Abstract
Environmental change may cause phenotypic changes that are inherited across generations through transgenerational plasticity (TGP). If TGP is adaptive, offspring fitness increases with an increasing match between parent and offspring environment. Here we test for adaptive TGP in somatic growth and metabolic rate in response to temperature in the clonal zooplankton Daphnia pulex. Animals of the first focal generation experienced thermal transgenerational ‘mismatch’ (parental and offspring temperatures differed), whereas conditions of the next two generations matched the (grand)maternal thermal conditions. Adjustments of metabolic rate occurred during the lifetime of the first generation (i.e. within-generation plasticity). However, no further change was observed during the subsequent two generations, as would be expected under TGP. Furthermore, we observed no tendency for increased juvenile somatic growth (a trait highly correlated with fitness in Daphnia) over the three generations when reared at new temperatures. These results are inconsistent with existing studies of thermal TGP, and we describe how previous experimental designs may have confounded TGP with within-generation plasticity and selective mortality. We suggest that the current evidence for thermal TGP is weak. To increase our understanding of the ecological and evolutionary role of TGP, future studies should more carefully identify possible confounding factors.
Keywords: maternal effects, climate change, epigenetics, respiration, between generation, acclimation
1. Introduction
Environmental change may cause phenotypic changes that are inherited across generations in the absence of concurrent changes in DNA sequences. More specifically, under transgenerational plasticity (TGP) the offspring environmental reaction norm changes in interaction with the parental [1], or even grandparental environment [2,3]. If environmental conditions are correlated across generations, parental environments may serve as a reliable cue for the optimal offspring gene expression, and TGP may be adaptive [4,5]. In this case, TGP represents active phenotypic plasticity, where phenotypes are optimized according to the environment experienced by the parents, in order to maximize the increase (or minimize the decline) in fitness when environments change [6,7]. However, plasticity may also occur in passive forms [8], in which case it is not a mechanism that has evolved to increase fitness. In this case, it is rather a phenotypic response to environmental conditions that organisms cannot prevent through evolution. Such responses might occur due to resource limitation or environmental stress (sensu [8,9]). Thus, to understand the ecological role of TGP it is not sufficient to demonstrate its presence; one also needs to evaluate whether it confers fitness benefits and preferably link this mechanism to specific patterns in environmental variation. With predictable environmental variation across generations, models that predict TGP could potentially be a special scenario in models on phenotypic tracking [10–13].
For ectothermic animals, metabolic activity (metabolic rates, commonly measured as oxygen consumption: VO2) is under strong control by the ambient temperature through passive plasticity [14,15]. However, studies of acclimation (within-generation) clearly demonstrate the ability to up- and downregulate metabolic rate in a more active way, presumably to counteract such passive responses [16,17]. The failure of adapting to new temperature regimes metabolically could inflict an excessive expenditure of energy or inadequate levels necessary to maintain important physiological functions, which in turn could affect fitness [18,19]. It is, therefore, a relevant quantitative trait to study for temperature-mediated TGP, especially as ecological impacts due to climate change become increasingly important.
Similar to the adaptive properties of within-generation phenotypic plasticity, a TGP response can potentially contribute to momentarily shield a population/species from extinction under changing thermal conditions and allow more time to adapt genetically [20,21]. Some studies suggest thermal TGP responses in somatic growth rates [22,23], but the role of metabolic adjustments remains unclear. Two recent studies in coral reef fish [24] and sticklebacks [25] investigated the role of TGP in metabolic capacity for ectotherms. However, the study design applied in those studies cannot rule out the possibility of genetic changes across generations due to effects of selective mortality and/or selective breeding (see Discussion for more details).
Here we test for thermal TGP responses in both metabolism and somatic growth using the crustacean Daphnia pulex. Daphnia sp. reproduce asexually during growth periods. By using this clonal animal as a model organism, genetic changes occurring from one generation to the next are minimized (being limited to arising new mutations), enabling TGP effects to be studied in isolation. The transparency of the animal and the ovoviviparous system allow maturation status to be assessed continuously, which permits a clear-cut distinction between maternal and offspring environment. We measured metabolic rate and somatic growth responses, the latter being a proxy for fitness [26], through three generations after being transferred to stable new temperatures. Thus, in this case there is a declining mismatch between offspring environment and environment experienced by previous generations when going from the first to the third generation. The interaction between environment in the current and previous generations are thus embedded in the study design and can be tested for by comparing performance in the three generations in the new environment. In the presence of adaptive TGP, we predict changes in metabolic rate across generations following a transfer to new temperatures, and corresponding increases in growth rate.
2. Material and methods
(a). Animal husbandry
Resting eggs (ephippia) of Daphnia pulex were sorted from sediment samples collected at Lake Asklundvatnet, central Norway (N 63.588, E 10.729), on 1 November 2013. Temperatures from this lake were logged at 0.5 m depth between 19 May and 9 September 2016. Mean temperature during this period was 17.6°C (range daily mean: 12–22.7°C; see electronic supplementary material, S1 for details). The ephippia were dried for one week on filter paper before cleansing and activation in a 5% hypochlorite solution for 5 min. After being rinsed thoroughly with distilled water, the ephippia were left to hatch in filtered lake water under continuous light at room temperature (22°C).
Resulting hatchlings were kept individually in separate 250 ml jars where they propagated by asexual reproduction. The animals were kept at a density of 10 animals in 250 ml glass jars and fed three times a week with Shellfish Diet 1800® (Reed Mariculture Inc.) at a final concentration of 2 × 105 cells ml−1, corresponding to approximately 3.4 mg carbon (C) l−1. If only juveniles were present, half of this concentration was used. The shellfish diet consisted of four different marine microalgae: Isochrysis sp., Pavlova sp., Tetraselmis sp., and Thalossiosira pseudonana. Culling of the populations down to 10 individuals took place once a week when the medium was replaced.
These populations (hereafter clones) were kept for 8–15 months (22–30 generations) in climate cabinets at 17°C on a 16 light (L) : 8 dark (D) light regime prior to the experiments. This extensive period ensured common garden settings, i.e. no environmental effects on phenotypes from the wild should remain. For sexually reproducing organisms such prolonged artificial rearing could result in evolutionary changes. For asexual organisms, however, the genetic background should stay more constant, being limited to new mutations and possibly within-clone selection at an epigenome level [27]. Although one cannot completely rule out these two selective forces, fixation of new advantageous mutations was made less likely by the random selection of individuals during the weekly culling. Furthermore, in the case of within-clone selection of epigenetic changes, the multiple-generation rearing at 17°C of our clones would be expected to produce patterns of gene expression that maximizes fitness at that temperature, and hence potentially increasing the ability to detect TGP (i.e. first generation offspring reared at a different temperature should perform worse than subsequent generations, see Study design, transgenerational plasticity). The artificial medium used was a modified selenium dioxide version of Aachener Daphnia medium (ADaM) [28].
(b). Study design, transgenerational plasticity
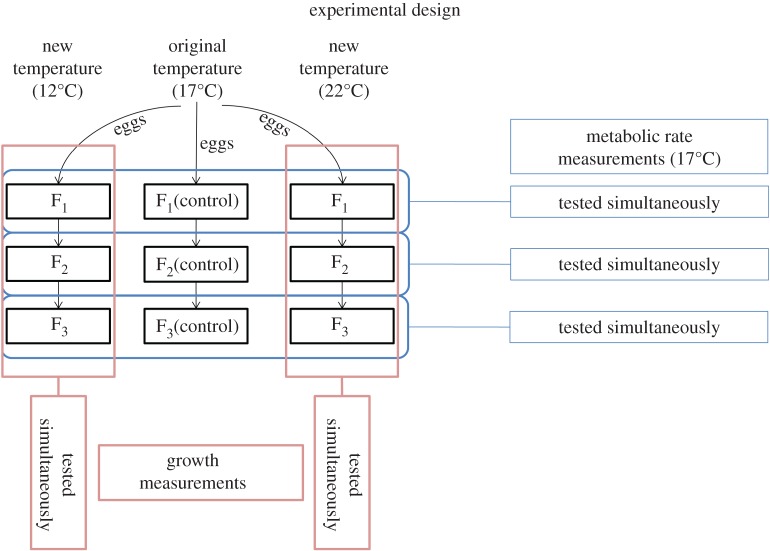
Five of the hatched clones from Lake Asklundvatnet (abbreviated laboratory names: BP, HF, LP, PM, WH) were used for both metabolic and growth experiments. The parental generation consisted of adult females that had ovulated (released the eggs into their brood chamber within 24 h) for the second or third time. These parents were selected from each clone at their original temperature (17°C) and transferred to a stable 12, 17, or 22°C temperature regime (figure 1). Feeding regimes were developed to ensure ad libitum conditions while at the same time preventing overfeeding at the colder temperature (overfeeding in Daphnia cultures causes water quality problems). Based on a pilot study it was found that this was achieved by adjusting food rations down or up by 20% relative to that given at 17°C (described above) for the 12 and 22°C treatments.
Figure 1.
Schematic of the experimental design. Metabolic rate (at 17°C) and growth (at 12°C and 22°C) were measured for 440 and 368 animals, respectively. Five unique clones of D. pulex were represented in each temperature treatment, and represented approximately equally at all factorial levels. This number of levels and animals yields approximately 10 inds. clone−1 generation−1 temperature−1 for metabolism measurements and approximately 13 inds. clone−1 generation−1 temperature−1 for growth measurements. (Online version in colour.)
The subsequent first clutch born by the transferred animals thus became the F1 generation. Individuals of this generation thus lived their entire life post-hatching as well as the majority of their post-ovulation egg stage at the new temperature, but were conceived and spent their pre-ovulation (i.e. gonadal) life at the original temperature (17°C). Upon onset of reproduction by the F1 generation (producing F2), the clone's first week of offspring were discarded to avoid selecting for any potential mutations on age at maturation. Thus, the individuals that comprised the F2 generation were randomly selected from offspring born after one week of clonal reproduction. Epigenetic effects from the grandparental generation (all reared at 17°C) may also affect the F2 generation, which required an additional F3 generation to be tested. The F3 generation was created in the same manner as the F2 generation.
(c). Study design, maternal effects
One potential caveat with our design relates to maternal effects. Specifically, under TGP we predict F2 to perform better (i.e. grow faster) than F1 in a new environment. However, if growth rate is influenced by an additive maternal effect, and offspring from mothers reared at 17° grow faster than those from mothers reared at both lower and higher temperatures, predictions may change. Under this scenario, this would give a positive effect on F1 in the new environments compared with F2, and hence counteract the predicted effect of TGP. Thus, a separate experiment was conducted to test for such maternal effects. In this, mothers that had been born and developed at the high and low temperatures (12 and 22°C) were transferred to the intermediate temperature (17°C). The growth rates until maturation of their resulting offspring was compared with that of offspring from mothers born and developed at the intermediate temperature. As in the main experiment, the transferred animals were mothers that had ovulated within the previous 24 h.
(d). Metabolic measurements
Oxygen consumption of one animal from each of the five clones from all three temperatures of a given generation (15 in total) was tested in each replicate run at a common temperature of 17°C (figure 1). This allowed us to quantify metabolic responses of being reared at new temperatures for one, two, or three generations. Ten replicate runs were conducted per generation. The body lengths (BL in millimetres, measured from the base of the caudal spine to the apex of the eye) of the animals were measured to the nearest 0.01 mm using the software ImageJ [29]. Total dry weight (DW in milligrams) of each individual was calculated using the formula DW = 0.0084BL2.58 [30]. The number of eggs in the brood chamber and their development stage were also recorded for each individual. Eggs were defined as late in the development process if it was possible to observe the eyes inside the brood chamber (i.e. development stage 4–5 in [31]), and early if not. Individuals were excluded from the dataset (n = 56) if we could not determine the contents of the brood chamber or where the individual had an ephippium present.
Oxygen consumption rates were measured in a sealed glass micro plate equipped with planar oxygen sensor spots with optical isolation glued onto the bottom of 128–148 µl (mean: 138 µl) wells (Loligo® Systems, Denmark) integrated with a 24 channel fluorescence-based respirometry system, the SDR SensorDish® Reader (PreSens, Germany). Such optode respirometry is known for its simplicity, high throughput, and high temporal resolution and sensitivity [32]. The reader was placed inside a Memmert Peltier-cooled incubator IPP (Memmert GmbH, Germany) that kept a stable 17°C temperature. Adult daphnids (BL 1.7–2.9 mm) were measured individually in their respective wells. Pressure influenced the readings greatly, which meant that the measurements had to be done without any lid on the chambers. Oxygen content was, therefore, measured immediately following the placement of animals into the wells, after which the wells were sealed using a silicone lid, which was suppressed by a lead weight to keep the wells airtight. At the end of the experimental period this lid was removed before taking the final oxygen measurements. The experimental period (30–60 min) depended on the experimental temperature, balancing sufficient consumption (approx. 10–25% reduction in O2) while being well clear of hypoxia (greater than 4 mg O2 l−1). Nine of the 24 wells functioned as controls, using a representative amount of medium (ADaM) from which the animals originated in addition to fresh ADaM. The mean change of controls was accounted for when calculating oxygen consumption of the daphnids. Oxygen consumption rate was expressed as µg O2 h−1.
Individual oxygen consumption rates (VO2) were first standardized to a common dry body mass (DW) equal to the mean of all individuals (n = 440, 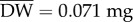 ) and using our observed relationship between individual DW and VO2 (VO2 = 2.2 × DW0.97; r2 = 0.23). The metabolic scaling exponent (0.97, s.e. 0.08) matches exponents reported in other studies of Daphnia [33], and did not differ between the different temperature treatments (electronic supplementary material, table S2-1, ANOVA, p = 0.1). Thus, size-standardized metabolic rate (sVO2) for a given individual i was calculated as:
) and using our observed relationship between individual DW and VO2 (VO2 = 2.2 × DW0.97; r2 = 0.23). The metabolic scaling exponent (0.97, s.e. 0.08) matches exponents reported in other studies of Daphnia [33], and did not differ between the different temperature treatments (electronic supplementary material, table S2-1, ANOVA, p = 0.1). Thus, size-standardized metabolic rate (sVO2) for a given individual i was calculated as:
| 2.1 |
Based on these size-standardized oxygen consumption rates (equation (2.1)), we calculated relative values (equation (2.2)) of metabolic rates for individuals reared at the new temperatures as:
| 2.2 |
where indices j and k represent generation and clone, respectively. The relative metabolic rate thus quantifies the up- or downregulation of metabolism for animals reared at new temperatures compared with their clonal counterparts being maintained at 17°C. The distributions of the individual observations, sorted by clone, temperature, and generation (sVO2, equation (2.2)), can be viewed in electronic supplementary material, S3.
(e). Growth measurements
Somatic growth (G) was calculated as:
| 2.3 |
where Bw1 and Bw2 is dry mass (milligrams) at the start (mean 3.1 × 10−3 mg, standard error (s.e.) 4.3 × 10−4 at 12°C, mean 2.9 × 10−3 mg, s.e. 6.9 × 10−4 at 22°C) and end (mean 4.6 × 10−2 mg, s.e. 6.9 × 10−3 at 12°C, mean 3.8 × 10−2 mg, s.e. 4.8 × 10−3 at 22°C) of the growth period, respectively, and t is the duration of the growth period in days (mean 13.2, s.e. 1.2 days at 12°C, mean 9.45, s.e. 2.31 days at 17°C, and mean 5.3, s.e. 0.4 days at 22°C). In the TGP experiment, all generations were tested simultaneously within each of the two new temperatures, 12°C and 22°C (figure 1). Similarly, all the animals in the maternal effect experiment were tested simultaneously at 17°C. All animals were size measured within 24 h after birth, visually inspected for maturation status one to three times daily, and measured once again as soon as they reached maturity. Maturation is defined as the first appearance of eggs in the brood chamber (ovulation). This measure of somatic growth is tightly correlated with the intrinsic population growth rate r [26], and thus represents a measure of fitness. Protocols for feeding and measurements followed those used in the metabolism experiment (see above), except that animals were reared individually in 50 ml plastic tubes.
(f). Statistics
When analysing metabolism; temperature during development, number of generations since the transfer to the new temperature, clone identity (all categorical variables), and number of eggs at early or late embryonic stages (continuous variables) were included as fixed effects in the full model. To test for genotype × environment, TGP × environment, and genotype × TGP interactions the interactions between clone and temperature, generation and temperature, and clone and generation were also included. Replicate number (15 animals representing all clones and temperatures, within a generation, were tested simultaneously) was included as a random effect (categorical variable).
For the main somatic growth analyses (investigating TGP effects), fixed effects in the full model included the number of generations, clone identity (both categorical), as well as the interaction between these two variables. As we were unable to start growth experiments for all individuals on the exact same day, experiment start date (categorical variable) was tested as a random effect. Within-clutch correlations were also analysed, using clutch ID as a random intercept. For the experiment at 22°C, initial analyses suggested a bimodal distribution of growth rates which corresponded to the distributions of age at maturity (5 versus 6 days, mean 5.25, s.e. 0.46). Thus, considerable noise appeared to be due to an insufficient frequency of maturity checks at this temperature. As all clones and generations were represented among both age classes (5 versus 6 days), we included the number of days over which growth was measured as a fixed effect for this temperature treatment. For the subsequent 12°C experiment, the maturity status was checked more regularly, and in addition the range of ages at maturity was considerably larger (10–17 days, mean 13.2, s.e. 1.2 days). Thus, in this treatment no such effects were noted.
In the separate growth experiment at 17°C (investigating additive maternal effects), the number of generations variable was replaced with maternal temperature (categorical). Experimental start date was similarly treated as a random effect. However, in this experiment clutch ID was not used as a random effect for the maternal effect experiment, as only one to two individual(s) represented a given clutch. Maturation status was checked two to three times daily and growth showed no signs of statistical noise due to maturation time, suggesting that it was not too infrequent.
All statistical analyses were performed using the statistical software R v. 3.2.1 [34]. Linear mixed models were fitted using the lme4 package [35,36]. Models that included significant random effects had estimates fitted with restricted maximum likelihood (REML). Model selection was conducted using a backwards selection procedure where variables were removed sequentially until no further simplification could be made without causing a significant decrease in log-likelihood (i.e. log-likelihood ratio test [36], where the degrees of freedom were estimated by Satterthwaite approximation using the lmerTest package [37]). Thermal autocorrelation analysis of the study site was analysed using the acf package in R, using daily mean temperatures from the summer of 2016 (see electronic supplementary material, S2 for correlogram). For details regarding this analysis, see [38,39].
3. Results
(a). Metabolism
Overall across all generations, animals living at a lower temperature had upregulated their metabolism (mean sVO2 = 0.201 µg O2 h−1, s.e. 0.007), and animals living at higher temperatures had downregulated their metabolism (mean sVO2 = 0.142 µg O2 h−1, s.e. 0.007), compared with the control group being maintained at 17°C (mean sVO2 = 0.162 µg O2 h−1, s.e. 0.007).
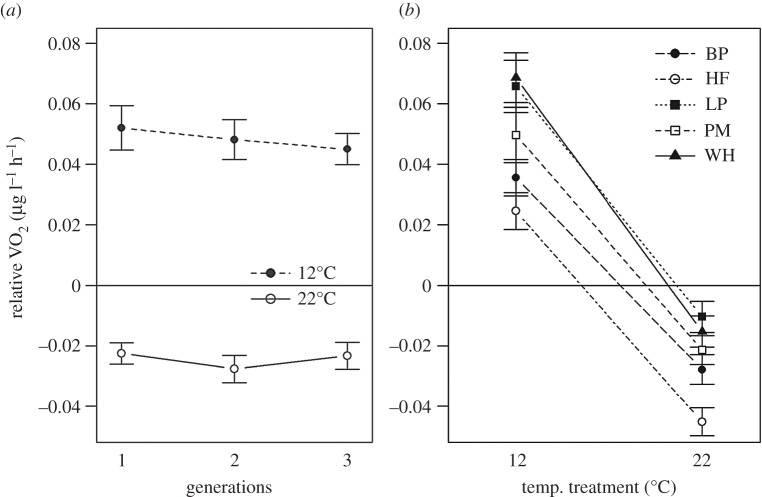
When analysing metabolic rate in individuals from new temperatures relative to that of their counterparts being kept at their original temperature (rVO2), the random effect of replicate number could not be removed from the model without causing a significant decrease in log-likelihood (p < 0.001). All interactions tested for could be removed (generation × temperature p = 0.23, generation × clone p = 0.09, clone×temperature p = 0.16, electronic supplementary material, S3). The effect of rearing temperature was significant (p < 0.001), whereas the number of generations kept at the new temperature had no effect (p = 0.73). Thus, adjustments of metabolic rate occurred during the first generation in a new thermal environment but then remained stable in subsequent generations (figure 2a). The final model also included a significant positive effect of the number of eggs at a late development stage (p < 0.001), increasing the oxygen consumption by 0.010 µg O2 h−1 egg−1 (s.e. 0.001). There was no such effect of number of eggs at an early development stage (p = 0.14). Finally, there was a minor significant effect of clone identity (p < 0.05, figure 2b).
Figure 2.
Size-standardized metabolic rate (rVO2, mean ± s.e., measured at 17°C) of D. pulex reared at two new temperatures for one, two, or three generations relative to controls being maintained at the original temperature (17°C). Deviations from the zero baselines thus indicate an upregulation (positive values) or downregulation (negative values) in VO2. (a) Generation-specific mean rVO2 across all clones; (b) clone-specific rVO2 means across all generations.
(b). Growth, transgenerational plasticity
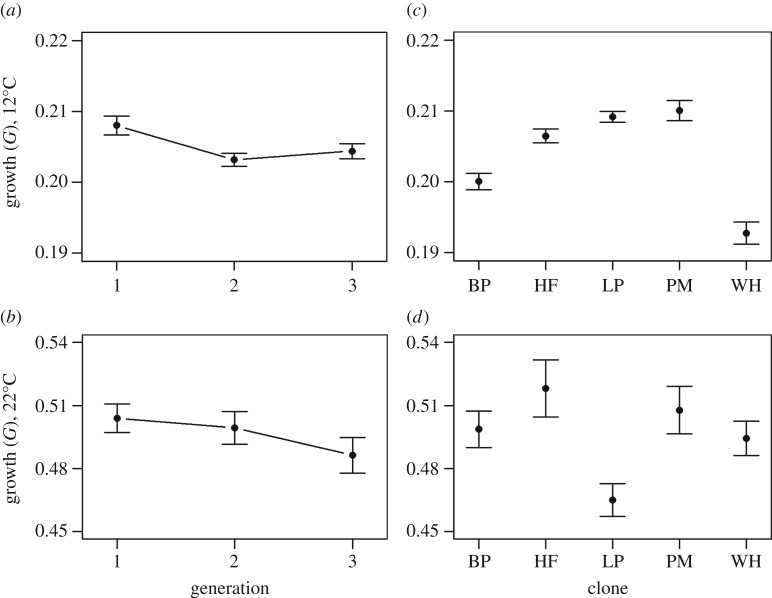
In the growth data for animals experiencing 12°C, there was no significant effect from using clutch ID as a random effect (p ∼ 1), whereas experiment start date effects were significant (p < 0.001). No significant interaction occurred between clones and TGP effects (p = 0.12). There was no general response from TGP, with approximately equal growth in all generations (figure 3a, p = 0.55). The different clones showed significant difference in growth at 12°C (figure 3b, p < 0.01). At 22°C, the effect of clutch ID was significant (p < 0.001), as was the effect of experimental start date (p < 0.01). The interaction between clone and generation number did not show a significant influence on the growth (p = 0.32). Genetic differences and TGP were absent, as neither the generation number variable nor clone variable proved significant in the final models (figure 3c,d, p = 0.10 and p = 0.12, respectively).
Figure 3.
Mean (±s.e.) growth until maturity (G, equation (2.3)) in D. pulex. (a,b) Growth, in the 1st and two subsequent generations after being introduced to new thermal environments; 12°C (a) and 22°C (b), with n = 205 and n = 162 animals, respectively. The ancestors had reproduced asexually for 12–15 months at an original temperature (17°C) prior to 1st generation. (c,d) Growth for the different clones at 12°C (c) and 22°C (d).
(c). Growth, maternal effects
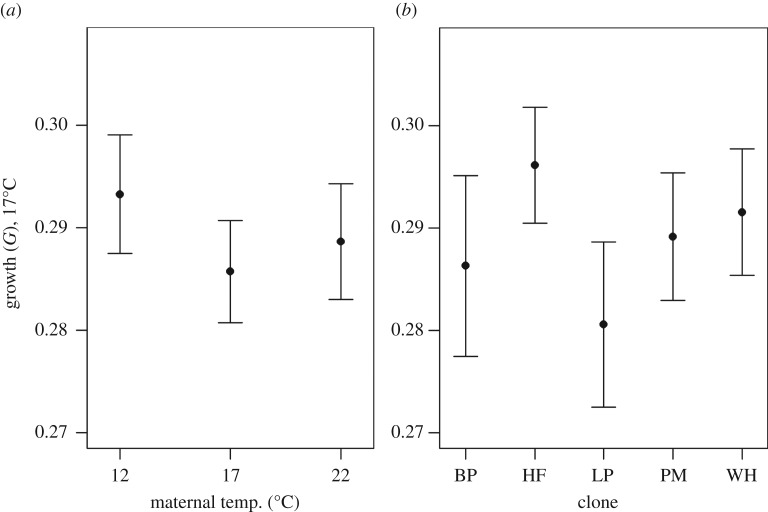
Temporal laboratory fluctuations (experiment replicate number, see Material and methods) were also present in the growth experiment at 17°C (p < 0.001). As in the analyses of growth at 12°C and 22°C, animals grew equally at 17°C regardless of maternal temperature (figure 4a, p = 0.46). There were no differences among the clones in growth (figure 4b, p = 0.65) at 17°C, nor any significant interaction between clone and maternal temperature (p = 0.28).
Figure 4.
Mean (±s.e.) growth until maturity (G, equation (2.3)) in D. pulex at 17°C (total n = 327 inds.; (a) 77–128 inds. temperature−1; (b) 58–71 inds. clone−1). The maternal generation (n = 226) were all conceived at 17°C, but spent their life (including embryogenesis) at different maternal temperatures.
4. Discussion
In this study, we tested for thermal TGP responses in Daphnia. We found no evidence for such effects, neither for metabolic rate nor for somatic growth rate. For the metabolic rates, up- and downregulation was observed for the low- and high-temperature treatments, respectively. These patterns are consistent with the cold-adaptation hypothesis, whereby ectothermic organisms living in cold environments are expected to upregulate their metabolism relative to those living in warmer environments when tested at a common temperature. This represents a mechanism to counteract the direct passive effect of reduced temperature on their ability to feed, grow, and reproduce [16,17]. However, the patterns were consistent for all three generations, such that the level of up- or downregulation was just as great for the first generation (having experienced only within-generation plasticity) as for the two subsequent generations (where additional TGP effects would be expected). Thus, the full possible extent of such adaptation was obtained through acclimation occurring during post-ovulation egg development and/or the juvenile stage. Consistent with this, we observed no tendency for increased somatic growth over the three generations when reared at new temperatures. Finally, we found no evidence for effects of temperatures experienced by mothers on the growth rate of their offspring. The negative results with respect to a TGP response was, therefore, not caused by counteracting additive maternal effects. As our measure of somatic growth rates is highly correlated with fitness (i.e. population growth rate r [26]), this also suggests an absence of thermal TGP responses in traits other than metabolic rate that would provide fitness benefits.
Our results are inconsistent with previous studies reporting the presence of thermal TGP responses in animals (e.g. [3,22–24]). One potential explanation of this discrepancy could be a difference in thermal autocorrelation patterns between study systems. One obvious prerequisite for expecting the evolution of TGP as an adaptive mechanism is that the environment experienced by mothers has some predictive value for the environments their offspring will encounter. In Lake Asklundvatnet, the water temperature on a given day is significantly positively correlated within a lag of up to 12 days, which is a period that exceeds the age of first reproduction during considerable periods of the summer (maturation time 5–14 days between 12°C and 22°C, electronic supplementary material, S1). Thus, the thermal properties of our study system are similar to the temperature autocorrelation patterns observed in other study systems, both where thermal TGP in ectotherms is shown to be present [40] and absent [39]. Both these marine study systems showed significant autocorrelation in temperature for 9 and 15 days, respectively, which were relevant biological timescales for the ectotherms studies.
In attempting to explain the discrepancies between our results and previous studies demonstrating thermal TGP, we suggest that one should consider the potential for confounding effects that may arise in tests of thermal TGP. The suggested confounding effects are not highlighted as to deny the existence of TGP, but intend to exemplify certain issues with commonly applied study designs in this field. This is necessary to improve the ability of future studies to disentangle TGP effects from other sources of variance. First, when animals are moved back and forth between temperatures to test for interactive effects, focal traits could be measured during different temporal periods due to logistical reasons. When measurements are disjointed on a temporal scale, significant differences might appear between treatments due to laboratory fluctuations (this study, [39]). Second, for highly fecund sexually reproducing organisms (such as those used in [3,22]) there is often extensive mortality either at birth or through life (e.g. approx. 50–75% in [3], 60–90% in [40]), and/or individuals might fail to mature and/or reproduce at certain temperatures [24]. Even if some members within each family survive and reproduce, it will remain unknown to what extent genetic composition among treatment groups and generations remain constant. For clonally reproducing organisms, effects of selective mortality/breeding can usually be excluded. To some degree, one may also exclude genetic changes in strains of sexually reproducing organisms that are fully homozygous (e.g. Arabidopsis thaliana [41]). Finally, studies that aim to apply a certain environmental treatment to the parental generation may inadvertently expose early stages of the offspring generation to the same treatment. This may be particularly important for organisms with short life cycles. For example, for studies of Daphnia, a period of 24 h post-birth may represent approximately 20% of the juvenile lifespan (and 100% of the embryonic development). Yet, it is not uncommon for experimental designs to allow F1 offspring periods of 12–24 h following birth in the same environment as the parental generation before transferring the offspring to the new environment, and then use the comparison of phenotypes of F1 from different parental environments as a test of TGP. This design has recently been used to show thermal TGP in growth/fitness for Daphnia [23]. However, an alternative explanation could be that poikilothermic individuals having completed their egg development as well as the initial period after birth at the same temperature as their mothers perform best at that temperature due to within-generation phenotypic plasticity. Late transfer of offspring and within-generation phenotypic plasticity could also potentially confound other studies that investigate the role of TGP in asexual animals with respect to environmental variables such as food concentration [42,43], pathogen abundance [43–45], exposure to toxic substances [46], predator presence [47], and temperature and salinity [48].
With our approach, where we transferred the parental generation to contrasting environments shortly after ovulation of eggs (rather than transferring their offspring at birth), and tested for changes in phenotypes of clones over the subsequent generations, we minimize the confounding effects identified above. To our knowledge, only in two previous studies have ‘pregnant’ Daphnia, instead of neonates, been introduced into a new environment to study TGP. These studies show TGP effects of maternal predation exposure on offspring defensive morphology [49], and maternal food and photoperiod exposure on ephippia production by offspring [50]. Thus, there seems to be a clear potential for evolution of adaptive TGP in Daphnia. The fact that we failed to find any differences among generations in the present study suggests that thermal TGP responses to maternal or grand maternal temperature are weak or absent with the given experimental settings. Within-generation phenotypic responses to temperature (e.g. metabolic rate (this study), haemoglobin concentrations [51]) could be sufficiently rapid, and the costs of responding rapidly sufficiently low, so that any additional TGP responses would be of marginal adaptive value.
In conclusion, our study provides strong evidence for within-generation phenotypic plasticity in Daphnia metabolic rates as a response to temperature regimes, but no indication that thermal TGP plays a role in improving fitness under changing temperatures. We suggest that future studies of thermal TGP should use designs that clearly separate between these two sources of variation, as well as avoiding effects of selective mortality, and caution that failing to do so may lead to an overly optimistic view on the ability of organisms to adapt to changing climates through TGP.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank D. Ebert and J. Stillman for information on culturing Daphnia sp., V. Yashchenko for culture maintenance, E. Fossen, E.G. Ofstad, K. Räsänen, and C. Pelabon for comments.
Data accessibility
The data used for this article is available at the Dryad Digital Depository: http://dx.doi.org/10.5061/dryad.fp00p [52].
Authors' contributions
Ø.N.K. participated in the study design, method development, animal husbandry, experimental procedures, data and statistical analysis, and writing of the manuscript. C.B. helped with the study design, method development, statistical analysis, and manuscript revisions. S.E. initiated the project, developed the study design and methods, revised the manuscript, and participated in the data and statistical analysis. All authors gave final approval for publication.
Competing interests
The authors have no competing interests to declare.
Funding
The study received funding from The Research Council of Norway, projects 230482, and was partly supported by the Research Council of Norway through its Centres of Excellence funding scheme, project no. 223257/F50 and the Norwegian University of Science and Technology (NTNU).
References
- 1.Salinas S, Brown SC, Mangel M, Munch SB. 2013. Non-genetic inheritance and changing environments. Non-Genetic Inheritance 1, 38–50. ( 10.2478/ngi-2013-0005) [DOI] [Google Scholar]
- 2.Herman JJ, Sultan SE, Horgan-Kobelski T, Riggs C. 2012. Adaptive transgenerational plasticity in an annual plant: grandparental and parental drought stress enhance performance of seedlings in dry soil. Integr. Comp. Biol. 52, 77–88. ( 10.1093/Icb/Ics041) [DOI] [PubMed] [Google Scholar]
- 3.Shama LNS, Wegner KM. 2014. Grandparental effects in marine sticklebacks: transgenerational plasticity across multiple generations. J. Evol. Biol. 27, 2297–2307. ( 10.1111/jeb.12490) [DOI] [PubMed] [Google Scholar]
- 4.Galloway LF, Etterson JR. 2007. Transgenerational plasticity is adaptive in the wild. Science 318, 1134–1136. ( 10.1126/science.1148766) [DOI] [PubMed] [Google Scholar]
- 5.Fox CW, Mousseau TA. 1998. Maternal effects as adaptations for transgenerational phenotypic plasticity in insects. In Maternal effects as adaptations (eds Mousseau TA, Fox CW), pp. 159–177. New York, NY: Oxford University Press. [Google Scholar]
- 6.Via S, Gomulkiewicz R, Dejong G, Scheiner SM, Schlichting CD, Vantienderen PH. 1995. Adaptive phenotypic plasticity—consensus and controversy. Trends Ecol. Evol. 10, 212–217. ( 10.1016/S0169-5347(00)89061-8) [DOI] [PubMed] [Google Scholar]
- 7.Forsman A. 2015. Rethinking phenotypic plasticity and its consequences for individuals, populations and species. Heredity 115, 276–284. ( 10.1038/hdy.2014.92) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell DL, Galloway LF. 2007. Plasticity to neighbour shade: fitness consequences and allometry. Funct. Ecol. 21, 1146–1153. ( 10.1111/j.1365-2435.2007.01327.x) [DOI] [Google Scholar]
- 9.Dorn LA, Pyle EH, Schmitt J. 2000. Plasticity to light cues and resources in Arabidopsis thaliana: testing for adaptive value and costs. Evolution 54, 1982–1994. ( 10.1111/j.0014-3820.2000.tb01242.x) [DOI] [PubMed] [Google Scholar]
- 10.Tufto J. 2000. The evolution of plasticity and nonplastic spatial and temporal adaptations in the presence of imperfect environmental cues. Am. Nat. 156, 121–130. ( 10.1086/303381) [DOI] [PubMed] [Google Scholar]
- 11.Reed TE, Waples RS, Schindler DE, Hard JJ, Kinnison MT. 2010. Phenotypic plasticity and population viability: the importance of environmental predictability. Proc. R. Soc. B 277, 3391–3400. ( 10.1098/rspb.2010.0771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Botero CA, Weissing FJ, Wright J, Rubenstein DR. 2015. Evolutionary tipping points in the capacity to adapt to environmental change. Proc. Natl Acad. Sci. USA 112, 184–189. ( 10.1073/pnas.1408589111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tufto J. 2015. Genetic evolution, plasticity, and bet-hedging as adaptive responses to temporally autocorrelated fluctuating selection: a quantitative genetic model. Evolution 69, 2034–2049. ( 10.1111/evo.12716) [DOI] [PubMed] [Google Scholar]
- 14.Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. 2001. Effects of size and temperature on metabolic rate. Science 293, 2248–2251. ( 10.1126/science.1061967) [DOI] [PubMed] [Google Scholar]
- 15.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. 2004. Toward a metabolic theory of ecology. Ecology 85, 1771–1789. ( 10.1890/03-9000) [DOI] [Google Scholar]
- 16.White CR, Alton LA, Frappell PB. 2012. Metabolic cold adaptation in fishes occurs at the level of whole animal, mitochondria and enzyme. Proc. R. Soc. B 279, 1740–1747. ( 10.1098/rspb.2011.2060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke A. 2003. Costs and consequences of evolutionary temperature adaptation. Trends Ecol. Evol. 18, 573–581. ( 10.1016/j.tree.2003.08.007) [DOI] [Google Scholar]
- 18.Pörtner HO, et al. 2001. Climate induced temperature effects on growth performance, fecundity and recruitment in marine fish: developing a hypothesis for cause and effect relationships in Atlantic cod (Gadus morhua) and common eelpout (Zoarces viviparus). Cont. Shelf Res. 21, 1975–1997. ( 10.1016/S0278-4343(01)00038-3) [DOI] [Google Scholar]
- 19.Pörtner HO. 2002. Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp. Biochem. Physiol. 132, 739–761. ( 10.1016/S1095-6433(02)00045-4) [DOI] [PubMed] [Google Scholar]
- 20.Pfennig DW, Wund MA, Snell-Rood EC, Cruickshank T, Schlichting CD, Moczek AP. 2010. Phenotypic plasticity's impacts on diversification and speciation. Trends Ecol. Evol. 25, 459–467. ( 10.1016/j.tree.2010.05.006) [DOI] [PubMed] [Google Scholar]
- 21.Price TD, Qvarnström A, Irwin DE. 2003. The role of phenotypic plasticity in driving genetic evolution. Proc. R. Soc. B 270, 1433–1440. ( 10.1098/rspb.2003.2372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salinas S, Munch SB. 2012. Thermal legacies: transgenerational effects of temperature on growth in a vertebrate. Ecol. Lett. 15, 159–163. ( 10.1111/j.1461-0248.2011.01721.x) [DOI] [PubMed] [Google Scholar]
- 23.Walsh MR, Whittington D, Funkhouser C. 2014. Thermal transgenerational plasticity in natural populations of Daphnia. Integr. Comp. Biol. 54, 822–829. ( 10.1093/Icb/Icu078) [DOI] [PubMed] [Google Scholar]
- 24.Donelson JM, Munday PL, McCormick MI, Pitcher CR. 2012. Rapid transgenerational acclimation of a tropical reef fish to climate change. Nat. Clim. Change 2, 30–32. ( 10.1038/Nclimate1323) [DOI] [Google Scholar]
- 25.Shama LNS, Strobel A, Mark FC, Wegner KM. 2014. Transgenerational plasticity in marine sticklebacks: maternal effects mediate impacts of a warming ocean. Funct. Ecol. 28, 1482–1493. ( 10.1111/1365-2435.12280) [DOI] [Google Scholar]
- 26.Lampert W, Trubetskova I. 1996. Juvenile growth rate as a measure of fitness in Daphnia. Funct. Ecol. 10, 631–635. ( 10.2307/2390173) [DOI] [Google Scholar]
- 27.Jablonka E. 2013. Epigenetic inheritance and plasticity: the responsive germline. Prog. Biophys. Mol. Biol. 111, 99–107. ( 10.1016/j.pbiomolbio.2012.08.014) [DOI] [PubMed] [Google Scholar]
- 28.Kluttgen B, Dulmer U, Engels M, Ratte HT. 1994. ADaM, an Artificial Fresh-Water for the Culture of Zooplankton. Water Res. 28, 743–746. ( 10.1016/0043-1354(94)90157-0) [DOI] [Google Scholar]
- 29.Rasband WS. 1997–2014 ImageJ, US. Bethesda, MD. See http://imagej.nih.gov/ij/ .
- 30.Gorokhova E, Kyle M. 2002. Analysis of nucleic acids in Daphnia: development of methods and ontogenetic variations in RNA-DNA content. J. Plankton Res. 24, 511–522. ( 10.1093/plankt/24.5.511) [DOI] [Google Scholar]
- 31.Threlkeld ST. 1979. Estimating Cladoceran birth-rates—importance of egg mortality and the egg age distribution. Limnol. Oceanogr. 24, 601–612. ( 10.4319/lo.1979.24.4.0601) [DOI] [Google Scholar]
- 32.Szela TL, Marsh AG. 2005. Microtiter plate, optode respirometry, and inter-individual variance in metabolic rates among nauplii of Artemia sp. Mar. Ecol. Prog. Ser. 296, 281–289. ( 10.3354/meps296281) [DOI] [Google Scholar]
- 33.Glazier DS. 2006. The 3/4-power law is not universal: evolution of isometric, ontogenetic metabolic scaling in pelagic animals. Bioscience 56, 325–332. ( 10.1641/0006-3568(2006)56325:%5BTplinu%5D.2.0.Co;2) [DOI] [Google Scholar]
- 34.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 35.Bates D, Maechler M, Bolker BM, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 36.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R, p. 574 New York, NY: Springer Science. [Google Scholar]
- 37.Kuznetsova A, Brockhoff PB, Christensen RHB.2016. lmerTest: tests in linear mixed effects models. See http://CRAN.R-project.org/package=lmerTest .
- 38.Legendre P, Legendre L. 2012. Chapter 12–Ecological data series. In Developments in environmental modelling (eds Pierre L, Louis L), pp. 711–783. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 39.Burgess SC, Marshall DJ. 2011. Temperature-induced maternal effects and environmental predictability. J. Exp. Biol. 214, 2329–2336. ( 10.1242/jeb.054718) [DOI] [PubMed] [Google Scholar]
- 40.Shama LNS. 2015. Bet hedging in a warming ocean: predictability of maternal environment shapes offspring size variation in marine sticklebacks. Glob. Change Biol. 21, 4387–4400. ( 10.1111/gcb.13041) [DOI] [PubMed] [Google Scholar]
- 41.Suter L, Widmer A. 2013. Phenotypic effects of salt and heat stress over three generations in Arabidopsis thaliana. PLoS ONE 8, 12 ( 10.1371/journal.pone.0080819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LaMontagne JM, McCauley E. 2001. Maternal effects in Daphnia: what mothers are telling their offspring and do they listen? Ecol. Lett. 4, 64–71. ( 10.1046/j.1461-0248.2001.00197.x) [DOI] [Google Scholar]
- 43.Mitchell SE, Read AF. 2005. Poor maternal environment enhances offspring disease resistance in an invertebrate. Proc. R. Soc. B 272, 2601–2607. ( 10.1098/rspb.2005.3253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Little TJ, O'Connor B, Colegrave N, Watt K, Read AF. 2003. Maternal transfer of strain-specific immunity in an invertebrate. Curr. Biol. 13, 489–492. ( 10.1016/S0960-9822(03)00163-5) [DOI] [PubMed] [Google Scholar]
- 45.Gustafsson S, Rengefors K, Hansson LA. 2005. Increased consumer fitness following transfer of toxin tolerance to offspring via maternal effects. Ecology 86, 2561–2567. ( 10.1890/04-1710) [DOI] [Google Scholar]
- 46.Vandegehuchte MB, Lemiere F, Janssen CR. 2009. Quantitative DNA-methylation in Daphnia magna and effects of multigeneration Zn exposure. Comp. Biochem. Phys. C 150, 343–348. ( 10.1016/j.cbpc.2009.05.014) [DOI] [PubMed] [Google Scholar]
- 47.Walsh MR, Cooley F, Biles K, Munch SB. 2015. Predator-induced phenotypic plasticity within- and across-generations: a challenge for theory? Proc. R. Soc. B 282, 20142205 ( 10.1098/Rspb.2014.2205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen X, Stillman JH. 2012. Multigenerational analysis of temperature and salinity variability affects on metabolic rate, generation time, and acute thermal and salinity tolerance in Daphnia pulex. J. Therm. Biol. 37, 185–194. ( 10.1016/j.jtherbio.2011.12.010) [DOI] [Google Scholar]
- 49.Agrawal AA, Laforsch C, Tollrian R. 1999. Transgenerational induction of defences in animals and plants. Nature 401, 60–63. ( 10.1038/43425) [DOI] [Google Scholar]
- 50.Alekseev V, Lampert W. 2001. Maternal control of resting-egg production in Daphnia. Nature 414, 899–901. ( 10.1038/414899a) [DOI] [PubMed] [Google Scholar]
- 51.Lamkemeyer T, Zeis B, Paul RJ. 2003. Temperature acclimation influences temperature-related behaviour as well as oxygen-transport physiology and biochemistry in the water flea Daphnia magna. Can. J. Zool. 81, 237–249. ( 10.1139/Z03-001) [DOI] [Google Scholar]
- 52.Kielland ØN, Bech C, Einum S. 2017. No evidence for thermal transgenerational plasticity in metabolism when minimizing the potential for confounding effects. Dryad Digital Repository. ( 10.5061/dryad.fp00p) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used for this article is available at the Dryad Digital Depository: http://dx.doi.org/10.5061/dryad.fp00p [52].