Abstract
Mammals that possess elaborate antipredator defences such as body armour, spines and quills are usually well protected, intermediate in size, primarily insectivorous and live in simple open environments. The benefits of such defences seem clear and may relax selection on maintaining cognitive abilities that aid in vigilance and predator recognition, and their bearers may accrue extensive production and maintenance costs. Here, in this comparative phylogenetic analysis of measurements of encephalization quotient and morphological defence scores of 647 mammal species representing nearly every order, we found that as lineages evolve stronger defences, they suffer a correlated reduction in encephalization. The only exceptions were those that live in trees—a complex three-dimensional world probably requiring greater cognitive abilities. At the proximate level, because brain tissue is extremely energetically expensive to build, mammals may be trading off spending more on elaborate defences and saving by building less powerful brains. At the ultimate level, having greater defences may also reduce the need for advanced cognitive abilities for constant assessment of environmental predation risk, especially in simple open environments.
Keywords: armour, spines, quills, encephalization quotient, brain size, spray
1. Introduction
Much attention has been paid to energetic trade-offs involved in mammalian encephalization and the variation in relative sizes of brains. Brains are metabolically expensive [1,2] owing to the implications for cognitive and behavioural performance, which have been demonstrated empirically in artificial selection experiments [3,4]. While many studies have examined traits that might favour increased encephalization and cognition (e.g. sociality, extractive foraging and diet) especially in primates, few focus on traits that might favour decephalization (reduction in relative brain size). Natural selection undoubtedly acts on brain size in a myriad of ways, and no single (or even a few) factors can explain the majority of variation in brain size across all mammals. In this study, however, we explore one such factor, demonstrating an evolutionary correlation between reductions in brain size and the evolution of elaborate morphological defences in mammals, and we propose two hypotheses that may explain why it exists.
All organisms face energetic trade-offs: allocating more energy to the construction and maintenance of one structure or function at the expense of another. For example, the horns produced for sexual combat by male dung beetles come at a cost of reductions in neighbouring structures (antennae, eyes or wings) [5]. Across mammals, the positive relationship between relative brain size and basal metabolic rate is statistically significant but weak [6]. The ‘expensive brain hypothesis’ [2,7], however, simply predicts that species that invest more in larger brains compensate by reducing energy allocation to some other energetically expensive function or structure in the body (e.g. muscle mass, fat storage and gonads), and some correlative evidence supports this [8,9]. Some mammals construct and maintain specialized morphological defences and body armour (e.g. armadillos, porcupines and echidnas); given that these structures have greater mass than a thin coat of fine hair, we can assume that they come with greater energetic costs of production, maintenance and carrying/bearing these structures during locomotion [10–12] (pangolin scales may comprise up to one-third of their entire body mass) that must be repaid somehow. One way to repay this debt would be through a reduction in brain size.
A variety of defences have evolved particularly in insectivorous mammals of intermediate size living in exposed habitats, including body armour, spines, quills and noxious sprays [12]. These armoured species, however, also have lower metabolic rates than non-armoured counterparts, who frequently rely on rapid escape to avoid predation (e.g. saltation: [10]). At the ultimate level of investigation, therefore, defended species living in simple terrestrial environments, while they may flee as a first option, may be able to afford to be less vigilant of their surroundings while foraging and rely on their defence to deter predators during close-up confrontations. This may relax selection favouring expensive, high-powered brains to, for example, detect, recognize and assess predator motivation or to navigate complex environments as many arboureal species do (e.g. Primates). In fact, some arboureal and aquatic mammals appear to show an increase in relative brain size [13], possibly as result of the need to navigate in three dimensions (3D) or remember the location of valuable food/fruit trees. Similarly, bats that forage in open habitats have undergone a reduction in brain size, while brain size in bats that feed in more complicated environments increased, suggesting that a bigger brain is needed for the ability to manoeuver in a dense 3D habitat [14].
There is great debate about how best to measure intelligence and cognitive ability in vertebrates with examples including the relative sizes of the whole brain or the cortex, degree of encephalization, number of cortical neurons or conduction velocity [15]. To conduct a large-scale comparative study of the evolution of intelligence and defences across hundreds of species of mammals, we are limited to measures for which data are available and comparable across most species, which in this case includes whole brain size and encephalization. Encephalization quotient (EQ) quantifies a species' brain mass relative to its body size using an allometric equation, which makes it useful for cross-group comparisons [16]. Species with high EQs have a brain mass higher than expected for its body mass and are predicted to be more intelligent or have greater cognitive capacity (but see [13,15]). For example, humans have an EQ of 7.4–7.8, meaning that the human brain is seven times bigger than would be expected from our body mass [15]. A recent study used comparative analyses to provide adaptive explanations for the evolution of encephalization in mammals: increased encephalization in odontocete whales, carnivores and primates correlates with emergent social complexity and decephalization in folivorous primates to allow for reallocation of energy to build and maintain a larger gut [17]. Finally, Benson-Amram et al. [18] found that relative brain size was a reliable predictor of the ability of mammalian carnivores in problem solving using a puzzle box.
Both the ‘expensive tissue’ and ‘relaxed selection’ hypotheses predict that as mammal lineages evolve more elaborate, robust defences (e.g. spines, quills, armour and sprays), there will be a correlated reduction in relative brain size. We aimed here to test whether this relationship actually exists and discuss its prevalence and origins in each defended taxon. We conducted a large-scale comparative phylogenetic study of the evolution of EQ and defensive ability across mammals in 647 species for which we found published data on brain size [17] or for which we were able to measure brain size directly from museum specimens; we used previously published quantitative scores of defensive ability, diet and habitat openness [12]. Every terrestrial mammal order is represented in the dataset (electronic supplementary material, table S1; i.e. Pinnipedia, Cetacea, Sirenia were not included) and every morphologically defended mammalian taxon is represented.
2. Material and methods
(a). Data collection
The data for this study consisted of brain mass and body mass data of 602 species from the existing published literature [17,19–23] and direct measurements taken from post-mortem museum specimens. We only included published data for species with a defence score from [12]. Using only intact skulls, we measured the cranial capacity of 198 specimens across 45 species from the mammal collections at California State University, Long Beach, the Natural History Museum of Los Angeles County and the National Museum of Natural History of the Smithsonian Institution (electronic supplementary material, table S1). We attempted to measure at least five female specimens and five male specimens of each species and took the mean of the measurements to get an average cranial capacity for the species, but key species to this study with fewer specimens were measured as well. No zoo specimens or domesticated species were included. To measure cranial capacity, we poured 3 mm glass beads into the crania through the foramen magnum and then measured the volume of the beads to the nearest millilitre using a graduated cylinder. We converted this volume to mass (g) by multiplying the volume by 1.036 g ml−1 [17]. In all, we collected brain mass data on 647 species for our analyses. We used previously published continuous defence scores from [12]. Continuous defence scores more accurately reflect the true degree of defence that these morphologies provide than a discrete (0/1) score would provide (e.g. do we call spiny rats with their sharp but flexible spines as defended as an armadillo or as weak as a mouse?) and we argue that using a continuous predictive factor to explain variation in an obviously continuous response trait (EQ) is most appropriate. Defence scores are on a scale of 0–48 and are calculated based on sub scores of spine length (1–3), spine thickness (1–4), the amount of the body covered by spines or armour (body: 0–3, head: 0/1, tail 0/1), and whether the species can further protect itself by rolling into a ball (0/1). We used their ‘defence score 1’, which for species with spines and quills was 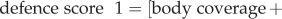
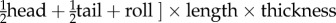 . For species with plates/scales that lack length and thickness scores, the equation was
. For species with plates/scales that lack length and thickness scores, the equation was 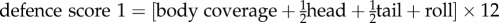 . For Carnivores with defensive sprays, we grouped them into four categories (0: does not use noxious odour in defence; 1: able to secrete/ooze foul anal secretions when alarmed; 2: able to squirt noxious secretions in a stream; 3: able to aim a stream of noxious secretions at the predator) and multiplied the category number by 16 so the best sprayers received a score of 48 and other levels of spraying were spread evenly across the defence scale. Additionally, we scored each species by defence type (0 = no defence, 1 = spines or quills, 2 = noxious spray, 3 = body armour).
. For Carnivores with defensive sprays, we grouped them into four categories (0: does not use noxious odour in defence; 1: able to secrete/ooze foul anal secretions when alarmed; 2: able to squirt noxious secretions in a stream; 3: able to aim a stream of noxious secretions at the predator) and multiplied the category number by 16 so the best sprayers received a score of 48 and other levels of spraying were spread evenly across the defence scale. Additionally, we scored each species by defence type (0 = no defence, 1 = spines or quills, 2 = noxious spray, 3 = body armour).
We also used previously published diet and habitat openness scores from [12]: species that were scored as either primarily insectivorous (1) or not primarily insectivorous (0) based on dietary data from [24], and habitat openness was calculated on a scale from 0 to 1 based on what types of habitats species were found in [25] and an openness score (0–1) for each habitat type.
(b). Encephalization quotient
To calculate EQ, following Boddy et al. [17] we log-transformed both brain mass and body mass and ran a linear regression analysis of the latter on the former, from which we obtained the slope (0.746) and y-intercept (−1.262), which were nearly identical to those obtained by Boddy et al. [17]. Based on the allometric formula described by Boddy et al. [17], E = kPα, where E = brain mass, P = body mass, k = 10y-intercept, and the allometric exponent α = slope, we were able to use the formula EQ = brain mass/(0.0547 × body mass0.746) to calculate EQ from the measured brain masses and species body masses. Boddy et al. [17] found this standard calculation of EQ to be highly correlated with phylogenetically corrected calculations of EQ, so for the sake of comparisons with past EQ studies and because it would be inappropriate to double-phylogenetically correct EQ values for the subsequent analysis with defence scores, we chose to use standard EQ values in our phylogenetic analyses. Finally, we log-transformed EQ to satisfy assumptions of normality. The complete dataset of species brain masses, body masses, EQ values, defence scores, diet and habitat openness used in the analysis is available in the electronic supplementary material, table S1 and in the Dryad online repository.
(c). Statistical analyses
To test for the effect of defence score, defence type, habitat openness and insectivory on EQ, while accounting for the effect of phylogenetic relatedness between species, we conducted a phylogenetic ANCOVA using the gls function in the ‘nlme’ package [26] in R [27], where lambda is computed using maximum-likelihood methods. We used a pruned published mammal supertree [28] for all analyses except as noted below. Because species with defence type 0 all have defence scores of 0, we could not directly test the interaction between these two factors (the correlation matrix was singular). However, we desired to test whether the effects of defence on EQ were equal across defence types 1–3, so as a workaround solution, we added a small amount of variation to the defence scores of species with defence type 0. By adding random variation (drawn from a uniform distribution bounded by −2 and 2), we could run the ANCOVA style analysis and calculate means and variances for the effects of defence score within each of the defence type groups. We used a Tukey's test to determine if different defence types had significantly different slopes (see the electronic supplementary material). Because testing for the effects of defence on EQ is analogous to regressing defence on the residuals of brain mass on body mass and because there is disagreement in the literature regarding the validity of such practices [29], we also ran a phylogenetic ANCOVA of defence type, log body mass, defence score, habitat openness and insectivory simultaneously on log brain mass. We conducted ancestral state reconstructions of EQ, body mass and brain mass using parsimony methods in targeted clades with defended species in Mesquite [30]. We analysed defended groups separately because we found that the presence of extraordinarily large species heavily skewed size reconstructions over a complete tree and judged the reconstructions of targeted groups to be more accurate and representative of the probable actual sizes of those species based on what we know of the fossil record. We conducted such reconstructions using pruned trees of the following groups: Xenarthra, Macroscelidea, Eulipotyphla, Ferae (Carnivora + Pholidota) and Hystricognathi. Because the resolution of the Caniformia portion of the Fritz et al. [28] supertree was unsatisfactory and not in agreement with more recent targeted phylogenetic reconstructions [31,32], we used a composite Carnivora tree downloaded from 10KTrees [31] to reconstruct the ancestral states for this group.
3. Results
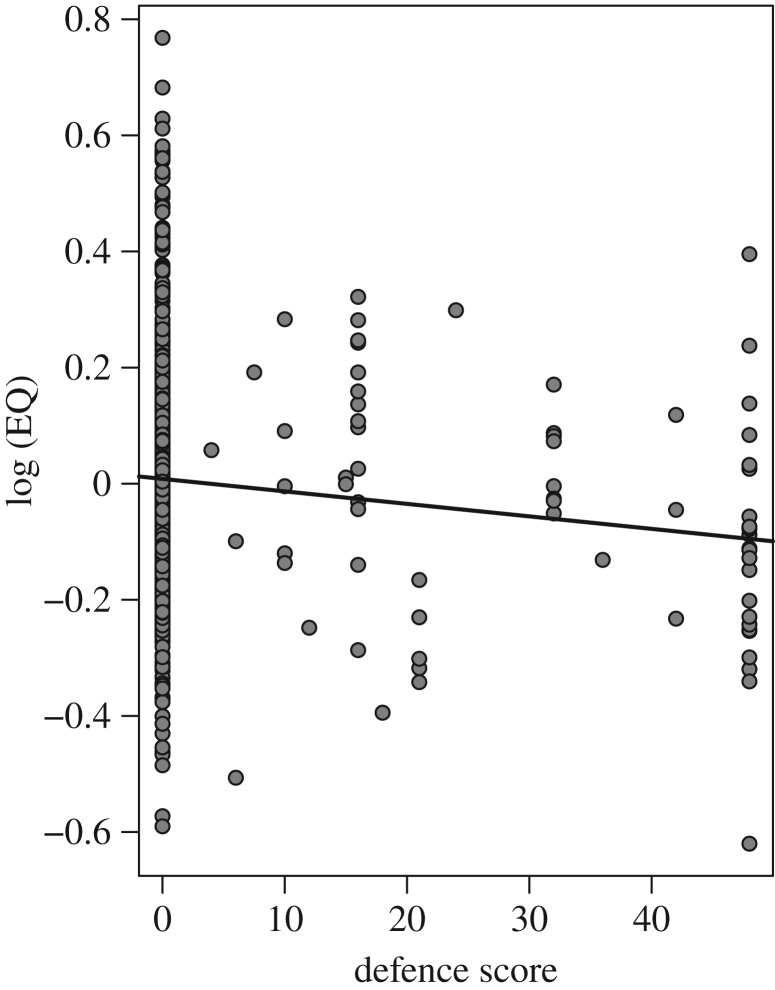
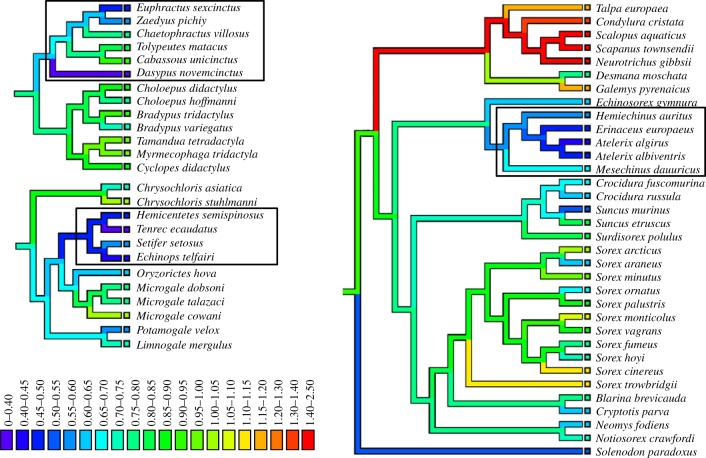
Phylogenetic ANCOVA showed that there was a statistically significant negative relationship between EQ and defensive ability across all mammal groups (λ = 0.85, t = −2.9390, p = 0.0034; figure 1; electronic supplementary material). Differences in the strength of this effect across the three defence types were not statistically significant (Tukey's test: q = 2.8466, p = 0.1166); i.e. all three types of defence produced similar negative relationships between defence score and EQ. Similarly, we found statistically significant effects of both log body mass (positive correlation) and defence (negative correlation) on log brain mass (λ = 0.927; log body mass: t = 60.7359, p < 0.0001; defence: t = −2.4430, p = 0.0148). Neither habitat openness nor insectivory had statistically significant effects on EQ in either the EQ or the log brain mass analysis (all p > 0.1538; see the electronic supplementary material). Ancestral state reconstructions of brain mass, body mass and EQ in targeted lineages showed that in five of the eight lineages where significant defences have evolved, there has been a moderate to strong coincident decline in EQ from the undefended ancestral node to the defended extant crown species (figure 2 and table 1).
Figure 1.
Relationship between defence score and log10 (EQ) across all mammals. As lineages evolve more robust defences, they become less encephalized.
Figure 2.
Ancestral state reconstructions of EQ in taxa with defended species. Branches are coloured according to EQ and boxes at branch tips indicate EQ of extant species. Clockwise from top left: Xenarthra (Dasypodidae in box), Eulipotyphla (Erinaceinae in box) and Afrosoricida (Tenrecinae in box).
Table 1.
Changes in body mass, brain mass and EQ in defended taxa. Ancestor taxon contains undefended clades sister to target defended taxon, and therefore lack the defence of the target taxon. (The numbers in italics call attention to dramatic declines in EQ in these particular groups.)
| undefended ancestor reconstruction | extant defended species averages | % change | |
|---|---|---|---|
| tenrecs | Tenrecidae | Tenrecinae (n = 4) | |
| brain mass (g) | 1.55 | 1.50 | −3% |
| body mass (g) | 232 | 319 | 38% |
| EQ | 0.651 | 0.438 | −33% |
| armadillos | Xenarthra | Cingulata (n = 6) | |
| brain mass (g) | 22.72 | 14.30 | −37% |
| body mass (g) | 4941 | 3801 | −23% |
| EQ | 0.773 | 0.629 | −19% |
| hedgehogs | Eulipotyphla | Erinaceinae (n = 5) | |
| brain mass (g) | 2.38 | 2.40 | 1% |
| body mass (g) | 356 | 407 | 14% |
| EQ | 0.910 | 0.525 | −42% |
| pangolins | Feraea | Pholidota (n = 3) | |
| brain mass (g) | 64.10 | 12.65 | −80% |
| body mass (g) | 17 800 | 3414 | −81% |
| EQ | 1.007 | 0.540 | −46% |
| skunks | Musteloidea | Mephitidae (n = 6) | |
| brain mass (g) | 55.91 | 12.60 | −77% |
| body mass (g) | 12 919 | 1549 | −88% |
| EQ | 1.368 | 1.16 | −15% |
| grisons polecats | Mustelidae | Ictonychinaeb (n = 4) | |
| brain mass (g) | 36.47 | 10.69 | −71% |
| body mass (g) | 6298 | 773 | −88% |
| EQ | 1.400 | 1.379 | −1% |
| NW porcupines | Hystricognathi | Erethizontidae (n = 4) | |
| brain mass (g) | 15.35 | 18.47 | 20% |
| body mass (g) | 4373 | 3133 | −28% |
| EQ | 0.834 | 1.049 | 26% |
| OW porcupines | Hystricognathi | Hystricidae (n = 8) | |
| brain mass (g) | 15.35 | 32.14 | 109% |
| body mass (g) | 4373 | 8386 | 92% |
| EQ | 0.834 | 0.956 | 15% |
aTaxon containing Orders Carnivora and Pholidota.
bSubfamily [32] containing Vormela, Ictonyx, Poecilogale, Lyncodon and Galictis.
4. Discussion
As morphological defences become more robust and impenetrable, EQ decreases and defended species become less intelligent. This relationship is expectedly weak given the great variation in EQ among mammals that is probably influenced by a variety of physiological and ecological factors and the paucity of clades with morphological defences, but it is consistent across defence types. Given this variation, we will discuss the evolution of EQ, defences and natural history in each lineage to understand the true magnitude of the effect of defences on EQ.
Table 1 demonstrates how both clades with dermal body armour showed strong declines in EQ from their non-armoured ancestors: armadillos (table 1; Cingulata) from basal Xenarthrans (sloths, anteaters and armadillos) and pangolins (Pholidota) from the common ancestor of Carnivora and Pholidota (Ferae). Dermal plating and scales are exceptionally heavy and probably energetically expensive to bear; a decline in relative brain size to pay this cost is unsurprising. Furthermore, both of these orders are myrmecophagous with slow metabolism, lumbering gaits and the ability to roll into a nearly impregnable ball when accosted. This tremendously effective defence renders pangolins and armadillos relatively safe from most predators, reducing selection favouring superior intelligence, detection and predator assessment.
Spiny insectivores also showed similar coincident declines in EQ with spine evolution: spiny tenrecs (Tenrecinae) from basal tenrecs (table 1: Tenrecidae) and Macroscelideans (EQ = 0.71), and spiny hedgehogs (Erinaceinae) from the common ancestor of shrews, moles and hedgehogs (table 1: Eulipotyphla). These species bear relatively thick spines for their body size, probably incurring significant production and transport costs. Ecologically, these small primitive insectivores share similar life histories and both rely somewhat on escape or aggression when initially encountering a potential predator. Hedgehogs will flinch and erect their spines or even run away if possible as a first choice for dealing with predators; it is only when physically touched that they will roll into a ball to protect the vulnerable areas of their body [33]. Similarly, tenrecs will flee from but also hiss and harass predators if confronted; streaked tenrecs may even buck their heads around in an effort to impale predators with the longer spines on the crest posterior to the head [34].
Noxious anal secretions are used in defence by a number of carnivore species [11,35,36]. Skunks have advanced ability to spray noxious anal gland secretions at predators and showed moderate declines in EQ: skunks (Mephitidae) from basal Musteloids (table 1; common ancestor of Mustelidae, Mephitidae, Procyonidae and Ailuridae) and Arctoids (Musteloidea plus Ursidae; EQ = 1.28). Striped and marbled polecats (Ictonychinae), which evolved their noxious sprays independently of skunks [35,36], were, however, equivocal (table 1). Early Musteloids evolved greater EQs, possibly to help with a stalking hunting style in complex habitats as body sizes decreased. The anal glands of spraying animals are several times larger than those of other carnivores, and the constant production of noxious thiols from the diet takes time and energy. Behaviourally, striped skunks (Mephitis mephitis) are fairly fearless omnivores that exhibit a variety of defensive behaviours to deter predators and use spraying only as a last resort [37]. Adults are often less aware of their surroundings until confronted by a predator at close range. Noxious Ictonychines did not suffer a drop in EQ, possibly owing to their highly predatory carnivorous diet, which may require greater cognitive problem solving ability in order to detect prey and hunt successfully, relative to omnivorous skunks.
The exceptions to the defence-EQ trend are the porcupines (table 1). Both families of porcupines have shown small increases in EQ from their non-defended ancestors: Old World (OW) porcupines (Hystricidae) and New World (NW) porcupines (Erethizontidae) have higher EQs than the basal Hystricognath rodent ancestor. There is considerable variation in quill length and thickness among the porcupines with the more arboureal Erethizontids having many more lightweight, short, narrow (but barbed) quills and some of the more terrestrial Hystricids having far more robust, heavier, long, thick quills. Energetic expenditure on defence is, therefore, highly variable in these groups. Similar to armadillos and pangolins, porcupines are among the largest of the defended mammals and may be limited in their ability to flee from most larger predators unlike tenrecs, hedgehogs, or even skunks and polecats. Both families show great variation in EQ. Kruska [13] found that arboureal species, particularly in Carnivora, Rodentia and Primates, have higher EQs than their terrestrial relatives. Erethizontid porcupines are arboureal and therefore: (i) must remain lightweight making heavy dermal armour impractical, and (ii) must be able to navigate a more complex 3D world, similar to primates and marsupials, making greater cognitive ability useful. Hystricids vary in life history: the climbing forest dwellers (Trichys, Atherurus) have greater EQs (0.93–1.98) while the lumbering, terrestrial, desert/savannah dwelling Hystrix have greatly reduced EQs (0.5–0.8; except Hystrix sumatrae: 1.2). Stankowich & Campbell [12] also found porcupines to be the exception to the rule that intermediate size, insectivory and living in exposed habitats selects for defensive morphologies: their arboureal and herbivorous nature is fairly unique among defended species. Given the unique (semi)arboureal lifestyle of most porcupines that may favour greater cognitive ability, these small increases in EQ are not entirely surprising.
Porcupines are not the only defended mammals to show greater EQs. While some cases of simultaneous gains in EQ and defence score may result from measurement error or insufficient sample sizes within a species, a few are more noteworthy. Spotted skunks (Spilogale) are effective aposematic sprayers and have much greater EQs (1.37–1.72) than other skunks (0.77–1.06). Most other Mephitids lumber on the ground and hide in burrows, but spotted skunks are smaller and more arboureal (with more potential predators), possibly necessitating greater cognitive ability to navigate in the trees similar to porcupines. This increased EQ may be driven, however, by a decrease in body mass rather than brain size. Our reconstructions show that the common ancestor of Spilogale had a body mass of 717 g while the common ancestor of all Mephitids had a mass of 3045 g, a decline of 76%, while the coincident decline in brain mass was only 55% (18.9–8.5 g). Increased EQ may be, therefore, a by-product of a disproportional decrease in body size compared to the decrease in brain size.
Interestingly, decephalization may also be associated with a shift towards a less social and more insectivorous or at least a less predatory or herbivorous lifestyle; although, we found no significant effect of insectivory in our analyses. There are no social defended mammals, and armadillos, echidnas, pangolins, hedgehogs and tenrecs are all insectivorous, which may require less cognitive power than predatory or extractive foragers (tool use to collect embedded seeds/nuts, insects or even fossorial prey). Several studies have argued that, among Primates, enhanced sociality and extractive foraging favoured the evolution of enhanced cognitive abilities [38,39]. Insectivory and solitary living, however, are common among many non-defended mammal groups and probably describe the first true mammals. Also, while it is more likely that evolution has more commonly moved in the opposite direction (enhanced cognition correlated with greater sociality and more advanced predatory hunting or foraging behaviours), perhaps we should consider adding these two traits to a growing list that evolve together in defended mammals: intermediate body size, reduced brain size, open habitat use, reduced metabolic rate and enhanced defences [10,12].
Studies of EQ have always used body mass to correct for brain size when estimating encephalization; however, we recognize that this may have introduced bias into our analysis, given that the evolution of some morphological defences involve a significant increase in body mass, or at the very least mass per unit volume (e.g. dermal plates). It is nearly impossible, however, to tease apart mass added from the weapon and overall body mass added because we do not know the mass of all 647 species without their skin, and body volumes are not available. We contend, however, that spending this extra energy on mass should come at a metabolic cost of not building a relatively larger brain as well.
Our analyses show clear correlated decreases in EQ with the evolution of enhanced morphological defences in mammals. At the proximate level, there may be an energetic trade-off between building, maintaining and carrying defensive armour and building and maintaining expensive brain tissue. Other ‘expensive’ morphological traits in mammals like cranial weapons, large tusks, or even blubber may also result in similar reductions in EQ, but these remain to be tested. At the ultimate level, well-defended species might rely less on enhanced cognition for predator detection/recognition/escape and sacrifice larger brains to save energy. We do not consider the expensive tissue and relaxed selection hypotheses to be mutually exclusive or even competing, but simply on different levels of analysis [40,41]. Despite exceptional cases where defended species are arboureal and must navigate complex 3D habitats (e.g. porcupines and spotted skunks), every other instance of a de novo evolution of body armour, spines, quills or sprays also shows a simultaneous decrease in EQ. Future studies should focus on how different parts of the brain evolve with antipredator defences and different lifestyles (e.g. habitat, diet, locomotion and social structure) in order to help explain the huge variation that exists within and between mammalian taxa.
Supplementary Material
Acknowledgements
We thank T. Caro for comments on a previous version of the manuscript and B. Allen and D. Johnson for advice on the statistical analysis. We thank James Dines at the Los Angeles County Museum of Natural History Museum and Esther Langan at the Smithsonian Institution National Museum of Natural History for access to collections and support.
Data accessibility
Data available through the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.tr7m5 [42].
Authors' contributions
T.S. conceptualized the project, advised on data collection, conducted all statistical analyses and wrote the first draft of the manuscript. A.R. collected the new data on brain size and helped edit and revise the manuscript.
Competing interests
We have no competing interests.
Funding
This research was supported by funds from California State University Long Beach, College of Natural Sciences and Mathematics.
References
- 1.Aschoff J, Günther B, Kramer K. 1971. Energiehaushalt und temperaturregulation. Munich, Germany: Urban & Schwarzenberg. [Google Scholar]
- 2.Isler K, van Schaik CP. 2009. The expensive brain: a framework for explaining evolutionary changes in brain size. J. Hum. Evol . 57, 392–400. ( ) [DOI]
- 3.Kotrschal A, Rogell B, Bundsen A, Svensson B, Zajitschek S, Brännström I, Immler S, Maklakov AA, Kolm N. 2013. Artificial selection on relative brain size in the guppy reveals costs and benefits of evolving a larger brain. Curr. Biol. 23, 168–171. ( 10.1016/j.cub.2012.11.058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Bijl W, Thyselius M, Kotrschal A, Kolm N. 2015. Brain size affects the behavioural response to predators in female guppies (Poecilia reticulata). Proc. R. Soc. B 282, 20151132 ( 10.1098/rspb.2015.1132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emlen DJ. 2001. Costs and the diversification of exaggerated animal structures. Science 291, 1534 ( 10.1126/science.1056607) [DOI] [PubMed] [Google Scholar]
- 6.Isler K, van Schaik CP. 2006. Metabolic costs of brain size evolution. Biol. Lett. 2, 557–560. ( 10.1098/rsbl.2006.0538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aiello LC, Wheeler P. 1995. The expensive-tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr. Anthropol. 36, 199–221. ( 10.1086/204350) [DOI] [Google Scholar]
- 8.Isler K, van Schaik C. 2006. Costs of encephalization: the energy trade-off hypothesis tested on birds. J. Hum. Evol. 51, 228–243. ( 10.1016/j.jhevol.2006.03.006) [DOI] [PubMed] [Google Scholar]
- 9.Navarrete A, van Schaik CP, Isler K. 2011. Energetics and the evolution of human brain size. Nature 480, 91–93. ( 10.1038/nature10629) [DOI] [PubMed] [Google Scholar]
- 10.Lovegrove BG. 2001. The evolution of body armor in mammals: plantigrade constraints of large body size. Evolution 55, 1464–1473. ( 10.1111/j.0014-3820.2001.tb00666.x) [DOI] [PubMed] [Google Scholar]
- 11.Stankowich T. 2012. Armed and dangerous: predicting the presence and function of defensive weaponry in mammals. Adapt. Behav. 20, 34–45. ( 10.1177/1059712311426798) [DOI] [Google Scholar]
- 12.Stankowich T, Campbell LA. 2016. Living in the danger zone: exposure to predators and the evolution of spines and body armor in mammals. Evolution 70, 1501–1511. ( 10.1111/evo.12961) [DOI] [PubMed] [Google Scholar]
- 13.Kruska DCT. 2005. On the evolutionary significance of encephalization in some eutherian mammals: effects of adaptive radiation, domestication, and feralization. Brain Behav. Evol. 65, 73–108. ( 10.1159/000082979) [DOI] [PubMed] [Google Scholar]
- 14.Safi K, Seid MA, Dechmann DKN. 2005. Bigger is not always better: when brains get smaller. Biol. Lett. 1, 283–286. ( 10.1098/rsbl.2005.0333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roth G, Dicke U. 2005. Evolution of the brain and intelligence. Trends Cogn. Sci. 9, 250–257. ( 10.1016/j.tics.2005.03.005) [DOI] [PubMed] [Google Scholar]
- 16.Jerison HJ. 1973. Evolution of the brain and intelligence. New York, NY: Academic Press. [Google Scholar]
- 17.Boddy AM, McGowen MR, Sherwood CC, Grossman LI, Goodman M, Wildman DE. 2012. Comparative analysis of encephalization in mammals reveals relaxed constraints on anthropoid primate and cetacean brain scaling. J. Evol. Biol. 25, 981–994. ( 10.1111/j.1420-9101.2012.02491.x) [DOI] [PubMed] [Google Scholar]
- 18.Benson-Amram S, Dantzer B, Stricker G, Swanson EM, Holekamp KE. 2016. Brain size predicts problem-solving ability in mammalian carnivores. Proc. Natl Acad. Sci. USA 113, 2532–2537. ( 10.1073/pnas.1505913113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenberg JF, Wilson DE. 1981. Relative brain size and demographic strategies in didelphid marsupials. Am. Nat. 118, 1–15. ( 10.1086/283796) [DOI] [Google Scholar]
- 20.Eisenberg JF, Wilson DE. 1978. Relative brain size and feeding strategies in the Chiroptera. Evolution 32, 740–751. ( 10.2307/2407489) [DOI] [PubMed] [Google Scholar]
- 21.McNab BK, Eisenberg JF. 1989. Brain size and its relation to the rate of metabolism in mammals. Am Nat 13, 157–167. ( 10.1086/284907) [DOI] [Google Scholar]
- 22.Mace GM, Harvey PH, Clutton-Brock TH. 1981. Brain size and ecology in small mammals. J. Zool. 193, 333–354. ( 10.1111/j.1469-7998.1981.tb03449.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenberg JF. 1981. The mammalian radiations. Chicago, IL: University of Chicago. [Google Scholar]
- 24.Kissling WD, Dalby L, Fløjgaard C, Lenoir J, Sandel B, Sandom C, Trøjelsgaard K, Svenning J-C. 2014. Establishing macroecological trait datasets: digitalization, extrapolation, and validation of diet preferences in terrestrial mammals worldwide. Ecol. Evol. 4, 2913–2930. ( 10.1002/ece3.1136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.IUCN. 2011. The IUCN Red List of Threatened Species. See http://www.iucnredlist.org.
- 26.Pinheiro J, Bates D, DebRoy S, Sarkar D, R Development Core Team. 2012. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-103. [Google Scholar]
- 27.R Core Team. 2012. R: a language and environment for statistical computing (ver. 2.14.12). (2.8.0 edn) Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 28.Fritz SA, Bininda-Emonds ORP, Purvis A. 2009. Geographical variation in predictors of mammalian extinction risk: big is bad, but only in the tropics. Ecol. Lett. 12, 538–549. ( 10.1111/j.1461-0248.2009.01307.x) [DOI] [PubMed] [Google Scholar]
- 29.Freckleton RP. 2002. On the misuse of residuals in ecology: regression of residuals vs. multiple regression. J. Anim. Ecol. 71, 542–545. ( 10.1046/j.1365-2656.2002.00618.x) [DOI] [Google Scholar]
- 30.Maddison WP, Maddison DR. 2015. Mesquite: a modular system for evolutionary analysis. (3.04 edn). [Google Scholar]
- 31.Arnold C, Matthews LJ, Nunn CL. 2010. The 10kTrees website: a new online resource for primate phylogeny. Evol. Anthropol. 19, 114–118. ( 10.1002/evan.20251) [DOI] [Google Scholar]
- 32.Sato JJ, Wolsan M, Prevosti FJ, D'Elía G, Begg C, Begg K, Hosoda T, Campbell KL, Suzuki H. 2012. Evolutionary and biogeographic history of weasel-like carnivorans (Musteloidea). Mol. Phylogen. Evol. 63, 745–757. ( 10.1016/j.ympev.2012.02.025) [DOI] [PubMed] [Google Scholar]
- 33.Reeve N. 1994. Hedgehogs, 313 p. London, UK: T. & A.D. Poyser Natural History. [Google Scholar]
- 34.Eisenberg JF, Gould E. 1970. The tenrecs: a study in mammalian behavior and evolution, 138 p. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 35.Stankowich T, Caro T, Cox M. 2011. Bold coloration and the evolution of aposematism in terrestrial carnivores. Evolution 65, 3090–3099. ( 10.1111/j.1558-5646.2011.01334.x) [DOI] [PubMed] [Google Scholar]
- 36.Stankowich T, Haverkamp PJ, Caro T. 2014. Ecological drivers of antipredator defenses in carnivores. Evolution 68, 1415–1425. ( 10.1111/evo.12356) [DOI] [PubMed] [Google Scholar]
- 37.Larivière S, Messier F. 1996. Aposematic behaviour in the striped skunk, Mephitis mephitis. Ethology 102, 986–992. ( 10.1111/j.1439-0310.1996.tb01176.x) [DOI] [Google Scholar]
- 38.Parker ST. 2015. Re-evaluating the extractive foraging hypothesis. New Ideas Psychol. 37, 1–12. ( 10.1016/j.newideapsych.2014.11.001) [DOI] [Google Scholar]
- 39.Dunbar RIM. 1992. Neocortex size as a constraint on group size in primates. J. Hum. Evol. 22, 469–493. ( 10.1016/0047-2484(92)90081-J) [DOI] [Google Scholar]
- 40.Sherman PW. 1988. The levels of analysis. Anim. Behav. 36, 616–619. ( 10.1016/S0003-3472(88)80039-3) [DOI] [Google Scholar]
- 41.Tinbergen N. 1963. On aims and methods of ethology. Z. Tierpsychol. 20, 410–433. ( 10.1111/j.1439-0310.1963.tb01161.x) [DOI] [Google Scholar]
- 42.Stankowich T, Romero AN. 2017 doi: 10.5061/dryad.tr7m5. Data from: The correlated evolution of antipredator defences and brain size in mammals. Dryad Digital Repository. ( ) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available through the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.tr7m5 [42].