Abstract
Recurrent or chronic adenotonsillar infections mainly affect children and frequently involve otherwise healthy subjects. Therefore, having excluded systemic immunological deficiencies, this disease may be due to a local dysfunction of the epithelial structures at either the rhino or oropharyngeal level. The aim of the present investigation was to analyze structural and immunological aspects of tonsils and adenoids in subjects who underwent adenotonsillectomy because of recurrent inflammatory episodes with fever. Histological studies and analyses of the cytokine patterns were carried out in palatine tonsils and adenoid samples from 105 patients who underwent adenoidectomy and bilateral extracapsular tonsillectomy for chronic inflammatory hypertrophy of these organs; 46 of the 105 cases examined presented hyperkeratosis of the crypt epithelium; in the remaining 59, the epithelium was hyperplastic with no signs of keratosis. Scanning electron microscopy revealed a continuous epithelial surface of polygon-shaped flattened cells with fissures towards the cryptic depressions. Titration of interleukin-1β and tumor necrosis factor alpha in serum and tissues demonstrated higher concentrations in the adenotonsillar specimens, whereas the rise in interleukin-6 was more modest.
There are still some controversies among specialists in internal medicine, pediatrics, and otorhinolaryngologists concerning the diagnostic and therapeutic approach to recurrent and chronic inflammatory conditions of adenoids and tonsils in children.
Over the last few decades, immunobiological techniques have allowed the identification of tonsillar cells responsible for inflammatory immune reactions (2, 5). Recurrent or chronic adenotonsillar infections mainly affect children and frequently involve otherwise healthy subjects. Therefore, having excluded systemic immunological deficiencies, this disease may be due to a local dysfunction of the epithelial structures, at either the rhino or oropharyngeal level.
Several authors (4, 6, 8) have tried to explain why and how a modification in the balance between the local immunological function of the host and the infectious agents would lead to a clinical process characterized by recurrent inflammatory events.
The complex histological configuration of the parenchyma is fundamental for the uptake and presentation of antigens to the subepithelial immunocompetent cells. This allows the whole organ to act as a functional unit and hence to play an important role in fighting microorganisms. It may therefore be hypothesized that persistent local inflammatory reactions in adenotonsillitis may, with time, lead to histomorphological changes and functional deficiencies in defense barriers (3).
The aim of the present investigation was to analyze structural and immunological aspects of tonsils and adenoids in subjects who underwent adenotonsillectomy because of recurrent inflammatory episodes with fever.
MATERIALS AND METHODS
Patients.
We selected 105 children affected by chronic inflammatory hypertrophy of palatine tonsils and adenoids which had not responded to previous medical treatments; 54 males and 51 females, aged between 4 and 18 years, were enrolled in the study.
All patients underwent adenoidectomy and bilateral extracapsular tonsillectomy, under general anesthesia. Signed written consent was obtained from all patients.
Histological investigations.
Each tonsil was divided into three parts. These were prepared for study under the light microscope as well as the scanning electron microscope and for immunohistochemical studies.
Light microscopy.
Specimens were fixed in 10% buffered formalin (phosphate buffer according to Lillie), at room temperature, for 24 h, and then fixed in paraffin and processed according to routine techniques of light microscopy.
Slices 5 to 8 μm thick were stained with hematoxylin and eosin, Azan Mallory trichrome stain, and Gomori silver stain.
Scanning electron microscopy.
Specimens were fixed in 2.5% aldehyde glutaraldehyde (in phosphate buffer according to Sorensen at 4°C) for 48 h.
These were then rinsed in phosphate buffer for 30 min and in running water for 90 min, at room temperature. Postfixation was carried out with OsO4 (1.33%), for 2 h, with further rinsing in phosphate buffer for 20 min. This was followed by dehydration in an increasing series of alcohol solutions and critical point drying by means of six cycles of 10 min each.
Once metallization in gold had been carried out by the Edwards Sputtering method, the samples were observed under the scanning electron microscope (Cambridge 250 Stereoscan) at 20 kV.
Immunohistochemistry.
The 5-μm sections were processed according to the peroxidase-antiperoxidase method for the detection of immunoglobulin E, immunoglobulin A, and J chains. Inhibition of endogenous peroxidase was performed prior to dehydration, for 5 min at room temperature, by means of a hydrogen peroxide solution at 3% in methanol. Following dehydration, trypsinization was carried out for 5 min in an aqueous solution of trypsin at 0.1% and calcium chloride at 0.4%.
To detect J chains, the sections were subjected to preincubation in a 0.4 M urea solution, at pH 5.2, for 1 h at 4°C. This was followed by immunohistochemical detection by means of the peroxidase-antiperoxidase method, with polyclonal antibodies (rabbit anti-human immunoglobulin A, Ortho code 00460; rabbit anti-human immunoglobulin G, Ortho code 00461; and J chain, Ortho code 00453).
Nuclear contrast staining was then carried out with Mayer hematoxylin for 2 min. The samples were then mounted in an aqueous medium and observed under a Zeiss FO-M12 photomicroscope.
Cytokine and immunoglobulin detection.
In a randomly selected group of 10 children, 1 g of adenoid and 1 g of tonsil tissue, homogenized in 2 ml of physiological solution, were centrifuged at 3,000 rpm, for 10 min.
Several kinds of immunoglobulins were assayed simultaneously with a simple radial immunodiffusion technique (LC-Partigen, Boehring, Germany).
Levels of interleukin-1β, interleukin-6, and tumor necrosis factor alpha were determined in the supernatant, with a quantitative enzyme-linked immunosorbent assay (Quantikine; R and D Systems, Minneapolis, Minn.). The same method was also applied to the serum. The lowest detectable values in the standard curve, according to the manufacturer, were as follows: interleukin-1β, 4 pg/ml; tumor necrosis factor alpha, 12 pg/ml; and interleukin-6, 3 pg/ml.
Detection of interleukin-2 and interleukin-4 was carried out by determining the mRNA with reverse transcription-PCR (RT-PCR) (7).
Total RNA was extracted from adenotonsillar cell homogenates with organic solvents such as guanidine, isocyanate, and phenol-chloroform. Reverse transcription was then carried out with Moloney murine leukemia virus reverse transcriptase (8).
Clontech products (Advantage RT for PCR kit) were employed for the reverse transcriptase reaction. The reverse transcriptase specimens were incubated at 37°C for 30 min. The cDNA so obtained was subjected to amplification cycles, with the specific primers for the PCR, in a DNA thermal cycler (Perkin-Elmer Cetus, Norwalk, Conn.). The amplification conditions were as follows: 94°C for 1 min, 58°C for 45 s, and 72°C for 1 min, for a total of 35 cycles. Clontech and Promega products were used for this reaction.
The amplification products for specific interleukin-2 and interleukin-4 were subjected to electrophoresis on a 3% agarose gel stained with ethyl bromide and revealed by means of transillumination under UV rays.
RESULTS
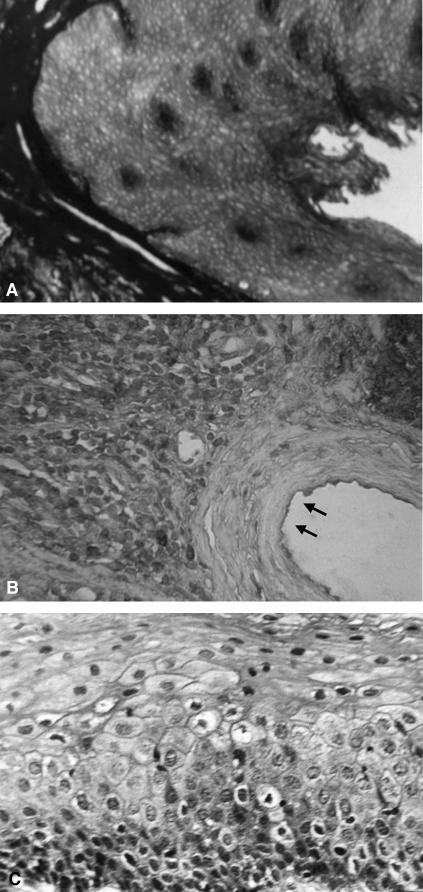
Out of 105 patients examined, 46 presented hyperkeratosis of the epithelium in the bottom of the crypt (Fig. 1). This pattern was present throughout the tonsil tissue. In the other 59 cases, the epithelium presented a hyperplastic appearance, without any clear signs of keratosis.
FIG. 1.
(A) Normal tonsillar crypt. (B) Hematoxylin-eosin staining of chronically inflamed human tonsils. Hyperkeratosis of the epithelium is detectable in the bottom of the crypts (arrows). (C) Hematoxylin-eosin staining of chronically inflamed human tonsils. Hyperkeratosis of the cryptic epithelium (higher magnification).
Follicular hyperplasia was consistently present and was more evident in the germinal centers; since the plasma cells are found mainly in the cryptoreticular area, the hyperplasia appeared to be due to proliferation of B lymphocytes. A significant hyperplasia of the mantle zone was observed only in 10 cases.
Furthermore, immunoglobulin G-positive B lymphocytes were present in all specimens mainly in the germinal center, with only a few found in the follicular mantle.
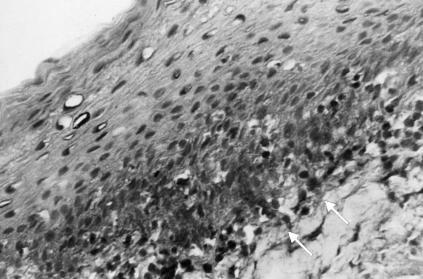
Immunoglobulin A-positive B lymphocytes were observed in 43% of the samples and were mainly localized close to the cryptoreticular epithelium, whereas only a few were detected within the germinal centers (Fig. 2).
FIG. 2.
Immunohistochemical staining for immunoglobulin A in chronically inflamed human tonsils. Immunoglobulin A-positive B lymphocytes are mainly localized close to the cryptoreticular epithelium (arrows).
In 29 of 105 cases examined (27.6%), B lymphocytes with intracytoplasmic staining for the J chain were detected. T lymphocytes, on the other hand, were present in all the samples examined and were localized primarily in the mantle zone of the lymphatic follicles between the crypt fundus and the germinal center. Only in a few samples were T lymphocytes found in the extrafollicular area.
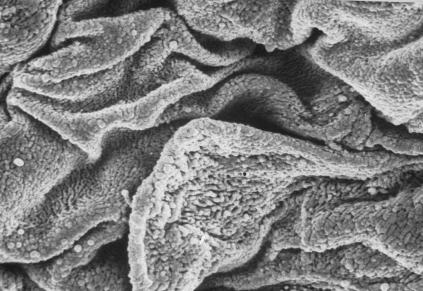
Scanning electron microscopy revealed a continuous epithelial surface of polygon-shaped flattened cells, with fissures towards the cryptic depressions.
With higher magnification, the cell surfaces presented an irregular appearance due to the presence of numerous small folds (Fig. 3).
FIG. 3.
Scanning electron microscopy of chronically inflamed human tonsils. Polygonal flattened cells presenting fissures tending towards the cryptic depressions are easily identifiable. Cell surfaces are irregular because of numerous small folds.
The cell edges within the crypt were raised, with small superficial folds, and the bottom of the crypts showed a raised, dome-shaped epithelium, the diameter of which ranged between 10 and 30 μm.
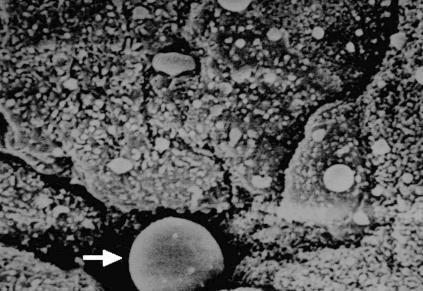
In some crypts, apart from disseminated epithelial cells present in various amounts, other cellular elements were also visible on the fundus (plasma cells and lymphocytes) (Fig. 4). No M cells were detected in the tonsils or on the surface of the crypts.
FIG. 4.
Scanning electron microscopy of chronically inflamed human tonsils. Plasma cells, lymphocytes and disseminated epithelial cells were visible on the fundus of crypts (arrow).
The interleukin-1β concentration was raised in all the specimens studied (mean value ± standard deviation: tonsils, 258.5 ± 16.8 pg/ml; adenoids, 187.6 ± 88.7 pg/ml) compared with the serum level (6.5 ± 3.6 pg/ml) (P < 0.0001) (Table 1).
TABLE 1.
Concentrations of cytokines in tonsils, adenoids, and serum
| Sample | Mean concn (pg/ml) ± SD
|
||
|---|---|---|---|
| Interleukin-1β | Interleukin-6 | Tumor necrosis factor alpha | |
| Tonsils | 258.5 ± 16.8 | 27.9 ± 15.3 | 138.7 ± 49.7 |
| Adenoids | 187.6 ± 88.7 | 9.2 ± 3.7 | 142.3 ± 42.9 |
| Serum | 6.5 ± 3.6 | 2.1 ± 1.2 | 2.9 ± 2.0 |
The concentrations of interleukin-6 were also raised compared with the plasma level (2.1 ± 1.2 pg/ml), the mean values (± standard deviation) being 27.9 ± 15.3 pg/ml in tonsil tissue and 9.2 ± 3.7 pg/ml in adenoid tissue; the difference between the two tissues and plasma was statistically significant (P < 0.0001) (Table 1).
The concentrations of tumor necrosis factor alpha were significantly raised in both homogenates (serum level, 2.9 ± 2.0 pg/ml), the mean values (± standard deviation) being 138.7 ± 49.7 pg/ml and 142.3 ± 42.9 pg/ml in tonsils and adenoids, respectively (P < 0.0001) (Table 1).
mRNA for interleukin-2 and interleukin-4 was detected only in two cases.
DISCUSSION
Whereas chronic inflammation of palatine tonsils in children usually leads to tissue hyperplasia and hypertrophy, in adults it is associated with sclerosis of the above-mentioned structures.
Histological analyses of the epithelial component demonstrated the presence, in the base of the crypts, of a constant hyperplasia and, in 44% of cases, of a hyperparakeratosis.
This observation gave rise to the hypothesis that epithelial modification caused impaired antigen uptake and this, in turn, led to the recurrence of inflammatory processes with an increasingly severe hyperparakeratosis, triggering a vicious circle for which tonsillectomy was the only curative solution.
Immunohistochemical examination revealed a persistent hyperplasia of B lymphocytes (often immunoglobulin G positive), which were more evident in the germinal centers, and a rather constant decrease in the T-cell compartment.
The observation of a negative J chain in 73% of the specimens would appear to confirm that chronic inflammation of the Waldeyer ring structures results in the inhibition or in a decrease in the production of secretory immunoglobulin A. This is not unexpected inasmuch as it is correlated with changes in the glandular epithelium responsible for the production of the secretory fragment. The modifications observed under scanning electron microscopy may be due to deregulation of the normal intercellular connections that, in turn, may be caused by the intraepithelial infiltration of the lymphocytes originating from the germinal centers below.
Our data do not support the hypothesis that M cells exist at the level of the reticular crypt epithelium, which act either as an antigen-presenting cell or as a carrier of foreign particles (9). We did not find any differentiated M cells.
The identification of high tissue concentrations of tumor necrosis factor alpha, interleukin-1β, and interleukin-6 is an expression of local hyperproduction or overproduction, due to monocyte-macrophage activation, caused by repeated stimulation by the pathogenic agents. The presence of high levels of these cytokines, nevertheless, instead of inducing an increase in immunological efficiency, may have a damaging effect on these mechanisms in the long term. These mediators, in fact, induce the activation and proliferation of endothelial cells and fibroblasts, which may result, with time, in the progressive replacement of immunologically active tissue with fibrotic tissue.
The first sign of a deficit in the activation of the immune system could be represented by the small quantity of mRNAs for interleukin-2 and interleukin-4 detected in our population, suggesting a defective activation of Th1 and Th2 lymphocytes.
The results from this study offer the opportunity for a series of extremely promising investigations even if the mechanisms responsible for chronic inflammation in some subjects and the persistence, in others, of an immunological equilibrium remain to be clarified.
REFERENCES
- 1.Alms, W. J., L. B Elwert, and B. White. 1996. Simultaneous quantification of cytokine mRNAs by reverse transcriptase-polymerase chain reaction using multiple internal standard cRNAs. Diagn. Mol. Pathol. 5:88-97. [DOI] [PubMed] [Google Scholar]
- 2.Awaya, K. 1969. The aging of secondary nodules in the relation of the postnatal development and involution of the human palatine tonsils. Acta Haematol. Jpn. 32:127-134. [PubMed] [Google Scholar]
- 3.Bellussi, L., S. Norscini, E. Gaudio, and D. Passali. 1992. Modificazioni istomorfologiche indotte da flogosi tonsillari ricorrenti. Acta Otorinol. Ital. 12:95-106. [PubMed] [Google Scholar]
- 4.Bernstein, J. M., S. Sendor, and J. Wactawski-Wende. 1992. Antigen-presenting cells in the nasopharyngeal tonsil. A quantitative immunohistochemical study, p. 80-90. In G. B. Galioto (ed.), Tonsils: a clinical oriented update. Karger, Basel, Switzerland. [DOI] [PubMed]
- 5.Brandtzaeg, P. 1992. Humoral immune response patterns of human mucosae: induction and relation to bacterial respiratory tract infections. J. Infect. Dis. 165:S167-S176. [DOI] [PubMed] [Google Scholar]
- 6.Brandtzaeg, P., L. Surjan, and P. Berdal. 1987. Immunoglobulin E systems of human tonsils. Control subjects of various ages: quantification of immunoglobulin -producing cells, tonsillar morphometry and serum immunoglobulin concentrations. Clin. Exp. Immunol. 31:367-372. [PMC free article] [PubMed] [Google Scholar]
- 7.Chomczynski, P., and N. Sacchi. 1987. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 8.Flanagan, J. G., and T. H. Rabbits. 1982. Arrangement of human immunoglobulin heavy chain constant region genes implies evolutionary duplication of a segment containing y, e, and a gene. Nature 300:709-713. [DOI] [PubMed] [Google Scholar]
- 9.Gallo, O., D. Bani, L. Rucci, and O. Fini-Storchi. 1991. Does the epithelium play a central role in the immune function of rhinopharyngeal tonsils? An immunocytometrical and ultrastructural study. Int. J. Pediatr. Otorhinolaryngol. 22:219-229. [DOI] [PubMed] [Google Scholar]