Abstract
Mammals commonly communicate olfactorily via urine. However, the extent to which they communicate via dung, another waste product, is unknown. Behavioural studies suggest that mammals can obtain information from dung odours but are unclear about the information transmitted. Moreover, an understanding of the volatile organic compounds (VOCs) released from dung is limited. To address this, we analysed the odours emitted from the dung of free-ranging white rhinos, and found that 2,3-dimethylundecane signalled an individual's sex, heptanal discriminated age class, nonane defined male territorial status and 2,6-dimethylundecane indicated female oestrous state. To validate these findings, we artificially reproduced key elements of the territorial and oestrous odour profiles (i.e. profiles likely to elicit behavioural responses from receivers). We then exposed free-ranging territorial males to these odours. In response, males elicited behaviours associated with the specific odours (e.g. territorial male (potential threat): reduced latency in assuming vigilance; oestrous female (potential mate): increased investigation). These results indicate that the VOCs identified from the dung of free-ranging individuals do transmit key information. Moreover, as white rhinos of all ages and sexes defecate communally, middens probably act as information centres. Furthermore, as many other mammals defecate communally, olfactory communication via dung odours is likely a widespread phenomenon.
Keywords: Ceratotherium simum, middens, olfactory communication, volatile compounds
1. Introduction
Olfactory signals are widely used by mammalian species [1–4] and the importance of urine in mammalian olfactory communication is well established [5–8]. Yet it is not clear to what extent dung, another waste product, relays specific information about individuals. There are examples of dung odours indicating oestrus in cows (Bos taurus) [9] and horses (Equus caballus) [10]. However, these are domesticated animals, and wild animals remain understudied. A large amount of information can be obtained from dung samples; for example, hormone metabolites indicating stress [11] or signalling dominance [12]. Yet it is unclear if these conditions are represented in the volatile organic compounds (VOCs) emitted from dung (i.e. odour). It has been suggested that the reason mammals defecate communally is to communicate olfactorily [13,14]. However, data on the VOCs emitted from dung are limited, especially in species using communal defecation sites (i.e. middens).
A wide range of mammals (e.g. oribi antelope (Ourebia ourebi) [1]; coyote (Canis latrans) [2]) use communal defecation sites and it is thought that these middens may play a key role in olfactory communication. For example, male and female Arabian gazelles (Gazella arabica) use middens for different purposes, males for territorial defence and females for social group communication [15]. Bushbuck (Tragelaphus scriptus) females leave information (i.e. defecate/urinate) at middens and males respond to this information (i.e. overmark) [13]. White rhinos (Ceratotherium simum) have poor eyesight and rely heavily on olfactory signals [14]. As white rhinos of all ages and sexes defecate in middens, it is possible that these middens act as information centres for male–male, female–male and female–female communication. For example, territorial males mark middens by performing a kicking action with their back legs before and after defecation in the centre of a midden, whereas females do not perform this action and have been observed to defecate at the edge of a midden [14]. Further, middens are found throughout a white rhino territory and not localized at boundaries [16], suggesting that they are used for more than territory demarcation. Therefore, middens may act as information centres and hold records of territory ownership and the reproductive state of females in the area.
Recently, a behavioural study suggested that white rhinos recognize the sex of the depositor from dung odours [17]. However, to date no study has quantified the VOCs released from white rhino dung, nor the information that is relayed by these VOCs. To address this, we aimed to determine the information transmitted (i.e. sex, age, territorial status of males and reproductive state of females) in the dung odour profiles of wild, free-ranging white rhinos. Having achieved this, we then experimentally validated the VOC profiles by artificially replicating key elements of the odours. If our odour profiles were correct, we expected them to elicit behavioural responses from wild, free-ranging territorial males (i.e. the individuals most likely to respond to the odours [18]). We expected territorial males to interpret our replicated odour of a territorial male as a potential rival (i.e. male–male communication) and thus adjust their behaviour accordingly. Specifically, we expected territorial males to increase their visitation to the midden. This would allow them to monitor the presence or absence of the novel male and provide the territory holder with an opportunity to defecate in the midden to reaffirm territory ownership [14,18]. Similarly, we expected the territorial males to increase their frequency of defecation at the midden to reaffirm this ownership. Finally, as many disputes over territorial ownership can be determined by fighting [14,18], we expected the territorial males to have a reduced latency in assuming a vigilance posture in response to detecting the odour of a novel territorial male. With regard to the replicated odour of an oestrous female (i.e. female–male communication), we expected territorial males to identify this as an indicator of a potential mate. Territorial males spend more time sniffing the dung of oestrous females compared with the dung of other individuals [14]. As a result, we expected the territorial males in our study to spend more time sniffing the artificial oestrous female odour compared with other odours. In addition, we expected them to increase their frequency of visitation to the midden to reaffirm the presence of our ‘oestrous female’. However, as male white rhinos do not ordinarily overmark females [14], we did not expect their defecation frequency to change. Additionally, as there is no threat from the presence of an oestrous female, we did not expect a reduction in their latency in assuming a vigilance posture. Our study ultimately explores the VOCs used in white rhino olfactory communication and exposes the role of communal defecation. Moreover, it creates a platform for the further exploration of the theoretical and practical applications of VOCs.
2. Material and methods
(a). Collection of dung odours
We conducted our study in the 896 km2 Hluhluwe-iMfolozi Park, KwaZulu-Natal, South Africa. Here we collected 150 dung odour samples from wild, free-ranging white rhinos varying in sex (adult male n = 61, adult female n = 46), age (adult n = 107, subadult n = 28, calf n = 15) and state (adult territorial male n = 32 and adult non-territorial male n = 29, adult oestrous female n = 9 and adult non-oestrous female n = 37) between June 2012 and November 2014 using headspace extraction. To do this, we used a dynamic headspace extraction method [19] to collect air for 25 min from approximately 800 g (one bolus) of fresh (less than 5 min old) dung enclosed in a polyacetate bag using a micro-air sampler (Supelco PAS-500) with a realized flow rate of 150 ml min−1. VOCs emitted from the dung were captured in a small thermodesorption trap filled with 1 mg of Tenax and 1 mg of Carbotrap. We collected each sample from a different individual, achieved by recording variations in horn shape, skin folds and other distinguishing characteristics.
(b). Identification of characteristics
The age of each individual was categorized into calf (0–2 years), subadult (2–7 years) or adult (more than 7 years) based on body size and horn development [20]. Territorial male white rhinos are solitary and perform specific marking behaviours, including spray urination and dung kicking [18,21]. Thus, we classified adult males performing these behaviours as being territorial, and adult males not displaying these behaviours as non-territorial. We identified oestrous females via the behavioural reactions of adult males. For white rhinos, there is a consort period where a territorial male moves with an oestrous female for several days. During this time, he follows her closely, restricts her movement beyond his territory boundary and makes several mounting attempts [14]. During sampling we observed males performing these behaviours with a number of adult females, as well as having visible erections and attempting to mount these females. Moreover, we observed four of the ten sampled oestrous females mating during the study. We identified non-oestrous females as adult females without an attached adult male.
(c). Gas chromatography-mass spectrometry analysis of dung odours
We analysed thermodesorption traps using gas chromatography-mass spectrometry (GC-MS). We carried out analysis on a Bruker 450 GC with a 30 m × 0.25 mm internal diameter (film thickness 0.25 µm) Varian VF-5 ms column, connected to a Varian VF-1 ms column (11 m × 0.25 mm internal diameter, film thickness 0.25 µm) coupled to a Bruker 300 quadrupole mass spectrometer in electron-impact ionization mode at 70 eV. Thermodesorption traps were placed in a Varian 1079 injector equipped with a chromatoprobe thermal desorption device [19]. The flow of helium carrier gas was 1 ml min−1. We held the injector at an initial temperature of 250°C for 20 min. The split vent was programmed to start with a 10 : 1 split for 2 min and then to switch to splitless mode for 2 min to allow for thermal desorption, followed by a 100 : 1 split after 4.2 min to clean the injector. After an initial temperature at 45°C the temperature of the GC oven was increased to 260°C at 7°C min−1 and, after reaching 260°C, held at this temperature for a total run time of 35 min. We identified VOCs using Varian Workstation software with the NIST 2011 mass spectral library (NIST/EPA/NIH Mass Spectral Library, data version: NIST 2011; Microsoft Search software v. 2.0d). We verified the identification of VOCs with retention times of authentic standards and published Kovats indices wherever possible (electronic supplementary material, table S1).
(d). Implementation of artificial dung odours
After GC-MS analysis, we artificially created three dung odour treatments: (i) territorial male, (ii) oestrous female and (iii) control, comprising a subset of VOCs based on the raw data and statistical importance from this study (table 1). The top-ranked VOCs for territorial and oestrous state were nonane and 2,6-dimethylundecane, respectively. The proportion contribution of these VOCs to their respective odours was difficult to discriminate per state (e.g. average proportion nonane for territorial 0.005 versus non-territorial 0.007). As a result, we were concerned about how to recreate this to accurate and sufficient levels. We then looked at the data more closely and found large variations in the proportion of hydrocarbon acids. Because the importance of acids in olfactory communication has been noted [8,22–24] and territorial state VOC importance included several acids, we decided to focus our attention on acids to recreate a subset of the dung odours. We selected acetic acid, for example, due to its relative proportions in both male and female odours. Specifically, acetic acid proportions displayed the largest difference between territorial and non-territorial males compared with any other acid. With regard to females, acetic acid was the only acid occurring in higher proportions in oestrous odours compared with non-oestrous odours.
Table 1.
The volatile compounds (aliquots) used in the artificial dung odours of territorial male, oestrous female and control 2 (common herbivore dung odours). These substances were mixed together and then 1 ml of the solution added to 1 l of water to create a mixture in which we soaked the artificial bolus.
| VOC name | functional group | territorial male treatment | oestrous female treatment | control treatment 2 |
|---|---|---|---|---|
| phenol | benzenoid | 1 | 1 | 1 |
| acetophenone | benzenoid | 1 | 1 | 1 |
| p-cresol | benzenoid | 1 | 1 | 1 |
| m-cresol | benzenoid | 1 | 1 | 1 |
| nonanal | hydrocarbon aldehyde | 1 | 1 | — |
| decanal | hydrocarbon aldehyde | 1 | 1 | — |
| acetic acid | hydrocarbon acid | 3 | 1 | — |
| butyric acid | hydrocarbon acid | 3 | 1 | — |
| isobutyric acid | hydrocarbon acid | 3 | 1 | — |
| 2-methylbutyric acid | hydrocarbon acid | 3 | 1 | — |
| (E)-caryophyllene | sesquiterpene | 1 | 1 | — |
We used two controls. First, the grass bolus (see below) soaked in water in case any VOCs were released by the addition of a liquid. Second, common plant-based herbivore dung odours (table 1), to test if the white rhinos would respond to any new, novel odour within the midden. We wanted to collect data in the most natural conditions possible so as to mimic natural processes and thus create a realistic understanding of odours in the wild. However, in an attempt to control parameters, we deposited the artificial odours during wet-season months (November–March) where midday temperature and humidity were consistent around the average 35°C and 75%, respectively. By using fresh dung odour profiles for our artificial dung odours (see below), we were able to simulate fresh dung being deposited into a midden. The baseline measures in our study represent normal behaviours without olfactory manipulation.
In order to validate the VOCs used in white rhino olfaction, we exposed free-ranging territorial males to the artificial treatments. We chose to investigate the treatments from a territorial male perspective as they should elicit the greatest responses to the odours and thus provide the most observable reactions to validate the treatments. Specifically, as territorial males would perceive a rival (i.e. territorial treatment) as a threat, they should show more of a response than either non-territorial males or adult females as they are not defending any resources [25]. Similarly, territorial males should show a greater response to the oestrous treatment over non-territorial males as they monopolize mating [25], and should thus exhibit behaviours to find and successfully mate with the perceived oestrous female. In contrast, we would not expect territorial males to change their behaviour in response to the odour of a subordinate male as a threat or the odour of a non-oestrous female as a breeding opportunity [14]. Thus, we did not generate the profiles of these individuals; if we had, we would not be able to determine whether a ‘non-reaction’ was because they did not to react to the odour profiles or that our artificial profiles were incorrect.
To expose a territorial male to the artificial odour, we placed an artificial dung bolus soaked in an artificial odour mixture (1 ml artificial odour solution mixed with 1 l water) into a midden. We simulated a white rhino dung bolus by using a woven ball of dried Digitaria eriantha grass (150 × 90 × 90 mm approximately) and soaking this bolus in the mixture of 1 ml artificial odour solution and 1 l water (i.e. artificial VOC odour profile) for 1 min prior to deposition in the midden. We used one or three aliquots of the different compounds in our artificial odour solutions (table 1) as these volumes, coupled with the surface area of our artificial dung boluses, provided the most natural emission of the odour profile compared with natural dung odours collected over time. To determine this, we compared the similarity between the peaks on the chromatograms from the natural and artificial dung odours. We did this for odour samples taken at 0, 6, 12, 24, 48 and 72 h after defecation. From the similarity in the changes of the peaks over this time period, we concluded that the emission rate of the quantities administered reflected natural emission rates found in the field. In an attempt to further control parameters, we used the same grass species (D. eriantha) for all artificial boluses. We selected ten middens, each with a different resident territorial male (i.e. n = 10 territorial males) identified via variations in horn shape and size. Each male received repeated treatments at random intervals over the experimental period. We aimed for each male to receive each experimental odour four times. However, as behavioural observations were limited to three days after artificial odour deposition, many of the males did not visit the experimental middens during a designated odour period, and thus were not exposed to those odours. Nevertheless, in total, each male received an average two artificial territorial treatments and two artificial oestrous treatments (see table 2 for a detailed breakdown).
Table 2.
Number of treatment exposures per individual territorial male.
| mumber of treatment exposures |
||||
|---|---|---|---|---|
| territorial male ID | territorial | oestrous | control | water |
| M0006T | 3 | 1 | 1 | 2 |
| M0132T | 2 | 2 | 0 | 0 |
| M0142T | 2 | 1 | 1 | 1 |
| M0127T | 4 | 3 | 1 | 1 |
| M0128T | 3 | 1 | 1 | 1 |
| M0129T | 1 | 3 | 1 | 1 |
| M0131T | 3 | 3 | 1 | 1 |
| M0113T | 3 | 2 | 1 | 1 |
| M0079T | 1 | 0 | 0 | 1 |
| M0136T | 0 | 3 | 1 | 1 |
Artificial boluses were placed in natural locations within the middens, mirroring normal white rhino behaviour (i.e. in the centre for territorial male odour and at the edge for oestrous female odour [14]). Similarity in the changes of the peaks on the chromatograms (see above) showed that both the natural white rhino dung odours and our synthetic odour mixtures lasted for approximately 48 h. Thus, to ensure that we covered the entire 48 h emission period, we extended our behavioural data collection beyond 48 h to 3 days (i.e. 72 h) after we deposited the artificial dung bolus containing the replicated VOC odour profiles into the middens. We carried out the experiment and baseline observations during the wet season of October 2014–March 2015 and created an identification profile for each adult rhino (e.g. horn shape and size, ear notches) so that we could record individual behaviours.
To determine behavioural changes in territorial male response to the replicated VOC odour profiles, we explored four aspects of behaviour: (i) visitation frequency to the midden, (ii) defecation frequency at the midden, (iii) duration of sniffing events and (iv) latency to a vigilance posture. We used motion-triggered infrared ‘no-glow’ video recording camera traps (either a Cuddeback Black Flash E3 or Cuddeback Attack Black Flash 1194 model) placed approximately 3 m from the edge of the midden. This provided a sufficient field of view and allowed us to record the different behavioural reactions. We used ‘no-glow’ cameras as they do not emit visible light or have a flash, creating minimal disturbance at the midden and, therefore, allowing us to capture natural behaviour. We programmed the cameras to record 30 s videos at each trigger with a 1 s delay before becoming active again and obtained the behavioural data of the territorial males' responses from the videos.
(e). Statistical analysis
Absolute concentration is subject to variability across samples, therefore we used relative abundance of a VOC within a sample (i.e. proportion) for statistical analyses. In order to determine the characteristic odour profiles of sex, age class and territorial/oestrous status of adult males/females, we analysed the proportion of VOCs emitted from white rhino dung using a random forests classification algorithm [26] within the R package randomForest [27]. VOC datasets contain more variables than samples; for example, headspace samples from this study contained up to 150 VOCs and each VOC acts as a single variable. As a result, we are limited in our ability to use the entire dataset for analyses. Therefore, we used random forests, a classification algorithm with features making it well suited to the analysis of VOC datasets. For example, it allows for more variables than samples, it does not overfit the data, it has high classification efficiency and it can create a minimal set of variables which can be used as group predictors. For each iteration we used default parameters for both number of permutations (ntree = 500) and the number of randomly selected predictor variables at each split (mtry = √ p, where p = number of variables) as outputs were unchanged when adjusting these parameters. We calculated a classification accuracy for each random forest using out-of-bag error rates. In order to provide an interpretation of the best predictors (i.e. VOCs) for each characteristic from the random forest, we calculated a measure of variable importance using the importance function of the randomForest package and the metric mean decrease in accuracy (MDA) [28]. The MDA is the increase in the percentage of times the outcome is misclassified when the variable is randomized. Therefore, a higher MDA means less misclassification and thus greater accuracy, and ultimately indicates higher variable importance to the classification of a characteristic. All VOCs representing undigested waste plant material were removed from the analysis [29,30]. For the age class analysis all white rhino samples were used (n = 135). For sex and territorial/oestrous status, we limited our samples to only adult white rhinos (n = 92).
For the behavioural responses, we calculated the visitation and defecation frequencies by the number of visits or defecations divided by the number days the camera was active. We defined sniffing events as standing still with the nose less than 20 cm above ground and nostril flares. We calculated the duration of the sniffing event as the number of seconds from nose less than 20 cm to nose more than 20 cm above ground and events were separated by 2 s. We calculated the latency to assuming a vigilance posture as the number of seconds from the start of the sniffing event until vigilance posture was assumed (i.e. head up, standing still and ears rotating). If no vigilance posture was assumed, then a default 300 s was recorded. We recorded behaviours using open source behavioural coding software CowLog [31]. We compared each aspect of behaviour with baseline behaviours without odour manipulation and the artificial profiles with one another. As data were not normally distributed, we analysed them using non-parametric Kruskal–Wallis with a post hoc Dunn's test. We performed all statistics in RStudio v. 0.99.491 for Windows [32] and created all figures using SigmaPlot v. 8.0 for Windows.
3. Results
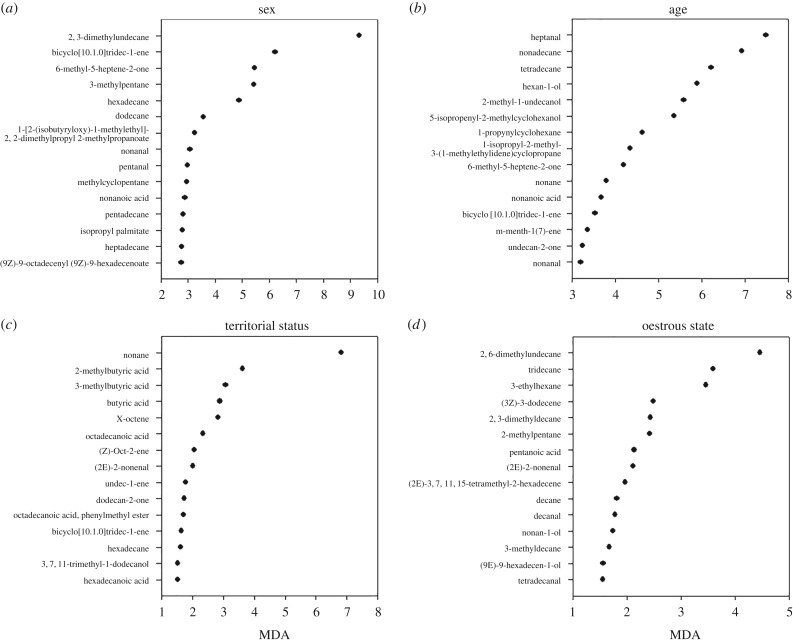
In our first experiment, we found that the sex, age class, male territorial and female oestrous state of an individual white rhino could be determined from the VOC profile of its dung. In total, we identified 225 VOCs emitted from the dung of white rhinos, classified within 13 functional groups (electronic supplementary material, table S1). Using a random forests classification algorithm, we identified the most important VOCs for distinguishing each characteristic. The random forest was most successful at differentiating sex (classification accuracy of 77.17%) with 2,3-dimethylundecane identified as the most important VOC for classifying this characteristic (figure 1a). In addition, heptanal was the most important VOC for discriminating age class (figure 1b), with a classification accuracy of 68.89%. With regard to defining male territorial state, we found that nonane was the most important VOC (figure 1c), with a classification accuracy of 55.93%. Finally, 2,6-dimethylundecane was the most important VOC for defining oestrous state in females (figure 1d), with a classification accuracy of 72.73%. Therefore, we were able to successfully determine exact odour profiles and effectively indicate the differences between each state.
Figure 1.
The importance of volatile organic compounds (VOCs) distinguishing (a) sex, (b) age, (c) territorial state of adult males and (d) oestrous state of adult females emitted from white rhino dung. Importance was based on mean decrease in accuracy (MDA). Only the top 15 compounds are presented in the figure.
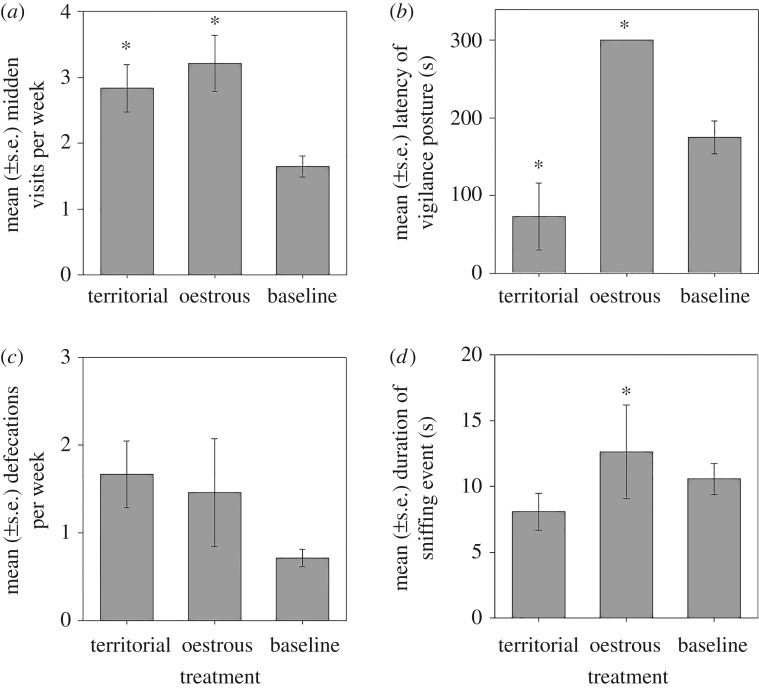
In our second experiment, during the replicated territorial male odour treatment, resident territorial males responded by significantly increasing visitation frequency to the midden (H(4) = 13.036, p = 0.002; figure 2a) and decreasing latency to vigilance posture (H(4) = 10.686, p = 0.012; figure 2b) compared with baseline behaviour (i.e. normal behaviours with no olfactory manipulation). They did not change their frequency of defecation (H(4) = 3.586, p = 0.125; figure 2c) or the duration of their sniffing events (H(4) = 6.134, p = 0.458; figure 2d). In response to the replicated oestrous female odour treatment, territorial males significantly increased the duration of sniffing events (H(4) = 6.134, p = 0.011; figure 2d) and increased their visitation frequency to the midden (H(4) = 13.036, p = 0.001; figure 2a). The latency to vigilance posture increased (i.e. they did not assume a vigilance posture; H(4) = 10.686, p = 0.024; figure 2b) and they did not adjust frequency of defecation (H(4) = 3.586, p = 0.398; figure 2c) compared with baseline behaviour.
Figure 2.
Territorial male (a) midden visits per week, (b) latency of the vigilance posture, (c) midden defecations per week and (d) duration of sniffing events during odour manipulation of replicated territorial male, replicated oestrous female and no odour manipulation (baseline). Asterisks indicate significant difference to baseline.
Comparing the territorial males' behavioural reactions with each of the artificial odour profiles, we found that the midden visitation frequency of territorial males was 15% greater in response to the artificial oestrous female odour compared with the artificial territorial male odour, although non-significant (H(4) = 13.036, p = 0.259). Furthermore, they spent significantly more time sniffing the artificial dung odour of an oestrous female (H(4) = 6.134, p = 0.021) and showed a longer latency to vigilance posture (H(4) = 10.686, p < 0.001) compared with that of the artificial territorial male odour. There was no difference in the defecation frequency (H(4) = 3.586, p = 0.243) between the oestrous and territorial odour treatments.
Finally, territorial males did not respond to our control odours compared with baseline behaviours (control 1: visitation frequency H(4) = 13.036, p = 0.218; latency to vigilance posture H(4) = 10.686, p = 0.409; defecation frequency H(4) = 3.586, p = 0.496; duration of sniffing event H(4) = 6.134, p = 0.483; control 2: visitation frequency H(4) = 13.036, p = 0.110; latency to vigilance posture H(4) = 10.686, p = 0.417; defecation frequency H(4) = 3.586, p = 0.163; duration of sniffing event H(4) = 6.134, p = 0.248).
4. Discussion
Many mammals transmit information via their urine [5–8]; however, the extent to which they transmit information via their dung is unclear. Some behavioural studies have suggested that mammals can identify sex [17], age [33] or oestrous state [34] from dung odours. However, none have indicated whether a wide range of information (e.g. sex, age, territorial status and oestrous state) is transmitted in the dung odour of a single species, nor identified the VOCs that transmit this information. Here we show that white rhinos transmit information on sex, age, territorial status and oestrous state via the VOCs emitted from their dung. Moreover, we identify the specific VOCs responsible for transmitting this information. This is a first for a species using communal defecation sites. Finally, we produced artificial odour blends representing a territorial male and an oestrous female, comprising key VOCs from these odour profiles. We then used these artificial odours to elicit specific behaviours associated with the different odours from free-ranging territorial males.
Odour differences can be a result of genetic distinctions; for instance, the presence of an X or Y chromosome produces a unique odour signature [35]. Age class differences are probably due to hormonal variations and immature physical development, where diet and gut development also influence odours [36,37]. Bacteria play an important role in mammalian olfactory communication [38]. The fermentation hypothesis proposes that the symbiotic bacteria living within scent glands break down organic material and produce VOCs that ultimately contribute to mammalian recognition cues [39]. The variation in composition and abundance of these bacterial communities then creates a unique individual odour, thereby allowing recognition by other individuals [40]. Although the fermentation hypothesis was developed for mammals that scent mark with specialized glands, it has been suggested that it could be applied to mammals that mark with faeces or urine [38]. The interaction between bacteria and hormones can also affect odours directly via their presence within glands. For example, differences in the anal gland microbiota of both male and female meerkats (Suricata suricatta) occur only after individuals reach sexual maturity, suggesting that reproductive hormones have a role in determining host bacterial communities [41]. Sex differences in the microbiota of adults have also been identified in greater sac-winged bats (Saccopteryx bilineata) [42] and white-tailed deer (Odocoileus virginianus) [43]. This interaction can also affect odours via the breakdown of hormone metabolites post excretion, for example microbes mediate the timed release of semiochemicals from the urine of male musth elephants (Loxodonta africana, Elephas maximus) [44]. Bacteria and hormones can also have an indirect effect on odours via behaviour modification. Higher testosterone levels can cause increased locomotor activity [45] and increased metabolic rate [46]. Both of these have subsequent effects on feeding, and therefore gut content, ultimately leading to changes in bacterial activity which could lead to differences in odours. Territorial males have significantly higher concentrations of faecal testosterone than non-territorial (subordinate) adult males [12]. Therefore, for adult male white rhinos achieving territorial status, the subsequent associated increase in testosterone [12] may affect microbiota directly or indirectly. However, the random forests algorithm was least accurate at distinguishing the territorial state of males. This may be due to territory acquisition taking up to 5 years for an adult male [14]. Thus, there could be an uncertain period before adult males obtain their own territory, but are physically able to do so (i.e. higher testosterone levels). Interestingly, nonane, ranked the most important VOC, is not currently cited in relation to dominance or territory ownership. Yet some of the other VOCs identified (e.g. 2- and 3-methylbutyric acid; figure 1c) are the same VOCs suggested to represent dominance in other mammals [47].
Examples of female oestrous odours come primarily from urine and vaginal secretions [8,48–50], while those obtained from dung odours of wild animals are limited. With regard to female white rhinos, we found a decrease, and in most cases a complete disappearance, in the proportion of several VOCs emitted during oestrus. Specifically, oestrous females emitted lower proportions of alkanes and alcohols. These same functional groups have been identified with roles in signalling reproductive state in the odours of domestic cow faeces [9]. However, most studies of oestrous odours have identified a higher concentration or sudden appearance of VOCs during oestrus. A potential explanation for the lower proportion of VOCs emitted from oestrous female white rhino dung could be due to the fermentation–absorption process in hindgut fermenters [51]. VOCs can be absorbed in the hindgut before they are released with faecal matter, and this can contribute significantly to host energy requirements [52,53]. White rhinos are very efficient in the absorption of VOCs in the hindgut [54], and ovulation, gestation and lactation are energetically costly to females [55]. As the absorption of VOCs may be under hormonal control [52], hormones may indirectly affect dung odours where oestrous females may be absorbing higher levels of VOCs during oestrus in preparation for the subsequent strain on body condition. Further, as with males, this may be related to hormone-induced behaviour and its subsequent impact on bacteria, where females could have reduced food intake during oestrus [56].
Territory holders must manage both potential threats and mating opportunities in order to defend their territory and increase their fitness [18]. Using artificial odours comprising key elements of the complete odour profile, we showed how novel olfactory information could stimulate predicted behaviours [21]. Specifically, territorial males responded repeatedly to the replicated odour of a novel territorial male as a potential rival. They did this by increasing their visitation to the midden and reducing their latency in assuming a vigilance posture. Increasing midden visitation probably allows territorial males the opportunity to reassess the odour and the presence of the rival [57,58]. Shorter latency in assuming a vigilance posture, however, permits the territorial males to prepare for a potential aggressive encounter with another male [58]. In contrast with our expectations, the defecation frequency of territorial males did not increase in response to our artificial territorial male odour profile. We suggest that this is due to the limited availability of dung as a marking resource [1]. Despite this, overall, the behavioural responses of the territorial males to our artificial VOC profiles suggest that the key VOC elements that we identified from free-ranging white rhinos are the key VOCs that signal territorial ownership in white rhino males.
In response to our replicated oestrous female odour (i.e. a potential mate) territorial males showed repeated high levels of interest, which was unaffected by the deposition of dung by other individuals in the midden. In line with observations of free-ranging males responding to the dung odours of oestrous females [14], the territorial males in our study increased their duration of sniffing events of the artificial oestrous female odour. These males also increased their visitation frequency to the midden. Our manipulations did not provide an odour trail away from the midden for the males to follow [14], thus we suggest that this behaviour probably indicates a reaffirmation of the presence of our ‘oestrous female’. The combination of these results suggests that, as with the scent profile of territorial males, the key VOCs we identified for oestrous females are an accurate reflection of an oestrous female odour profile.
Overall, the territorial males showed greater interest in the artificial oestrous female odour (i.e. more frequent midden visitation, more time spent sniffing) compared with our artificial territorial male odour. Males establish territories to defend high-quality resources so that they can monopolize mating opportunities [25]. Thus, the greater behavioural response to oestrous odours could indicate males looking to maximize their breeding opportunities. It is possible that the VOCs used in our odour replication study may simply indicate sex, and not specifically territorial or oestrous state. Yet territorial males do not show interest in non-oestrous females (e.g. extensive smelling of their dung), and tolerate subordinate males living within their territories (i.e. they do not see them as a threat) [14]. Thus, if our artificial odours signalled only the sex of an individual, then it would have been unlikely for the territorial males to have reacted as dramatically as they did to our artificial oestrous female odour (i.e. increased the duration of their sniffing events) and our artificial territorial male odour (i.e. reducing their latency in assuming a vigilance posture). Thus, we feel confident that our artificial odours do in fact transmit information about territorial status and oestrous state.
Identifying the information transmitted in dung odours is essential for understanding how communally defecating mammalian species communicate. Despite recent progress in the field of mammalian olfactory communication, examples of specific odour profiles with subsequent bioassays in the wild are rare. Yet not all the VOCs in the odour profiles transmit information. To determine which VOCs are important requires confirmation of behavioural responses towards a substance in a bioassay, similar to our study. Our study shows that the composition of white rhino dung odours differ with sex, age, territorial status and oestrous state. Thus, we show that dung, a waste product, is a valuable medium for transmitting biological information for male–male, female–male and potentially female–female communication. Based on these results, we propose that white rhinos use middens to deposit and extract a wealth of biological information. If correct, then this helps explain the phenomenon of communal defecation in a large number of mammalian species [1–3]. Finally, the success of our replicated odour profiles in eliciting desired behavioural responses provides a platform for further research into the theoretical and practical applications of VOCs for a wide range of mammalian species.
Supplementary Material
Acknowledgement
We thank Dr Adam Shuttleworth for processing the GC-MS samples and Chris Kelly (WildlifeACT Fund) for use of the camera traps. Norman Owen-Smith, Steve Johnson and two anonymous reviewers provided valuable comments on earlier drafts.
Ethics
The study followed local animal ethics guidelines and was granted ethics clearance from the University of KwaZulu-Natal Animal Research Ethics Committee.
Data accessibility
The data supporting this article can be obtained from the Dryad Digital Repository [59].
Authors' contributions
C.M. expanded the initial idea, collected the data and carried out the statistical analyses; A.J. helped to develop the initial project, processed GC-MS samples and produced artificial dung odours; A.M.S. created the initial project and further developed it with the co-authors. All authors discussed the results, wrote the manuscript and gave final approval for publication.
Competing interests
We declare that we have no competing interests.
Funding
This research was funded by the National Research Foundation (grant 77582).
References
- 1.Brachares JS, Arcese P. 1999. Scent marking in a territorial African antelope: II. The economics of marking with faeces. Anim. Behav. 57, 11–17. ( 10.1006/anbe.1998.0942) [DOI] [PubMed] [Google Scholar]
- 2.Ralls K, Smith D. 2004. Latrine use by San Joaquin kit foxes (Vulpes macrotis mutica) and coyotes (Canis latrans). West N. Am. Nat. 64, 544–547. [Google Scholar]
- 3.Roper TJ, Conradt L, Butler J, Christian SE, Ostler J, Schmid TK. 1993. Territorial marking with faeces in badgers (Meles meles): a comparison of boundary and hinterland latrine use. Behaviour 127, 289–307. ( 10.1163/156853993X00074) [DOI] [Google Scholar]
- 4.Burgener N, Dehnhard M, Hofer H, East M. 2009. Does anal gland scent signal identity in the spotted hyaena?. Anim. Behav. 77, 707–715. ( 10.1016/j.anbehav.2008.11.022) [DOI] [Google Scholar]
- 5.Achiraman S, Archunan G. 2005. 3-Ethyl-2,7-dimethyl octane, a testosterone dependent unique urinary sex pheromone in male mouse (Mus musculus). Anim. Reprod. Sci. 87, 151–161. ( 10.1016/j.anireprosci.2004.11.001) [DOI] [PubMed] [Google Scholar]
- 6.Hollister-Smith JA, Alberts SC, Rasmussen LEL. 2008. Do male African elephants, Loxodonta africana, signal musth via urine dribbling? Anim. Behav. 76, 1829–1841. ( 10.1016/j.anbehav.2008.05.033) [DOI] [Google Scholar]
- 7.Būda V, Mozūraitis R, Kutra J, Borg-Karlson A. 2012. p-Cresol: a sex pheromone component identified from the estrous urine of mares. J. Chem. Ecol. 38, 811–813. ( 10.1007/s10886-012-0138-2) [DOI] [PubMed] [Google Scholar]
- 8.Archunan G, Rajagopal T. 2013. Detection of estrus in Indian blackbuck: behavioural, hormonal and urinary volatiles evaluation. Gen. Comp. Endocrinol. 181, 156–166. ( 10.1016/j.ygcen.2012.11.012) [DOI] [PubMed] [Google Scholar]
- 9.Sankar R, Archunan G. 2008. Identification of putative pheromones in bovine (Bos taurus) faeces in relation to estrus detection. Anim. Reprod. Sci. 103, 149–153. ( 10.1016/j.anireprosci.2007.04.014) [DOI] [PubMed] [Google Scholar]
- 10.Kimura R. 2001. Volatile substances in feces, urine and urine-marked feces of feral horses. Can. J. Anim. Sci. 81, 411–420. ( 10.4141/A00-068) [DOI] [Google Scholar]
- 11.Barja I, Silván G, Martínez-Fernández L, Illera J. 2011. Physiological stress responses, fecal marking behavior, and reproduction in wild European pine martens (Martes martes). J. Chem. Ecol. 37, 253–259. ( 10.1007/s10886-011-9928-1) [DOI] [PubMed] [Google Scholar]
- 12.Rachlow J, Berkeley E, Berger J. 1998. Correlates of male mating strategies in white rhinos (Ceratotherium simum). J. Mammal. 79, 1317–1324. ( 10.2307/1383023) [DOI] [Google Scholar]
- 13.Wronski T, Apio A, Plath M. 2006. The communicatory significance of localised defecation sites in bushbuck (Tragelaphus scriptus). Behav. Ecol. Sociobiol. 60, 368–378. ( 10.1007/s00265-006-0174-4) [DOI] [Google Scholar]
- 14.Owen-Smith N. 1973. The behavioural ecology of the white rhinoceros. Madison, WI: University of Wisconsin. [Google Scholar]
- 15.Wronski T, Apio A, Plath M, Ziege M. 2013. Sex differences in the communicatory significance of localized defecation sites in Arabian gazelles (Gazella arabica). J. Ethol. 31, 129–140. ( 10.1007/s10164-012-0357-6) [DOI] [Google Scholar]
- 16.Kretzschmar P, Ganslosser U, Goldschmid A, Aberham A. 2001. Stimulation of territorial and mating behaviour by faecal samples: a comparative study on behaviour of captive and free-living white rhinoceros. In International elephant and rhino research symposium (ed. Schwammer H.), pp. 299–302. Vienna, Austria: Schüling Verlag. [Google Scholar]
- 17.Cinková I, Policht R. 2014. Discrimination of familiarity and sex from chemical cues in the dung by wild southern white rhinoceros. Anim. Cogn. 18, 385–392. ( 10.1007/s10071-014-0810-8) [DOI] [PubMed] [Google Scholar]
- 18.Owen-Smith N. 1971. Territoriality in the white rhinoceros (Ceratotherium simum) Burchell. Nature 231, 294–296. ( 10.1038/231294a0) [DOI] [PubMed] [Google Scholar]
- 19.Amirav A, Dagan S. 1997. A direct sample introduction device for mass spectrometry studies and GC-MS analysis. Eur. J. Mass Spectrom. 3, 105–111. ( 10.1255/ejms.27) [DOI] [Google Scholar]
- 20.Hillman-Smith AKK, Owen-Smith N, Anderson JL, Hall-Martin AJ, Selaladi JP. 1986. Age estimation of white rhinoceros Ceratotherium simum. J. Zool. Lond. A 210, 355–379. ( 10.1111/j.1469-7998.1986.tb03639.x) [DOI] [Google Scholar]
- 21.Owen-Smith RN. 1975. The social ethology of the white rhinoceros Ceratotherium simum (Burchell 1817). Z. Tierpsychol. 38, 337–384. ( 10.1111/j.1439-0310.1975.tb02010.x) [DOI] [Google Scholar]
- 22.Albone ES, Perry GC. 1975. Anal sac secretion of the red fox, Vulpes vulpes; volatile fatty acids and diamines: implications for a fermentation hypothesis of chemical recognition. J. Chem. Ecol. 2, 101–111. ( 10.1007/BF00988029) [DOI] [Google Scholar]
- 23.Apps P, Mmualefe L, McNutt JW. 2012. Identification of volatiles from the secretions and excretions of African wild dogs (Lycaon pictus). J. Chem. Ecol. 38, 1450–1461. ( 10.1007/s10886-012-0206-7) [DOI] [PubMed] [Google Scholar]
- 24.Nielsen BL, Jerôme N, Saint-Albin A, Thonat C, Briant C, Boue F, Rampin O, Maurin Y. 2011. A mixture of odorant molecules potentially indicating oestrus in mammals elicits penile erections in male rats. Behav. Brain Res. 225, 584–589. ( 10.1016/j.bbr.2011.08.026) [DOI] [PubMed] [Google Scholar]
- 25.Clutton-Brock TH, Harvey PH. 1978. Mammals, resources and reproductive strategies. Nature 17, 191–195. ( 10.1038/273191a0) [DOI] [PubMed] [Google Scholar]
- 26.Breiman L. 2001. Random forests. Mach. Learn. 45, 5–32. ( 10.1023/A:1010933404324) [DOI] [Google Scholar]
- 27.Liaw A, Wiener M. 2012. Classification and regression by randomForest. R News 2, 18–22. [Google Scholar]
- 28.Strobl C, Boulesteix AL, Zeileis A, Hothorn T. 2007. Bias in random forest variable importance measures: Illustrations, sources and a solution. BMC Bioinform. 8, 25 ( 10.1186/1471-2105-8-25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gershenzon J, Croteau R. 1991. Herbivores: their interactions with secondary plant metabolites, 2nd edn San Diego, CA: Academic Press. [Google Scholar]
- 30.Ishida T. 2005. Biotransformation of terpenoids by mammals, microorganisms, and plant-cultured cells. Chem. Biodivers. 2, 569–590. ( 10.1002/cbdv.200590038) [DOI] [PubMed] [Google Scholar]
- 31.Hänninen L, Pastell M. 2009. CowLog: open source software for coding behaviors from digital video. Behav. Res. Methods 41, 472–476. ( 10.3758/BRM.41.2.472) [DOI] [PubMed] [Google Scholar]
- 32.R Core Team 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 33.Linklater W, Mayer K, Swaisgood R. 2013. Chemical signals of age, sex and identity in black rhinoceros. Anim. Behav. 85, 671–677. ( 10.1016/j.anbehav.2012.12.034) [DOI] [Google Scholar]
- 34.Ghosal R, Seshagiri P, Sukumar R. 2012. Dung as a potential medium for inter-sexual chemical signaling in Asian elephants (Elephas maximus). Behav. Processes 91, 15–21. ( 10.1016/j.beproc.2012.04.010) [DOI] [PubMed] [Google Scholar]
- 35.Yamazaki K, Beauchamp GK, Matsuzaki O, Bard J, Thomas L, Boyse EA. 1986. Participation of the murine X and Y chromosomes in genetically determined chemosensory identity. Proc. Natl Acad. Sci. USA 83, 4438–4440. ( 10.1073/pnas.83.12.4438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kean EF, Muller CT, Chadwick EA. 2011. Otter scent signals age, sex, and reproductive status. Chem. Senses 36, 555–564. ( 10.1093/chemse/bjr025) [DOI] [PubMed] [Google Scholar]
- 37.Macdonald EA, Fernandez-Duque E, Evans S, Hagey LR. 2008. Sex, age, and family differences in the chemical composition of owl monkey (Aotus nancymaae) subcaudal scent secretions. Am. J. Primatol. 70, 12–18. ( 10.1002/ajp.20450) [DOI] [PubMed] [Google Scholar]
- 38.Archie EA, Theis KR. 2011. Animal behaviour meets microbial ecology. Anim. Behav. 82, 425–436. ( 10.1016/j.anbehav.2011.05.029) [DOI] [Google Scholar]
- 39.Albone ES, Eglinton G, Walker JM, Ware GC. 1974. The anal sac secretion of the red fox (Vulpes vulpes): its chemistry and microbiology. A comparison with the anal sac secretion of the lion (Panthera leo). Life Sci. 14, 387–400. ( 10.1016/0024-3205(74)90069-1) [DOI] [PubMed] [Google Scholar]
- 40.Gorman ML. 1976. A mechanism for individual recognition by odour in Herpestes auropunctatus (Carnivora: viverridae). Anim. Behav. 24, 141–145. ( 10.1016/S0003-3472(76)80107-8) [DOI] [Google Scholar]
- 41.Leclaire S, Nielsen JF, Drea CM. 2014. Bacterial communities in meerkat anal scent secretions vary with host sex, age, and group membership. Behav. Ecol. 24, 996–1004. ( 10.1093/beheco/aru074) [DOI] [Google Scholar]
- 42.Voigt CC, Caspers B, Speck S. 2005. Bats, bacteria, and bat smell: sex-specific diversity of microbes in a sexually selected scent organ. J. Mammal. 86, 745–749. ( 10.1644/1545-1542(2005)086%5B0745:BBABSS%5D2.0.CO;2) [DOI] [Google Scholar]
- 43.Alexy KJ, Gassett JW, Osborn DA, Miller KV, Russell SM. 2003. Bacterial fauna of the tarsal tufts of white-tailed deer (Odocoileus virginianus). Am. Midland Nat. 149, 237–240. ( 10.1674/0003-0031(2003)149%5B0237:BFOTTT%5D2.0.CO;2) [DOI] [Google Scholar]
- 44.Goodwin TE, et al. 2012. Chemical signals of elephant musth: temporal aspects of microbially-mediated modifications. J. Chem. Ecol. 38, 81–87. ( 10.1007/s10886-011-0056-8) [DOI] [PubMed] [Google Scholar]
- 45.Ellis GB, Turek FW. 1983. Testosterone and photoperiod interact to regulate locomotor activity in male hamsters. Hormones Behav. 17, 66–75. ( 10.1016/0018-506X(83)90016-8) [DOI] [PubMed] [Google Scholar]
- 46.Buchanan KL, Evans MR, Goldsmith AR, Bryant DM, Rowe LV. 2001. Testosterone influences basal metabolic rate in male house sparrows: a new cost of dominance signalling? Proc. R. Soc. Lond. B 268, 1337–1344. ( 10.1098/rspb.2001.1669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tobey J, Nute T, Bercovitch F. 2009. Age and seasonal changes in the semiochemicals of the sternal gland secretions of male koalas (Phascolarctos cinereus). Aust. J. Zool. 57, 111–118. ( 10.1071/ZO08090) [DOI] [Google Scholar]
- 48.Jemiolo B, Miller KV, Wiesler D, Jelinek I, Novotny M, Marchinton RL. 1995. Putative chemical signals from white-tailed deer (Odocoileus virginianus). Urinary and vaginal mucus volatiles excreted by females during breeding season. J. Chem. Ecol. 21, 869–879. ( 10.1007/BF02033467) [DOI] [PubMed] [Google Scholar]
- 49.Mozūraitis R, Būda V, Kutra J, Borg-Karlson AK. 2012. p- and m-Cresols emitted from estrous urine are reliable volatile chemical markers of ovulation in mares. Anim. Reprod. Sci. 130, 51–56. ( 10.1016/j.anireprosci.2011.12.008) [DOI] [PubMed] [Google Scholar]
- 50.Klemm WR, Hawkins GN, De Los Santos E. 1987. Identification of compounds in bovine cervico-vaginal mucus extracts that evoke male sexual behavior. Chem. Senses 12, 77–87. ( 10.1093/chemse/12.1.77) [DOI] [Google Scholar]
- 51.Titus E, Ahearn G. 1992. Vertebrate gastrointestinal fermentation: transport mechanisms for volatile fatty acids. Am. J. Physiol. 262, R547–R553. [DOI] [PubMed] [Google Scholar]
- 52.Marty J, Vernay M. 1984. Absorption and metabolism of the volatile fatty acids in the hind-gut of the rabbit. Br. J. Nutr. 51, 265–277. ( 10.1079/BJN19840031) [DOI] [PubMed] [Google Scholar]
- 53.Daly K, Proudman CJ, Duncan SH, Flint HJ, Dyer J, Shirazi-Beechey SP. 2012. Alterations in microbiota and fermentation products in equine large intestine in response to dietary variation and intestinal disease. Br. J. Nutr. 107, 989–995. ( 10.1017/S0007114511003825) [DOI] [PubMed] [Google Scholar]
- 54.Steuer P, Südekum K, Müller D, Franz R, Kaandorp J, Clauss M, Hummel J. 2011. Is there an influence of body mass on digesta mean retention time in herbivores? A comparative study on ungulates. Comp. Biochem. Physiol. A 160, 355–364. ( 10.1016/j.cbpa.2011.07.005) [DOI] [PubMed] [Google Scholar]
- 55.Gittleman J, Thompson S. 1988. Energy allocation in mammalian reproduction. Am. Zool. 28, 863–875. ( 10.1093/icb/28.3.863) [DOI] [Google Scholar]
- 56.Eckel LA, Houpt TA, Geary N. 2000. Spontaneous meal patterns in female rats with and without access to running wheels. Physiol. Behav. 70, 397–405. ( 10.1016/S0031-9384(00)00278-X) [DOI] [PubMed] [Google Scholar]
- 57.Kepecs A, Uchida N, Mainen Z. 2006. The sniff as a unit of olfactory processing. Chem. Senses 31, 167–179. ( 10.1093/chemse/bjj016) [DOI] [PubMed] [Google Scholar]
- 58.Marino A. 2012. Indirect measures of reproductive effort in a resource-defense polygynous ungulate: territorial defense by male guanacos. J. Ethol. 30, 83–91. ( 10.1007/s10164-011-0299-4) [DOI] [Google Scholar]
- 59.Marneweck C, Jürgens A, Shrader AM. 2017. Data from: Dung odours signal sex, age, territorial and oestrous state in white rhinos. Dryad Digital Repository. ( 10.5061/dryad.5b45b) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this article can be obtained from the Dryad Digital Repository [59].