Abstract
Purpose
Physician reported symptomatic late rectal injury occurs in about 5-25% of patients treated with radiation therapy for prostate cancer, depending on the treatment technique. Patients, however, report clinically meaningful declines in bowel/rectal function regardless of the technique used. Lovastatin has been shown to protect mice from late radiation injury. This study was designed to determine if lovastatin might reduce the incidence of late rectal injury in patients receiving radiation therapy for prostate cancer.
Methods and Materials
Patients with adenocarcinoma of the prostate receiving radiotherapy with curative intent were eligible. A portion of the rectum had to receive at least 60 Gy. Gastrointestinal functioning was assessed using both physician reported and patient reported instruments at baseline and at prescribed intervals during and after treatment. Lovastatin (20-80 mg/d) was started on day 1 of radiation and continued for 12 months. Patients were followed for an additional 12 months. The primary endpoint was physician reported rectal toxicity ≥Grade 2 during the first 2 years after treatment.
Results
20/53 (38%) patients developed Grade 2 or higher toxicity during the 2-year follow-up period. Seventeen patients had one or more unresolved GI symptom at the end of 2 years, 3 (6%) of which were grade 2 and none were of higher grade.
Conclusions
The primary endpoint of the study was not met. Lovastatin, as administered in this trial, did not reduce the incidence of Grade 2 or higher rectal toxicity compared to historical controls.
Keywords: radiation proctitis, radiation therapy, complications, prostate cancer, statins
Introduction
The tolerance of normal tissues limits the dose of radiation therapy (RT) that can be used in the treatment of carcinoma of the prostate 1. The rectum is the primary dose limiting structure. Two clinical forms of rectal injury have been described: early and late 2,3. Early rectal injury typically develops during radiation therapy, and occurs to some degree in most patients. The condition is generally self-limited, but may predispose patients to the development of late injury 4. Late rectal injury occurs months to years after pelvic RT, and can occur in about 25% of patients 5. Studies using the more modern approaches of intensity modulated or image guided radiation therapy have physician reported late rectal toxicity ranging from 5-20% 6,7, however, patients generally report meaningful declines in bowel quality of life 8. Although late injury is less common than early injury, it is often more concerning, since it may be irreversible, difficult to manage and have a major impact on a patient’s lifestyle 2. Thus, preventing the occurrence of radiation induced rectal injury is an important goal.
While advances in treatment delivery technologies have improved dose distribution, they are unlikely to ever eliminate the risk of rectal injury, given the proximity of the rectum to the prostate. Thus, the development of alternative approaches to the prevention and management of this problem remain necessary.
The 3-Hydroxy-methylglutaryl coenzyme-A (HMG Co-A) reductase inhibitor lovastatin has been shown to protect mice from the late effects of radiation therapy 9. The most likely mechanisms of action are related to the anti-inflammatory and anti-fibrotic effects of statins9,10. These observations laid the foundation to prospectively evaluate the ability of this drug to protect against the development of late rectal injury in a group of patients receiving high-dose radiation therapy for prostate cancer.
Materials and Methods
Patient eligibility
The study was approved by the institutional review boards of all participating institutions, Virginia Commonwealth University, Southside Regional Medical Center and the McGuire Veterans Affairs Medical Center, and in accordance with an assurance filed with and approved by the U.S. Department of health and Human services. All patients signed written informed consent prior to enrollment. The trial was registered with Clinicaltrials.gov (registration number: NCT00580970). This was an open label, single arm study.
Eligible patients were those with a histologically proven diagnosis of adenocarcinoma of the prostate to be treated with radiation therapy with curative intent. Patients may have had prior radical prostatectomy and prior or concurrent androgen deprivation was allowed. The planned total dose to the planning target volume had to be at least 60 Gy such that a portion of the rectum received a minimum exposure of at least 60 Gy 11. Other requirements included age ≥18 years, Karnofsky Performance Status (KPS) ≥70, no history of prior radiotherapy to the prostate or rectum, no evidence of distant metastases, no active liver or muscle disease, adequate renal function, no contraindications to an HMG-coA-reductase inhibitor, not currently taking an inhibitor of cytochrome P450 3A4, and no major medical or psychiatric illnesses that would preclude completion of treatment or follow-up.
Pretreatment evaluation
All patients underwent a history and physical including assessment of KPS and clinical stage. Baseline genitourinary, gastrointestinal and sexual function were assessed using both patient reported data (EPIC [Expanded Prostate Cancer Index Composite: urinary, bowel, sexual, hormonal modules and overall satisfaction], IIEF [International Index of Erectile Function]) 12-14 and physician reported data (CTCAE v3 [Common Terminology Criteria for Adverse Events Version 3: genitourinary and gastrointestinal adverse events])15. Baseline laboratory studies (Complete Blood Counts [CBC], Creatine Kinase [CK}, metabolic panel, liver panel and lipid profile) were obtained. Patients were registered after the pretreatment evaluation was completed and eligibility confirmed.
Radiation therapy
Contouring of organs
For patients receiving external beam radiation (EBRT), 3 fiducial markers were placed transperineally under ultrasound guidance and approximately 1 week later, a planning CT was obtained (3 mm slice intervals) without contrast. The prostate, seminal vesicles, bladder, penile bulb and femoral heads were contoured in their entirety. The rectal wall was contoured from the anus to the inferior margin of the SI joints. In patients receiving treatment to the pelvic nodes, the small and large bowels were contoured to the top of the L4 vertebral body. The internal iliac, external iliac and common iliac lymph nodes were contoured. For the purpose of dose-volume histogram (DVH) calculations, the volume of rectum extending from 1 cm below to 1 cm above the inferior and superior field margins, respectively, was included. In patients whose superior field border extended above the rectum, the most superior contour defined the upper border of the rectum.
For patients receiving brachytherapy, a Foley catheter was inserted to define the urethra and a transrectal ultrasound study was performed to define the prostate, rectum and bladder contours. All brachytherapy patients also underwent a post-implant CT scan for dosimetry purposes on which the prostate and seminal vesicles were contoured in their entirety and the rectum was contoured from the anus to the inferior margin of the SI joints. For the purpose of DVH calculations, the volume of rectum extending from 1 cm below to 1 cm above the most inferior and superior placed source, respectively, defined the rectum.
Definitions of target volumes
All patients receiving EBRT were treated with intensity modulated radiation therapy (IMRT). Target volumes were defined as follows. Prostate: GTV (Gross Target Volume)=prostate; CTV (Clinical Target Volume)=GTV; PTV (Planning Target Volume)=CTV+5-10 mm in all directions except 3-5 mm posteriorly. Seminal vesicles: GTV=seminal vesicles; CTV=GTV; PTV=CTV+5-10 mm in all directions. Lymph nodes: GTV=nodes+vessels; CTV=GTV; PTV=CTV+5-15 mm, but PTV may be adjusted to respect departmental normal tissue guidelines for IMRT. For brachytherapy, target volumes were defined as follows. Prostate: GTV=prostate; CTV=GTV; PTV=CTV+0-3mm laterally and anteriorly, CTV+0-5mm cranially and caudally, and CTV+0mm posteriorly.
Radiation doses
The doses to target volumes and normal tissues were determined using established departmental guidelines for both EBRT and brachytherapy. For EBRT, 1.8-2 Gy fractions were used. If lymph nodes were treated, doses of 45-50 Gy were used. Doses to the prostate were 78-79 Gy. For brachytherapy delivered as a boost, doses were 90 Gy using Pd103 or 100-110 Gy using I125 given after EBRT (45-46 Gy). For brachytherapy used alone, doses were 124 Gy using Pd103 and 145 Gy using I125. Dose constraints for the rectum for EBRT alone were: 39 Gy to ≤50%, 56 Gy to ≤30%, 70 Gy to ≤20% and 76.4 Gy to ≤5%. For brachytherapy alone, rectal dose was limited to 100 Gy (maximum point dose). For combined EBRT plus brachytherapy, the permissible doses from each modality were reduced proportionally. For example, if a patient was to receive 46 Gy to the prostate plus seminal vesicles with a boost of 90 Gy using Pd103 to the prostate, the permissible dose to ≤20% of the rectum was 70 Gy x(46Gy÷78Gy) and the permissible maximum point dose from brachytherapy was reduced to 100 Gy x(90 Gy÷124 Gy).
Drug therapy
Lovastatin was the HMG-CoA-reductase inhibitor used in this study. For patients not on a statin, the dose was 20mg/d PO. Patients already on lovastatin continued on the drug at their dose established at the time of enrollment. Patients on a different statin were switched to lovastatin (20-80 mg/d) with the approval of their primary care physician, with the intent of maintaining calculated low-density lipoproteins below 130mg/dl. Lovastatin was started on the first day of radiation (either EBRT or on the day of brachytherapy) and continued for 12 months. If necessary for the management of hyperlipidemia, a statin was continued beyond that point at the discretion of the primary care physician, otherwise it was discontinued. Pill counts were not performed to verify compliance with the lovastatin regimen.
Other therapies
Chemotherapy was not permissible. Androgen deprivation therapy (neoadjuvant, concurrent and/or adjuvant) was permissible and was used at the discretion of the attending physicians. Supportive care to manage side effects developing during radiation was administered as needed.
Patient assessments
Patients receiving external beam radiation were seen at least weekly during treatment. At each visit, the patients were assessed for toxicity using both patient reported and physician reported instruments. If musculoskeletal symptoms were noted, a CK was obtained, and if hepatic toxicity was suspected a liver panel was drawn. After completing external beam or undergoing brachytherapy, all patients were evaluated at weeks 4, 8, then months 4, 6, 9, 12, 15, 18, 21 and 24. At each follow-up visit, patients were assessed for toxicity using both patient reported (IIEF and EPIC) and physician reported instruments (CTCAE V3). A KPS was ascertained. CK and hepatic panels were drawn if symptoms were detected.
Modification of statin or radiation therapy dosing
Lovastatin was discontinued if transaminases became elevated ≥3 times above the upper limit of normal or if CK levels became elevated. For other side effects thought to be due to lovastatin, if ≤ Grade 2, the dose was lowered to 10 mg/day, and if ≥ Grade 3, the drug was stopped and the patient was removed from the study. Radiation treatment interruptions were permitted, but were to be kept to a minimum, and criteria for a treatment break were up to the attending physician. Once radiation commenced, changes in fractionation were not permitted. Patients were to remain on lovastatin during any treatment breaks.
Evaluation of rectal bleeding
Hematochezia during treatment was noted, but not further evaluated unless severity exceeded Grade 2 (CTCAE V3). Grade 3 or higher bleeding required flexible sigmoidoscopy. Bleeding occurring more than 3 months after completion of treatment required flexible sigmoidoscopy.
Statistical considerations
The primary endpoint of the study was the proportion of physician reported rectal toxicity ≥Grade 2 during the first 2 years after treatment. If a patient developed more than 1 symptom of rectal toxicity, only the highest-grade toxicity for each symptom was counted. Symptoms starting in the acute period and unresolved beyond 90 days post treatment are considered late toxicity. The rate of rectal injury overall, based on a review of the literature available at the time of study initiation, was estimated to be 30% 16, and a 50% reduction was predicted with the use of lovastatin based on the level of protection observed in preclinical experiments9,10,17. For a 5% level of significance, and a power of 83%, 53 patients were required (one-stage design, since it was felt that lovastatin would not increase the risk of rectal injury). Patients who did not complete at least 6 months of lovastatin treatment were deemed ineligible for the purpose of assessing late rectal toxicity and additional patients were enrolled in order to achieve the accrual goal of 53 evaluable patients. The secondary endpoints of patient reported outcomes were also obtained concurrently with physician reported outcomes, using the validated and reliable EPIC and IIEF instruments as described above 12-15 with scores compared to baseline at each time point. The patients completed paper versions of the instruments at the time of the clinic visit. All analysis was done in statistical software R v3.0.2 and SAS v 9.4.
Results
Patient characteristics
Between May 10, 2007 and May 30, 2013, 73 consecutive patients enrolled in the study. A total of 20 patients were ineligible for analysis (patient non-compliance: n=7; intolerance of lovastatin, n=7; physician choice, n=4; development of a metastatic second primary cancer, n=1; death from myocardial infarction before completing 6 months of lovastatin therapy, n=1), resulting in a total of 53 evaluable patients.
For the 53 evaluable patients, the median age was 63 years (range 47-80 years). Thirty-one (58%) were Caucasian and 22 (42%) were African American, reflecting the demographics of the population served at the participating centers. The median follow-up for evaluable patients was 26 months, and only 2 patients were followed for less than 24 months (20 and 23 months, respectively). Forty-five (85%) were treated with external beam radiation without brachytherapy, 2 (4%) were treated with external beam radiation plus brachytherapy and the remaining 6 (11%) were treated with brachytherapy alone. Seventeen patients (32%) received androgen deprivation therapy in conjunction with radiation.
Gastrointestinal toxicity (CTCAE v3)
Late rectal bleeding developed in 14 (26%) patients. This toxicity was Grade 1 in 13 patients and Grade 2 in 1 patient. In 7/14 patients, rectal bleeding resolved by the end of the 2-year follow-up period. None of the patients in whom rectal bleeding persisted experienced toxicity higher than Grade 1.
Other late rectal toxicities, e.g., diarrhea, developed in 47 patients. Of these toxicities, 27 were Grade 1, 19 were Grade 2 and 1 was Grade 3. By the end of the 2-year follow-up period, 30/47 patients experience resolution of their symptoms. Of the 17 patients with persistent symptoms, 14 were grade 1 and 3 were Grade 2.
Considering all late rectal toxicities, 20/53 (38%) developed Grade 2 or higher late rectal toxicity during the 2 year follow-up period after RT. This incidence is not lower than the anticipated incidence of 30% based on the literature available at the time of study implementation.
Three patients were scored as having both unresolved bleeding and another unresolved GI symptom. Thus, a total of 17 patients had one or more unresolved GI symptom at the end of the 2 year follow-up period, 3 (6%) of which were grade 2.
Patient reported outcomes
In terms of bowel function, patients reported a significant decline in function from baseline at months 18 and 24 (Figure 1). However, with respect to bowel bother, there was a significant increase in bother at 1 month after treatment, but at no other time point was there a significant change from baseline (Figure 2).
Figure 1.
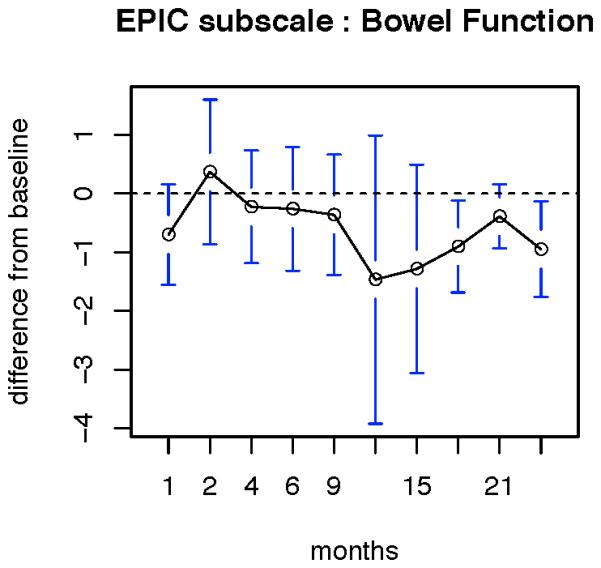
patient reported change in bowel function. Data are reported as the mean values at each time point. Error bars represent standard error of mean x2.96. Significant declines in bowel function compared to baseline were reported at 18 and 24 months.
Figure 2.
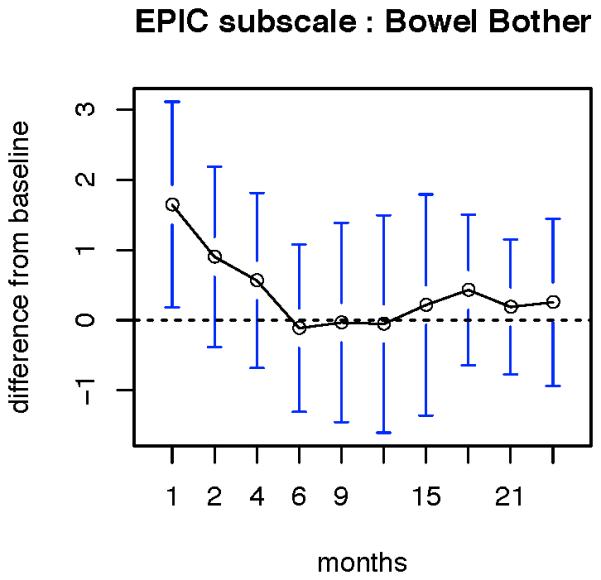
patient reported change in bowel bother. Data are reported as the mean values at each time point. Error bars represent standard error of mean x2.96. A significant increase in bowel bother was noted only in the first month after completing radiation.
Discussion
To our knowledge, this is the first prospective clinical trial evaluating the potential utility of a statin to prevent or ameliorate late radiation induced rectal injury from the treatment of prostate cancer. Late Grade 2 rectal injury was common in this study, occurring in 38% at some point during the 2-year follow-up period after RT. Persistent toxicity beyond 2 years, was uncommon, occurring in only 6%. Patients reported a significant decline in bowel function at 18 and 24 months after RT, but no change in bowel bother from baseline beyond 1 month. Thus, the findings from this study do not support the hypothesis that lovastatin may be effective in reducing the risk of late rectal toxicity from radiation therapy.
Late complications after pelvic radiation therapy, recently termed “pelvic radiation disease (PRD)” 18, are more common than generally appreciated, adversely affecting the quality of life of up to 50% of patients receiving this treatment 18. As the number of cancer survivors increases, its prevalence is likely to increase. PRD has been estimated to be more common than Crohn’s disease 18, yet few resources are expended on PRD and little progress has been made in the understanding and management of this condition. While technological improvements in tumor targeting and radiation delivery have reduced radiation exposures to surrounding normal tissues, this improved accuracy has not eliminated the development of late complications, particularly in relatively radiosensitive organs such as the rectum 18-20. Thus, there remains a need to explore biological strategies to reduce rectal injuries, especially as the beneficial effect for higher doses of radiation in the treatment of prostate cancer are becoming more apparent. Despite persistent worries about the risks of acute and late rectal toxicities, which remain the primary dose-limiting concern 21, higher doses are being more widely utilized, thereby placing more patients at risk for these complications.
Recent work into the underlying molecular mechanisms of PRD may help physicians to develop new strategies to reduce the frequency and severity of this problem. Radiation injury in the bowel is a multicellular response leading to dysfunctional wound healing 22,23. Fibrosis is a key component of this injury 10,24,25, accompanied by excessive accumulation of the fibrogenic mediator Connective Tissue Growth Factor (CTGF) in the stroma of the injured bowel 26. The Rho/Rho kinase pathway has been shown to be an important mediator of CTGF expression 10,26. Statins inhibit Rho isoprenylation 10, leading to decreased CTGF and collagen production in vitro 10,27. In addition, statins have been shown to protect against both late radiation induced lung and intestinal injury in mice, without effecting the anti-tumor activity of radiotherapy 9,10. A recent retrospective analysis of 308 patients treated for various pelvic malignancies, including prostate cancer, showed that the use of statins alone or in combination with angiotensin converting enzyme inhibitors during radiotherapy was associated with reduced gastrointestinal toxicities both acutely and at 1 year after radiotherapy 28. These authors suggested a stronger protective effect from the non-lipophilic statin, pravastatin, as this class of statins in not cleared as readily by the liver as the lipophilic statins, and concentrate more readily in peripheral tissues 28.
The data reported herein do not support the hypothesis that there may be a benefit to the use of lovastatin, but the study can be criticized for several reasons. First, there have been technological improvements since the trial was opened, that may facilitate dose reduction to the rectum, in particular the recent approval of an implantable spacer to separate the rectum from the prostate 29. However, this device is designed to be used only in the region between the prostate and the rectum, and would offer no protection to rectum or other portions of bowel above or below this region. Second, prediction of the potential benefit from lovastatin was based upon preclinical results and may have been overestimated. In addition, the toxicity rates for radiotherapy to the prostate that were available in the literature at the time the study was opened nearly 10 years ago may be higher than currently expected. However, the recent widespread adoption of intensity modulated radiation therapy has led to the use of higher radiation doses than were utilized in most of these older studies, and the rectum remains the dose limiting structure21. Third, one could criticize the study design, for permitting a multitude of radiation therapy approaches, which may have introduced too many confounding variables, by including a broad variety of patients (e.g., those treated with EBRT alone, brachytherapy alone, EBRT plus brachytherapy, post-prostatectomy patients who received lower doses of EBRT, with or without pelvic nodal irradiation, with or without androgen deprivation).. However, in practice, all prostate cancer patients undergoing curative radiation therapy will receive a dose to a portion of the rectum that is associated with a non-zero risk of late injury, and might potentially benefit from inclusion in a study of this nature. Forth, lovastatin is lipophilic and may not be as effective as the non-lipophilic statins, however, unlike many other statins, it has the advantage of in vivo data demonstrating its efficacy as a radio-protector, and it is very inexpensive, thus eliminating a potential barrier to participation for many of our patients. Other, more potent statins should be studied in clinical trials. Fifth, the side effect profile of statins overlaps to some extent with that of radiation therapy (e.g., diarrhea and flatulence), which may make assessment of radiation related rectal toxicity more difficult, and which adds to the importance of global patient reported outcomes in trying to determine whether these drugs are worth pursuing further in this capacity. Sixth, the study permitted a wide range of lovastatin doses, which was necessary to maintain lipid control, but may have made assessment of efficacy as a radiation protector more difficult. Also, pill counts were not required, which may have compromised assessment of patient compliance. Seventh, no dose volume histogram (DVH) data were gathered prospectively, although strict DVH requirements were in routine use throughout the department. Unfortunately, one of the participating centers is no longer part of our network, so these data cannot be retrieved for 10% of the patients, compromising the ability to assess the impact of these parameters on the outcome. Finally, the follow-up in this study was limited to 2 years after radiation and it is possible that additional rectal side effects may appear in these patients at a later date. However, the vast majority of rectal injuries will develop within this time frame 30, so this duration of follow-up should permit a reasonable estimate of rectal toxicity in this patient population.
In summary, the HMG Co-A reductase inhibitor, lovastatin, was not effective as an agent to reduce the frequency and severity of late radiation induced rectal injury when administered concurrently and for 1 year following radiation therapy. Based on preclinical data, however, other more potent members of this class of agents deserve further study as radiation protectors.
Acknowledgments
Funding: Supported by the Department of Radiation Oncology and the Massey Cancer Center (2P30CA016059-31), Virginia Commonwealth University
Footnotes
Disclaimers: the authors have no conflicts of interest to disclose
References
- 1.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int. J. Rad. Oncol. Biol. Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 2.Andreyev HJ. Gastrointestinal problems after pelvic radiotherapy: the past, the present and the future. Clin Oncol (R Coll Radiol) 2007;19:790–9. doi: 10.1016/j.clon.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Michalski JM, Gay H, Jackson A, et al. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76:S123–9. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peach MS, Showalter TN, Ohri N. Systematic Review of the Relationship between Acute and Late Gastrointestinal Toxicity after Radiotherapy for Prostate Cancer. Prostate Cancer. 2015;2015:624736. doi: 10.1155/2015/624736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauer-Jensen M, Wang J, Denham JW. Bowel injury: current and evolving management strategies. Semin Radiat Oncol. 2003;13:357–71. doi: 10.1016/s1053-4296(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 6.Mohammed N, Kestin L, Ghilezan M, et al. Comparison of acute and late toxicities for three modern high-dose radiation treatment techniques for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:204–12. doi: 10.1016/j.ijrobp.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Spratt DE, Pei X, Yamada J, et al. Long-term survival and toxicity in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2013;85:686–92. doi: 10.1016/j.ijrobp.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray PJ, Paly JJ, Yeap BY, et al. Patient-reported outcomes after 3-dimensional conformal, intensity-modulated, or proton beam radiotherapy for localized prostate cancer. Cancer. 2013;119:1729–35. doi: 10.1002/cncr.27956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams JP, Hernady E, Johnston CJ, et al. Effect of administration of lovastatin on the development of late pulmonary effects after whole-lung irradiation in a murine model. Radiat Res. 2004;161:560–7. doi: 10.1667/rr3168. [DOI] [PubMed] [Google Scholar]
- 10.Haydont V, Bourgier C, Pocard M, et al. Pravastatin Inhibits the Rho/CCN2/extracellular matrix cascade in human fibrosis explants and improves radiation-induced intestinal fibrosis in rats. Clin Cancer Res. 2007;13:5331–40. doi: 10.1158/1078-0432.CCR-07-0625. [DOI] [PubMed] [Google Scholar]
- 11.Tucker SL, Dong L, Michalski JM, et al. Do intermediate radiation doses contribute to late rectal toxicity? An analysis of data from radiation therapy oncology group protocol 94-06. Int J Radiat Oncol Biol Phys. 2012;84:390–5. doi: 10.1016/j.ijrobp.2011.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang P, Szymanski KM, Dunn RL, et al. Expanded prostate cancer index composite for clinical practice: development and validation of a practical health related quality of life instrument for use in the routine clinical care of patients with prostate cancer. J Urol. 2011;186:865–72. doi: 10.1016/j.juro.2011.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 14.Wei JT, Dunn RL, Litwin MS, et al. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 15.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–81. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 16.Giordano SH, Lee A, Kuo YF, et al. Late gastrointestinal toxicity after radiation for prostate cancer. Cancer. 2006;107:423–32. doi: 10.1002/cncr.21999. [DOI] [PubMed] [Google Scholar]
- 17.Ostrau C, Hulsenbeck J, Herzog M, et al. Lovastatin attenuates ionizing radiation-induced normal tissue damage in vivo. Radiother Oncol. 2009;92:492–9. doi: 10.1016/j.radonc.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Andreyev HJ, Wotherspoon A, Denham JW, et al. "Pelvic radiation disease": new understanding and new solutions for a new disease in the era of cancer survivorship. Scand J Gastroenterol. 2011;46:389–97. doi: 10.3109/00365521.2010.545832. [DOI] [PubMed] [Google Scholar]
- 19.Bentzen SM, Constine LS, Deasy JO, et al. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys. 2010;76:S3–9. doi: 10.1016/j.ijrobp.2009.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Showalter TN, Wages NA, Ohri N. Strategic evaluation of interventions to prevent consequential late proctitis after prostate radiation therapy: new clinical trial designs should be considered. Cancer Biol Ther. 2014;15:361–4. doi: 10.4161/cbt.27822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budaus L, Bolla M, Bossi A, et al. Functional outcomes and complications following radiation therapy for prostate cancer: a critical analysis of the literature. Eur Urol. 2012;61:112–27. doi: 10.1016/j.eururo.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 22.Barcellos-Hoff MH, Costes SV. A systems biology approach to multicellular and multi-generational radiation responses. Mutat Res. 2006;597:32–8. doi: 10.1016/j.mrfmmm.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Rodemann HP, Bamberg M. Cellular basis of radiation-induced fibrosis. Radiother Oncol. 1995;35:83–90. doi: 10.1016/0167-8140(95)01540-w. [DOI] [PubMed] [Google Scholar]
- 24.Martin M, Lefaix J-L, Delanian S. TGF-ß1 and radiation fibrosis: a master switch and a specific target? Int J Radiat Oncol Biol Phys. 2000;47:277–290. doi: 10.1016/s0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 25.Okunieff P, Rubin P, Williams J, et al. TGFß1 is an important contributing factor in the development of radiation induced fibrosis. Int J Radiat Oncol Biol Phys. 2000;48(Suppl):246. [Google Scholar]
- 26.Vozenin-Brotons MC, Milliat F, Sabourin JC, et al. Fibrogenic signals in patients with radiation enteritis are associated with increased connective tissue growth factor expression. Int J Radiat Oncol Biol Phys. 2003;56:561–72. doi: 10.1016/s0360-3016(02)04601-1. [DOI] [PubMed] [Google Scholar]
- 27.Eberlein M, Heusinger-Ribeiro J, Goppelt-Struebe M. Rho-dependent inhibition of the induction of connective tissue growth factor (CTGF) by HMG CoA reductase inhibitors (statins) Br J Pharmacol. 2001;133:1172–80. doi: 10.1038/sj.bjp.0704173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wedlake LJ, Silia F, Benton B, et al. Evaluating the efficacy of statins and ACE-inhibitors in reducing gastrointestinal toxicity in patients receiving radiotherapy for pelvic malignancies. Eur J Cancer. 2012;48:2117–24. doi: 10.1016/j.ejca.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 29.Mariados N, Sylvester J, Shah D, et al. Hydrogel Spacer Prospective Multicenter Randomized Controlled Pivotal Trial: Dosimetric and Clinical Effects of Perirectal Spacer Application in Men Undergoing Prostate Image Guided Intensity Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys. 2015;92:971–7. doi: 10.1016/j.ijrobp.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 30.Peeters ST, Hoogeman MS, Heemsbergen WD, et al. Rectal bleeding, fecal incontinence, and high stool frequency after conformal radiotherapy for prostate cancer: normal tissue complication probability modeling. Int J Radiat Oncol Biol Phys. 2006;66:11–9. doi: 10.1016/j.ijrobp.2006.03.034. [DOI] [PubMed] [Google Scholar]


