Abstract
Simple diagnostic tests are needed for the detection of norovirus (NoV) outbreaks. Salivary antibody assays provide an attractive alternative to collecting and testing serum or stool samples. Antibodies to Norwalk virus (NV) in oral fluid samples were compared with NV antibodies in serum collected from 38 volunteers challenged with NV inoculum. Pre- and postchallenge (day 4, 8, 14, and 21) saliva and serum samples were examined by enzyme immunoassay (EIA) using recombinant NV antigen. Of 18 infected subjects (those who shed NV in stool or who demonstrated immunoglobulin G [IgG] seroconversion), 15 (83%) had ≥4-fold increases in NV-specific salivary IgA and 15 (83%) had ≥4-fold increases in NV-specific salivary IgG when prechallenge and postchallenge saliva samples were compared. When the results of the IgA and IgG assays were combined, all 18 infected subjects showed ≥4-fold increases in NV-specific salivary IgG or IgA postchallenge titers compared to their prechallenge titers. One of 19 uninfected subjects had a ≥4-fold increase in NV-specific salivary IgG. The sensitivity of the combined assay results was 100%, and the specificity was 95%. NV-specific salivary IgA titers peaked around 14 days postchallenge. NV-specific salivary IgG and serum IgG titers continued to rise through 21 days postchallenge. The application of this EIA to an elementary school outbreak indicated that 67% of the subjects with confirmed infections had >4-fold rises in anti-NoV IgA when an antigen in the same genetic cluster as the outbreak virus was used. This is the first documented mucosal antibody response to NoV in children. This EIA provides a useful approach for diagnosing NoV outbreaks.
Norwalk virus (NV) is the prototype of a large group of enteric viruses that are the leading cause of acute epidemic gastroenteritis in adults and school-age children in the United States (16). The characterization of the complete NV genome (22, 24) and of the genomes of several related viruses (28) established that these viruses should be classified in the family Caliciviridae. Based on sequence analyses of the polymerase and capsid genes, the genetically diverse human caliciviruses are presently classified into two distinct genera, Norovirus (NoV) and Sapovirus (International Committee on Taxonomy of Viruses Index of Viruses [http://www.ncbi.nlm.nih.gov/ICTVdb/Ictv/index.htm]). The NoVs are further divided into two genogroups (I and II) (25, 37). Despite intensive efforts, the NoVs and other human caliciviruses have not been successfully propagated in cell culture, and no animal models have been identified.
NoV cases and outbreaks are being reported with increasing frequency in the United States (7) and Europe (18a, 31) due to improved PCR-based diagnostic assays. However, the collection of appropriate stool and serum specimens for diagnosis remains challenging. Diagnosis of NoV infection is based primarily on detecting virus particles in stool specimens by direct electron microscopy, immunoelectron microscopy, amplification of viral nucleic acid in stool samples by reverse transcription (RT)-PCR, or measurement of a rise in virus-specific serum antibody titer by enzyme immunoassay (EIA) (27). A new commercial EIA for the detection of virus antigen in feces has been evaluated, but the sensitivity of this assay for diagnosing an NoV infection is only 55% when RT-PCR is the reference assay (47). All these approaches require the collection of fecal specimens within the first few days of illness or of acute- and convalescent-phase sera. Historically, limitations to these assays have included a low concentration of virus particles (44, 49), poor detection limits (>104 to 105 particles/ml), and a limited supply of natural viral antigen for serological testing and developing reagents (21). Since the development of recombinant NV-like (rNV) particles (23, 24), much progress has been made in the development of sensitive EIAs to detect NV-specific immunoglobulin A (IgA), IgG, and IgM in serum (2, 17, 18, 36) and fecal IgA in stool (39).
While many EIAs have been described for the measurement of virus-specific antibodies in serum, the detection of antibodies in body fluids other than serum is a method that has been relatively unexplored but which has practical benefits. Parry et al. (41) first reviewed the use of saliva as a noninvasive alternative to serum for detecting virus-specific antibodies. Subsequently, there have been reports of EIAs that detect salivary antibodies specific to human immunodeficiency virus (15, 33); hepatitis A, B, and C viruses (5, 38, 42, 52); measles, mumps, and rubella viruses (13, 43, 53); dengue virus (8); poliovirus (19); and rotavirus (54). The collection of blood requires trained personnel, is time-consuming, and carries a risk of needlestick injuries (11). In contrast, saliva collection is easy and rapid, requires little training, eliminates the risk of needlestick injuries, is appropriate for both children and adults, and is suitable for nonclinical settings. Measuring NV-specific antibodies in saliva is an attractive, less-invasive alternative to testing serum and can provide valuable information about both the mucosal immune response and the humoral immune response.
The objectives of this study were (i) to develop an EIA to quantitatively detect NV-specific IgG and IgA in saliva and (ii) to verify that the EIA can accurately diagnose NV infection in 38 volunteers challenged with NV.
MATERIALS AND METHODS
Studies of NV infectivity in human volunteers.
Serum and saliva samples were collected preinoculation and at days 4, 8, 14, and 21 postdosing from 38 adults challenged with the 8FIIa NV inoculum (C. L. Moe, D. Rhodes, S. Pusek, F. Tseng, W. Heizer, C. Kapoor, B. Gilliam, M. Harb, P. Stewart, S. Miller, M. Sobsey, J. Herrmann, N. Blacklow, and R. Calderon, Abstr. 98th Gen. Meet. Am. Soc. Microbiol., poster C-384, 1998). Saliva specimens were collected by asking the volunteers to spit directly into a sterile 50-ml polypropylene test tube. Saliva samples were stored at −20°C until testing.
EIA to detect NV-specific IgG in serum.
The titers of anti-NV IgG in serum specimens were determined by an EIA with rNV capsid antigen, as described by Monroe et al. (36), with alkaline phosphatase-conjugated goat anti-human IgG (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.). Net absorbance was calculated for each sample as the mean value in the duplicate antigen-coated wells minus the mean value in the duplicate antigen-negative wells. NV seroconversion was defined as a ≥4-fold increase in NV-specific serum IgG units based on an assigned concentration of total NoV IgG units in the reference serum (36).
EIA to detect NV-specific IgG and IgA in saliva.
Polyvinyl microtiter plates (Dynatech Laboratories, Inc., Chantilly, Va.) were coated with either 75 μl of rNV antigen (4 μg/ml in 0.025 M Tris-buffered saline [TBS; pH 7.4]) (antigen-positive wells) or 0.025 M TBS alone (antigen-negative wells) and incubated at room temperature for 4 h. Wells were washed twice with TBS with 0.05% Tween 20 (TBS-T) and blocked with 185 μl of 5% BLOTTO (Carnation nonfat milk) in TBS overnight at 4°C. After the wells were washed six times with TBS-T, duplicate dilutions of test saliva (1:16; 75 μl/well, diluted in 1% BLOTTO-TBS) were added to two antigen-coated wells and two antigen-negative wells, and the plates were incubated for 2 h at 37°C. After six washes with TBS-T, bound antibody was detected by the addition of alkaline phosphatase-conjugated goat anti-human IgG or IgA (Kirkegaard & Perry Laboratories, Inc.) (1:1,000 dilution in 1% BLOTTO-TBS, 75 μl/well) and incubated for 2 h at 37°C. After six washes with TBS-T, 100 μl of p-nitrophenylphosphate substrate (Sigma, St. Louis, Mo.) in diethanolamine buffer (pH 9.8) was added to each well. The plates were incubated in the dark at room temperature for 90 min before the optical density was determined as the ratio of the absorbance values at 405 and 630 nm (MR 5000 microplate reader; Dynatech Laboratories, Inc.).
Known concentrations (3.1, 6.2, 12.5, 25, 50, and 100 ng/ml) of human IgG and IgA (Sigma) were included on each plate to allow for construction of a standard curve. The concentration of anti-NV salivary antibody was determined by comparison of the optical densities at 405 nm of the samples to that of the known antibody standard curve. Results are reported as nanograms of NV-specific IgG or IgA per milliliter. Using the convention for seroconversion, we defined NV-specific salivary conversion as a ≥4-fold increase in NV-specific salivary IgG or IgA concentration in postchallenge saliva compared to that in prechallenge saliva.
Data analysis.
The four-parameter log-logistic model was used to approximate the sigmoidal dose-response relationship for the known antibody standard curve (36, 45). The coefficient of variation (10) was used to evaluate between-run and within-run assay precision. The coefficient of variation for between-run precision was based on eight replicate tests using 16.7, 50, and 150 ng of IgG/ml for the human IgG3(κ) standard and 33.3, 100, and 300 ng of IgA/ml for the human IgA standard. The coefficient of variation for within-run precision was based on 15 replicate tests of three concentrations of the IgG (50, 16.7, and 5.6 ng/ml) and IgA (100, 33.3, and 11.1 ng/ml) standards.
NV-specific salivary antibody responses were compared to the anti-NV serum antibody response as the reference assay to determine infection status. Infection was defined as detection of NV RNA by RT-PCR in more than one stool sample in the first 5 days postchallenge or a >4-fold rise in NV-specific serum IgG in day 21 postchallenge serum compared to that in prechallenge serum (Moe et al., Abstr. 98th Gen. Meet. Am. Soc. Microbiol.). The sensitivity of the salivary EIA was calculated as the percentage of salivary conversions among infected subjects. The specificity was expressed as the percentage of negative salivary conversion test results among infected subjects. Both serum IgG units and salivary antibody concentrations were log transformed prior to analysis.
RESULTS
Assay optimization.
Initial experiments optimized the concentration of the rNV used as coating antigen, the dilution of the test saliva, and the dilution of the conjugated secondary antibody to provide the best discrimination between known infected and uninfected individuals. Saliva samples from infected volunteers were tested at dilutions ranging from 1:4 to 1:256. Subsequently, a single test dilution (1:16) was chosen because it provided the best assay sensitivity and specificity and consistently demonstrated a ≥4-fold increase in NV-specific salivary IgG or IgA when preexposure saliva samples and saliva samples collected 21 days postchallenge from NV-infected volunteers were compared.
Assay precision.
Calculation of the coefficients of variation provided a measure of the precision of the assay between runs and within runs at three concentrations of the immunoglobulin standards. The coefficient of variation for between-run precision ranged from 8.0 to 16.8 for the salivary IgA EIA and 0.6 to 12.8 for the salivary IgG EIA (data not shown). The within-run coefficients of variation for IgA and IgG salivary EIAs ranged from 13.6 to 18.4 and 1.9 to 8.7, respectively (data not shown). The precision of our salivary EIAs was comparable to that reported for salivary antibody detection assays for other viruses. Madsen et al. (32) reported a 15.6% within-run coefficient of variation for an enzyme-linked immunosorbent assay used to screen and quantitate IgG antibodies to rubella virus, and Friedman (13) reported a 19.3% within-run coefficient of variation for the detection of mumps virus-specific IgA antibodies in saliva.
Salivary antibody response to NV infection.
Eighteen (47%) of the 38 volunteers had ≥4-fold rises in NV-specific serum IgG by 21 days postchallenge and were classified as infected. All infected volunteers also shed NV (detected by RT-PCR) in more than one stool sample in the first 5 days postchallenge. No individuals who shed NV failed to demonstrate seroconversion. Seroconversion occurred in 13 of 18 (72%) infected volunteers between 5 and 8 days postchallenge (Table 1). Three of the remaining five volunteers showed seroconversion by day 14, and the remaining two demonstrated seroconversion by day 21. The NV-specific salivary IgA response occurred earlier than the salivary and serum IgG responses. Of the 18 infected volunteers, a total of 15 (83%) had ≥4-fold increases in NV-specific salivary IgA, and 6 of these volunteers showed ≥4-fold increases in NV-specific salivary IgA by 4 days postchallenge (Table 1). The anti-NV salivary IgG response was generally of a greater magnitude than the salivary IgA response and usually occurred several days later, with 56% of the infected volunteers showing >4-fold increases in anti-NV salivary IgG titer by day 8 postchallenge (Table 1). For the 18 infected volunteers, the average ratio of the highest postchallenge NV-specific antibody titer (from day 8, 14, or 21) to the prechallenge anti-NV titer was 78.1 (range, 4.2 to 250) for serum IgG, 47.2 (range, 1.4 to 205) for salivary IgG, and 25.1 (range, 1.0 to 74) for salivary IgA (Table 2). Among the 18 infected volunteers, NV-specific serum IgG and salivary IgG titers started to rise by day 4 postchallenge and continued to rise through day 21 postchallenge (Table 3). The geometric mean concentration of NV-specific salivary IgG increased from 26 ng/ml (range, 5 to 383) at baseline to 47 ng/ml on day 4 postchallenge, 102 ng/ml on day 8, 237 ng/ml on day 14, and 398 ng/ml on day 21. The geometric mean NV-specific salivary IgA titers were somewhat higher than the salivary IgG titers; however, the titer peaked at day 14 postchallenge (Table 3). For the 18 infected volunteers, the geometric mean NV-specific salivary IgA concentration was 46 ng/ml (range, 11 to 199) at baseline, increased to 80, 252, and 338 ng/ml by days 4, 8, and 14, respectively, and then decreased to 269 ng/ml by day 21 postchallenge.
TABLE 1.
Detection of NV-specific antibodies in serum and saliva specimens collected from 18 infected volunteers
| Antibody | No. (cumulative %) of subjects with ≥4-fold increase in antibodies on daya:
|
|||
|---|---|---|---|---|
| 4 | 8 | 14 | 21 | |
| NV-specific serum IgG | 0 (0) | 13 (72) | 3 (89) | 2 (100) |
| NV-specific salivary antibody | ||||
| IgA | 6 (33) | 3 (50) | 4 (72) | 2 (83) |
| IgG | 3 (17) | 7 (56) | 3 (72) | 2 (83) |
Day postchallenge at which ≥4-fold increase in antibody titer was first detected, in comparison to prechallenge specimen titer.
TABLE 2.
NV-specific antibody reactivity in serum and saliva specimens
| Antibody | Antibody reactivitya
|
|||
|---|---|---|---|---|
| Infectected volunteers (n = 18)
|
Uninfected volunteers (n = 20)
|
|||
| Range | Mean | Range | Mean | |
| Serum IgG | 4.2-250 | 78.1 | 0.04-3.4 | 1.1 |
| Salivary | ||||
| IgG | 1.4-205 | 47.2 | 0.2-4.4 | 1.2 |
| IgA | 1.0-74 | 25.1 | 0.5-2.6 | 1.2 |
Antibody reactivity is defined as the ratio of NV-specific serum IgG units or NV-specific salivary IgG or IgA concentration at highest postchallenge titer versus prechallenge titer. Specimens were collected from 38 NV-challenged volunteers.
TABLE 3.
Geometric mean antibody units in 18 infected subjects
| Collection time | Antibody units
|
||
|---|---|---|---|
| Serum IgGa | Salivaryb
|
||
| IgG | IgA | ||
| Prechallenge | 8,129 | 26 | 46 |
| Postchallenge, day: | |||
| 4 | 8,499 | 47 | 80 |
| 8 | 50,216 | 102 | 252 |
| 14 | 89,989 | 237 | 338 |
| 21 | 130,903 | 398 | 269 |
Expressed in NV-specific IgG units of a standard control serum.
Expressed in nanograms per milliliter by using a standard curve of known antibody concentration.
Comparison of serum and salivary EIAs.
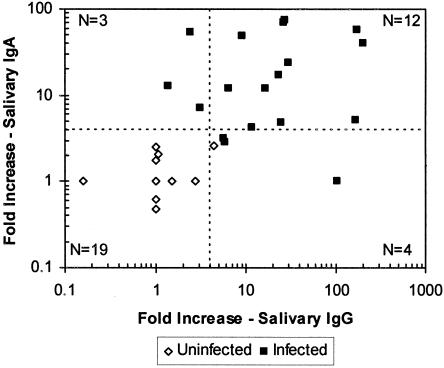
There was good agreement between NV-specific salivary IgA and salivary IgG responses in individual volunteers. With ≥4-fold increases in salivary NV-specific IgG or IgA considered indicative of NV infection, 12 volunteers demonstrated salivary conversions in both the IgG and IgA assays, and 19 volunteers did not demonstrate conversions in either assay, yielding a total of 31 (82%) volunteers with concordant results (Fig. 1). Three (8%) of the salivary specimen pairs showed salivary IgA conversions but not salivary IgG conversions. Four (11%) salivary specimen pairs showed salivary IgG conversions but not salivary IgA conversions.
FIG. 1.
Comparison of NV-specific salivary IgA and IgG conversions. Each symbol represents the increase (n-fold) in antibody response in paired saliva specimens (prechallenge and day 21 postchallenge) from a single individual that were assayed for both NV-specific IgA and IgG. The broken vertical and horizontal lines indicate the fourfold minimum increases that define an infection. Points to the right of the broken vertical line represent salivary conversions defined by a ≥4-fold increase in salivary IgG. Points above the horizontal broken line represent salivary conversions defined by a ≥4-fold increase in IgA. N, number of saliva specimens represented in each quadrant of the plot.
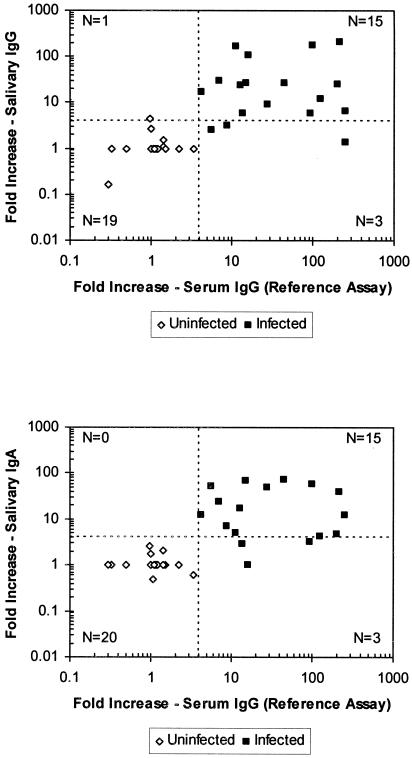
There was overall agreement between serum and salivary IgG responses for 34 of 38 (89%) volunteers (Fig. 2, top panel) and between serum IgG and salivary IgA responses for 35 of 38 (92%) volunteers (Fig. 2, bottom panel). We detected >4-fold rises in NV-specific salivary IgG and IgA titers in 15 (83%) of the 18 volunteers who had ≥4-fold rises in NV-specific serum IgG titers. Compared to the reference assays for NV-specific serum IgG, the EIA for NV-specific salivary IgA and IgG had 83% sensitivity. None of the 20 uninfected subjects demonstrated seroconversion. Nineteen had negative salivary IgG tests, and all 20 had negative salivary IgA tests, indicating specificities of 95 and 100% for the NV-specific salivary IgG and IgA EIAs, respectively. One uninfected volunteer had a 4.4-fold increase in anti-NV salivary IgG but did not show seroconversion or have a >4-fold increase in anti-NV salivary IgA.
FIG. 2.
Comparison of NV-specific seroconversions and salivary conversions. Each symbol represents the increase (n-fold) in antibody response in paired (prechallenge and day 21 postchallenge) serum and saliva specimens from a single individual that were assayed for both NV-specific serum IgG and NV-specific salivary IgA and IgG. The broken vertical and horizontal lines indicate the fourfold minimum increases that define infection. Points to the right of the broken vertical lines represent seroconversions defined by a ≥4-fold increase in serum IgG. Points above the horizontal broken line represent salivary conversions defined by a ≥4-fold increase in salivary IgG (top panel) or IgA (bottom panel). N, number of saliva specimens represented in each quadrant of the plot.
Application of salivary EIA to NoV outbreak investigations.
In order to evaluate the use of the salivary EIA for NoV outbreak investigations, we compared the anti-NV salivary titers in samples from day 4 postchallenge to the titers in the samples from day 14 postchallenge because those days seemed like the most feasible sample collection times in an outbreak investigation. Fourteen of the 18 infected subjects had >4-fold rises in NV-specific antibody titers (IgA or IgG), giving an assay sensitivity of 78% (data not shown). Of the four subjects who did not have a salivary conversion during this time interval, two had early rises in anti-NV salivary IgA or IgG titer (by day 4 postchallenge) and the other two had late rises in anti-NV salivary IgA or IgG titer (by day 21). Only 1 of the 20 uninfected subjects had a >4-fold rise in anti-NV salivary antibody titer, giving an assay specificity of 95%.
In a trial application of this assay to an NoV outbreak with approximately 100 cases in an elementary school, we were able to collect stool specimens and paired acute- and convalescent-phase saliva samples from nine infected subjects, one exposed control subject, and four unexposed control subjects (Table 4). The infected subjects were seven schoolchildren who were willing to provide specimens, one adult, and the school nurse. The exposed control subject was a schoolchild who was not ill, and the unexposed control subjects were Health Department employees who investigated the outbreak. Acute-phase saliva was collected 0 to 4 days after the onset of symptoms (mean, 2.8 days). Convalescent-phase saliva was collected 18 to 37 days after onset (mean, 22.9 days). NoV RNA could be detected in stool specimens from eight of nine infected subjects by RT-PCR, and sequencing and phylogenetic analyses of the 81-bp PCR product of the polymerase gene indicated that for the seven children, the virus was a genogroup I NoV in the Desert Shield virus (DSV) cluster (GI/3). The school nurse was infected with a genogroup II NoV and became ill 5 days after the children. None of the Health Department employees were infected. Ten saliva specimens were tested against both the rNV antigen and a recombinant antigen from a DSV-like virus (VA98115; generously provided by Xi Jiang). Four additional specimens (including one from an RT-PCR-confirmed genogroup I NoV case) were of insufficient volume for both assays and were tested only with the NV antigen. For the RT-PCR-confirmed genogroup I NoV-infected subjects, two had >4-fold rises in anti-NoV salivary IgA titer with the NV protein and four had >4-fold rises in anti-NoV salivary IgA titer with the DSV-like protein. Two subjects with confirmed infections had >4-fold rises in anti-DSV-like salivary IgG titer. The median rises in anti-DSV-like salivary antibody titer for six NoV genogroup I-infected subjects were 17.1 for IgA and 2.7 for IgG. None of the paired saliva specimens collected from the six people with RT-PCR-negative stools showed a >4-fold rise in anti-DSV-like or anti-NV salivary IgA or IgG. These results suggest that the salivary IgA assay with the DSV-like antigen was effective for detecting a genogroup I NoV outbreak.
TABLE 4.
RT-PCR and salivary EIA results from an elementary school outbreak investigation
| RT-PCR resulta | Salivary EIA resultb
|
||
|---|---|---|---|
| NV antigen IgAc | DSV-like antigend
|
||
| IgA | IgG | ||
| NoV positive | |||
| Genogroup I (n = 7) | 2/7 (29) | 4/6 (67) | 2/6 (33) |
| Genogroup II (n = 1) | 0/1 | NT | NT |
| NoV negative (n = 6) | 0/6 | 0/4 | 0/4 |
RT-PCR results from stool specimens.
Expressed as number of specimens positive for indicated antibody/total number of specimens tested. Percentages are given in parentheses. Positivity was indicated by a >4-fold rise in salivary antibody titer between acute- and convalescent-phase samples. NT, not tested.
IgA test using NV antigen.
IgA and IgG tests using DSV-like antigen. For these data, some samples were not tested because of insufficient sample volume.
DISCUSSION
NoVs are the leading cause of acute epidemic gastroenteritis. Currently, diagnosis rests on the detection of viral RNA in stool within the first few days of symptoms or evidence of a fourfold rise in anti-NoV serum IgG in acute and convalescent-phase sera, and it is often challenging to collect satisfactory clinical specimens in outbreak investigations to confirm the cause and extent of the outbreak. Our overall goal was to develop an EIA to diagnose recent NoV infection and NoV outbreaks based on the detection of a rise in NoV-specific salivary IgA or IgG in a high proportion of subjects. With saliva specimens from volunteers in an NV challenge study, our results indicate that NV-specific IgG and IgA salivary conversion is a reliable indicator of NV infection. Our EIA for NV-specific salivary IgA and IgG had a sensitivity of 83% and a specificity of 95 to 100%. The magnitude of the rise in NV-specific salivary antibody concentrations in infected volunteers was substantial (means, 47.2 for IgG and 25.1 for IgA), although not as great as the mean rise in NV-specific serum IgG (78.1). In our application of the assay to a limited number of specimens from an elementary school outbreak, 67% of the infections could be detected by a >4-fold rise in anti-NoV IgA by use of an antigen that was in the same genetic cluster as the outbreak virus. This is the first documented mucosal antibody response to NoVs in children.
Since the 1980s, there have been a growing number of reports of the development and application of salivary antibody assays to diagnose viral infections (1, 3, 4, 6, 12, 14, 20, 29, 38, 40, 43, 46, 52). The sensitivity of our EIA for NV-specific salivary IgA and IgG conversion (83%) is similar to that reported for antirotavirus salivary IgA conversion in adult volunteers challenged with human rotavirus (75% by 13 to 27 days postchallenge) (54). Most previous salivary assays have simply detected the presence of virus-specific salivary antibodies and reported high sensitivity and specificity. However, because most of the volunteers had anti-NV salivary IgA (95%) and IgG (71%) in their baseline samples, we used our EIA to examine the rise in NV-specific salivary antibodies following infection. This approach requires paired specimens from the same individual.
Our results from the NV challenge studies on the time course of various components of the host immune response provide guidance on timing specimen collection for accurate diagnosis. We observed that the average NV-specific salivary IgA levels in infected volunteers peaked at day 14 postchallenge and decreased by day 21 (Table 3). This pattern was also observed in the salivary IgA response to rotavirus in infected adult volunteers (54). Some of our results suggested that the salivary IgA response started quite early, because six subjects had ≥4-fold rises in NV-specific salivary IgA by day 4 postchallenge. However, when we retested the specimens from three of these subjects for which there was sufficient sample volume, we found that the ratio of NV-specific salivary IgA to total salivary IgA on day 1 versus that on day 4 postchallenge indicated that there was no salivary conversion by day 4. This finding confirms a recent report (30) that, in infected volunteers, there can be an initial release of total salivary IgA in the first 5 days postchallenge but that (i) the ratio of the NV-specific IgA to total salivary IgA remains relatively constant or shows only a modest rise during this time and (ii) most of the new NV-specific salivary antibodies are made 5 to 14 days postchallenge. In contrast, NV-specific salivary and serum IgG levels continued to increase through day 21 postchallenge. NV seroconversion usually occurred around day 8 postchallenge.
Because the magnitude of the NV-specific salivary antibody increase and the timing of the peak levels differ among infected individuals (data not shown), it is not possible to diagnose an NV infection with a single sample. However, by comparing anti-NV salivary antibody titers of acute-phase samples collected as early as possible during infection to those of convalescent-phase specimens collected around 14 days postexposure, it should be possible to reliably detect the majority of NV infections by this approach. With the day 4 and 14 postchallenge saliva samples from volunteers, the sensitivity of this salivary antibody assay for detecting NV infection (78%) is better than that reported for a commercially available NoV antigen detection EIA (71.4% when six or more specimens from an outbreak were tested), and the specificity of the salivary assay (95 to 100%) is similar to that of the commercial antigen detection EIA (100% when six or more specimens from an outbreak were tested) (47).
The clinical sensitivity of the salivary antibody EIA and the overall consistency of our findings suggest that this is a robust assay despite a range in antibody levels and sample integrity. Salivary antibody levels are influenced by the flow rate of saliva (35). A variety of factors, including food ingestion, diet, sensory stimulation, drugs, smoking, stress, exercise, and degree of hydration, can influence the salivary flow and antibody concentrations (9, 48, 50, 55). Our study tested almost 200 saliva samples collected from 38 volunteers over a 2.5-year study period, and some of these samples had been stored at −20°C for up to 5 years, which may have caused some degradation (43, 51).
The evidence that salivary antibody concentrations can be influenced by many extrinsic factors may explain some of the individual fluctuations that we observed in NV-specific salivary IgA and IgG antibody concentrations over time and some of the discrepancies between anti-NV IgG seroconversion and anti-NV salivary antibody conversions. Three volunteers who showed seroconversion did not show corresponding fourfold increases in NV-specific salivary IgG, and three different volunteers who showed seroconversion did not have fourfold increases in NV-specific salivary IgA. However, if the results of both the salivary IgG and the salivary IgA conversions are combined, all of the NV-infected volunteers were correctly classified, and one volunteer who did not show seroconversion and who did not shed virus would have been incorrectly classified as infected by the NV-specific salivary IgG EIA.
Salivary antibody assays for other viruses have been used for a variety of research and surveillance applications, including hepatitis A virus outbreak investigations (4), poliovirus and rotavirus vaccine studies (6, 14), measles virus surveillance studies (3), and studies of host immune responses to viral infection (46). Salivary assays are also useful for populations in developing countries, pediatric populations, and difficult-to-serve-populations, such as intravenous drug users. From both clinical and epidemiological perspectives, diagnostic assays based on saliva or another oral fluid offer many advantages over serum-based assays (40). Collecting saliva specimens is far easier than collecting stool or serum specimens and is associated with fewer refusals by subjects to provide specimens. It is more convenient and less expensive because it is possible to train subjects to collect specimens from themselves and does not require a trained phlebotomist. In our experience, saliva collection was well accepted by healthy volunteers in clinical trials and children involved in NoV outbreaks.
The epidemiological significance of this study is that the detection of NV-specific antibodies in saliva is a reliable, noninvasive method for the diagnosis of NV infection that could be well suited for outbreak investigations and that could lead to greater recognition of NoV outbreaks. Although our knowledge of the role of NoVs in epidemic gastroenteritis has increased dramatically since the advent of RT-PCR techniques and recombinant NoV antigens, epidemic NoV infections are probably greatly underestimated, because infected persons often do not seek medical attention and are often reluctant to provide stool or serum specimens. While the incidence of individual NoV infections may be underestimated by this assay, a salivary antibody assay with a pool of representative recombinant NoV antigens should prove useful for detecting or confirming a NoV outbreak if multiple specimens are available for testing. Assays for salivary antibodies may also be useful for population-based prospective studies of endemic NoV infection. The collection of saliva specimens at 2-week intervals would capture the rapid rise and fall of NoV-specific salivary antibody titers and could be used to estimate the incidence of NoV infection in longitudinal studies. This salivary antibody assay together with analyses of histo-blood group antigens and salivary binding studies could lead to major advances in our knowledge of NoV susceptibility and infection.
Acknowledgments
This work was supported by the USDA-CSREES National Needs Fellowship Program in Food and Agricultural Sciences; the USDA-CSREES National Research Initiative Competitive Grants Program, Ensuring Food Safety Division (grant 98-35201-62161); the U.S. Environmental Protection Agency's Science to Achieve Results (STAR) program (grant R826139); NIH grant AI 65299; U.S. EPA Cooperative Agreement R82936501; the General Clinical Research Centers Program, National Institutes of Health, National Center for Research Resources (grant RR00046); and NIH grant AI 57788 to M.K.E.
We thank Erin-Joi Collins McNeal, Hannah Cluck, Deanne Rhodes, Fan-Chen Tseng, Rukmini Bagchee, and Susan Pusek for technical assistance. We are grateful to Jean-Marie Maillard and Newt MacCormack of the North Carolina Department of Health and Human Services for inviting us to assist with the outbreak investigation. Additionally, we thank John Monahan for his help with the statistical analysis of the data and Juan Leon for his thoughtful comments.
REFERENCES
- 1.Aiyar, J., M. K. Bhan, N. Bhandari, R. Kumar, P. Raj, and S. Sazawal. 1990. Rotavirus-specific antibody response in saliva of infants with rotavirus diarrhea. J. Infect. Dis. 162:1383-1384. [DOI] [PubMed] [Google Scholar]
- 2.Brinker, J. P., N. R. Blacklow, M. K. Estes, C. L. Moe, K. J. Schwab, and J. E. Herrmann. 1998. Detection of Norwalk virus and other genogroup 1 human caliciviruses by a monoclonal antibody, recombinant-antigen-based immunoglobulin M capture enzyme immunoassay. J. Clin. Microbiol. 36:1064-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, D. W., M. E. Ramsay, A. F. Richards, and E. Miller. 1994. Salivary diagnosis of measles: a study of notified cases in the United Kingdom, 1991-3. BMJ 308:1015-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bull, A. R., K. J. Kimmance, J. V. Parry, and K. R. Perry. 1989. Investigation of an outbreak of hepatitis A simplified by salivary antibody testing. Epidemiol. Infect. 103:371-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron, S. O., K. S. Wilson, T. Good, J. McMenamin, B. McCarron, A. Pithie, and R. Fox. 1999. Detection of antibodies against hepatitis C virus in saliva: a marker of viral replication. J. Viral Hepat. 6:141-144. [DOI] [PubMed] [Google Scholar]
- 6.Carlsson, B., S. Zaman, L. Mellander, F. Jalil, and L. A. Hanson. 1985. Secretory and serum immunoglobulin class-specific antibodies to poliovirus after vaccination. J. Infect. Dis. 152:1238-1244. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 2003. Norovirus activity—United States, 2002. Morb. Mortal. Wkly. Rep. 52:41-45. [PubMed] [Google Scholar]
- 8.Cuzzubbo, A. J., D. W. Vaughn, A. Nisalak, S. Suntayakorn, J. Aaskov, and P. L. Devine. 1998. Detection of specific antibodies in saliva during dengue infection. J. Clin. Microbiol. 36:3737-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawes, C. 1993. Considerations in the development of diagnostic tests on saliva. Ann. N. Y. Acad. Sci. 694:265-269. [DOI] [PubMed] [Google Scholar]
- 10.Deshpande, S. S. 1996. Assay development, evaluation, and validation, p. 275-359. In S. S. Deshpande (ed.), Enzyme immunoassays, from concept to product development. Chapman and Hall, New York, N.Y.
- 11.Emmons, W. W. 1997. Accuracy of oral specimen testing for human immunodeficiency virus. Am. J. Med. 102(4A):15-20. [DOI] [PubMed] [Google Scholar]
- 12.Friedman, M. G. 1981. Salivary IgA antibodies to mumps virus during and after mumps. J. Infect. Dis. 143:617. [DOI] [PubMed] [Google Scholar]
- 13.Friedman, M. G. 1982. Radioimmunoassay for the detection of virus-specific IgA antibodies in saliva. J. Immunol. Methods 54:203-211. [DOI] [PubMed] [Google Scholar]
- 14.Friedman, M. G., B. Segal, R. Zedaka, B. Sarov, M. Margalith, R. Bishop, and R. Dagan. 1993. Serum and salivary responses to oral tetravalent reassortant rotavirus vaccine in newborns. Clin. Exp. Immunol. 92:194-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallo, D., J. R. George, J. H. Fitchen, A. S. Goldstein, and M. S. Hindahl. 1997. Evaluation of a system using oral mucosal transudate for HIV-1 antibody screening and confirmatory testing. JAMA 277:254-258. [PubMed] [Google Scholar]
- 16.Glass, R. I., J. Noel, T. Ando, R. Fankhauser, G. Belliot, A. Mounts, U. D. Parashar, J. S. Bresee, and S. S. Monroe. 2000. The epidemiology of enteric caliciviruses from humans: a reassessment using new diagnostics. J. Infect. Dis. 181(Suppl. 2):S254-S261. [DOI] [PubMed] [Google Scholar]
- 17.Graham, D. Y., X. Jiang, T. Tanaka, A. R. Opekun, H. P. Madore, and M. K. Estes. 1994. Norwalk virus infection of volunteers: new insights based on improved assays. J. Infect. Dis. 170:34-43. [DOI] [PubMed] [Google Scholar]
- 18.Gray, J. J., X. Jiang, P. Morgan-Capner, U. Desselberger, and M. K. Estes. 1993. Prevalence of Norwalk virus antibodies in England: detection by enzyme-linked immunosorbent assay using baculovirus-expressed Norwalk virus capsid antigen. J. Clin. Microbiol. 31:1022-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Health Protection Agency. 2002. Norovirus (Norwalk-like virus) increase in 2002. CDR Wkly. Vol. 12, no. 51. [Online.] http:www.hpa.org.uk/cdr/PDFfiles/2002/cdr5102.pdf.
- 19.Herremans, M. M. P. T., A. M. van Loon, J. H. J. Reimerink, H. C. Rümke, H. G. A. M. van der Avoort, T. G. Kimman, and M. P. G. Koopmans. 1997. Poliovirus-specific immunoglobulin A in persons vaccinated with inactivated poliovirus vaccine in The Netherlands. Clin. Diagn. Lab. Immunol. 4:499-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herremans, T. M., J. H. Reimerink, A. M. Buisman, T. G. Kimman, and M. P. Koopmans. 1999. Induction of mucosal immunity by inactivated poliovirus vaccine is dependent on previous mucosal contact with live virus. J. Immunol. 162:5011-5018. [PubMed] [Google Scholar]
- 21.Jaykus, L.-A. 2000. Detection of human enteric viruses in foods, p. 137-163. In Y. H. Hui, S. A. Sattar, K. D. Murrell, W.-K. Nip, and P. S. Stanfield (ed.), Foodborne disease handbook, vol. 2. Viruses, parasites, and HACCP, 2nd ed. Marcel Dekker, Inc., New York, N.Y. [Google Scholar]
- 22.Jiang, X., D. Y. Graham, K. Wang, and M. K. Estes. 1990. Norwalk virus genome cloning and characterization. Science 250:1580-1583. [DOI] [PubMed] [Google Scholar]
- 23.Jiang, X., M. Wang, D. Y. Graham, and M. K. Estes. 1992. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J. Virol. 66:6527-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang, X., M. Wang, K. Wang, and M. K. Estes. 1993. Sequence and genomic organization of Norwalk virus. Virology 195:51-61. [DOI] [PubMed] [Google Scholar]
- 25.Jiang, X., W. D. Cubitt, T. Berke, W. Zhong, X. Dai, S. Nakata, L. K. Pickering, and D. O. Matson. 1997. Sapporo-like human caliciviruses are genetically and antigenically diverse. Arch. Virol. 142:1813-1827. [DOI] [PubMed] [Google Scholar]
- 26.Johnson, P. C., J. J. Mathewson, H. L. Dupont, and H. B. Greenberg. 1990. Multiple-challenge study of host susceptibility to Norwalk gastroenteritis in US adults. J. Infect. Dis. 161:18-21. [DOI] [PubMed] [Google Scholar]
- 27.Kapikian, A. Z., M. K. Estes, and R. M. Chanock. 1996. Norwalk group of viruses, p. 783-810. In B. N. Fields, D. M. Knipe, P. M. Howley, et al. (ed.), Virology, 3rd ed. Raven Press, New York, N.Y.
- 28.Lambden, P. R., E. O. Caul, C. R. Ashley, and I. N. Clarke. 1993. Sequence and genome organization of a human small round-structured (Norwalk-like) virus. Science 259:516-519. [DOI] [PubMed] [Google Scholar]
- 29.Lamey, P. J., A. Nolan, E. A. Follett, I. Coote, T. W. MacFarlane, D. H. Kennedy, A. Connell, and J. V. Parry. 1996. Anti-HIV antibody in saliva: an assessment of the role of the components of saliva, testing methodologies and collection systems. J. Oral Pathol. Med. 25:104-107. [DOI] [PubMed] [Google Scholar]
- 30.Lindesmith, L., C. Moe, S. Marionneau, N. Ruvoen, X. Jiang, L. Lindblad, P. Stewart, J. LePendu, and R. Baric. 2003. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 9:548-553. [DOI] [PubMed] [Google Scholar]
- 31.Lopman, B. A., M. H. Reacher, Y. Van Duijnhoven, F. X. Hanon, D. Brown, and M. Koopmans. 2003. Viral gastroenteritis outbreaks in Europe, 1995-2000. Emerg. Infect. Dis. 9:90-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madsen, R. D., A. P. Witheiler, S. R. Coates, and D. F. Rippe. 1983. Enzygnost-Rubella: an enzyme immunoassay for screening and quantitation of antibodies to rubella virus. Am. J. Clin. Pathol. 79:206-210. [DOI] [PubMed] [Google Scholar]
- 33.Malamud, D. 1997. Oral diagnostic testing for detecting human immunodeficiency virus-1 antibodies: a technology whose time has come. Am. J. Med. 102(4A):9-14. [DOI] [PubMed] [Google Scholar]
- 34.Matsui, S. M., and H. B. Greenberg. 2000. Immunity to calicivirus infection. J. Infect. Dis. 181(Suppl. 2):S331-S335. [DOI] [PubMed] [Google Scholar]
- 35.Miletic, I. D., S. S. Schiffman, V. D. Miletic, and E. A. Sattely-Miller. 1996. Salivary IgA secretion rate in young and elderly persons. Physiol. Behav. 60:243-248. [DOI] [PubMed] [Google Scholar]
- 36.Monroe, S. S., S. E. Stine, X. Jiang, M. K. Estes, and R. I. Glass. 1993. Detection of antibody to recombinant Norwalk virus antigen in specimens from outbreaks of gastroenteritis. J. Clin. Microbiol. 31:2866-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakata, S., S. Honma, K. Numata, K. Kogawa, S. Ukae, N. Adachi, X. Jiang, M. K. Estes, Z. Gatheru, P. M. Tukei, and S. Chiba. 1998. Prevalence of human calicivirus infections in Kenya as determined by enzyme immunoassays for three genogroups of the virus. J. Clin. Microbiol. 36:3160-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochnio, J. J., D. W. Scheifele, M. Ho, and L. A. Mitchell. 1997. New, ultrasensitive enzyme immunoassay for detecting vaccine- and disease-induced hepatitis A virus-specific immunoglobulin G in saliva. J. Clin. Microbiol. 35:98-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okhuysen, P. C., X. Jiang, L. Ye, P. C. Johnson, and M. K. Estes. 1995. Viral shedding and fecal IgA response after Norwalk virus infection. J. Infect. Dis. 171:566-569. [DOI] [PubMed] [Google Scholar]
- 40.Parry, J. V. 1993. Simple and reliable salivary tests for HIV and hepatitis A and B virus diagnosis and surveillance. Ann. N. Y. Acad. Sci. 694:216-233. [DOI] [PubMed] [Google Scholar]
- 41.Parry, J. V., K. R. Perry, and P. P. Mortimer. 1987. Sensitive assays for viral antibodies in saliva: an alternative to tests on serum. Lancet ii:72-75. [DOI] [PubMed] [Google Scholar]
- 42.Parry, J. V., K. R. Perry, S. Panday, and P. P. Mortimer. 1989. Diagnosis of hepatitis A and B by testing saliva. J. Med. Virol. 28:255-260. [DOI] [PubMed] [Google Scholar]
- 43.Perry, K. R., D. W. Brown, J. V. Parry, S. Panday, C. Pipkin, and A. Richards. 1993. Detection of measles, mumps, and rubella antibodies in saliva using antibody capture radioimmunoassay. J. Med. Virol. 40:235-240. [DOI] [PubMed] [Google Scholar]
- 44.Pether, J. V., and E. O. Caul. 1983. An outbreak of food-borne gastroenteritis in two hospitals associated with a Norwalk-like virus. J. Hyg. 91:343-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plikaytis, B. D., S. H. Turner, L. L. Gheesling, and G. M. Carlone. 1991. Comparisons of standard curve-fitting methods to quantitate Neisseria meningitidis group A polysaccharide antibody levels by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 29:1439-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramachandran, M., A. Vij, R. Kumar, B. K. Das, J. R. Gentsch, M. K. Bhan, and R. I. Glass. 1998. Lack of maternal antibodies to P serotypes may predispose neonates to infections with unusual rotavirus strains. Clin. Diagn. Lab. Immunol. 5:527-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richards, A. F., B. Lopman, A. Gunn, A. Curry, D. Ellis, H. Cotterill, S. Ratcliffe, M. Jenkins, H. Appleton, C. I. Gallimore, J. J. Gray, and D. W. Brown. 2003. Evaluation of a commercial ELISA for detecting Norwalk-like virus antigen in faeces. J. Clin. Virol. 26:109-115. [DOI] [PubMed] [Google Scholar]
- 48.Rider, M. S., J. Achterberg, G. F. Lawlis, A. Goven, R. Toledo, and J. R. Butler. 1990. Effect of immune system imagery on secretory IgA. Biofeedback Self-Regul. 15:317-333. [DOI] [PubMed] [Google Scholar]
- 49.Riepenhoff-Talty, M., H. J. Barrett, B. A. Spada, and P. L. Ogra. 1983. Negative staining and immune electron microscopy as techniques for rapid diagnosis of viral agents. Ann. N. Y. Acad. Sci. 420:391-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stone, A. A., D. S. Cox, H. Valdimarsdottir, L. Jandorf, and J. M. Neale. 1987. Evidence that secretory IgA antibody is associated with daily mood. J. Pers. Soc. Psychol. 52:988-993. [DOI] [PubMed] [Google Scholar]
- 51.Tencer, J., H. Thysell, K. Andersson, and A. Grubb. 1994. Stability of albumin, protein HC, immunoglobulin G, kappa- and lambda-chain immunoreactivity, orosomucoid and alpha 1-antitrypsin in urine stored at various conditions. Scand. J. Clin. Lab. Investig. 54:199-206. [DOI] [PubMed] [Google Scholar]
- 52.Thieme, T., P. Yoshihara, S. Piacentini, and M. Beller. 1992. Clinical evaluation of oral fluid samples for diagnosis of viral hepatitis. J. Clin. Microbiol. 30:1076-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vyse, A. J., D. W. G. Brown, B. J. Cohen, R. Samuel, and D. J. Nokes. 1999. Detection of rubella virus-specific immunoglobulin G in saliva by an amplification-based enzyme-linked immunosorbent assay using monoclonal antibody to fluorescein isothiocyanate. J. Clin. Microbiol. 37:391-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ward, R. L., K. A. Pax, J. R. Sherwood, E. C. Young, G. M. Schiff, and D. I. Bernstein. 1992. Salivary antibody titers in adults challenged with a human rotavirus. J. Med. Virol. 36:222-225. [DOI] [PubMed] [Google Scholar]
- 55.Watson, R. R., D. N. McMurray, P. Martin, and M. A. Reyes. 1985. Effect of age, malnutrition and renutrition on free secretory component and IgA in secretions. Am. J. Clin. Nutr. 42:281-288. [DOI] [PubMed] [Google Scholar]