Abstract
High-protein diets shorten lifespan in many organisms. Is it because protein digestion is energetically costly or because the final products (the amino acids) are harmful? To answer this question while circumventing the life-history trade-off between reproduction and longevity, we fed sterile ant workers on diets based on whole proteins or free amino acids. We found that (i) free amino acids shortened lifespan even more than proteins; (ii) the higher the amino acid-to-carbohydrate ratio, the shorter ants lived and the lower their lipid reserves; (iii) for the same amino acid-to-carbohydrate ratio, ants eating free amino acids had more lipid reserves than those eating whole proteins; and (iv) on whole protein diets, ants seem to regulate food intake by prioritizing sugar, while on free amino acid diets, they seem to prioritize amino acids. To test the effect of the amino acid profile, we tested diets containing proportions of each amino acid that matched the ant's exome; surprisingly, longevity was unaffected by this change. We further tested diets with all amino acids under-represented except one, finding that methionine, serine, threonine and phenylalanine are especially harmful. All together, our results show certain amino acids are key elements behind the high-protein diet reduction in lifespan.
Keywords: free amino acids, lifespan, nutrition, protein, argentine ants
1. Introduction
What animals eat—and how much—strongly affects their health and longevity [1,2]. Modest calorie restriction is well known to increase lifespan across a range of organisms (review in [3]), but under ad libitum feeding conditions the balance of macronutrients is more important than calorie intake per se [4]. Notably, the ratio of protein to carbohydrate strongly affects longevity and measures of late life health. Diets containing a high protein-to-carbohydrate ratio reduce lifespan in a diversity of organisms [2,5–7], suggesting that conserved mechanisms underlie this sensitivity to high-protein, low-carbohydrate diets.
It remains unclear how high-protein, low-carbohydrate diets shorten lifespan. One hypothesis relates to protein digestion, which is energetically costly [8,9] and produces potentially toxic nitrogen waste products [10]. Another possibility is that absorbed amino acids stimulate signalling pathways that regulate lifespan, such as the canonical target of rapamycin (TOR) pathway [11–13]. A trade-off between longevity and reproduction complicates attempts to understand the effect of high-protein diets on lifespan; while decreasing longevity, high-protein diets improve the reproductive output of individuals [5,7,14,15].
To avoid the complication of reproduction, we used Argentine ant (Linepithema humile) workers, which are sterile—only the queens reproduce. Like many other organisms, ant workers die prematurely on high-protein diets [6,16–19]. To find out why, we first focused on the potential toxicity of protein digestion. We hypothesized that bypassing protein digestion would extend workers' lives. To test this hypothesis, we first examined the performance (survival, foraging effort and lipid storage) of ants fed on diets containing either whole proteins or an equivalent amount of each free amino acid, presuming a complete protein digestion. Second, because ant larvae perform an important digestive function inside the colony [16], we investigated their role in the digestion of high-protein diets for whole protein and free amino acid based diets. Third, aiming to explore the effect of the balance and interactions among amino acids, we fed ant workers with a diet based on a direct translation of the Argentine ant exome (all the exons of the ant genome [20]). We hypothesized that this diet could better fit the ant needs and therefore reverse the negative effects of high-protein diets. Finally, and to begin to consider the isolated roles of single amino acids, we fed ants on 20 diets where one amino acid was over-represented compared with the others.
The purpose of this study was to understand the underlying mechanisms behind the high-protein diet toxicity. Our results showed that an excess of certain amino acids is key to this toxicity.
2. Material and methods
See the electronic supplementary material for an extended version of the methods.
(a). Species studied and rearing conditions
We used foragers of the invasive Argentine ant, L. humile. In the field, these ants feed on dead insects and honeydew rich in sugars and amino acids [21]. Ant foragers were collected in Toulouse (France) two weeks before every experiment and were provided with a mixed diet of vitamin-enriched diet and water every 2 days [22] (modified from [23]).
For the experiments, we created subcolonies and housed them in experimental nests comprising an opaque and humid box connected to a foraging arena.
All ants were kept in a 14–10 light–dark cycle and at room temperature (25 ± 2°C).
(b). Synthetic diets
(i). Full mixture diets: whole protein and amino acid diets
To examine the role of protein digestion in the high-protein diet toxicity, we designed 22 diets (see recipes in the electronic supplementary material) varying in their amino acid source (whole proteins, PWP, or free amino acids, PAA—11 diets per amino acid source), their protein-to-carbohydrate ratios (P : C—5 : 1, 3 : 1, 1 : 1, 1 : 3, 1 : 5 or 1 : 10) and their protein plus carbohydrate concentrations ([P + C]—0.17, 0.10, 0.5 or 0.3 g g−1). The protein content of the ‘whole protein diets' consisted of a mixture of casein, whey powder and white egg proteins as in [6,18,19]. The ‘free amino acid diet’ contained the same amount of each amino acid as found in the whole protein diet, but in the form of free amino acids instead of whole proteins. We used glucose as the carbohydrate source. All diets also contained egg yolk, vitamins, sodium, choline chloride and ascorbic acid, and were presented at 4.5 : 1 ratio of dry mass in a 1% agar solution (see [22] for preparation details).
To further explore the role of amino acids composition, we prepared another diet based on a direct translation of the entire Argentine ant exome. This new mix could theoretically better fit the ant needs and reverse the negative effect of high-protein diets. The Argentine ant exome proportion was calculated as the median of the frequency of each amino acid produced in a hypothetical translation of all genes (courtesy of M. Piper). Using this new amino acid composition, we designed two diets at the same concentration ([P + C] = 0.10 g g−1) but varying in their P : C ratios (5 : 1 and 1 : 5).
(ii). Single amino acid mixture diets
To understand the role of single amino acids in the toxicity of high-protein diets, we designed 20 different synthetic diets identical to the 5 : 1 PAA : C diet but adding only one of the 20 free amino acids, and replacing the rest with 1% agar. As a result, the diets contained all free amino acids present in the egg yolk (around 0.0001 g g−1, calculated using [24]; see the electronic supplementary material) plus the one added (0.0006–0.0106 g g−1). One supplementary control for this experiment consisted of an artificial diet without amino acid (‘no AA’) except the ones from the egg yolk.
For full recipes and ingredient manufacturers, see the electronic supplementary material.
(c). Experiments
(i). Experiment 1: role of amino acid source on ant lifespan, feeding and lipid storage
In a first experiment to investigate the possible toxic effects of protein digestion, we fed subcolonies of Argentine ant workers with artificial diets with either whole proteins or free amino acids. Subcolonies (208) of approximately 200 ants (mean ± s.d. 196.3 ± 14.9) were confined to a single nutritional diet from the beginning to the end of the experiment (defined as either when all ants from the same subcolony were dead or when only 10% of the initial number of ants remained alive). The nutritional diet could be any of the described whole protein or free amino acid diets. Each diet was tested on 5–16 subcolonies. Water and fresh food were available and renewed every 2 days. Ants never collected all the food offered before it was renewed. As a control, 13 subcolonies were food deprived and received only water.
First, we counted the number of dead ants every 2 days until the end of the experiment to estimate ant lifespan. Second, we froze all the dead ants for lipid content analysis, as described in [17,18]. The lipid contents were measured at different survival points (when 0.9, 0.7, 0.5, 0.3 and 0.1 of the ants were still alive; total n = 690, five to seven subcolonies per survival point and diet, 23 diets). Third, we recovered from the foraging arena all food pellets, which are leftovers of the original food jettisoned by the ants. Lastly, we took pictures of the foraging arena 5 min, 2 h and 16 h after supplying the food and counted the number of ants eating on each picture for 74 days (or, if the subcolonies did not survive that long, until the 10% of the ants remained alive). We calculated the proportion of ants eating as the number of ants eating divided by number of ants still alive. The proportion of ants eating after 5 min reflects the initial attractiveness of the food [25], whereas the proportions of ants eating 2 and 16 h later reflect longer-term homeostatic responses and hence the amount of food they need to collect to satisfy colony requirements (electronic supplementary material, figure S1) [6]. The foraging effort was estimated as the proportion of ants eating 2 h after offering fresh food. The approximate nutrient collection for the colony derived from the foraging effort was calculated using the following equation (inspired from [18]):
 |
2.1 |
(ii). Experiment 2: effect of larvae presence on ant lifespan
Ants and larvae continuously exchange food via trophallaxis, and have different appetites for proteins and carbohydrates; workers use carbohydrates as their main energy source while larvae need proteins to develop [26]. Under high-protein diets, colonies with larvae live longer than those without, because larvae help with protein digestion [16]. To explore the effect of larvae on ant lifespan, we observed the survival of ants eating high-protein diets based on either whole proteins or free amino acids with and without larvae. Twenty-four subcolonies of approximately 100 ants (mean ± s.d. 100.5 ± 7.0) with or without larvae were restricted to either 5 : 1 PWP : C or 5 : 1 PAA : C from the beginning to the end of the experiment (defined as in experiment 1). For the 12 subcolonies with larvae, we introduced 0.025 g of larvae (40–50 larvae) into the nest. We fed the ants every 2 days and followed their survival as in experiment 1.
(iii). Experiment 3: effect of amino acid composition on ant lifespan
If high-protein diet toxicity is due to amino acid composition, changing this composition would be expected to affect ant lifespan. Two hundred ants were isolated in individual nests and constrained to a single free amino acid diet varying in the amino acid profile (‘casein-whey-egg’ diet or ‘Argentine ant exome’ diet) and in the protein to carbohydrate ratio (1 : 5 or 5 : 1 PAA : C, [P + C] = 0.10 g g−1) from the beginning to the end of the experiment. Here, the end of the experiment was defined as either when all ants under the same diet were dead or when just 10% of the initial number of ants remained alive. Each diet was tested on 50 ants. We fed the ants every 2 days and followed their survival as in experiment 1.
(iv). Experiment 4: effect of single amino acids on ant lifespan
In this last experiment, we aimed to dissect the individual roles of each of amino acid in protein diet toxicity. This is a 20-dimensional mixture problem, and testing all of the possible main and interactive effects would be a vast undertaking. Instead, we proceeded to analyse the effect of diets comprising all amino acids under-represented except one. A total of 1200 ants were isolated as in experiment 3). Each ant was constrained to one of 24 nutritional diets (20 single amino acid diets and four controls: food deprivation, 5 : 1 PAA : C, 1 : 5 PAA : C or No AA) from the beginning to the end of the experiment (defined as in experiment 3). Each diet was tested on 50 ants. We fed the ants every 2 days and followed their survival as in experiment 1.
(d). Statistics
Survival analysis was performed using a log rank test to compare two groups or Cox proportional hazards regression model considering censured data. Ants observed in the same subcolony were clustered together for the survival analysis. Nutritional response landscapes for survival, foraging effort and lipid proportion were evaluated with surface regression models. To compare the amount of food pellets left in whole protein diets and in amino acid diets we used a permutation test. The rest of the data were analysed with linear mixed-effects models with subcolony as a random factor. Statistics are described in more details in the electronic supplementary material.
3. Results
(a). Role of amino acid source on ant lifespan, feeding and lipid storage
We compared the performance of ants under 11 whole protein and 11 free amino acid diets. We used nutritional geometry [1] to map the performance as response landscapes onto arrays of protein versus carbohydrate concentrations in the dietary treatments.
(i). Whole proteins versus free amino acids effects on ant lifespan
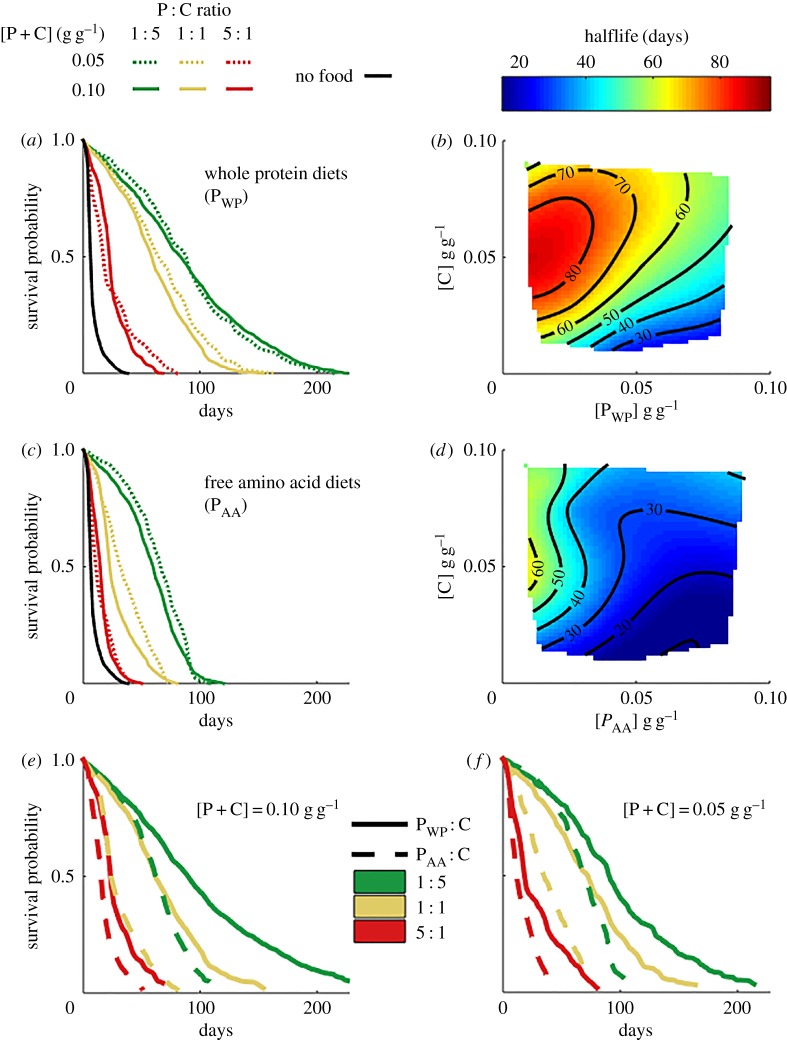
The higher the proportion of protein relative to carbohydrate in the diet, the higher the mortality rate (p < 0.001; electronic supplementary material, table S1), for both whole protein (figure 1a,b; electronic supplementary material, table S2 and figure S2) and free amino acid diets (figure 1c,d; electronic supplementary material, table S3 and figure S2). The amino acid source had a strong effect on mortality: ants fed on free amino acid diets lived half as long as those feeding on whole proteins (p < 0.001; electronic supplementary material, table S1; figure 1a–d). Nutrient concentration ([P + C]) had no effect on survival (p = 0.123; figure 1a,c; electronic supplementary material, table S1 and figure S2).
Figure 1.
Role of amino acid source on ant lifespan. (a) Survival curves of six whole protein diets differing in PWP : C ratio and [PWP + C] concentration. (b) Half-life of ants on all the 11 whole protein diets tested as a function of [C] and [PWP] in those diets (R2 of surface regression = 0.971). (c) Survival curves of six free amino acid diets differing in PAA : C ratio and [PAA + C] concentration. (d) Half-life of ants on all the 11 amino acid diets tested as a function of [C] and [PAA] in those diets (R2 of surface regression = 0.872). (e) Reorganization of survival curves in (a,c) for the six diets at [P + C] = 0.10 g g−1 differing in P : C ratio and source of amino acids (whole proteins, PWP, or free amino acids, PAA). (f) Reorganization of survival curves in (a,c) for the six diets at [P + C] = 0.05 g g−1 differing in P : C ratio and source of amino acids (whole proteins, PWP, or free amino acids, PAA).
A closer inspection of the lifespan of ants under the different diets suggests a consistent scaling relationship between whole protein and free amino acid diets, with the impacts of free amino acid diets being the same as those of a fivefold quantity of whole protein (figure 1e,f). Hence, ants fed free amino acid in a 1 : 5 PAA : C ratio survived similarly to those ants eating whole proteins in a 1 : 1 PWP : C ratio. This was the case for the two concentrations we tested ([P + C] = 0.10 and 0.05 g g−1; figure 1f,g). The ants fed 1 : 1 PAA : C diet, also survived more similarly to ants eating the richer protein diet 5 : 1 PWP : C than ants eating 1 : 1 PWP : C for both concentrations (figure 1e,f). Therefore, bypassing digestion did not help ants to survive better on a high protein, lower carbohydrate diet; rather it provided an approximately five times more potent dose effect.
(ii). Whole proteins versus free amino acids effects on collective foraging behaviour
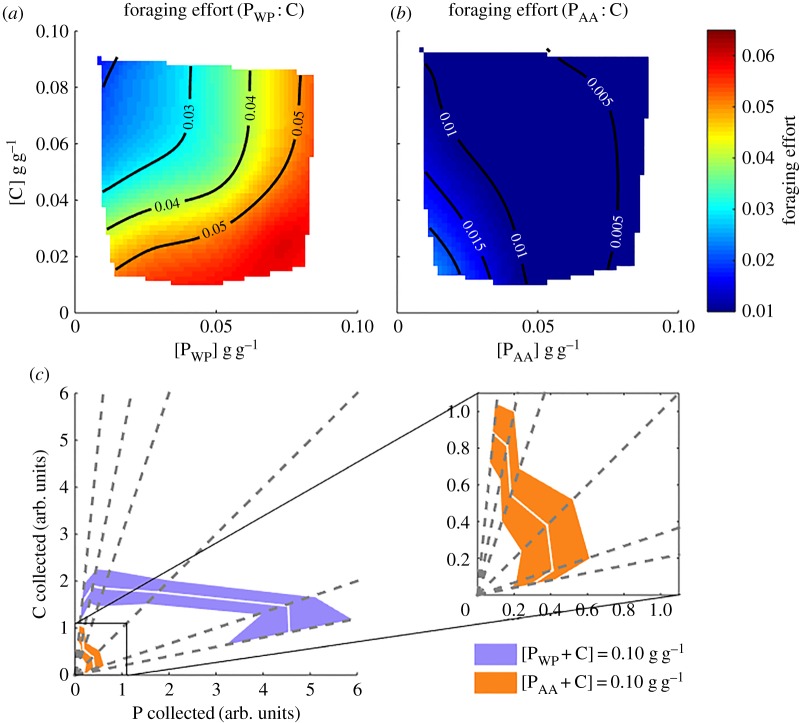
One possible explanation for ants dying faster on free amino acid diets is that they eat less and suffer consequences of under-nutrition (electronic supplementary material, figure S1). When ants were fed on whole protein diets (figure 2a; electronic supplementary material, table S4), their foraging effort was greater the higher the P : C ratio (p < 0.001; electronic supplementary material, table S5), with nutrient concentration per se having no effect (p = 0.676). In contrast, ants on free amino acid diets (figure 2b; electronic supplementary material, table S6) increased foraging as the food P : C ratio decreased (p < 0.001; electronic supplementary material, table S7). Ants feeding on whole proteins foraged more than ants feeding on free amino acids (p < 0.001; electronic supplementary material, table S8). Ants compensated for sugar scarcity in high PWP : C ratio diets by foraging more, therefore achieving roughly the same final amount of carbohydrates collected, regardless of their concentration in the diet (figure 2c). However, when fed amino acid diets this strategy was not seen (figure 2c inset): instead, the higher the P : C ratio, the less the ants foraged, such that the quantity of amino acid rather than the quantity of carbohydrate was largely conserved.
Figure 2.
Role of amino acid source on collective foraging behaviour. (a) Foraging effort (proportion of ants eating 2 h after we supplied fresh food) in whole protein diets as a function of [C] and [PWP] (R2 of surface regression = 0.758). (b) Foraging effort in free amino acid diets as a function of [C] and [PAA] (R2 of surface regression = 0.717). (c) Estimated carbohydrate and whole protein/free amino acid collection for diets where [P + C] = 0.10 g g−1 (white solid lines: mean values, purple patch: standard deviation around the mean for whole protein diets, orange: standard deviation around the mean for free amino acid diets). Inset: zoom of the estimated carbohydrate and free amino acid collection for free amino acid diets. Data are represented as mean ± s.d. Approximate [C] and [P] collection was multiplied by 103 for visualization purposes.
We observed that ants left waste pellets in the foraging arena. The amount of pellets increased as the dietary P : C ratio increased (p < 0.001; electronic supplementary material, table S9). Ants left, on average, 20 times more pellets on whole protein than on free amino acid diets (p < 0.001).
(iii). Whole proteins versus free amino acids effects on lipid reserves
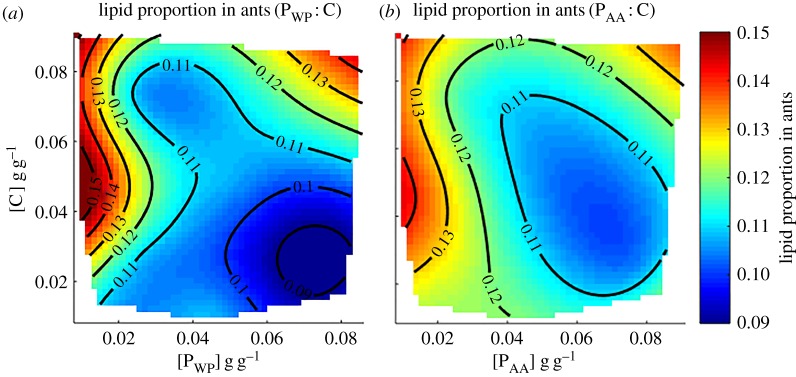
Given that ants collected less free amino acid than whole protein diets, they might have died faster in free amino acid diets because they did not consume enough sugar and instead had to deplete their lipid reserves [19]. We found that ants on whole protein diets (figure 3a; electronic supplementary material, table S10) had less body lipid the higher the P : C ratio (p < 0.001; electronic supplementary material, table S11). For ants on free amino acid diets (figure 3b; electronic supplementary material, table S12), the ratio had no influence (p = 0.227; electronic supplementary material, table S13) but the higher the nutrient concentration [P + C], the fatter the ants (p = 0.032; electronic supplementary material, table S13). Unexpectedly, ants fed free amino acid diets were on average fatter than those eating whole proteins (p = 0.002; electronic supplementary material, table S14).
Figure 3.
Role of amino acid source on lipid storage. (a) Lipid proportion in ants in whole protein diets as a function of [C] and [PWP] (R2 of surface regression = 0.439). (b) Lipid proportion in ants in free amino acid diets as a function of [C] and [PAA] (R2 of surface regression = 0.109).
(b). Effect of larvae presence on ant lifespan
To explore the effect of larvae on colony survival, we monitored subcolonies of ants eating high-protein diets with and without larvae. We found that larvae increased the ant survival, regardless of the amino acid source (p = 0.038; figure 4; electronic supplementary material, table S15).
Figure 4.
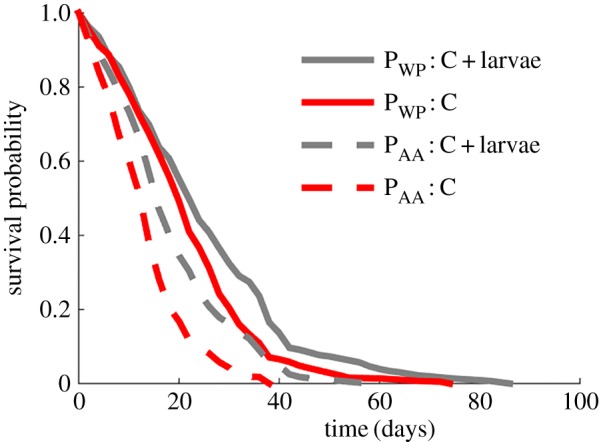
Effect of larvae presence on ant lifespan. Survival curves of subcolonies feeding on 5 : 1 PWP : C or PAA : C ratio diets ([P + C] = 0.10 g g−1) with and without larvae. Each curve represents six subcolonies.
(c). Effect of amino acid composition on ant lifespan
The following experiment was conducted on isolated ants, and it is important to note that the response of isolated ants to dietary P : C ratio is qualitatively similar to that of subcolonies (electronic supplementary material, figure S3 and table S16).
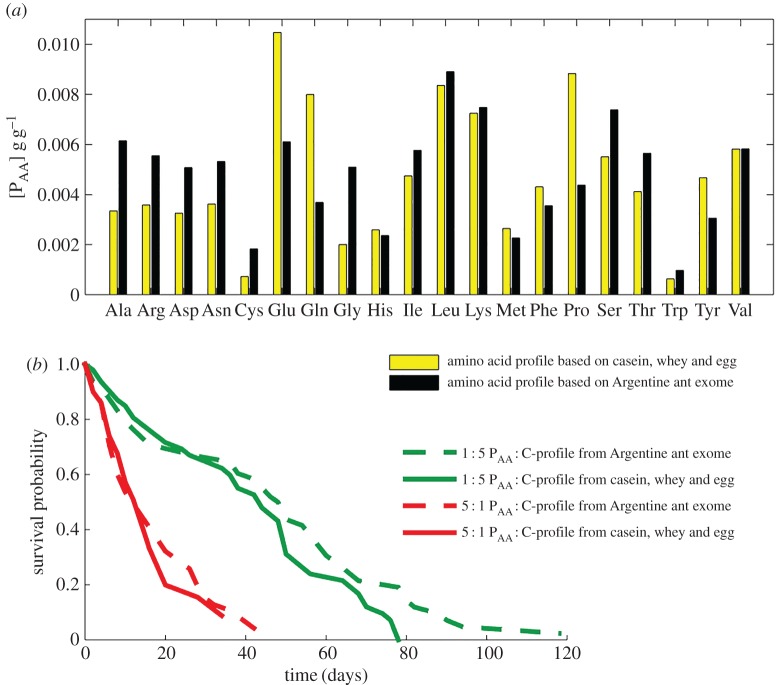
To explore whether amino acid balance modulates high-protein diets toxicity, we fed ants with the previous free amino acid diets and new ones matching the translation of Argentine ant exome. The ‘Argentine ant exome’ diet had a higher proportion of glutamic acid, glutamine or proline but lower proportions of alanine, cysteine or serine than the ‘casein-whey-egg’ diet (figure 5a). We hypothesized that the ‘Argentine ant exome’ diet would not produce the deleterious effect of high-protein diets because it could theoretically better fit the ant need for amino acids. However, we found no statistically significant difference in the survival between those two free amino acids compositions, regardless of the P : C ratio (figure 5b; p = 0.130; electronic supplementary material, table S17).
Figure 5.
Effect of amino acid composition on ant lifespan. (a) Comparison of the amino acid profile of the ‘casein-whey-egg’ and ‘Argentine ant exome’ free amino acid diets. The composition across ratios is proportional, 5 : 1 diets are plotted as an example. (b) Survival curves for isolated ants fed on free amino acid diets at [PAA + C] = 0.10 g g−1 and varying in their PAA : C ratios and their proportion of amino acids. We have tested 50 ants for each diet. Ala, alanine; Arg, arginine; Asn, asparagine; Asp, aspartic acid; Cys, cysteine; Gln, glutamine; Glu, glutamic acid; Gly, glycine; His, histidine; Ile, isoleucine; Leu, leucine; Lys, lysine; Met, methionine; Phe, phenylalanine; Pro, proline; Ser, serine; Thr, threonine; Trp, tryptophan; Tyr, tyrosine; Val, valine.
(d). Effect of single amino acids on ant lifespan
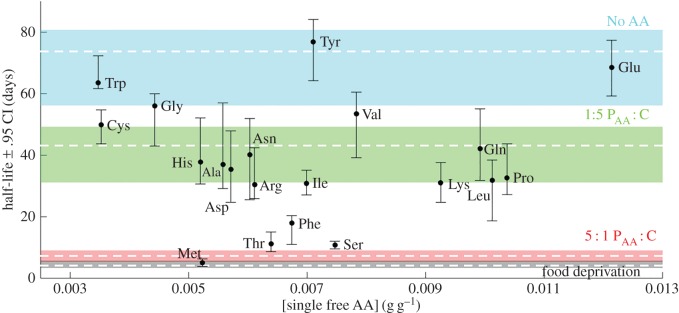
After finding that free amino acids were more toxic than whole proteins, we investigated the individual roles of each of them. Ants lived the longest in the ‘no AA’ diet (P : C ratio ≈ 1 : 6, [P + C] = 0.02 g g−1), indicating little need for amino acids; and, as expected, ‘food deprivation’ and 5 : 1 PAA : C dramatically shortened ant survival (figure 6). Most of the single amino acid diets shortened lifespan with respect to ‘no AA’ diet, except glutamate, tyrosine and tryptophan (figure 6; electronic supplementary material, table S18). Among those that shortened lifespan, four were especially harmful: phenylalanine, serine, threonine and methionine (figure 6). Methionine shortened lifespan by as much as the 5 : 1 PAA : C and ‘food deprivation’ diets (p = 0.597 and p = 0.115; electronic supplementary material, table S19–S20). The differences in concentration between amino acids do not explain the differences in amino acid toxicity (p = 0.440; electronic supplementary material, table S21). In the single amino acid, the total amino acid concentration is much lower than that of the 5 : 1 PAA : C diet (on average, 1/20), because we added only one amino acid instead of 20. Accordingly, the PAA : C ratios were much lower than 5 : 1 (approx. between 1 : 6 and 2 : 3). It is remarkable that such low concentrations of a single amino acid can have such a dramatic effect.
Figure 6.
Effect of single amino acids on ant lifespan. Half-life of isolated ants on every single amino acid diet as a function of the single free amino acid concentration. Diets where no amino acid was added (no AA), 1 : 5 PAA : C, 5 : 1 PAA : C and starvation (Food deprivation) are plotted as coloured bands for comparison. We have tested 50 ants for each diet. Data are represented as half-life ± 95% CI. Ala, alanine; Arg, arginine; Asn, asparagine; Asp, aspartic acid; Cys, cysteine; Gln, glutamine; Glu, glutamic acid; Gly, glycine; His, histidine; Ile, isoleucine; Leu, leucine; Lys, lysine; Met, methionine; Phe, phenylalanine; Pro, proline; Ser, serine; Thr, threonine; Trp, tryptophan; Tyr, tyrosine; Val, valine.
4. Discussion
We have found that, for sterile Argentine ant workers, bypassing the possible harmful side effects of protein digestion (by providing free amino acids directly) did not increase the survival on high-protein diets; on the contrary, it increased mortality, producing an approximately five times more potent dose effect. Digested proteins are absorbed in the insect midgut as small peptides or amino acids [27,28]; however, ant workers have a reduced enzymatic presence in their midguts [29,30] that might limit their capacity to break down proteins into amino acids. Ants eating whole proteins might then absorb just a small proportion of amino acids, whereas those eating free amino acids amino might potentially absorb all of them. The increased mortality under free amino acid diets indicates that the ultimate products of protein digestion, the amino acids, are responsible for the high-protein diet toxicity [2,5–7].
An excess in amino acids might increase potentially toxic nitrogen waste products [10] or over-stimulate nutrient-sensing pathways that regulate lifespan (such as the TOR pathway [11–13]). The gut microbiota, which is important for ant nutrition [31] and sensitive to dietary macronutrients [32], might also play an important role in the physiological response to the diet. Additionally, ant workers do not seem to need proteins in their diet (figure 6) [6,26] and might have a reduced expression of hexamerins (storage proteins) [33], leading to a less efficient way to deal with the amino acid excess under high-protein diets. Ant larvae, on the contrary, have higher levels of protease activity [30] and hexamerin expression [33]. Indeed, we found that, regardless of the amino acid source, colonies with larvae lived longer under high-protein diets than those without. This suggests that larvae process the amino acid excess under high-protein diets.
In addition to the effects on lifespan, we found that ants showed a very different foraging strategy when presented with whole proteins or free amino acids. Similarly to other ants [6,16–18], Argentine ants compensated for the insufficient amount of sugar in high-protein diets by collecting more food, thereby stabilizing sugar collection across a wide range of P : C ratios, irrespective of the excess of protein ingested. However, for free amino acid diets, ants followed the opposite rule: the more amino acids in the food, the less food the ants collected. Alternatively, instead of adapting their foraging strategy to the free amino acid presence, ants could be intoxicated by the amino acid excess and therefore eat less. However, the strategy for free amino acid diets was established on the first day of the experiments (electronic supplementary material, figure S4), just 2 h after the first food administration, whereas significant differences in survival appeared only after 4 days for 5 : 1 treatments and after 30 days for the 1 : 5 treatments. This suggests that ants might stop collecting food once the target for amino acid has been reached, irrespective of the shortfall of carbohydrate ingested.
The underlying mechanisms supporting a different strategy for free amino acid and for whole protein foods might be twofold. First, ants might not be able to ‘detect’ the presence of whole proteins in the high-protein diet and perceive it more as a diluted carbohydrate diet. Indeed, ants increase their consumption of food when faced with highly diluted carbohydrate diet [25]. Free amino acid taste receptors have not yet been described in ants, but they presumably exist given that they are widespread among insects [34,35] and that ants react differently to food sources presenting free amino acids [36,37]. In a previous study, we have shown that, when given the choice between 5 : 1 PWP : C versus 5 : 1 PAA : C, Argentine ants prefer free amino acid diets over whole protein diets [38]. The perception of free amino acids, through their corresponding taste receptors, might underlie their nutritional regulation strategy via satiation effects. Work on locusts has shown that an elevated level of amino acids in the blood triggers protein repletion and a desensitization of taste receptors detecting the amino acids in the food [39]. According to such a mechanism, when ants have eaten sufficient free amino acids, they would stop feeding irrespective of the amount of sugar ingested. This strategy might be similar to the human regulation of protein intake [40].
Second, the free amino acid mixtures might provide a greater effective dose to the ants than whole proteins and therefore elicit an early satiation. We observed that ants left food pellets outside the nest when fed whole protein diets, a behaviour also seen in other ant species [16,17]. As in previous studies, the amount of pellets was higher when the protein-to-carbohydrate ratio was high. Chemical analysis of pellets have revealed that ants are able to separate the proteins from the sugar and discard the excess proteins as pellets [16,17]. Interestingly, when ants were confined to free amino acid diets, they left almost no pellets. This observation suggests that while ants were able to manipulate the protein content before digestion of whole protein diets, they could not do it when the diet was based on free amino acids. This confirms that amino acid diets provided a greater effective dose of amino acids to the ants than the whole protein diets.
To examine the physiological response of ants to the various diets, we measured their lipid storage. Some species of ants respond to high-protein diets by increasing their lipid reserves as a possible strategy to survive sugar scarcity [17,18] and others, as observed here in the Argentine ant, see their lipid reserves decrease [19,41,42]. In our experiment, ants collected less free amino acid than whole protein diet, but they presented on average a higher body mass proportion of lipids than those feeding on whole proteins. Therefore, the cause of death cannot be the result of the depletion of the lipid reserves. We might speculate that lipid reserves in ants on free amino acid diets were higher because (i) ants had a lower activity rate owing to diet toxicity [43], (ii) they synthesized lipids from amino acids or (iii) they died before lipid depletion occurs.
The amino acid composition of food might be expected to modulate the response to high-protein diets as it is known to affect vital functions [44]. Here, we have shown that two different amino acid profiles have similar effects on ant survival (even though the ‘exome’ diet profile was especially designed to anticipate the amino acid requirement of the ants). Thus, we further explored the individual role of each amino acid on the high-protein toxicity and we found that the over-representation of methionine (Met), serine (Ser), threonine (Thr) and phenylalanine (Phe) were especially harmful. These amino acids when in excess decrease growth and/or lifespan in a wide variety of life forms such as unicellular algae (Met, Thr and Ser [45]), yeasts (Met, Thr and Ser [46–48]), roundworms (Phe [49]), flies (Met [50,51]), rodents (Met, Thr, Ser and Phe [46,52–54]), and even cancer tumour cells (Met and Phe [55]) and other human cells (Met [46]). Methionine is proposed to be related to oxidative stress processes [56]. Interestingly, injections of phenylalanine in rats induce metabolic disorders also associated with oxidative stress [57] and, in the African honeybee, the use of an antioxidant reduces the life-shortening effect of foods with an excess of free essential amino acids [58]. Despite their toxicity at high concentrations, these amino acids are essential (Met, Thr, Phe) or conditionally essential (Ser) for insects [59]. Indeed, some ants prefer sugar solutions enriched with these amino acids over solutions of pure sugar [36].
5. Conclusion
This study suggests us a potential role of amino acids on the regulatory feeding strategy of ants and has showed that amino acids are key elements triggering the reduction of lifespan under high-protein diets. Furthermore, we have identified four amino acids that are especially harmful when present at high concentrations relative to other amino acids and whose toxicity had been independently reported in organisms from yeasts to mammals.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank A Pérez-Escudero, J Gautrais, JFA Traniello and AP Hoadley for fruitful discussions and constructive comments on the manuscript; R. Shayan, E. Biyong, M. Lanter, A. Felden and D. Vogel for their help during the experiments; M. Piper for providing the Argentine ant exome proportion of amino acids; and the Laplace Institute (Toulouse, France) for hosting S.A. during part of this project. We also thank the critical comments of three anonymous referees.
Data accessibility
Data are available at http://dx.doi.org/10.5061/dryad.d1hg5 [60].
Authors' contributions
A.D. conceived the study. S.A. and A.D. designed the study. S.A., S.Bo., S.Ba., S.L., C.P. and G.L. performed the experiments. S.A. performed data analysis and wrote the first draft of the manuscript, S.J.S. and A.D. contributed to revisions. A.D. secured finding. All authors gave final approval for publication.
Competing interests
No competing interests.
Funding
S.A. was supported by an ANR 11 JSV7 009 01 and a Marie Skłodowska-Curie Individual Fellowship (660976). The research was supported by a grant from the ‘Agence Nationale de la Recherche’, reference no. JSV7-0009-01.
References
- 1.Simpson SJ, Raubenheimer D. 2012. The nature of nutrition: a unifying framework from animal adaptation to human obesity. Princeton, NJ: Princeton University Press.
- 2.Le Couteur DG, Solon-Biet S, Cogger VC, Mitchell SJ, Senior A, de Cabo R, Raubenheimer D, Simpson SJ. 2015. The impact of low-protein high-carbohydrate diets on aging and lifespan. Cell. Mol. Life Sci. 73, 1237 ( 10.1007/s00018-015-2120-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fontana L, Partridge L. 2015. Promoting health and longevity through diet: from model organisms to humans. Cell 161, 106–118. ( 10.1016/j.cell.2015.02.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piper MDW, Partridge L, Raubenheimer D, Simpson SJ. 2011. Dietary restriction and aging: a unifying perspective. Cell Metab. 14, 154–160. ( 10.1016/j.cmet.2011.06.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JWO, Taylor PW, Soran N, Raubenheimer D. 2008. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc. Natl Acad. Sci. USA 105, 2498–2503. ( 10.1073/pnas.0710787105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dussutour A, Simpson SJ. 2012. Ant workers die young and colonies collapse when fed a high-protein diet. Proc. R. Soc. B 279, 2402–2408. ( 10.1098/rspb.2012.0051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solon-Biet SM, et al. 2015. Macronutrient balance, reproductive function, and lifespan in aging mice. Proc. Natl Acad. Sci. USA 112, 201422041 ( 10.1073/pnas.1422041112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westerterp KR, Wilson SA, Rolland V. 1999. Diet induced thermogenesis measured over 24 h in a respiration chamber: effect of diet composition. Int. J. Obes. Relat. Metab. Disord. 23, 287–292. ( 10.1038/sj.ijo.0800810) [DOI] [PubMed] [Google Scholar]
- 9.Halton TL, Hu FB. 2004. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J. Am. Coll. Nutr. 23, 373–385. ( 10.1080/07315724.2004.10719381) [DOI] [PubMed] [Google Scholar]
- 10.Wright P. 1995. Review nitrogen excretion: three end products, many physiological roles. J. Exp. Biol. 281, 273–281. [DOI] [PubMed] [Google Scholar]
- 11.Pankaj K, Brian Z. 2004. TOR pathway: linking nutrient senseing to life span. Sci. Aging Knowl. Environ. 2004, 1–7. ( 10.1126/sageke.2004.36.pe34.TOR) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simpson SJ, Raubenheimer D. 2009. Macronutrient balance and lifespan. Aging (Albany, NY) 1, 875–880. ( 10.18632/aging.100098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emran S, Yang M, He X, Zandveld J, Piper MDW. 2014. Target of rapamycin signalling mediates the lifespan-extending effects of dietary restriction by essential amino acid alteration. Aging (Albany, NY) 6, 390–398. ( 10.18632/aging.100665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson MM, Evans HM. 1953. Relation of dietary protein levels to reproduction in the rat. J. Nutr. 51, 71–84. [DOI] [PubMed] [Google Scholar]
- 15.Hunt J, Brooks R, Jennions MD, Smith MJ, Bentsen CL, Bussière LF. 2004. High-quality male field crickets invest heavily in sexual display but die young. Nature 432, 1024–1027. ( 10.1038/nature03084) [DOI] [PubMed] [Google Scholar]
- 16.Dussutour A, Simpson SJ. 2009. Communal nutrition in ants. Curr. Biol. 19, 740–744. ( 10.1016/j.cub.2009.03.015) [DOI] [PubMed] [Google Scholar]
- 17.Cook SC, Eubanks MD, Gold RE, Behmer ST. 2010. Colony-level macronutrient regulation in ants: mechanisms, hoarding and associated costs. Anim. Behav. 79, 429–437. ( 10.1016/j.anbehav.2009.11.022) [DOI] [Google Scholar]
- 18.Bazazi S, Arganda S, Moreau M, Jeanson R, Dussutour A. 2016. Responses to nutritional challenges in ant colonies. Anim. Behav. 111, 235–249. ( 10.1016/j.anbehav.2015.10.021) [DOI] [Google Scholar]
- 19.Dussutour A, Poissonnier L-A, Buhl J, Simpson SJ. 2016. Resistance to nutritional stress in ants: when being fat is advantageous. J. Exp. Biol. 219, 824–833. ( 10.1242/jeb.136234) [DOI] [PubMed] [Google Scholar]
- 20.Smith CD, et al. 2011. Draft genome of the globally widespread and invasive Argentine ant (Linepithema humile). Proc. Natl Acad. Sci. USA 108, 5673–5678. ( 10.1073/pnas.1008617108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodring J, Wiedemann R, Fischer MK, Hoffmann KH. 2004. Honeydew amino acids in relation to sugars and their role in the establishment of ant-attendance hierarchy in eight species of aphids feeding on tansy (Tanacetum vulgare). Physiol. Entomol. 29, 311–319. ( 10.1111/j.0307-6962.2004.00386.x) [DOI] [Google Scholar]
- 22.Dussutour A, Simpson SJ. 2008. Description of a simple synthetic diet for studying nutritional responses in ants. Insect. Soc. 55, 329–333. ( 10.1007/s00040-008-1008-3) [DOI] [Google Scholar]
- 23.Bhatkar A, Whitcomb WH. 1970. Artificial diet for rearing various species of ants. Florida Entomol. 53, 229–232. ( 10.2307/3493193) [DOI] [Google Scholar]
- 24.Nimalaratne C, Lopes-Lutz D, Schieber A, Wu J. 2011. Free aromatic amino acids in egg yolk show antioxidant properties. Food Chem. 129, 155–161. ( 10.1016/j.foodchem.2011.04.058) [DOI] [Google Scholar]
- 25.Dussutour A, Simpson SJ. 2008. Carbohydrate regulation in relation to colony growth in ants. J. Exp. Biol. 211, 2224–2232. ( 10.1242/jeb.017509) [DOI] [PubMed] [Google Scholar]
- 26.Hölldobler B, Wilson EO.1990. The ants. Cambridge, MA: Harvard University Press.
- 27.Chown S, Nicolson S. 2004. Insect physiological ecology: mechanisms and patterns. Oxford, UK: Oxford University Press. ( 10.1093/acprof:oso/9780198515494.001.0001) [DOI]
- 28.Roman G, Meller V, Wu KH, Davis RL. 1998. The opt1 gene of Drosophila melanogaster encodesa proton-dependent dipeptide transporter. Am. J. Physiol. 275, C857–C869. [DOI] [PubMed] [Google Scholar]
- 29.Ricks BL, Vinson SB. 1972. Digestive enzymes of the imported fire ant, Solenopsis richteri (Hymenoptera: Formicidae). Ann. Entomol. Soc. Am. 15, 135–138. ( 10.1111/j.1570-7458.1972.tb00217.x) [DOI] [Google Scholar]
- 30.Erthal M, Peres Silva C, Ian Samuels R. 2007. Digestive enzymes in larvae of the leaf cutting ant, Acromyrmex subterraneus (Hymenoptera: Formicidae: Attini). J. Insect Physiol. 53, 1101–1111. ( 10.1016/j.jinsphys.2007.06.014) [DOI] [PubMed] [Google Scholar]
- 31.Russell JA, Moreau CS, Goldman-Huertas B, Fujiwara M, Lohman DJ, Pierce NE. 2009. Bacterial gut symbionts are tightly linked with the evolution of herbivory in ants. Proc. Natl Acad. Sci. USA 106, 21 236–21 241. ( 10.1073/pnas.0907926106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faith JJ, et al. 2011. Predicting a human gut microbiota's response to diet in gnotobiotic mice. Science 333, 101–104. ( 10.1126/science.1206025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wheeler DE, Martinez T. 1995. Storage proteins in ants (Hymenoptera:Formicidae). Comp. Biochem. Physiol. Part B, Biochem. 112, 15–19. ( 10.1016/0305-0491(95)00035-7) [DOI] [PubMed] [Google Scholar]
- 34.Hallem EA, Dahanukar A, Carlson JR. 2006. Insect odor and taste receptors. Annu. Rev. Entomol. 51, 113–135. ( 10.1146/annurev.ento.51.051705.113646) [DOI] [PubMed] [Google Scholar]
- 35.Thorne N, Chromey C, Bray S, Amrein H. 2004. Taste perception and coding in Drosophila. Curr. Biol. 14, 1065–1079. ( 10.1016/j.cub.2004.05.019) [DOI] [PubMed] [Google Scholar]
- 36.Lanza J, Krauss B. 1984. Detection of amino acids in artificial nectars by two tropical ants, Leptothorax and Monomorium. Oecologia 63, 423–425. ( 10.1007/BF00390676) [DOI] [PubMed] [Google Scholar]
- 37.Blüthgen N, Fiedler K. 2004. Preferences for sugars and amino acids and their conditionality in a diverse nectar-feeding ant community. J. Anim. Ecol. 73, 155–166. ( 10.1111/j.1365-2656.2004.00789.x) [DOI] [Google Scholar]
- 38.Arganda S, Nicolis SC, Perochain A, Péchabadens C, Latil G, Dussutour A. 2014. Collective choice in ants: the role of protein and carbohydrates ratios. J. Insect Physiol. 69, 19–29. ( 10.1016/j.jinsphys.2014.04.002) [DOI] [PubMed] [Google Scholar]
- 39.Simpson C, Simpson S, Abisgold J. 1990. The role of various amino acids in the protein compensatory response of Locusta migratoria. Symp. Biol. Hungarica 39, 39–46. [Google Scholar]
- 40.Simpson SJ, Batley R, Raubenheimer D. 2003. Geometric analysis of macronutrient intake in humans: the power of protein? Appetite 41, 123–140. ( 10.1016/S0195-6663(03)00049-7) [DOI] [PubMed] [Google Scholar]
- 41.Shik JZ, Kay AD, Silverman J. 2014. Aphid honeydew provides a nutritionally balanced resource for incipient Argentine ant mutualists. Anim. Behav. 95, 33–39. ( 10.1016/j.anbehav.2014.06.008) [DOI] [Google Scholar]
- 42.Feldhaar H. 2014. Ant nutritional ecology: linking the nutritional niche plasticity on individual and colony-level to community ecology. Curr. Opin. Insect Sci. 5, 25–30. ( 10.1016/j.cois.2014.09.007) [DOI] [PubMed] [Google Scholar]
- 43.Poissonnier LA, Simpson SJ, Dussutour A. 2014. Observations of the egg white injury in ants. PLoS ONE 9, 1–10. ( 10.1371/journal.pone.0112801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grandison RC, Piper MDW, Partridge L. 2009. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 462, 1061–1064. ( 10.1038/nature08619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oda Y, Nakano Y, Kitaoka S. 1982. Utilization and toxicity of exogenous amino acids in Euglena gracilis. Microbiology 128, 853–858. ( 10.1099/00221287-128-4-853) [DOI] [Google Scholar]
- 46.Johnson JE, Johnson FB. 2014. Methionine restriction activates the retrograde response and confers both stress tolerance and lifespan extension to yeast, mouse and human cells. PLoS ONE 9, e97729 ( 10.1371/journal.pone.0097729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Z, Song L, Liu SQ, Huang D. 2013. Independent and additive effects of glutamic acid and methionine on yeast longevity. PLoS ONE 8, e79319 ( 10.1371/journal.pone.0079319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirisola MG, Taormina G, Fabrizio P, Wei M, Hu J, Longo VD. 2014. Serine- and threonine/valine-dependent activation of PDK and Tor orthologs converge on Sch9 to promote aging. PLoS Genet. 10, e1004113 ( 10.1371/journal.pgen.1004113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edwards C, Canfield J, Copes N, Brito A, Rehan M, Lipps D, Brunquell J, Westerheide SD, Bradshaw PC. 2015. Mechanisms of amino acid-mediated lifespan extension in Caenorhabditis elegans. BMC Genet. 16, 8 ( 10.1186/s12863-015-0167-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Troen AM, French EE, Roberts JF, Selhub J, Ordovas JM, Parnell LD, Lai CQ. 2007. Lifespan modification by glucose and methionine in Drosophila melanogaster fed a chemically defined diet. Age (Omaha). 29, 29–39. ( 10.1007/s11357-006-9018-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee BC, Kaya A, Ma S, Kim G, Gerashchenko MV, Yim SH, Hu Z, Harshman LG, Gladyshev VN. 2014. Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino-acid status. Nat. Commun. 5, 1–12. ( 10.1038/ncomms4592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richie JP, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA. 1994. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J. 8, 1302–1307. [DOI] [PubMed] [Google Scholar]
- 53.Muramatsu K, Odagiri H, Morishita S, Takeuchi H. 1971. Effect of excess levels of individual amino acids on growth of rats fed casein diets. J. Nutr. 101, 1117–1126. [DOI] [PubMed] [Google Scholar]
- 54.Orentreich N, Matias JR, DeFelice A, Zimmerman JA. 1993. Low methionine ingestion by rats extends life span. J. Nutr. 123, 269–274. [DOI] [PubMed] [Google Scholar]
- 55.Kulcsár G, Gaál D, Kulcsár PI, Schulcz Á, Czömpöly T. 2013. A mixture of amino acids and other small molecules present in the serum suppresses the growth of murine and human tumors in vivo. Int. J. Cancer 132, 1213–1221. ( 10.1002/ijc.27756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koc A, Gasch AP, Rutherford JC, Kim HY, Gladyshev VN. 2004. Methionine sulfoxide reductase regulation of yeast lifespan reveals reactive oxygen species-dependent and -independent components of aging. Proc. Natl Acad. Sci. USA 101, 7999–8004. ( 10.1073/pnas.0307929101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kienzle Hagen ME, Pederzolli CD, Sgaravatti AM, Bridi R, Wajner M, Wannmacher CMD, Wyse ATS, Dutra-Filho CS. 2002. Experimental hyperphenylalaninemia provokes oxidative stress in rat brain. Biochim. Biophys. Acta 1586, 344–352. ( 10.1016/S0925-4439(01)00112-0) [DOI] [PubMed] [Google Scholar]
- 58.Archer CR, Köhler A, Pirk CWW, Oosthuizen V, Apostolides Z, Nicolson SW. 2014. Antioxidant supplementation can reduce the survival costs of excess amino acid intake in honeybees. J. Insect Physiol. 71, 78–86. ( 10.1016/j.jinsphys.2014.10.006) [DOI] [PubMed] [Google Scholar]
- 59.Boudko DY. 2012. Molecular basis of essential amino acid transport from studies of insect nutrient amino acid transporters of the SLC6 family (NAT-SLC6). J. Insect. Physiol. 58, 433–449. ( 10.1016/j.jinsphys.2011.12.018.Molecular) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arganda Carreras S, Bouchebti S, Bazazi S, Le Hesran S, Puga C, Latil G, Simpson SJ, Dussutour A. 2017. Data from: Parsing the life-shortening effects of dietary protein: effects of individual amino acids. Dryad Digital Repository. ( 10.5061/dryad.d1hg5) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available at http://dx.doi.org/10.5061/dryad.d1hg5 [60].