose tamoxifen-treated mice also developed SPEM within 3 days of treatment with scattered proliferative SPEM cells identified. The effect could be reversed 2–3 weeks after drug withdrawal. The pattern of SPEM induction by high-dose tamoxifen resembles that induced by DMP-777 and also is ameliorated by omeprazole. However, there is evidence that tamoxifen causes more inflammation than DMP-777.
Oral administration of L635
L635 is a chiral molecular cousin of DMP-777, which retains parietal cell protonophore activity, but lacks the neutrophil elastase inhibitory capacity.34 L635 treatment causes rapid induction of parietal cell loss and SPEM in the presence of a prominent inflammatory milieu. The rapid induction of oxyntic atrophy combined with a strong inflammatory infiltrate in the gastric mucosa leads to accelerated development of a highly proliferative and intestinalized SPEM lineage within 3 days of treatment7 that is remarkably similar to SPEM observed after infection with H felis.37 M2 macrophages drive the proliferation and intestinalization of SPEM in L635-treated mice.70 Therefore, L635 treatment provides a rapid model for the development of highly proliferative intestinalizing SPEM.
Gastrin-Related Genetic Models
Located in the antrum, G cells produce gastrin to both regulate gland homeostasis and promote acid secretion from parietal cells, primarily through the stimulation of histamine release from enterochromaffin-like cells.71, 72 Gastrin also functions as a growth factor for pit cells and exerts anti-apoptotic activity at the transcriptional level.73, 74, 75 In the stomach, endogenous gastrin levels increase in response to parietal cell loss and increase epithelial cell proliferation and foveolar hyperplasia.76
Insulin-gastrin transgenic mice
The insulin-gastrin mouse model uses the mouse insulin promoter to drive expression of a human gastrin transgene, producing moderate increases in circulating gastrin levels. The insulin-gastrin mice on the FVB background develop SPEM and then submucosal lesions in the gastric corpus that express TFF2 by 20 months of age.4 Metaplastic changes are accelerated in the setting of Helicobacter infection.4 Although the cause of parietal cell loss is unclear, a chronic state of acid hypersecretion may contribute to the oxyntic atrophy.
Gastrin null mice
Gastrin null mice develop antral tumors by 12 months of age.77, 78 In the corpus, the mucosa generally is thinner, with fewer parietal cells present. DMP-777 treatment in gastrin null mice causes an accelerated induction of SPEM in 1–3 days, as compared with 10 days in wild-type mice.10, 25 However, gastrin null mice do not develop foveolar hyperplasia, which typically occurs as a result of oxyntic atrophy, consistent with the concept that gastrin is a major driver of foveolar hyperplasia.
Genetic Mouse Models of Parietal Cell loss
Several mouse models induce changes spontaneously, consistent with the development of SPEM in the corpus of the stomach. These models may initiate with a normal mucosa and then develop increasing levels of atrophy and metaplasia.
Claudin 18 null mice
Claudin 18 levels are high in the normal stomach, but prominently are reduced in the metaplastic stomach.79 Hayashi et al79 examined the impact of claudin-18 loss in the stomachs of claudin 18 null mice. These mice showed few parietal cells at birth and never developed a normal complement of parietal cells during the postnatal period. Instead, claudin 18 null mice showed proliferative SPEM that was associated with a significant neutrophil infiltrate and increases in IL1β expression. The claudin 18 null stomachs also showed high levels of the SPEM marker HE-4 (WFDC2). Although the metaplastic SPEM lineages in the claudin 18 null mice were highly proliferative, these mice were studied only up to 16 weeks of age, at which time no invasive or dysplastic lesions were reported. Thus, claudin 18 is necessary for normal parietal cell development and gland homeostasis.
Kruppel-like factor 4 null mice
Kruppel-like factor 4 is down-regulated in gastric cancers, and loss of Kruppel-like factor 4 is associated with poor prognosis.80, 81 In the Kruppel-like factor 4 null mouse, the stomach shows marked oxyntic atrophy from birth and both foveolar hyperplasia and mucous cell metaplasia.82 Some intrinsic factor–expressing cells remain at the bases of corpus glands that are dominated by a TFF2-expressing mucous cell lineage. Although no dual labeling for intrinsic factor and TFF2 was performed, the staining for TFF2 appeared at the base of the glands, consistent with the suggestion that these glands represented SPEM. Interestingly, however, the investigators noted that there was little proliferation among the SPEM lineages and even at up to a year of age, no intestinal metaplasia, dysplasia, or invasive lesions developed. They also noted no inflammation. Thus, this model resembles the stable benign metaplasia after long-term administration of DMP-777.34
Runx3 null mice
Runx3 is a transcription factor that is highly expressed in chief cells with lower levels of expression in surface cells and mucous neck cells.83 RUNX3 is a gastric tumor suppressor in human beings.84 Runx3 null mice develop marked TFF2-expressing SPEM lineages in the corpus.85 Interestingly, Runx3 null mice do maintain parietal cells, although they appear small and acid secretion is variable. Proliferative cells are observed throughout SPEM. In some mice, intestinalized SPEM can be observed with expression of Muc2 and Cdx2 in deep gland regions, but no goblet cells appear to be present. These findings are consistent with an association of Runx3 loss with intestinalization.85, 86 Although Runx3 null mice do not develop any evidence of dysplasia or penetrating glands, they do show increased susceptibility to N-Methyl-N-Nitrosourea (MNU)-induced dysplasia in the corpus. Thus, this model suggests that regulation of intestinalization focused in chief cell lineages may promote metaplastic progression without complete parietal cell loss. The phenotype of this model also may show aspects of mucous neck cell hyperplasia accentuated by the small size of remaining parietal cells.
H/K-cholera toxin mouse
Increased cyclic adenosine monophosphate is the principal driver of parietal cell acid secretion mediated through activation of the H2-histamine receptor.87 Lopez-Diaz et al88 examined a model of chronic acid overproduction in the H/K-cholera toxin mouse. This mouse showed increased acid secretion from an early age, as would be expected for parietal cells with increased expression of cholera toxin and increased cyclic adenosine monophosphate production. Although the mice showed a normal compendium of cell lineages in the gastric corpus at 2 months of age, by 7 months of age the mice developed increasing levels of parietal cell loss until loss was severe by 15–16 months of age. The parietal cell loss at the later time points was associated with the presence of SPEM. The loss of parietal cells appears to relate to the development of autoantibodies against parietal cell antigens, especially H/K-ATPase. This model therefore mimics the pathogenesis of autoimmune gastritis. No invasive glands were noted in these mice at the later stages, although measures of proliferation were not determined.
Models of Signaling Pathway Activation
Several studies conducted on characterizing gene and protein expression of metaplasia in the murine and human stomachs have yet to identify a dominant signaling pathway driving neoplasia.25, 37, 89 However, different signaling pathway aberrations in gastric cancer have been characterized, identifying KRAS, fibroblast growth factor receptor 2, epidermal growth factor receptor (EGFR), ERBB2, and MET using single-nucleotide polymorphism arrays.90 These and other signaling pathways have been investigated for their role in the development of metaplasia.
Mitogen-activated protein kinase/ERK
Up-regulation of phospho-ERK in metaplasia has been noted in both human beings and mice.43, 91 However, Ras mutations have been observed in only approximately 9% of gastric cancers. Nevertheless, recent gene-profiling studies have noted a signature for up-regulation of Kras activity in more than 40% of intestinal-type human gastric cancers.92, 93 Choi et al43 recently examined the induction of metaplasia in Mist1CreERT2;LSL-KRas(G12D) mice (Mist1-Kras mice). After tamoxifen induction, these mice expressed activated Kras in gastric chief cells, and after 4 weeks developed proliferative metaplasia. Three months after induction, intestinal goblet cells expressing MUC2 were evident, progressing to invasive glands 4 months after induction. Importantly, treatment of the mice with a MEK inhibitor, selumetinib, caused arrest of the metaplasia and allowed recrudescence of normal gastric lineages. These normal lineages were derived from normal progenitor cells and not the metaplastic lineages. The normal gastric lineages appeared to cause extrusion of the metaplastic glands from the gastric mucosa into the lumen. These findings indicate that activation of Ras is involved at all of the key steps in the development of metaplasia, from transdifferentiation of chief cells into SPEM, to further differentiation of intestinal metaplasia and the promotion of invasive changes.
Hayakawa et al94 have suggested that Mist1CreERT2;LSL-KRas(G12D) mice develop metaplasia from scattered isthmal Mist1-expressing cells. This interpretation is at odds with previous studies in K19-KRas(G12D)-expressing mice that showed a phenotype dominated by foveolar hyperplasia.95 Matsuo et al96 recently confirmed this phenotype of induced foveolar hyperplasia after targeting of KRas expression to the isthmal progenitor cells using the Runx1 eR1 promoter. In that investigation, occasional chief cells also were targeted by eR1, and active KRas in those cells appeared to give rise to SPEM. Thus, the majority of evidence suggests that Ras activation in chief cells leads to the development of SPEM.
TGFβ and Bone Morphogenic Proteins
SMAD3 is a critical signaling protein downstream of TGFβ-receptor activation. Smad3 null mice were first characterized extensively for the spontaneous development of colonic adenocarcinoma.97 Although development of colon cancers required colonization of animals with Helicobacter hepaticus, observations at the Jackson Laboratories (Bar Harbor, ME) indicated that gastric tumors evolved in the absence of any infection. Detailed analysis of these mice subsequently showed that Smad3 null mice developed SPEM by 6 months of age and invasive lesions in the proximal corpus by 10 months of age.98 Invasive lesions, thought to be derived from the first gland of the corpus, generally showed TFF2-positive cells along with large numbers of DCLK1-positive tuft cells. These results suggested that loss of SMAD3 can lead to the development of proximal gastric neoplasia.
H/K-noggin mouse
Shinohara et al99 sought to evaluate the role of BMP signaling in the differentiation of gastric lineages in the H/K-noggin mouse. Inhibition of BMP signaling through overexpression of Noggin resulted in a reduction of parietal cell numbers and expansion of SPEM lineages expressing TFF2. Although there was increased proliferation in the mucosa, most of this proliferation was abolished when the transgenic mice were crossed onto a gastrin null background, a finding consistent with a role for proliferation in foveolar hyperplasia in these mice rather than SPEM.75 Importantly, the overexpression of Noggin in the stomach caused an augmentation of metaplastic changes after infection with H felis or H pylori.100 These findings suggest that BMP signaling generally is anti-inflammatory and that loss of this signaling may promote metaplasia development.
EGFR and EGFR ligands
The role of EGFR and its ligands in alterations in gastric mucosal lineage differentiation has been well established. Overexpression of TGFα in the gastric corpus is associated with severe foveolar hyperplasia in Ménétrier disease in human beings.17, 101 Overexpression of TGFα in the stomachs of Metalothionein (MT)-TGFα mice leads to a similar phenotype.102, 103 These studies all suggested that overexpression of TGFα altered the differentiation of gastric progenitors to the production of surface mucous cells over gland cells (ie, parietal, mucous neck, and chief cells).
Nevertheless, other studies have suggested that alterations in EGFR activation can influence the induction of metaplasia. Waved-2 mice, which have an attenuating mutation of the EGFR, showed enhanced development of SPEM after loss of parietal cells induced by DMP-777.104 These effects seemed to be specific to particular EGFR ligands because although TGFα null mice showed no alteration in SPEM induction, amphiregulin null mice showed an enhanced induction of SPEM after DMP-777 treatment, similar to that observed in waved-2 mice.105 Just as interestingly, amphiregulin-deficient mice older than 1 year of age developed SPEM spontaneously.42 By 18 months of age, more than 40% of amphiregulin null mice showed parietal cell loss and both SPEM as well as goblet cell intestinal metaplasia. The goblet cell intestinal metaplasia evolved in glands also containing SPEM, and cells with intermediate morphology with dual expression of TFF2 and MUC2 were observed at the interface between SPEM and intestinal metaplasia lineages.42 These findings showed that EGFR-mediated signaling was involved in the evolution of metaplastic lineages.
Immune-Mediated Preneoplastic Mouse Models
Inflammation accompanies most models of metaplasia in the gastric mucosa. Insights into different aspects of the inflammatory response show the impact of immune cells in the progression of metaplasia.
H/K-ATPase-interferon-γ transgenic mice
The expression of the proinflammatory cytokine interferon (IFN)γ, typically expressed by T-helper (Th)1-polarized T cells, is increased in Helicobacter-infected mice. Transgenic overexpression of IFNγ using elements of the parietal cell–specific driver H/K-ATPase β (Atp4b) promoter caused a prominent inflammatory infiltrate, oxyntic atrophy, epithelial cell proliferation, and SPEM after 3.5 months. SPEM and immune infiltration still were present in transgenic (Tg)-IFNγ mice crossed to Rag1-/- mice that lacked mature T and B cells.106 Therefore, IFNγ alone can drive metaplastic changes in the corpus without the assistance of T or B cells.
Huntington interacting protein 1 related and Interferon gamma double-knockout mice
Huntington interacting protein 1 (Hip1) is required for normal parietal cell maturation.107 Parietal cells undergo apoptosis in Hip1-related (Hip1r-/-) mice and develop a robust inflammatory response and SPEM by 12 months of age. Double-knockout mice, null for both Hip1r and Ifng, have delayed metaplasia development, but mice at 12 months of age appear to have similar phenotypic SPEM as control Hip1R null mice. Therefore, IFNγ is not required for SPEM induction after parietal cell loss.23
TxA23 mice
Human autoimmune gastritis occurs when T cells target parietal cells for apoptosis in the stomach, causing robust metaplasia that predisposes affected individuals to endocrine carcinoids.108 Transgenic BALB/c mice engineered with CD4+ T cells that recognize the parietal-specific antigen H/K-ATPase develop SPEM by 2–4 months of age. By 12 months of age, TxA23 mice have extensive inflammation with areas of aberrant gland morphology and penetrating submucosal glands.109 The phenotype observed in this model may differ from most other models of metaplasia because these mice are on a BALB/c background.110, 111 Specifically, T cells in BALB/c mice express higher levels of IL4 than IFNγ, thereby driving a Th2-predominant response. Most investigations in the stomach use C57BL/6 mice, which tend toward a Th1 predominant response wherein T cells express higher levels of IFNγ. Therefore, the type of metaplasia in the TxA23 atrophic gastritis model perhaps is indicative of a Th2-driven environment paired with parietal cell loss.
H/K-ATPase-IL1β transgenic mice
Polymorphisms in the Il1b gene are observed in a variety of human, including gastric, adenocarcinomas. IL1β is part of the IL1 family of cytokines that function as alarmins by promoting the inflammatory response. IL1β expression increases in Helicobacter infections and also is associated with myeloid cell infiltration. Transgenic mice expressing human IL1β using the H/K-ATPase promoter develop spontaneous inflammation, oxyntic atrophy, and SPEM with invasive lesions at late time points. Helicobacter increases the susceptibility and speed at which gastric pathology develops in H/K-Il1b mice.112 Conversely, IL1-receptor antagonist treatment can inhibit the progression of metaplasia and inflammatory cell infiltration. Together, these findings support IL1β as a major driver of inflammatory cell recruitment that promotes the progression of metaplasia.
Stromal-derived factor 1 transgenic mice
Stromal-derived factor 1 (SDF1) typically is expressed by cancer-associated fibroblasts and is found to be up-regulated in Helicobacter-infected gastric mucosa. Transgenic mice expressing SDF1 using the H/K-ATPase promoter do not show recruitment of inflammatory cells, but show hyperproliferative gastric epithelial cells and focal areas of SPEM with dilated glands at late time points (18 months). H felis–infected SDF1-Tg mice develop accelerated pathology. Similarly, when Tg-SDF1 mice are crossed to proinflammatory Tg-IL1β mice there is increased epithelial cell proliferation and accelerated SPEM development in the corpus.113 These data indicate that although SDF1 does not drive recruitment of inflammatory cells, it does appear to promote epithelial changes and promote SPEM, especially in the setting of atrophic gastritis.
K19-C2mE
Cyclooxygenase 2 (PTGS2) frequently is overexpressed in gastric cancer. Oshima et al114 developed transgenic mice expressing Ptgs2 and mPGES-1 in progenitor cells, driven by elements from the cytokeratin 19 promoter. The resulting mice showed recruitment of M2 polarized macrophages into the mucosa, subsequently causing the development of SPEM. After 80 weeks, these mice progress to develop hyperplastic tumors. Treatment using the nonsteroidal anti-inflammatory drug meloxicam ameliorated the inflammation and reversed the metaplastic gland morphology. In an effort to determine the role of specific cytokines in this process, Tnfα and Il1r1-/- mice were crossed to K19-C2mE. Mice null for Tnfα had decreased inflammation, reducing metaplasia overall. However, no overt change in inflammation or metaplasia was observed in l1r1-/- mice. Therefore, cyclooxygenase 2 and prostaglandin E2 can drive metaplastic changes in the stomach through the recruitment of M2-polarized macrophages in a TNFα-dependent pathway.114
Gan mouse
Activated β-catenin activity, but not mutations in APC, is observed in 51% of human intestinal-type gastric cancers.115 K19-promoter–expressing cells driving Wnt1 expression caused suppression of normal gastric epithelial cell differentiation that was accompanied with increased proliferation and TFF2-positive cells. By 7 to 18 weeks, mice developed small preneoplastic lesions in the corpus. The Gan mice were developed by crossing K19-Wnt1 mice to K19-C2mE mice (described earlier), thereby activating Wnt signaling in progenitor cells with a coordinated immune cell infiltration. This results in highly proliferative SPEM that drives the rapid development of tumors in the corpus.116 Furthermore, amelioration of the inflammatory response using a CCL2 chemokine inhibitor or clodronate treatment to deplete macrophages causes tumor regression in Gan mice.117 Thus, activation of the Wnt signaling pathway can drive metaplasia formation, however, inflammation is necessary for metaplasia progression.
Exogenous IL11 treatment
IL11 belongs to the IL6 family of cytokines that induces signal transduction through the IL11RA. IL11 is not expressed during the early stages of an inflammatory response associated with Helicobacter infection, but gradually increases during chronic inflammation and intestinal metaplasia.118 IL11 expression normally is localized to parietal cells, therefore its expression in the stomach is lost when parietal cells die (eg, in the setting of oxyntic atrophy in response to Helicobacter infection). Therefore, the source of increased IL11 expression in chronic Helicobacter infection originates elsewhere. Nevertheless, exogenous treatment of IL11 in wild-type mice leads to parietal cell loss within 24 hours, an infiltration of polymorphonuclear cells, and the emergence of SPEM.65 Thus, it is hypothesized that IL11 is released by dying parietal cells to recruit inflammatory cells into the stomach and promote the development of SPEM.
Exogenous IL33 treatment
A member of the IL1 family of cytokines, IL33 is expressed normally in the gastric mucosa, localizing to cells in the surface cell zone. IL33 synergizes to promote a Th2 inflammatory response that activates innate and adaptive immune cells. IL33 generally is classified as an alarmin because of its release into the extracellular environment by necrotic or apoptotic cells.64 IL33-treated mice develop SPEM and a robust inflammatory response in the lungs, intestine, and stomach, indicating that IL33 is sufficient to recruit inflammatory cells and drive mucous production within respiratory and gut epithelial tissue.64, 119 However, IL33 is expressed only in the normal stomach by cells in the surface cell (upper pit/foveolar) zone in the corpus, and those cells are not thought to undergo cell death during Helicobacter infection. Therefore, although administration of IL33 is capable of recruiting inflammatory cells and inducing SPEM, it remains to be seen if endogenous IL33 functions in this capacity.
Conclusions
Data from mouse models indicate that the induction of metaplasia involves at least 2 phases. Induction of metaplasia first requires the loss of parietal cells. Parietal cell loss induced by Helicobacter infection requires the action of specific lymphocytic populations and specific immune modulatory molecules. In a second phase, after, or concomitant to, the loss of parietal cells, SPEM develops, at least in part, from transdifferentiation of chief cells. The expansion and maintenance of the metaplasia requires the chronic influence of M2-macrophage subclasses. The utilization of a number of drug-induced methods to ablate parietal cells acutely can bypass the initial inflammatory phase required for parietal cell loss. Similarly, genetic models, such as directed activation of Ras in chief cells, can bypass parietal cell loss to induce metaplasia via transdifferentiation. Constitutive activated Ras in these metaplastic cells seems to be sufficient to maintain and expand metaplasia, although Ras activation also may recruit macrophages to support the progression of metaplasia. Mouse models of immune modulator overexpression similarly can induce both parietal cell loss and SPEM induction. It seems evident that inflammatory mediators are required for induction of more aggressive and proliferative metaplasia.
What remains unclear from mouse models is the explicit connection between the metaplastic/atrophic lesions and progression of carcinogenesis. Thus far, none of the models of metaplasia progress to true metastatic, or even regionally invasive, adenocarcinoma. In our experience, even the mouse models termed dysplastic seem predominantly to be exuberantly proliferative metaplastic or reactive lesions. With the exception of the Mist1-Kras mouse and some aged mice on the amphiregulin null background, mouse models of metaplasia also have not faithfully recapitulated the induction of goblet cell–containing intestinal metaplasia. This could suggest that extensive intestinal metaplasia as seen in human beings is central to the development of adenocarcinoma. Alternatively, intestinal metaplasia in human beings may represent an adaptive response, occurring over years of chronic injury rather than a true preneoplastic lesion. Further studies are needed to discern the molecular mediators of key transition points along the metaplasia-to-carcinoma sequence: at the point of transdifferentiation of chief cells into SPEM, at the transition of SPEM into intestinal metaplasia, and at the point of true dysplastic transition from within the metaplastic milieu (SPEM or intestinal metaplasia). Moreover, the search will continue for further mouse models that can extend investigations to true models of gastric adenocarcinoma development.
Acknowledgment
The authors thank Hei-Yong (Grant) Lo for some of the H&E images.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Previously supported by the American Gastroenterological Association Institute Funderburg Award (J.R.G. and J.C.M.). Supported by grants from a Department of Veterans Affairs Merit Review Award (I01BX000930) and National Institutes of HealthRO1 DK071590 (J.R.G.); by National Institutes of Health National Research Service Award Predoctoral FellowshipF31 DK104600 (C.P.P.); by National Institutes of HealthDK105129, DK094989, and DK052574 to the Washington University Digestive Core Centers, and by a pre-Program Project Award from the Siteman Cancer Center Investment Program (J.C.M.).
Contributor Information
Jason C. Mills, Email: jmills@wustl.edu.
James R. Goldenring, Email: jim.goldenring@vanderbilt.edu.
References
- 1.Eidt S., Oberhuber G., Schneider A. The histopathological spectrum of type A gastritis. Pathol Res Pract. 1996;192:101–106. doi: 10.1016/S0344-0338(96)80203-2. [DOI] [PubMed] [Google Scholar]
- 2.Lennerz J.K.M., Kim S., Oates E.L. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia and carcinoma. Am J Pathol. 2010;177:1514–1533. doi: 10.2353/ajpath.2010.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox J.G., Wang T.C., Rogers A.B. Host and microbial constituents influence Helicobacter pylori-induced cancer in a murine model of hypergastrinemia. Gastroenterology. 2003;124:1879–1890. doi: 10.1016/s0016-5085(03)00406-2. [DOI] [PubMed] [Google Scholar]
- 4.Wang T.C., Dangler C.A., Chen D. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118:36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 5.Wang T.C., Goldenring J.R., Dangler C. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology. 1998;114:675–689. doi: 10.1016/s0016-5085(98)70581-5. [DOI] [PubMed] [Google Scholar]
- 6.Fox J.G., Li X., Cahill R.J. Hypertrophic gastropathy in Helicobacter felis-Infected wild type C57BL/6 mice and p53 hemizygous transgenic mice. Gastroenterology. 1996;110:155–166. doi: 10.1053/gast.1996.v110.pm8536852. [DOI] [PubMed] [Google Scholar]
- 7.Nam K.T., Lee H.-J., Sousa J.F. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aikou S., Fukushima Y., Ogawa M. Alterations in gastric mucosal lineages before or after acute oxyntic atrophy in gastrin receptor and H2 histamine receptor-deficient mice. Dig Dis Sci. 2009;54:1625–1635. doi: 10.1007/s10620-009-0832-2. [DOI] [PubMed] [Google Scholar]
- 9.Nozaki K., Weis V., Wang T.C. Altered gastric chief cell lineage differentiation in histamine-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1211–G1220. doi: 10.1152/ajpgi.90643.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nomura S., Yamaguchi H., Ogawa M. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G362–G375. doi: 10.1152/ajpgi.00160.2004. [DOI] [PubMed] [Google Scholar]
- 11.Mills J.C., Sansom O.J. Reserve stem cells: differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract. Sci Signal. 2015;8:re8. doi: 10.1126/scisignal.aaa7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldenring J.R., Nam K.T., Wang T.C. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology. 2010;138:2207–2210. doi: 10.1053/j.gastro.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karam S.M., Leblond C.P. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cells. Anat Rec. 1993;236:259–279. doi: 10.1002/ar.1092360202. [DOI] [PubMed] [Google Scholar]
- 14.Karam S.M., Leblond C.P. Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat Rec. 1993;236:280–296. doi: 10.1002/ar.1092360203. [DOI] [PubMed] [Google Scholar]
- 15.Willet S.G., Mills J.C. Stomach organ and cell lineage differentiation: from embryogenesis to adult homeostasis. Cell Mol Gastroenterol Hepatol. 2016;2:546–559. doi: 10.1016/j.jcmgh.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poynter D., Selway S.A., Papworth S.A. Changes in the gastric mucosa of the mouse associated with long lasting unsurmountable histamine H2 blockade. Gut. 1986;27:1338–1346. doi: 10.1136/gut.27.11.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dempsey P.J., Goldenring J.R., Soroka C.J. Possible role of transforming growth factor alpha in the pathogenesis of Menetrier's disease: supportive evidence form humans and transgenic mice. Gastroenterology. 1992;103:1950–1963. doi: 10.1016/0016-5085(92)91455-d. [DOI] [PubMed] [Google Scholar]
- 18.Coffey R.J., Washington M.K., Corless C.L. Menetrier disease and gastrointestinal stromal tumors: hyperproliferative disorders of the stomach. J Clin Invest. 2007;117:70–80. doi: 10.1172/JCI30491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neuburger P., Lewin M., Bonfils S. Parietal and chief cell populations in four cases of the Zollinger-Ellison syndrome. Gastroenterology. 1972;6:937–942. [PubMed] [Google Scholar]
- 20.Bredemeyer A.J., Geahlen J.H., Weis V.G. The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage. Dev Biol. 2009;325:211–224. doi: 10.1016/j.ydbio.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geahlen J.H., Lapid C., Thorell K. Evolution of the human gastrokine locus and confounding factors regarding the pseudogenicity of GKN3. Physiol Genomics. 2013;45:667–683. doi: 10.1152/physiolgenomics.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee M.P., Ravenel J.D., Hu R.J. Targeted disruption of the Kvlqt1 gene causes deafness and gastric hyperplasia in mice. J Clin Invest. 2000;106:1447–1455. doi: 10.1172/JCI10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z., Demitrack E.S., Keeley T.M. IFNgamma contributes to the development of gastric epithelial cell metaplasia in Huntingtin interacting protein 1 related (Hip1r)-deficient mice. Lab Invest. 2012;92:1045–1057. doi: 10.1038/labinvest.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldenring J.R., Nomura S. Differentiation of the gastric mucosa III. Animal models of oxyntic atrophy and metaplasia. Am J Physiol Gastrointest Liver Physiol. 2006;291:G999–G1004. doi: 10.1152/ajpgi.00187.2006. [DOI] [PubMed] [Google Scholar]
- 25.Nozaki K., Ogawa M., Williams J.A. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology. 2008;134:511–521. doi: 10.1053/j.gastro.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huh W.J., Khurana S.S., Geahlen J.H. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology. 2012;142:21–24 e7. doi: 10.1053/j.gastro.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keeley T.M., Samuelson L.C. Cytodifferentiation of the postnatal mouse stomach in normal and Huntingtin-interacting protein 1-related-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1241–G1251. doi: 10.1152/ajpgi.00239.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hattori T., Helpap B., Gedigk P. The morphology and cell kinetics of pseudopyloric glands. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;39:31–40. doi: 10.1007/BF02892834. [DOI] [PubMed] [Google Scholar]
- 29.Wright N.A., Pike C., Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. 1990;343:82–85. doi: 10.1038/343082a0. [DOI] [PubMed] [Google Scholar]
- 30.Engevik A.C., Feng R., Choi E. The development of spasmolytic polypeptide/TFF2-expressing metaplasia (SPEM) during gastric repair is absent in the aged stomach. Cell Mol Gastroenterol Hepatol. 2016;2:605–624. doi: 10.1016/j.jcmgh.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aihara E., Matthis A.L., Karns R.A. Epithelial regeneration after gastric ulceration causes prolonged cell type alterations. Cell Mol Gastroenterol Hepatol. 2016;2:625–647. doi: 10.1016/j.jcmgh.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldenring J.R., Nam K.T., Mills J.C. The origin of pre-neoplastic metaplasia in the stomach: chief cells emerge from the Mist. Exp Cell Res. 2011;317:2759–2764. doi: 10.1016/j.yexcr.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt P.H., Lee J.R., Joshi V. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639–646. [PMC free article] [PubMed] [Google Scholar]
- 34.Goldenring J.R., Ray G.S., Coffey R.J. Reversible drug-induced oxyntic atrophy in rats. Gastroenterology. 2000;118:1080–1093. doi: 10.1016/s0016-5085(00)70361-1. [DOI] [PubMed] [Google Scholar]
- 35.Nomura S., Baxter T., Yamaguchi H. Spasmolytic polypeptide expressing metaplasia to preneoplasia in H. felis-infected mice. Gastroenterology. 2004;127:582–594. doi: 10.1053/j.gastro.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 36.Weis V.G., Sousa J.F., LaFleur B.J. Heterogeneity in mouse SPEM lineages identifies markers of metaplastic progression. Gut. 2013;62:1270–1279. doi: 10.1136/gutjnl-2012-302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshizawa N., Takenaka Y., Yamaguchi H. Emergence of spasmolytic polypeptide-expressing metaplasia in Mongolian gerbils infected with Helicobacter pylori. Lab Invest. 2007;87:1265–1276. doi: 10.1038/labinvest.3700682. [DOI] [PubMed] [Google Scholar]
- 38.Wada T., Ishimoto T., Seishima R. Functional role of CD44v-xCT system in the development of spasmolytic polypeptide-expressing metaplasia. Cancer Sci. 2013;104:1323–1329. doi: 10.1111/cas.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weis V.G., Petersen C.P., Mills J.C. Establishment of novel in vitro mouse chief cell and SPEM cultures identifies MAL2 as a marker of metaplasia in the stomach. Am J Physiol Gastrointest Liver Physiol. 2014;307:G777–G792. doi: 10.1152/ajpgi.00169.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varon C., Dubus P., Mazurier F. Helicobacter pylori infection recruits bone marrow-derived cells that participate in gastric preneoplasia in mice. Gastroenterology. 2012;142:281–291. doi: 10.1053/j.gastro.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 41.Kang J.M., Lee B.H., Kim N. CDX1 and CDX2 expression in intestinal metaplasia, dysplasia and gastric cancer. J Korean Med Sci. 2011;26:647–653. doi: 10.3346/jkms.2011.26.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nam K.T., Lee H.J., Mok H. Amphiregulin-deficient mice develop spasmolytic polypeptide expressing metaplasia and intestinal metaplasia. Gastroenterology. 2009;136:1288–1296. doi: 10.1053/j.gastro.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi E., Hendley A.M., Bailey J.M. Expression of activated Ras in gastric chief cells of mice leads to the full spectrum of metaplastic lineage transitions. Gastroenterology. 2016;150:918–930. doi: 10.1053/j.gastro.2015.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leys C.M., Nomura S., Rudzinski E. Expression of Pdx-1 in human gastric metaplasia and gastric adenocarcinoma. Hum Pathol. 2006;37:1162–1168. doi: 10.1016/j.humpath.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Silberg D.G., Sullivan J., Kang E. Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology. 2002;122:689–696. doi: 10.1053/gast.2002.31902. [DOI] [PubMed] [Google Scholar]
- 46.Mutoh H., Hakamata Y., Sato K. Conversion of gastric mucosa to intestinal metaplasia in Cdx2-expressing transgenic mice. Biochem Biophys Res Commun. 2002;294:470–479. doi: 10.1016/S0006-291X(02)00480-1. [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi H., Goldenring J.R., Kaminishi M. Identification of spasmolytic polypeptide expressing metaplasia (SPEM) in remnant gastric cancer and surveillance postgastrectomy biopsies. Dig Dis Sci. 2001;47:573–578. doi: 10.1023/a:1017920220149. [DOI] [PubMed] [Google Scholar]
- 48.Tatematsu M., Tsukamoto T., Toyoda T. Effects of eradication of Helicobacter pylori on gastric carcinogenesis in experimental models. J Gastroenterol. 2007;42(Suppl 17):7–9. doi: 10.1007/s00535-006-1927-6. [DOI] [PubMed] [Google Scholar]
- 49.Shimizu T., Choi E., Petersen C.P. Characterization of progressive metaplasia in the gastric corpus mucosa of Mongolian gerbils infected with Helicobacter pylori. J Pathol. 2016;239:399–410. doi: 10.1002/path.4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Houghton J., Stoicov C., Nomura S. Gastric cancer originating from bone marrow derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 51.Washington M.K., Powell A.E., Sullivan R. Pathology of rodent models of intestinal cancer: progress report and recommendations. Gastroenterology. 2013;144:705–717. doi: 10.1053/j.gastro.2013.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Syder A.J., Karam S.M., Mills J.C. A transgenic mouse model of metastatic carcinoma involving transdifferentiation of a gastric epithelial lineage progenitor to a neuroendocrine phenotype. Proc Natl Acad Sci U S A. 2004;101:4471–4476. doi: 10.1073/pnas.0307983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu S., Yang M., Nam K.T. Mouse models of gastric carcinogenesis. J Gastric Cancer. 2014;14:67–86. doi: 10.5230/jgc.2014.14.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Correa P., Piazuelo M.B. The gastric precancerous cascade. J Dig Dis. 2012;13:2–9. doi: 10.1111/j.1751-2980.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitchell H.M. Epidemiology of infection. In: Mobley H.L.T., Mendz G.L., Hazell S.L., editors. Helicobacter pylori. Washington, DC: Physiology and Genetics. ASM Press; Washington (DC): 2001. [PubMed] [Google Scholar]
- 56.Halldorsdottir A.M., Sigurdardottir M., Jonasson J.G. Spasmolytic polypeptide expressing metaplasia (SPEM) associated with gastric cancer in Iceland. Dig Dis Sci. 2003;48:431–441. doi: 10.1023/a:1022564027468. [DOI] [PubMed] [Google Scholar]
- 57.Minegishi Y., Suzuki H., Arakawa M. Reduced Shh expression in TFF2-overexpressing lesions of the gastric fundus under hypochlorhydric conditions. J Pathol. 2007;213:161–169. doi: 10.1002/path.2221. [DOI] [PubMed] [Google Scholar]
- 58.Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554–3560. [PubMed] [Google Scholar]
- 59.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 60.Weis V.G., Goldenring J.R. Current understanding of SPEM and its standing in the preneoplastic process. Gastric Cancer. 2009;12:189–197. doi: 10.1007/s10120-009-0527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duckworth C.A., Burkitt M.D., Williams J.M. Murine models of Helicobacter (pylori or felis)-associated gastric cancer. Curr Protoc Pharmacol. 2015;69 doi: 10.1002/0471141755.ph1434s69. 14.34.1–35. [DOI] [PubMed] [Google Scholar]
- 62.Franco A.T., Johnston E., Krishna U. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 2008;68:379–387. doi: 10.1158/0008-5472.CAN-07-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee A., Fox J.G., Otto G. A small animal model of human Helicobacter pylori active chronic gastritis. Gastroenterology. 1990;99:1315–1323. doi: 10.1016/0016-5085(90)91156-z. [DOI] [PubMed] [Google Scholar]
- 64.Buzzelli J.N., Chalinor H.V., Pavlic D.I. IL33 is a stomach alarmin that initiates a skewed Th2 response to injury and infection. Cell Mol Gastroenterol Hepatol. 2015;1:19. doi: 10.1016/j.jcmgh.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Howlett M., Chalinor H.V., Buzzelli J.N. IL-11 is a parietal cell cytokine that induces atrophic gastritis. Gut. 2012;61:1398–1409. doi: 10.1136/gutjnl-2011-300539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roth K.A., Kapadia S.B., Martin S.M. Cellular immune responses are essential for the development of Helicobacter felis-associated gastric pathology. J Immunol. 1999;163:1490–1497. [PubMed] [Google Scholar]
- 67.Ogawa M., Nomura S., Car B.D. Omeprazole treatment ameliorates oxyntic atrophy induced by DMP-777. Dig Dis Sci. 2006;51:431–439. doi: 10.1007/s10620-006-3151-x. [DOI] [PubMed] [Google Scholar]
- 68.Saenz J.B., Burclaff J., Mills J.C. Modeling murine gastric metaplasia through tamoxifen-induced acute parietal cell loss. Methods Mol Biol. 2016;1422:329–339. doi: 10.1007/978-1-4939-3603-8_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seishima R., Wada T., Tsuchihashi K. Ink4a/Arf-dependent loss of parietal cells induced by oxidative stress promotes CD44-dependent gastric tumorigenesis. Cancer Prev Res (Phila) 2015;8:492–501. doi: 10.1158/1940-6207.CAPR-15-0025-T. [DOI] [PubMed] [Google Scholar]
- 70.Petersen C.P., Weis V.G., Nam K.T. Macrophages promote progression of spasmolytic polypeptide-expressing metaplasia following acute loss of parietal cells. Gastroenterology. 2014;146:1727–1738. doi: 10.1053/j.gastro.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ekblad E.B.M. Histamine: the sole mediator of pentagastrin-stimulated acid secretion. Acta Physiol Scand. 1985;125:135–143. doi: 10.1111/j.1748-1716.1985.tb07700.x. [DOI] [PubMed] [Google Scholar]
- 72.Royston C.M., Polak J., Bloom S.R. G cell population of the gastric antrum, plasma gastrin, and gastric acid secretion in patients with and without duodenal ulcer. Gut. 1978;19:689–698. doi: 10.1136/gut.19.8.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakajima T., Konda Y., Izumi Y. Gastrin stimulates the growth of gastric pit cell precursors by inducing its own receptors. Am J Physiol Gastrointest Liver Physiol. 2002;282:G359–G366. doi: 10.1152/ajpgi.00117.2001. [DOI] [PubMed] [Google Scholar]
- 74.Fjeldbo C.S., Bakke I., Erlandsen S.E. Gastrin upregulates the prosurvival factor secretory clusterin in adenocarcinoma cells and in oxyntic mucosa of hypergastrinemic rats. Am J Physiol Gastrointest Liver Physiol. 2012;302:G21–G33. doi: 10.1152/ajpgi.00197.2011. [DOI] [PubMed] [Google Scholar]
- 75.Todisco A., Mao M., Keeley T.M. Regulation of gastric epithelial cell homeostasis by gastrin and bone morphogenetic protein signaling. Physiol Rep. 2015;3:e12501. doi: 10.14814/phy2.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dockray G.J., Varro A., Dimaline R. The gastrins: their production and biological activities. Annu Rev Physiol. 2001;63:119–139. doi: 10.1146/annurev.physiol.63.1.119. [DOI] [PubMed] [Google Scholar]
- 77.Tomita H., Takaishi S., Menheniott T.R. Inhibition of gastric carcinogenesis by the hormone gastrin is mediated by suppression of TFF1 epigenetic silencing. Gastroenterology. 2011;140:879–891. doi: 10.1053/j.gastro.2010.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zavros Y., Eaton K.A., Kang W. Chronic gastritis in the hypochlorhydric gastrin-deficient mouse progresses to adenocarcinoma. Oncogene. 2005;24:2354–2366. doi: 10.1038/sj.onc.1208407. [DOI] [PubMed] [Google Scholar]
- 79.Hayashi D., Tamura A., Tanaka H. Deficiency of claudin-18 causes paracellular H+ leakage, up-regulation of interleukin-1beta, and atrophic gastritis in mice. Gastroenterology. 2012;142:292–304. doi: 10.1053/j.gastro.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 80.Hsu L.S., Chan C.P., Chen C.J. Decreased Kruppel-like factor 4 (KLF4) expression may correlate with poor survival in gastric adenocarcinoma. Med Oncol. 2013;30:632. doi: 10.1007/s12032-013-0632-6. [DOI] [PubMed] [Google Scholar]
- 81.Wei D., Gong W., Kanai M. Drastic down-regulation of Kruppel-like factor 4 expression is critical in human gastric cancer development and progression. Cancer Res. 2005;65:2746–2754. doi: 10.1158/0008-5472.CAN-04-3619. [DOI] [PubMed] [Google Scholar]
- 82.Katz J.P., Perreault N., Goldstein B.G. Loss of Klf4 in mice causes altered proliferation and differentiation and precancerous changes in the adult stomach. Gastroenterology. 2005;128:935–945. doi: 10.1053/j.gastro.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 83.Ogasawara N., Tsukamoto T., Mizoshita T. RUNX3 expression correlates with chief cell differentiation in human gastric cancers. Histol Histopathol. 2009;24:31–40. doi: 10.14670/HH-24.31. [DOI] [PubMed] [Google Scholar]
- 84.Yano T., Ito K., Fukamachi H. The RUNX3 tumor suppressor upregulates Bim in gastric epithelial cells undergoing transforming growth factor beta-induced apoptosis. Mol Cell Biol. 2006;26:4474–4488. doi: 10.1128/MCB.01926-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ito K., Chuang L.S., Ito T. Loss of Runx3 is a key event in inducing precancerous state of the stomach. Gastroenterology. 2011;140:1536–1546 e8. doi: 10.1053/j.gastro.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 86.Chuang L.S., Ito K., Ito Y. RUNX family: regulation and diversification of roles through interacting proteins. Int J Cancer. 2013;132:1260–1271. doi: 10.1002/ijc.27964. [DOI] [PubMed] [Google Scholar]
- 87.Chew C.S., Hersey S.J., Sachs G. Adenylate cyclase stimulation by histamine is coupled to acid secretion. Am J Physiol. 1980;238:G312–G320. doi: 10.1152/ajpgi.1980.238.4.G312. [DOI] [PubMed] [Google Scholar]
- 88.Lopez-Diaz L., Hinkle K.L., Jain R.N. Parietal cell hyperstimulation and autoimmune gastritis in cholera toxin transgenic mice. Am J Physiol Gastrointest Liver Physiol. 2006;290:G970–G979. doi: 10.1152/ajpgi.00461.2005. [DOI] [PubMed] [Google Scholar]
- 89.Sousa J.F., Ham A.J., Whitwell C. Proteomic profiling of paraffin-embedded samples identifies metaplasia-specific and early-stage gastric cancer biomarkers. Am J Pathol. 2012;181:1560–1572. doi: 10.1016/j.ajpath.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deng N., Goh L.K., Wang H. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut. 2012;61:673–684. doi: 10.1136/gutjnl-2011-301839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khurana S.S., Riehl T.E., Moore B.D. The hyaluronic acid receptor CD44 coordinates normal and metaplastic gastric epithelial progenitor cell proliferation. J Biol Chem. 2013;288:16085–16097. doi: 10.1074/jbc.M112.445551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tan I.B., Ivanova T., Lim K.H. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology. 2011;141:476–485. doi: 10.1053/j.gastro.2011.04.042. e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hayakawa Y., Ariyama H., Stancikova J. Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell. 2015;28:800–814. doi: 10.1016/j.ccell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ray K.C., Bell K.M., Yan J. Epithelial tissues have varying degrees of susceptibility to Kras(G12D)-initiated tumorigenesis in a mouse model. PLoS One. 2011;6:e16786. doi: 10.1371/journal.pone.0016786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matsuo J., Kimura S., Yamamura A. Identification of stem cells in the epithelium of the stomach corpus and antrum of mice. Gastroenterology. 2017;152:218–231. doi: 10.1053/j.gastro.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 97.Zhu Y., Richardson J.A., Parada L.F. Smad3 mutant mice develop metastatic colorectal cancer. Cell. 1998;94:703–714. doi: 10.1016/s0092-8674(00)81730-4. [DOI] [PubMed] [Google Scholar]
- 98.Nam K.T., O'Neal R., Lee Y.S. Gastric tumor development in Smad3-deficient mice initiates from forestomach/glandular transition zone along the lesser curvature. Lab Invest. 2012;92:883–895. doi: 10.1038/labinvest.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shinohara M., Mao M., Keeley T.M. Bone morphogenetic protein signaling regulates gastric epithelial cell development and proliferation in mice. Gastroenterology. 2010;139:2050–2060 e2. doi: 10.1053/j.gastro.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Takabayashi H., Shinohara M., Mao M. Anti-inflammatory activity of bone morphogenetic protein signaling pathways in stomachs of mice. Gastroenterology. 2014;147:396–406 e7. doi: 10.1053/j.gastro.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nomura S., Settle S.H., Leys C.M. Evidence for repatterning of the gastric fundic epithelium associated with Menetrier's disease and TGFalpha overexpression. Gastroenterology. 2005;128:1292–1305. doi: 10.1053/j.gastro.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 102.Goldenring J.R., Ray G.S., Soroka C.J. Overexpression of transforming growth factor-alpha alters differentiation of gastric cell lineages. Dig Dis Sci. 1996;41:773–784. doi: 10.1007/BF02213134. [DOI] [PubMed] [Google Scholar]
- 103.Sharp R., Babyatsky M.W., Takagi H. Transforming growth factor alpha disrupts the normal program of cellular differentiation in the gastric mucosa of transgenic mice. Development. 1995;121:149–161. doi: 10.1242/dev.121.1.149. [DOI] [PubMed] [Google Scholar]
- 104.Ogawa M., Nomura S., Varro A. Altered metaplastic response of waved-2 EGF receptor mutant mice to acute oxyntic atrophy. Am J Physiol Gastrointest Liver Physiol. 2006;290:G793–G804. doi: 10.1152/ajpgi.00309.2005. [DOI] [PubMed] [Google Scholar]
- 105.Nam K.T., Varro A., Coffey R.J. Potentiation of oxyntic atrophy-induced gastric metaplasia in amphiregulin-deficient mice. Gastroenterology. 2007;132:1804–1819. doi: 10.1053/j.gastro.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 106.Syu L.J., El-Zaatari M., Eaton K.A. Transgenic expression of interferon-gamma in mouse stomach leads to inflammation, metaplasia, and dysplasia. Am J Pathol. 2012;181:2114–2125. doi: 10.1016/j.ajpath.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jain R.N., Al-Menhali A.A., Keeley T.M. Hip1r is expressed in gastric parietal cells and is required for tubulovesicle formation and cell survival in mice. J Clin Invest. 2008;118:2459–2470. doi: 10.1172/JCI33569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Solcia E., Rindi G., Fiocca R. Distinct patterns of chronic gastritis associated with carcinoid and cancer and their role in tumorigenesis. Yale J Biol Med. 1992;65:793–804. discussion 827–829. [PMC free article] [PubMed] [Google Scholar]
- 109.Nguyen T.L., Khurana S.S., Bellone C.J. Autoimmune gastritis mediated by CD4+ T cells promotes the development of gastric cancer. Cancer Res. 2013;7:2117–2126. doi: 10.1158/0008-5472.CAN-12-3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Watanabe H., Numata K., Ito T. Innate immune response in Th1- and Th2-dominant mouse strains. Shock. 2004;22:460–466. doi: 10.1097/01.shk.0000142249.08135.e9. [DOI] [PubMed] [Google Scholar]
- 111.Hsieh C.S., Macatonia S.E., O'Garra A. T cell genetic background determines default T helper phenotype development in vitro. J Exp Med. 1995;181:713–721. doi: 10.1084/jem.181.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tu S., Bhagat G., Cui G. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shibata W., Ariyama H., Westphalen C.B. Stromal cell-derived factor-1 overexpression induces gastric dysplasia through expansion of stromal myofibroblasts and epithelial progenitors. Gut. 2013;62:192–200. doi: 10.1136/gutjnl-2011-301824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Oshima M., Oshima H., Matsunaga A. Hyperplastic gastric tumors with spasmolytic polypeptide-expressing metaplasia caused by tumor necrosis factor-alpha-dependent inflammation in cyclooxygenase-2/microsomal prostaglandin E synthase-1 transgenic mice. Cancer Res. 2005;65:9147–9151. doi: 10.1158/0008-5472.CAN-05-1936. [DOI] [PubMed] [Google Scholar]
- 115.Echizen K., Hirose O., Maeda Y. Inflammation in gastric cancer: interplay of the COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways. Cancer Sci. 2016;107:391–397. doi: 10.1111/cas.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oshima H., Matsunaga A., Fujimura T. Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology. 2006;131:1086–1095. doi: 10.1053/j.gastro.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 117.Oshima H., Hioki K., Popivanova B.K. Prostaglandin E(2) signaling and bacterial infection recruit tumor-promoting macrophages to mouse gastric tumors. Gastroenterology. 2011;140:596–607 e7. doi: 10.1053/j.gastro.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 118.Howlett M., Giraud A.S., Lescesen H. The interleukin-6 family cytokine interleukin-11 regulates homeostatic epithelial cell turnover and promotes gastric tumor development. Gastroenterology. 2009;136:967–977. doi: 10.1053/j.gastro.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 119.Pastorelli L., De Salvo C., Vecchi M. The role of IL-33 in gut mucosal inflammation. Mediators Inflamm. 2013;2013:608187. doi: 10.1155/2013/608187. [DOI] [PMC free article] [PubMed] [Google Scholar]