Abstract
Extensive studies have shown that the current assays used to identify cattle infected with Mycobacterium bovis or Mycobacterium avium subsp. paratuberculosis are not sufficiently sensitive and specific to detect all infected animals, especially animals recently infected with the pathogens. In the present report we show that these limitations might be overcome with a latex bead agglutination assay (LBAA). With the specific immunodominant epitope (ESAT6-p) of M. bovis, we developed an LBAA and enzyme immunoassay (EIA) for that purpose and compared them with the “gold standard” culture method and skin test for their efficacy in detecting bovine tuberculosis. When sera from control healthy cows (n = 10), M. avium subsp. paratuberculosis-positive cattle (naturally infected, n = 16; experimentally infected, n = 8), and M. bovis-positive cattle (naturally infected, n = 49;experimentally infected, n = 20) were applied to an EIA and an LBAA developed with ESAT6-p, the two tests showed similar sensitivity (97.1% by EIA, 95.7% by LBAA), high specificity (94.2% by EIA, 100% by LBAA), and a positive correlation (kappa value, 0.85; correlation rate, 93.2%; correlation coefficient, 0.64). Receiver operating characteristic analysis of EIA results and comparison with the culture method determined a suitable cutoff value at 0.469, with an area under the curve of 0.991 (95% confidence interval, 0.977 to 1.0). As LBAA didn't show any positive reactions with sera from uninfected control cows or M. avium subsp. paratuberculosis-infected cattle, which were confirmed to be free of M. bovis by culture or PCR, LBAA using the ESAT6-p can be a rapid and useful M. bovis diagnostic assay. The data suggest that rapid, sensitive, and specific assays can be developed with peptides containing immunodominant epitopes present in proteins uniquely expressed in M. bovis or M. avium subsp. paratuberculosis for differential diagnosis of cattle infected with M. bovis or M. avium subsp. paratuberculosis.
Mycobacterium bovis, the causative agent of bovine tuberculosis (bTB) (13), and Mycobacterium avium subsp. paratuberculosis, the causative agent of Johne's disease (paratuberculosis [pTB]), are of economic importance worldwide and a potential health hazard for animals and humans (9). Efforts to control these diseases have been difficult because of the lack of effective vaccines and the lack of diagnostic assays that can identify infected animals before the appearance of clinical disease. This has been a major problem in the control of pTB because infected animals begin to shed bacteria in feces and contaminate the environment well before signs of clinical disease. Difficulty in controlling the diseases has also been compounded by the presence of reservoirs of M. bovis and M. avium subsp. paratuberculosis in wild animal reservoirs (9, 11, 15, 17, 20). To address these problems, there is a need for a continued effort to develop diagnostic assays with greater sensitivity (Se) and specificity (Sp) that can be used in the field and laboratory, ideally assays that can be formatted for use with multiple species. Since the ESAT6 protein is secreted at an early or active phase of mycobacterial infection but not from M. bovis BCG-vaccinated animals (5, 22, 25, 26), synthetic ESAT6 peptides (ESAT6-p) were prepared to establish early specific detection of M. bovis serologically. In this report we show that a latex bead agglutination assay (LBAA) with synthetic peptides containing species-specific mycobacterial epitopes, ESAT6-p, may provide an approach to developing rapid diagnostic assays for bTB and pTB.
MATERIALS AND METHODS
Animals. (i) M. bovis-infected cattle.
Three different groups of bovine sera were used in this study. A herd surveillance program in Korea has been checking for bTB-positive cows among all the cattle in Korea by using skin tests and then following up with cultures from suspect cows at least two times a year. Within 10 days after a positive skin intradermal test reaction (skin thickness, over 5 mm) in this national herd check program, sera were obtained from 49 cows documented to be naturally infected with M. bovis, which was verified by culture of M. bovis from intestinal tissue or nasal and tracheal mucus at the time of necropsy. Although acute miliary TB with developing necrotic focus was observed in tracheobronchial and mediastinal lymph nodes of 10 cows, those cows were not thought to be in an advanced stage of bTB, as only a mild cough was observed as a clinical sign, without weight loss, swelling of lymph nodes in the head, or marked lymphadenomegaly with multiple nodular pale granulomas at necropsy. Additional sera were obtained from 20 calves (four groups of five calves each) experimentally infected with aerosol challenge of 103 or 105 CFU of either of two different strains of M. bovis, one isolated from white-tailed deer (strain 1315) and the other isolated from cattle (HC2005T). Serial samples of sera were collected at monthly intervals for 4 months pre- and postinfection (1 week preinfection and 27, 78, and 137 days postinfection) (18). Infection was confirmed in 19 calves by tuberculin skin test, isolation of M. bovis, and gross or microscopic tuberculous lesions in the lungs and tracheobronchial and mediastinal lymph nodes at the time of necropsy.
(ii) M. paratuberculosis-infected cattle.
Sera were collected from 16 cows naturally infected with M. avium subsp. paratuberculosis, as verified by clinical signs of advanced pTB or by use of the PARACHEK Johne's absorbed enzyme-linked immunoabsorbent assay (EIA; CSL Veterinary, Parkville, Victoria, Australia) and the isolation of M. avium subsp. paratuberculosis from the intestine at necropsy. Additionally, blood samples were obtained from three male Holstein calves inoculated intratonsillarly with the K10 strain of M. avium subsp. paratuberculosis (28) and five male Holstein calves exposed orally to a field strain (01-13665, Washington State University) of M. avium subsp. paratuberculosis. Those eight calves were not older than 1 year. The experimental M. avium subsp. paratuberculosis infection was confirmed by the peripheral M. avium subsp. paratuberculosis colonization detected by culture and IS900 PCR with feces and intestinal tissues in three intratonsillarly M. avium subsp. paratuberculosis-infected calves (28) and analysis of the immune response to M. avium subsp. paratuberculosis antigens in all eight calves (reference 28 and unpublished data). All of the M. avium subsp. paratuberculosis-positive cattle were confirmed to be free of M. bovis by M. bovis culture and PCR with intestinal tissue or fecal samples. The sera from naturally and experimentally infected cattle with M. avium subsp. paratuberculosis were included in this study to demonstrate there were no cross-reactive antibodies present in sera from M. avium subsp. paratuberculosis-infected cattle.
(iii) Negative controls.
Control sera were obtained from 10 cows from the Washington State University dairy herd, which is free of M. bovis and M. avium subsp. paratuberculosis.
Preparation of ESAT6-p-conjugated latex beads.
Latex beads [P(S/V-COOH); near soap free; 0.85 μm] were obtained from Bangs Labs Inc. The peptide sequence of ESAT6 (KGSGSMTEQQWNFAGIEAAASAIQG) known to contain an epitope recognized by antibodies from infected animals (7, 23) was synthesized as a single peptide with the peptide synthesizer ABI model 431A version 2.00 SSFmoc in the School of Molecular Biosciences at Washington State University (14). An extra lysine, glycine, and serine were added to the N-terminal end of ESAT6-p to enhance hydrophilicity and introduce a moiety for making an amide bond with the carboxylate groups on the beads (8).
Different concentrations of ESAT6-p were conjugated to beads by the following protocol and then tested for their capacity to agglutinate in the presence of sera from M. bovis-infected cattle. To conjugate the beads with ESAT6-p, the beads were washed four times, resuspended in an activation buffer (morpholineethanesulfonic acid buffer, pH 5.2), and then activated by mixing with 10 mM 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (Sigma, St. Louis, Mo.) and 5 mM sulfo-N-hydroxysuccinimide (Sigma) (24). After mixing for 30 min on a rotating wheel, the beads were washed and resuspended in coupling buffer (borate buffer, pH 8.5) and then reacted with different concentrations of ESAT6-p (dissolved in dimethyl sulfoxide). The bead mixture was placed on the rotating wheel, mixed for 18 h at room temperature, and then resuspended in a blocking solution (10 mM glycine) and mixed for an additional 30 min. In the final step, the beads were washed and then incubated for 3 h at room temperature in cold phosphate-buffered saline (PBS, pH 7.2) containing 1% bovine serum albumin and 0.05% Tween 20. The conjugated beads were stored at 4°C until used.
To standardize the assay, a checkerboard array was used in 96-well round-bottom tissue culture plates, with different concentrations of conjugated beads (50 μl/well in PBS) mixed with different concentrations of sera (50 μl/well) from uninfected control and M. bovis-infected animals (documented at necropsy and culture of M. bovis from tissue or from nasal and tracheal mucus). After determining the optimal conditions for obtaining antigen-specific agglutination, the LBAA was conducted with serial dilutions of sera from uninfected control cows and cattle infected with M. avium subsp. paratuberculosis or M. bovis.
Preparation of EIA for ESAT6-p.
To compare Se and Sp with the LBAA, an EIA was developed with ESAT6-p and standardized by checkerboard titration to determine the optimal concentration of ESAT6-p and serum. After developing the EIA with ESAT6-p, EIAs using all the sera samples were done once on the same day, and the identical sera from three healthy control cows, three M. avium subsp. paratuberculosis-infected cows, and three M. bovis-infected cows were applied repeatedly in every EIA plate in order to check the intra- and interassay variation of optical density (OD) values. Ninety-six-well flat-bottom EIA plates were coated with 0.25 μg of ESAT6-p in alkaline phosphatase binding buffer (0.05 M Tris; pH 9.5) at 4°C overnight, washed, and then blocked with 1% bovine serum albumin in 0.05 M carbonate buffer (pH 9.6) at 37°C for 2 h using standard procedures (4).
To conduct the assay, triplicate dilutions of sera (1/10, 1/50, 1/100, and 1/200) from the uninfected control and M. avium subsp. paratuberculosis- or M. bovis-infected animals were added to the plates (100 μl/well). The plates were incubated at room temperature or 37°C for 1 h, then washed in PBS, and reacted with 100 μl of a 1:25,000 dilution of biotinylated rabbit-anti bovine immunoglobulin G (H+L; Zymed) for 1 h. Following washing in PBS, the plates were reacted with 100 μl of substrate (p-nitrophenyl phosphate, disodium; Sigma) diluted in substrate buffer (1 mg/ml; pH 10.5). Following 30 min of incubation, 50 μl of 3 N NaOH was added to stop the reaction. Absorbance was read at 405 nm with an MCC340 EIA reader (Dynatech). The mean ratio of the OD of wells containing sera of infected animals to the OD of wells containing sera from uninfected animals (S/N) and standard error were determined by dividing the average OD of wells with sera from infected animals by the OD of wells containing sera from uninfected control animals.
EIA for antibody to M. avium subsp. paratuberculosis.
Serological responses against M. avium subsp. paratuberculosis were evaluated with the commercially available PARACHEK Johne's absorbed EIA (CSL Veterinary) with absorbed serum and HerdChek M. paratuberculosis (IDEXX Labs, Westbrook, Maine) to check possible M. avium subsp. paratuberculosis infection in M. bovis-infected cattle and ascertain serological positive responses in M. avium subsp. paratuberculosis-positive cattle used in this study. Briefly, absorption of serum was done by incubating 25 μl of test and control serum samples and 475 μl of the diluent in the kit at room temperature for 30 min. The EIA plates were incubated with 100 μl of absorbed test or control serum at 37°C for 1 h. After washing with wash buffer six times, the plates were reacted with 100 μl of freshly prepared 1× conjugate reagent (horseradish peroxidase-labeled antibovine immunoglobulin) at 37°C for 1 h, washed, and then reacted with freshly prepared 1× enzyme substrate solution (tetramethylbenzidine). When the yellow color appeared, 50 μl of enzyme stopping solution was added to stop the reaction. Absorbance was read at 450 nm with an MCC340 EIA reader (Dynatech). Plasma samples were considered positive if the OD from test samples was above the cutoff value (the mean of the negative controls plus 0.100), the OD from negative control was below 0.150, and the OD from the positive control was between 0.9 and 1.2. Duplicate serum samples were submitted to the diagnostic laboratory for evaluation with HerdChek M. paratuberculosis (IDEXX Labs).
Data analysis.
A suitable cutoff value for the EIA was determined with receiver operating characteristics (ROC) analysis (2) based on the results of the “gold standard” culture method and skin test after comparing Se and Sp at different cutoff values. The agreement among the result of the gold standard culture method, the LBAA, and the EIA was evaluated by the correlation rate (21) or the kappa value from a kappa test (1). The correlation between the OD value or S/N ratio from the EIA and the intensity of agglutination from the LBAA was based on the correlation coefficient from a Spearman rank correlation analysis (3). Commercially available software (Analyze-it) was used for all statistical analyses.
RESULTS
Standardization of the LBAA.
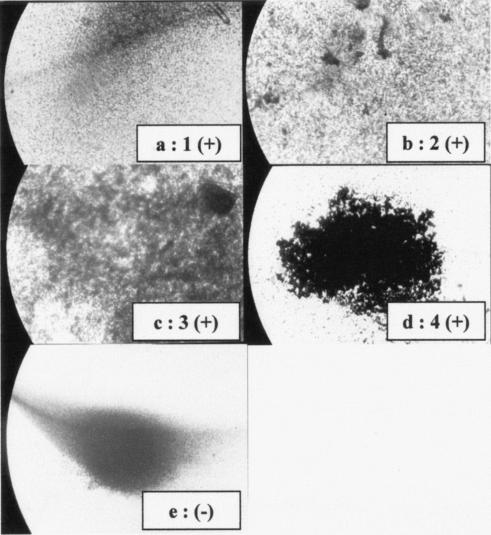
Testing of latex beads coated with different concentrations of ESAT6-p showed conjugation of 1 mg of beads with 45 μg of ESAT6-p was best for obtaining specific agglutination, with no agglutination evident with sera from uninfected control cows (Fig. 1). Examination of plates over a 4-h period revealed agglutination was evident with all test sera by 2 h. There was no change in the patterns of agglutination when the plates were incubated overnight at 4°C.
FIG. 1.
Representative pattern of agglutination of latex beads coated with ESAT6 and reacted with sera from an infected cow (a to d; positive agglutination scores of 1 to 4) and an uninfected cow (e; negative agglutination). An Image Analyzer (Olympus) and the Optimas 6.5 program were used to calculate and analyze the intensity of agglutination in 10 fields of 1 mm2 randomly selected in wells of the LBAA. Positive agglutination reactions were scored as follows: 1, 0 to 25%; 2, 26 to 50%; 3, 51 to 75%; and 4, 76 to 100%.
Se and Sp of EIA and LBAA.
After determining the optimal conditions for agglutination, sera from naturally or experimentally M. bovis-infected cattle were tested at four dilutions (1/5, 1/10, 1/20, and 1/40) along with sera from control healthy cows and M. avium subsp. paratuberculosis-infected cattle. Consistent results were obtained using a dilution of 1/20, and the resulting Se and Sp of LBAA are summarized in Table 1. The intensity of agglutination was calculated and analyzed in 10 fields of 1 mm2 randomly selected in wells of LBAA (19) by using an image analyzer (Olympus, Melville, N.Y.) and the Optimas 6.5 program. Agglutination was stronger with some sera than others, and agglutination was categorized as follows: score of 1, 0 to 25%; 2, 26 to 50%; 3, 51 to 75%; 4, 76 to 100% (Fig. 1). Although background agglutination was noted at higher concentrations of serum with some animals, no agglutination was evident with sera from control or M. avium subsp. paratuberculosis-infected cattle or from calves at the preinfection stage of aerosol M. bovis challenge.
TABLE 1.
Overall serological response of sera from naturally or experimentally M. bovis-infected cattle in EIA and LBAA utilizing ESAT6-p as coating antigen
| Assayb | Responsea to ESAT6-p in M. bovis-positive cattle
|
||
|---|---|---|---|
| Naturally infected cows (n = 49) | Experimentally inoculated calves (n = 20) | Total (n = 69) | |
| EIA | 49/49 (100) | 16/20 (80.0) | 65/69 (97.1) |
| LBAA | 46/49 (93.9) | 20/20 (100) | 66/69 (95.7) |
Results are expressed as number of sera positive/number of sera tested, with percent sensitivity shown in parentheses. Detailed information on how natural M. bovis infection was confirmed, how cattle were inoculated experimentally, and how a positive response in the EIA or LBAA was defined is provided in Materials and Methods.
There was no false-positive reaction of LBAA with sera from M. bovis-negative (naturally or experimentally M. avium subsp. paratuberculosis-infected or healthy) cattle, but one serum from the group of control healthy cows showed an OD of 0.469, which is the same as the selected cutoff value for the EIA used in this study.
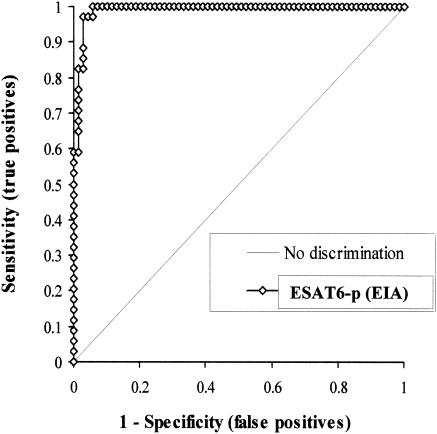
To compare Se and Sp and evaluate agreement between LBAA and EIA, the same sera were tested in an EIA format. As shown in Table 1, specific reactivity was obtained when sera were diluted to 1/50. Some sera yielded stronger reactions in EIA as well as in LBAA than other sera, as shown by the OD. After ROC analysis (Fig. 2), the area under the curve and standard error of the area under the curve were 0.991 (95% confidence interval [CI], 0.977 to 1.000) and 0.007, respectively. Among different cutoff values from the ROC analysis, a cutoff value of 0.469 was selected, as the Se of 97.1% and Sp of 94.2% at the chosen cutoff was optimal for our EIA. All M. bovis-positive sera from naturally M. bovis-infected cows confirmed by the culture of M. bovis at necropsy showed an OD which was greater than our selected cutoff value. Out of 20 bovine sera from experimentally M. bovis-inoculated calves, 4 sera showed a negative EIA result at the cutoff value.
FIG. 2.
ROC curves for EIA with ESAT6-p. Se and Sp were calculated as follows: Se = (number of serum samples showing positive culture and EIA results at each cutoff value)/(number of M. bovis culture positive results). Sp = (number of serum samples showing negative culture and EIA results at each cutoff value)/(number of M. bovis culture negative results). Both Se and Sp were based on the results of the M. bovis culture method as the standard and compared the two tests (culture versus EIA).
One calf experimentally infected with an aerosol challenge of 103 CFU of M. bovis isolated from cattle (HC2005T) didn't show any gross or microscopic findings, positive response at tuberculin skin test, or bacteriologic isolation in feces, lymph nodes, or other tissues (18). When sera from that calf were applied, sera from 27 days postinfection showed positive responses to ESAT6-p with the EIA and LBAA developed in this study, and other sera from 78 and 137 days following infection were also positive by LBAA with ESAT6-p.
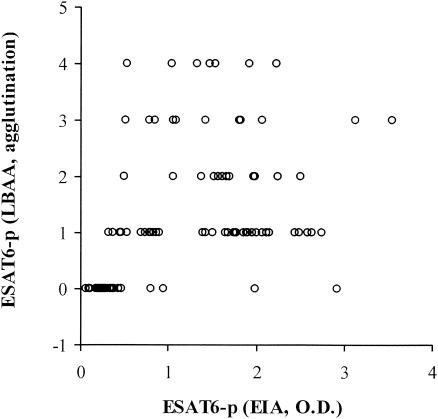
The correlation rate (21) or kappa value from the kappa test (1) was calculated in order to evaluate the agreement among results of the gold standard culture method, LBAA, and EIA. As a result of this evaluation, the correlation rates (and kappa values) between the gold standard culture method and EIA, between the culture method and LBAA, and between EIA and LBAA were 96.1% (kappa, 0.91), 97.1% (0.94), and 93.2% (0.85), respectively. After Spearman rank correlation analysis (3), the correlation coefficient between the OD value or S/N ratio from the EIA and the intensity of agglutination from the LBAA was 0.64 (95% CI, 0.51 to 0.74) (Fig. 3).
FIG. 3.
Spearman rank correlation analysis between the OD value or S/N ratio from the EIA and the intensity of agglutination from the LBAA. The two tests were positively correlated (P < 0.001), with a correlation coefficient of 0.64 (95% CI, 0.51 to 0.74).
EIA for antibody to M. avium subsp. paratuberculosis.
All of the sera from M. bovis-positive cattle (n = 69), control healthy cows (n = 10), and experimentally M. avium subsp. paratuberculosis-infected calves not older than 1 year (n = 8) showed a negative result with the PARACHEK Johne's absorbed EIA (CSL Veterinary) and the HerdChek M. paratuberculosis test (IDEXX Labs.). From the naturally M. avium subsp. paratuberculosis-infected cows (n = 16) that had clinical symptoms or showed a positive response with the Johne's skin test, two sera were negative for antibody to M. avium subsp. paratuberculosis.
DISCUSSION
In the early or active phase of infection, metabolically active mycobacteria secrete proteins such as ESAT-6 and CFP10 antigens, which were originally isolated from a highly stimulatory low-molecular-mass fraction of Mycobacterium tuberculosis filtrates and are located in the same operon (5, 22). They are very strongly recognized and induce gamma interferon release, and they are also found in recent converters to PPD positivity and in TB patients, but not in BCG-vaccinated or in unvaccinated individuals (25). Moreover, ESAT-6 (6 kDa) is virtually specific for M. tuberculosis complex; the gene is present in all isolates of M. tuberculosis and virulent M. bovis but is absent from all strains of BCG examined (22). Therefore, ESAT-6 and CFP10 are potential candidates for use in early detection as substitutes or as improved skin test antigens (26). In particular, ESAT-6 is an important T-cell antigen recognized by protective T cells in the early stages of M. tuberculosis infection in an animal model (22). Monoclonal antibody HYB76-8 specifically reacted with two peptides (p1-20 and p12-35) in the N-terminal region of the ESAT-6 protein, in which the p1-20 peptide (MTEQQWNFAGIEAAASAIQG) most strongly reacted with the monoclonal antibody HYB76-8 (6, 7). Thus, high specificity could be achieved with synthetic peptides, ESAT6-p, containing sequences from native proteins known to contain epitopes recognized by antibodies in sera from infected ani-mals.
Latex bead technology has been used successfully to develop simple, easy-to-use agglutination assays (12, 16, 27, 29, 30). Recombinant or synthetic peptides derived from species-specific M. bovis or M. avium subsp. paratuberculosis proteins that contain epitopes recognized by antibodies from infected animals could be used singly or multiplexed on a lysine polymer backbone by using currently available technology to detect antibodies specific for M. bovis or M. avium subsp. paratuberculosis (10).
In the present study we have demonstrated that an LBAA offers the potential of developing a relatively rapid assay for detecting animals infected with M. bovis. Serial samples of sera collected pre- and postinfection were applied to our developed EIA and LBAA in order to determine how early the serological response to ESAT6-p appears. Although all the sera from preinfection were negative by EIA and LBAA, 85, 100, and 100% of sera collected at 27, 78, and 137 days, respectively, following aerosol challenge with M. bovis showed a positive serological response to ESAT6-p by EIA or LBAA. Along with this early serological response to ESAT6-p in M. bovis-infected cattle, one of which showed a positive ESAT6-p response before fecal shedding, a tuberculin skin response, or histological changes, the high Se and Sp of EIA and LBAA and the high agreement between EIA and LBAA showed that the LBAA developed in our study can be a reliable and rapid M. bovis diagnostic assay. Importantly, the results suggest that a similar assay can be developed for M. avium subsp. paratuberculosis, following the identification of a specific and immunodominant epitope of M. avium subsp. paratuberculosis. An additional advantage that an LBAA offers is that the same assay can be used with multiple species, since no additional reagents are needed to develop the test. Further studies are now in progress to identify other peptides for use in the LBAA for M. bovis and M. avium subsp. paratuberculosis.
Acknowledgments
This study was funded in part by grants USDA-NRICGP 2002-35204-11688 and 2003-05165, USDA-APHIS-VS 03-9100-0788-GR, and 03-9100-07-GRT and intramural support from USDA Animal Health NV-00150. Further support was provided by the Research Institute of Veterinary Science, College of Veterinary Medicine, Seoul National University. The study was also supported by the Brain-Korea 21 project in Agricultural Biotechnology and the Washington State University Monoclonal Antibody Center.
REFERENCES
- 1.Altman, D. G. 1991. Practical statistics for medical research, p. 403-409. Chapman and Hall, London, England.
- 2.Beck, J. R., and E. K. Schultz. 1986. The use of relative operating characteristic (ROC) curves in test performance evaluation. Arch. Pathol. Lab. Med. 110:13-20. [PubMed] [Google Scholar]
- 3.Conover, W. J. 1980. Practical non-parametric statistics, 2nd ed., p. 252-256. John Wiley & Sons, New York, New York.
- 4.Crowther, J. R. 2000. The EIA guidebook. Methods Mol. Biol. 149:III-IV, 1-413. [DOI] [PubMed] [Google Scholar]
- 5.Dillon, D. C., M. R. Alderson, C. H. Day, T. Bement, A. Campos-Neto, Y. A. W. Skeiky, T. Vedvick, R. Badaro, S. G. Reed, and R. Houghton. 2000. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J. Clin. Microbiol. 38:3285-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harboe, M., T. Oettinger, H. G. Wiker, I. Rosenkrands, and P. Andersen. 1996. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in M. bovis BCG. Infect. Immun. 64:16-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harboe, M., A. S. Malin, H. S. Dockrell, H. G. Wiker, G. Ulvund, A. Holm, M. C. Jorgensen, and P. Andersen. 1998. B-cell epitopes and quantification of the ESAT-6 protein of Mycobacterium tuberculosis. Infect. Immun. 66:717-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermanson, G. T., A. K. Mallia, and P. K. Smith. 1992. Immobilized affinity ligand techniques. Academic Press, San Diego, Calif.
- 9.Hines, M. E., J. M. Kreeger, and A. J. Herron. 1995. Mycobacterial infections of animals: pathology and pathogenesis. Lab. Anim. Sci. 45:334-351. [PubMed] [Google Scholar]
- 10.Huang, W., B. Nardelli, and J. P. Tam. 1994. Lipophilic multiple antigen peptide system for peptide immunogen and synthetic vaccine. Mol. Immunol. 31:1191-1199. [DOI] [PubMed] [Google Scholar]
- 11.Hunter, D. L. 1996. Tuberculosis in free-ranging, semi-free-ranging and captive cervids. Rev. Sci. Tech. 15:171-181. [DOI] [PubMed] [Google Scholar]
- 12.Inzana, T. J., J. Todd, C. Koch, and J. Nicolet. 1992. Serotype specificity of immunological assays for the capsular polymer of Actinobacillus pleuropneumoniae serotypes 1 and 9. Vet. Microbiol. 31:351-362. [DOI] [PubMed] [Google Scholar]
- 13.Martin, S. W., R. A. Dietrich, P. Genho, W. P. Heuschele, R. L. Jones, M. Koller, J. D. Lee, H. C. Lopez, H. W. Moon, R. A. Robinson, P. L. Smith, and G. W. Williams. 1994. Livestock disease eradication: evaluation of the cooperative state-federal bovine tuberculosis eradication program. Board on Agriculture, National Research Council, National Academy of Sciences, Washington, D.C.
- 14.Merrifield, R. B. 1969. Solid-phase peptide synthesis. Adv. Enzymol. Relat. Areas Mol. Biol. 32:221-296. [DOI] [PubMed] [Google Scholar]
- 15.Mirsky, M. L., D. Morton, J. W. Piehl, and H. Gelberg. 1992. Mycobacterium bovis infection in a captive herd of Sika deer. J. Am. Vet. Med. Assoc. 200:1540-1542. [PubMed] [Google Scholar]
- 16.Nisengard, R. J., L. Mikulski, D. McDuffie, and P. Bronson. 1992. Development of a rapid latex agglutination test for periodontal pathogens. J. Periodontol. 63:611-617. [DOI] [PubMed] [Google Scholar]
- 17.Palmer, M. V., D. L. Whipple, J. B. Payeur, D. P. Alt, K. J. Esch, F. Bruning, and J. B. Kaneene. 2000. Naturally occurring tuberculosis in white-tailed deer. J. Am. Vet. Med. Assoc. 216:1921-1924. [DOI] [PubMed] [Google Scholar]
- 18.Palmer, M. V., W. R. Waters, and D. L. Whipple. 2002. Aerosol delivery of virulent Mycobacterium bovis to cattle. Tuberculosis 82:275-282. [DOI] [PubMed] [Google Scholar]
- 19.Perez, J., J. M. de las Mulas, F. C. De Lara, P. N. Gutierrez-Palomino, C. Becerra-Martel, and A. Martinez-Moreno. 1998. Immunohistochemical study of the local immune response to Fasciola hepatica in primarily and secondarily infected goats. Vet. Immunol. Immunopathol. 64:337-348. [DOI] [PubMed] [Google Scholar]
- 20.Schmitt, S. M., S. D. Fitzgerald, T. M. Cooley, F. Bruning, L. Sullivan, D. Berry, T. Carlson, R. B. Minnis, J. B. Payeur, and J. Sikarskie. 1997. Bovine tuberculosis in free-ranging white-tailed deer from Michigan. J. Wildl. Dis. 33:749-758. [DOI] [PubMed] [Google Scholar]
- 21.Silva, E. 2001. Evaluation of an enzyme-linked immunosorbent assay in the diagnosis of bovine tuberculosis. J. Vet. Microbiol. 78:111-117. [DOI] [PubMed] [Google Scholar]
- 22.Skjot, R. L. V., T. Oettinger, I. Rosenkrands, P. Ravn, I. Brock, S. Jacobsen, and P. Andersen. 2000. Comparative evaluation of low-molecular-mass proteins from Mycobacterium tuberculosis identifies members of the ESAT-6 family as immunodominant T-cell antigens. Infect. Immun. 68:214-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorensen, A. L., S. Nagai, G. Houen, P. Andersen, and A. B. Andersen. 1995. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 63:1710-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staros, J. V., R. W. Wright, and D. M. Swingle. 1986. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal. Biochem. 156:220-222. [DOI] [PubMed] [Google Scholar]
- 25.Ulrichs, T., P. Anding, S. Porcell, S. H. E. Kaufmann, and M. E. Munk. 2000. Increased numbers of ESAT-6- and purified protein derivative-specific gamma interferon-producing cells in subclinical and active tuberculosis infection. Infect. Immun. 68:6073-6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Pinxteren, L. A. H., P. Ravn, E. M. Agger, J. Pollock, and P. Andersen. 2000. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin. Diagn. Lab. Immunol. 7:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verloo, D., W. Holland, L. N. My, N. G. Thanh, P. T. Tam, B. Goddeeris, J. Vercruysse, and P. Buscher. 2000. Comparison of serological tests for Trypanosoma evansi natural infections in water buffaloes from North Vietnam. Vet. Parasitol. 20:87-96. [DOI] [PubMed] [Google Scholar]
- 28.Waters, W. R., J. M. Miller, M. V. Palmer, J. R. Stabel, D. E. Jones, K. A. Koistinen, E. M. Steadham, M. J. Hamilton, W. C. Davis, and J. P. Bannantine. 2003. Early induction of humoral and cellular immune responses during experimental Mycobacterium avium subsp. paratuberculosis infection of calves. Infect. Immun. 71:5130-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yam, W. C., M. L. Lung, and M. H. Ng. 1992. Evaluation and optimization of a latex agglutination assay for detection of cholera toxin and Escherichia coli heat-labile toxin. J. Clin. Microbiol. 30:2518-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto, A., M. Nakayama, M. Tashiro, T. Ogawa, and I. Kurane. 2000. Hydroxyapatite-coated nylon beads as a new reagent to develop a particle agglutination assay system for detecting Japanese encephalitis virus-specific human antibodies. J. Clin. Virol. 19:195-204. [DOI] [PubMed] [Google Scholar]