Abstract
Equine protozoal myeloencephalitis is a progressive neurologic disease of horses most commonly caused by infection with the apicomplexan parasite Sarcocystis neurona. Factors affecting neuroinvasion and neurovirulence have not been determined. We investigated the pathogenesis of infection with S. neurona in horses with severe combined immune deficiency (SCID). Two immunocompetent (IC) Arabian horses and two Arabian horses with SCID were infected orally with 5 × 105 sporocysts of S. neurona. Four IC horses and one SCID horse were infected intravenously (i.v.) with 5 × 108 merozoites of the WSU-1 isolate of S. neurona. Despite prolonged parasitemia and persistent infection of visceral tissues (skeletal muscle, cardiac muscle, lung, liver, and spleen) as demonstrated by PCR and culture, SCID horses did not develop neurologic signs after oral or i.v. infection. S. neurona was undetectable in the neuronal tissues of SCID horses by either PCR, immunohistochemistry, or culture. In contrast, although parasitemia was undetectable in orally infected IC horses and of only short duration in i.v. infected IC horses, four of six IC horses developed neurologic signs. S. neurona was detectable by PCR and/or culture of neural tissue but not visceral tissue of IC horses with neurologic disease. Infected SCID horses are unable to clear S. neurona from visceral tissues, but the infection does not result in neurologic signs; in contrast, IC horses rapidly control parasitemia and infection of visceral tissues but frequently experience neuroinvasion and exhibit clinical signs of neurologic disease.
Equine protozoal myeloencephalitis (EPM) is one of the most common neurologic disorders of horses in the Americas. Although there are occasional reports of neurologic disease in horses due to Neospora hughesii, EPM is most frequently associated with infection with the apicomplexan parasite Sarcocystis neurona (9). Despite recent advances in our understanding of the life cycle of S. neurona (1, 2, 10), very little is known about the pathogenesis of infection in horses (9). S. neurona can parasitize all regions of the equine central nervous system (CNS). The route of migration of the parasite from the time of ingestion of sporocysts to neuroinvasion is unknown. Studies of gamma interferon (IFN-γ) knockout (GKO) mice, which develop fulminant neurologic disease after infection, suggest that S. neurona may initially multiply in visceral tissues (6, 8).
The major goal of our laboratory is to investigate the pathogenesis of S. neurona infection in horses by determining steps in the progression of infection and the role of innate and adaptive immunity in the control of infection and neuropathology. GKO mice develop profound neurologic disease when they are infected with S. neurona (6, 8), suggesting that IFN-γ is crucial in the elimination of that infection in mice. IFN-γ is produced by lymphocytes during an adaptive Th1 immune response, and adaptive immune responses are presumed to be important in the protection of horses from S. neurona. However, in the absence of adaptive immune responses, mice are protected from Toxoplasma gondii infection, presumably through the production of IFN-γ by cells of the innate immune system (5, 13). Similarly, two mice with severe combined immune deficiency (SCID) experimentally infected with S. neurona did not develop neurologic disease (18). In a preliminary experiment, we infected an Arabian horse with SCID (19, 28) with sporocysts of S. neurona. The infected horse became parasitemic, and S. neurona merozoites were isolated from the blood on postinfection day (PID) 21 (16). This isolate was designated the WSU-1 strain of S. neurona. Although this SCID horse did not exhibit convincing neurologic deficits, S. neurona DNA was detectable by PCR after long-term culture of brain tissue obtained at necropsy.
In the present study, we further investigate the pathogenesis of S. neurona infection in SCID and immunocompetent (IC) horses. Horses were infected orally with sporocysts isolated from the feces of opossums or intravenously (i.v.) with merozoites of the WSU-1 strain of S. neurona. Parasitemia was monitored after infection by collection of blood samples for culture and PCR. Horses were monitored for the development of neurologic abnormalities. At the time of necropsy, tissues were collected for culture, PCR, immunohistochemistry (IHC), and histopathology to determine the presence of S. neurona and associated inflammatory lesions in peripheral tissues or the CNS.
MATERIALS AND METHODS
Experimental animals.
All horses were obtained from the Arabian herd at Washington State College of Veterinary Medicine and were the offspring of horses that were heterozygous for SCID (28). All mares were seronegative for antibodies to S. neurona, and foals were allowed to suckle colostrum immediately after birth. A complete blood count was performed on each foal 24 to 48 h after birth to identify immune status. Foals with blood lymphocyte counts of <200/μl were identified as probable SCID horses. Their immune status was subsequently confirmed by PCR for detection of the specific mutation in the DNA-dependent protein kinase gene. SCID foals were removed from their dams and placed in isolation stalls as soon as their status was confirmed. IC foals were weaned from their dams a few days prior to scheduled infection. All horses were anesthetized with xylazine (1.1 mg/kg of body weight i.v.) and ketamine (2.2 mg/kg i.v.) prior to infection for collection of cerebrospinal fluid (CSF) from the atlanto-occipital space. Serum and CSF from each horse were analyzed by Western blotting to confirm the absence of specific antibodies to S. neurona (Equine Biodiagnostics, Inc., Lexington, Ky.). SCID horses were maintained using procedures previously described (19), except that they did not receive trimethoprim-sulfa antibiotics. Horses were infected with S. neurona at 2 to 4 weeks of age.
Infection with S. neurona.
Sporocysts of S. neurona were obtained from the intestines and feces of wild opossums or opossums experimentally infected with S. neurona as previously described (1, 2, 4). Prior to infection, sporocysts were identified as S. neurona by a PCR protocol which differentiates S. neurona from the closely related organism Sarcocystis falcatula (27). Horses were infected orally with 5 × 105 sporocysts of S. neurona as previously described (16). For i.v. infections, merozoites of the previously described WSU-1 strain of S. neurona were harvested from culture (16). Merozoites were washed and resuspended in medium, and 5 × 108 were injected i.v. via the jugular vein. IC horses were anesthetized as described above after infection to obtain CSF for Western blotting to detect an antibody response to S. neurona. To assess parasitemia and seroconversion, blood for culture and Western blotting, respectively, was obtained by jugular venipuncture (16). Horses were monitored for development of neurologic abnormalities. All SCID horses and selected IC horses were euthanized by pentobarbital overdose, and a complete necropsy was performed to collect tissues (cerebral cortex, brain stem, spinal cord, spleen, liver, kidney, cardiac muscle, skeletal muscle, tongue, diaphragm, and adrenal gland) for culture, PCR, and histopathology. A CSF sample was obtained from the atlanto-occipital space of each horse immediately after euthanasia.
Identification of parasites in blood and tissue samples.
In order to assess the presence and duration of parasitemia and determine the tissue distribution of parasites, blood and tissue samples were obtained for PCR and culture as previously described (16). Samples were frozen at −70°C until processed for extraction of DNA, and PCR was performed to identify S. neurona as previously described (27). Purified DNA samples were amplified, and reaction products were analyzed by electrophoresis through an agarose gel stained with ethidium bromide. Controls consisted of a no-DNA control and either uninfected equine tissues or noninoculated cell cultures. Positive controls included DNA derived from cultured bovine turbinate cells infected with S. neurona. As an additional positive control, samples were reacted with primers that amplify a segment of the equine tumor necrosis factor alpha gene. Aliquots of blood or tissue were cultured on bovine turbinate cells as previously described (16). Cultures were maintained for 3 months or until cells were losing viability because of senescence or infection. At that time, cells were harvested, DNA was extracted, and the presence or absence of S. neurona was determined by PCR as previously described (16, 27).
Samples of liver, lung, spleen, brain, spinal cord, cardiac muscle, skeletal muscle, and tongue were obtained at necropsy, fixed in 10% neutral buffered formalin, embedded in paraffin, and stained with hematoxylin and eosin for routine histopathological evaluation. Slides were examined for evidence of inflammatory changes or parasites. Formalin-fixed, paraffin-embedded samples of CNS were also examined for the presence of S. neurona antigen by IHC using a polyclonal rabbit antiserum against the parasite. Control samples demonstrated that this antibody did not cross-react with related protozoal organisms, such as Neospora caninum (data not shown). Slides stained for IHC were examined by light microscopy for positive staining, indicative of parasite antigen in tissues.
RESULTS
Infection of IC horses. (i) Clinical outcome.
The clinical observations after oral and i.v. infection of IC horses are shown in Table 1. After oral infection, both IC horses remained systemically healthy and afebrile and their serum and CSF were antibody positive by PID 30. Horse IC1 exhibited ataxia and proprioceptive deficits of all four limbs beginning on PID 42. Clinical signs were improved from PID 48 through PID 66. This horse was not euthanized. At approximately 17 months postinfection, neurological examination revealed continuing mild proprioceptive deficits. However, the antibody to S. neurona was slightly positive in the serum and negative in the CSF at that time. Horse IC2 did not exhibit abnormal neurologic signs and was euthanized on PID 90.
TABLE 1.
Clinical outcomes of horses infected orally or i.v. with S. neuronaa
| Horse | Route of infection | Appearance of neurologic signs (PID) | Euthanasia (PID) |
|---|---|---|---|
| IC1 | p.o. | 42 | ND |
| IC2 | p.o. | Not observed | 90 |
| IC3 | i.v. | Not observed | 80 |
| IC4 | i.v. | 289 | 471 |
| IC5 | i.v. | 28 | 41 |
| IC6 | i.v. | 50 | 73 |
| SCID1 | p.o. | Not observed | 32 |
| SCID2 | p.o. | Not observed | 21 |
| SCID3 | i.v. | Not observed | 96 |
p.o., per os; ND, not done.
Horses IC3, IC4, and IC5 had a mild to moderate febrile response for 2 to 3 days after i.v. infection. Otherwise, they remained systemically healthy and afebrile throughout the infection period and became serum and CSF antibody positive after infection. Horses IC5 and IC6 developed neurologic signs at PIDs 28 and 50, respectively. They exhibited ataxia and proprioceptive deficits in all four limbs and remained neurologically abnormal until they were euthanized on PIDs 41 and 73, respectively. Horse IC3 did not develop signs of neurologic disease after infection. That horse was treated with dexamethasone at 15 mg intramuscularly once daily for 8 days (approximately 0.1 mg/kg) beginning on PID 72 and euthanized on PID 80.
Horse IC4 did not develop neurological abnormalities during the 73-day observation period after infection and was returned to the Washington State University research herd. At that time, the horse had strongly positive serum and CSF antibody responses to S. neurona as detected by Western blotting. At PID 289, the horse was observed to have moderate ataxia and proprioceptive deficits in all four limbs but was otherwise clinically healthy. Serum and CSF exhibited low positivity and weak positivity (as reported by the lab running the assay), respectively, for antibody to S. neurona. Standing lateral radiographs of the cervical spine were considered to be within normal limits. The horse was treated conservatively with stall and paddock rest. Neurological abnormalities persisted, and the horse was euthanized on PID 471. At the time of euthanasia, serum and CSF were weakly positive for antibodies to S. neurona. No cause for the neurological abnormalities was apparent on gross or histopathological examination of tissues obtained at necropsy. Culture of neural tissues was negative for DNA of S. neurona, but a sample of the spinal cord obtained at the level of the first thoracic vertebra was weakly positive by PCR.
(ii) Parasitemia.
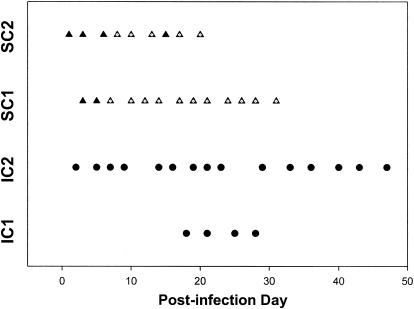
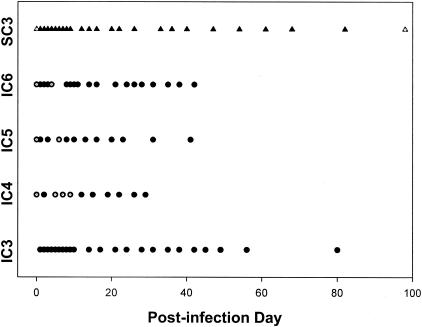
Blood was collected from all horses for parasite culture at frequent intervals throughout the infection period (Fig. 1 and 2). After oral infection, parasites were not detected in the blood of orally infected IC horses (IC1 and IC2) by PCR or culture at any time. After i.v. infection of horse IC3, parasites were not detected in the blood by direct PCR or culture at any time point. For subsequent i.v. infections (horses IC4, IC5, and IC6), a blood sample was obtained at 3 h postinfection to confirm the viability of the inoculum. The 3-h blood sample was culture positive for S. neurona in all horses. Parasites were cultured from the blood of horses IC4, IC5, and IC6 at several time points between PIDs 4 and 9 but not at PIDs 1 to 3 (Fig. 2).
FIG. 1.
Days of parasitemia for IC (circles) and SCID (triangles) horses after oral infection. Filled symbols, results for blood samples that were negative for S. neurona; nonfilled symbols, results for blood samples that were positive for S. neurona. SC, SCID.
FIG. 2.
Days of parasitemia for IC and SCID horses after i.v. infection. See the legend to Fig. 1 for a key.
(iii) Postmortem evaluation.
Tissues obtained from each horse at necropsy were examined by direct PCR, culture on bovine turbinate cells, histopathology, and IHC for evidence of S. neurona. Results are summarized in Table 2. All peripheral (non-CNS) tissues were negative for S. neurona in orally infected IC horses. Peripheral tissues from IC horses infected i.v. were negative for S. neurona by PCR and culture. However, CNS samples from horses IC4, IC5, and IC6 (the horses that developed neurological abnormalities after infection) were positive by PCR and/or culture. For all PCR analysis, negative (no DNA or uninfected tissues) and positive (tumor necrosis factor alpha gene amplification) controls yielded appropriate results. The only abnormality observed by light microscopy in tissues from IC horses was severe diffuse hepatic lipidosis in horse IC3, the horse that was treated with dexamethasone prior to euthanasia. The results of IHC of CNS tissues from all IC horses, with a polyclonal rabbit anti-S. neurona antiserum, were negative.
TABLE 2.
Identification of S. neurona in peripheral and CNS tissues from IC and SCID horses
| Horsea | Route of infectionb | PCR result for:
|
Culture result for:
|
||
|---|---|---|---|---|---|
| Peripheral tissues | CNS | Peripheral tissues | CNS | ||
| IC1 | p.o. | NDc | ND | ND | ND |
| IC2 | p.o. | − | − | − | − |
| IC3 | i.v. | − | − | − | − |
| IC4 | i.v. | ND | + | ND | − |
| IC5 | i.v. | − | + | − | − |
| IC6 | i.v. | − | − | − | + |
| SCID1 | p.o. | + | − | + | − |
| SCID2 | p.o. | + | − | + | − |
| SCID3 | i.v. | + | − | + | − |
Bold letters indicate horses that developed neurological abnormalities after infection.
p.o., per os.
ND, not done.
Infection of SCID horses. (i) Clinical outcome.
The clinical outcome after oral and i.v. infection of SCID horses is shown in Table 1. Oral infection of horses SCID1 and SCID2 resulted in no obvious neurologic signs; these horses were euthanized on PIDs 32 and 21, respectively. Horse SCID1 had a serum immunoglobulin G concentration of <400 mg/dl at 24 h of age and was treated i.v. with 1.5 liters of fresh plasma from his dam and intramuscularly with amikacin and sodium ceftiofur. A patent urachus and umbilical abscess developed. Antimicrobial therapy was continued until the horse was euthanized on PID 32 due to severe pneumonia. Horse SCID2 exhibited a dry cough and intermittent fever throughout the infection period. He was treated with sodium ceftiofur and/or gentamicin intermittently beginning on PID 14. However, the horse's condition continued to deteriorate, and he was euthanized on PID 21 due to severe pneumonia. Equine adenovirus, a common opportunistic pathogen of SCID horses, was isolated from samples of lung collected at necropsy (19). The sera and CSF of both SCID horses were antibody negative to S. neurona by Western blotting at the time of euthanasia.
Horse SCID3 was systemically healthy at the time of infection and did not exhibit a febrile reaction after i.v. injection of merozoites. This horse remained healthy throughout most of the infection period and was not treated with antimicrobial agents. The horse became persistently febrile at approximately PID 84, at which time he exhibited signs of respiratory tract disease that worsened until euthanasia on PID 95. Lung tissue obtained at necropsy was histologically normal. The horse remained neurologically normal on clinical examination and serum and CSF antibody negative to S. neurona by Western blotting throughout the experimental period.
(ii) Parasitemia.
Blood was collected from all horses for parasite culture at frequent intervals throughout the infection period (Fig. 1 and 2). Although direct PCR of DNA extracted from blood samples of SCID horses was negative at all time points, parasites were isolated from horses SCID1 and SCID2 by culture of blood samples on bovine turbinate cells on multiple days postinfection, beginning at PIDs 7 and 8, respectively. These parasites were morphologically indistinguishable from known isolates of S. neurona at the light microscopy level (data not shown). Amplification of DNA segments by use of primer pairs specific for S. neurona (27) confirmed the identity of all isolates (data not shown). Although the blood sample obtained from horse SCID3 at 3 h after i.v. infection was culture positive for parasites, confirming the viability of the inoculum administered to that horse, S. neurona was not isolated from any subsequent blood samples until PID 95, the day of euthanasia.
(iii) Postmortem evaluation.
Culture of peripheral tissues, but not CNS tissues, from horses SCID1 and SCID2 yielded S. neurona. Liver, spleen, and skeletal muscle from horse SCID1 and skeletal and cardiac muscle from horse SCID2 were culture positive. Horse SCID1 was PCR positive for S. neurona in lung, cardiac muscle, spleen, liver, skeletal muscle, kidney, and tongue. Only the lung tissue of horse SCID2 was PCR positive. Peripheral tissues of horse SCID3 (heart, lung, liver, spleen, and skeletal muscle) were PCR and/or culture positive, but CNS tissues from this horse were consistently negative. Histopathologic evaluation of hematoxylin- and eosin-stained tissue sections revealed bronchopneumonia in horses SCID1 and SCID2 and mild multifocal hepatic necrosis in horse SCID3. Horse SCID1 also had evidence of moderate follicular lymph node necrosis. The results of IHC of CNS tissues from all SCID horses, with a polyclonal rabbit anti-S. neurona antiserum, were negative.
DISCUSSION
The present work demonstrates that infected SCID horses are unable to terminate parasitemia or clear S. neurona from visceral tissues, but the infection does not result in clinical signs of neurologic disease; in contrast, IC horses rapidly control parasitemia and infection of visceral tissues but experience neuroinvasion and clinical signs of neurologic disease. This finding suggests that adaptive immune responses are important for the termination of parasitemia and control of parasite reproduction in peripheral tissues but are not necessary for the prevention of signs of neurologic disease after infection.
Arabian horses with SCID lack specific B- and T-cell responses because of a mutation in the DNA-dependent kinase catalytic subunit (23). These horses have been used experimentally to determine the role of adaptive immune responses in the pathogenesis of equine infectious anemia and equine piroplasmosis (babesiosis) (14, 20), and conditions for their maintenance have been described previously (19). The use of potentiated sulfonamide antimicrobials is recommended in these foals to prevent Pneumocystis carinii infection (19). However, because of the parasiticidal effect of these antimicrobials, they could not be used for the present study. Instead, two of the three SCID horses used in this study were treated with gentamicin or sodium ceftiofur when they showed signs of pneumonia. These drugs do not have significant parasiticidal activity and do not cross the intact equine blood-brain barrier in significant concentrations.
Despite recent advances in our understanding of the life cycle of S. neurona (1, 2, 10), very little is known about the pathogenesis of infection in horses (9). S. neurona can parasitize all regions of the equine CNS and may be found in neurons, mononuclear cells, and glial cells. Studies of GKO mice, which develop fulminant neurologic disease after infection, suggest that S. neurona strains initially multiply in intestinal tissues, with parasitemia occurring 1 to 8 days after infection (6, 7). We have previously reported the isolation of merozoites of S. neurona from the blood of a SCID horse after oral infection with sporocysts (16). The present report confirms the presence of persistent parasitemia in SCID horses after oral infection. The absence of detectable parasitemia in similarly infected IC horses suggests that parasitemia is controlled by specific immune responses.
After oral infection, SCID horses lived 21 to 98 days. At the time of necropsy, parasites were widely disseminated throughout the peripheral tissues of these horses, but there was no evidence of neuroinvasion or neuropathology. In contrast, S. neurona was detected in the CNS of IC horses with abnormal neurologic examination by PCR and/or culture. There was no evidence of pathology attributable to S. neurona infection (inflammation or parasites) in the CNS of any foal, and IHC was negative for S. neurona in all foals. It has been difficult for investigators to demonstrate pathology or parasites in the CNS after experimental infection of horses even when neurologic signs are evident. Saville et al. reported the presence of minimal numbers of or no inflammatory CNS lesions and no evidence of parasites in horses infected with S. neurona after transport stress (21). Fenger et al. reported mild to moderate inflammatory lesions consistent with S. neurona infection in three of five experimentally infected horses but no evidence of parasites (11). The present report provides the first evidence of parasites in the CNS of horses that develop neurologic disease after experimental infection.
Very little is known about innate or adaptive immune responses to S. neurona in horses. In murine models of infection, neurologic disease has been demonstrated only for GKO and nude mice (8, 18). IC BALB/c mice apparently clear infection without neural invasion (6, 8, 16). In nude and GKO mice, neuropathology is first evident at approximately 14 to 21 days postinfection (6, 7, 18), which is consistent with our conclusion that parasitemia precedes neural invasion. After oral infection, SCID horses demonstrated parasitemia as early as 7 to 8 days postinfection.
The lack of neuropathology in SCID horses may have been the result of low virulence of the inoculum or insufficient time between infection and euthanasia, but these possibilities were considered unlikely. Four of six IC horses in the present study developed neurologic abnormalities after infection with sporocysts or merozoites of S. neurona, suggesting that a lack of neurovirulence was not a significant factor in these experiments. In addition, merozoites of the WSU-1 strain of S. neurona used for the i.v. infections in the present study have previously been shown to be neurovirulent in GKO mice (16). SCID horses lived only 21 and 30 days after oral infection; however, this length of time is sufficient for neuropathology in susceptible mice and these horses were persistently parasitemic from PIDs 7 and 8. In addition, horse SCID3 lived for >90 days after i.v. infection with no neurological abnormalities or evidence of parasite invasion into the CNS, despite widespread dissemination of S. neurona through peripheral organs. Despite these considerations, we cannot eliminate the possibility that neurologic signs might have developed in SCID foals if they had survived longer after infection.
IC horses that developed neurologic disease first showed clinical signs at 28 to 289 days postinfection. Horse IC4 was first observed to demonstrate neurologic abnormalities at PID 289. However, this horse was not closely evaluated for neurologic disease after PID 73, and subtle abnormalities could have been present quite some time prior to presentation at PID 289. This horse had strong blood and CSF Western blot antibody responses to S. neurona at PID 68. At the time clinical signs were noticed, the CSF and serum antibody responses were much weaker and continued to decline throughout the observation period. The declining antibody response is inconsistent with current clinical guidelines for the diagnosis of EPM due to S. neurona infection (3, 9, 17). However, there was no other cause for neurologic disease evident at necropsy, and the spinal cord of this horse was positive for S. neurona by PCR.
It is possible that the lack of neurovirulence in SCID horses is a result of either protective innate immune responses or a requirement for adaptive immune responses in the pathogenesis of neurologic disease. GKO mice rapidly succumb to infection with S. neurona, suggesting that IFN-γ is crucial in elimination of this infection in mice (8, 22, 26). IFN-γ plays the main role in host resistance to toxoplasmic encephalitis (24, 25) and cryptoporidiosis (12) in mice and is produced by a variety of cell types, including CD8-positive T cells, Th1 cells activated during adaptive immune responses, γδ T cells, natural killer (NK) cells, and a population of non-T, non-NK cells in the brain. Mice with SCID and a BALB/c genetic background do not develop neurologic disease after infection with S. neurona unless they are treated with an antibody to block the effects of IFN-γ (22a). Similarly, doubly immunodeficient C57BL/6 SCID IFN-γ knockout mice are more likely to become moribund or die after infection with C. parvum than are mice carrying only the SCID mutation or only the IFN-γ knockout mutation (12).
Although SCID horses lack adaptive B- and T-cell responses, they should have functional innate immunity, including phagocytic and NK cell function, and it is possible that these innate responses are sufficient to control neural invasion by S. neurona. It is also possible that local production of IFN-γ by nonlymphoid cells in the CNS is the determining factor in preventing neurologic disease.
An alternative hypothesis is that B and/or T cells are required for neural invasion and/or neurovirulence. The method by which S. neurona gains entrance to the CNS of horses or mice is undetermined. SCID horses develop persistent parasitemia, consistent with the hypothesis that S. neurona gains entrance to the CNS across the blood-brain barrier. In support of this hypothesis, a model of experimental infection of horses in which the parasite was intracellular in host lymphocytes has recently been described (S. Ellison, T. Kennedy, and K. Brown, abstract presented at the American Association of Veterinary Parasitologists, Nashville, Tenn., 2002). It is possible that SCID horses and SCID mice fail to develop neurologic disease because parasites cannot gain access to the CNS in the absence of lymphocytes. However, we have shown that administration of anti-IFN-γ to SCID mice results in fulminant neurologic disease in the absence of T or B lymphocytes (our unpublished data).
Stress, especially transport stress, has been suggested to play a major role in the pathogenesis of infection with S. neurona in horses (21). The horses in the present study were stressed by weaning and isolation immediately prior to infection. In addition, horses SCID1 and SCID2 experienced continuous concurrent illness throughout the infection period. Horse SCID3 remained systemically healthy throughout most of the infection period, and parasitemia was demonstrable only at the time of euthanasia when the horse was suffering from a respiratory tract infection. It is possible that the stress of concurrent illness in horses SCID1 and SCID2 contributed to the ease with which parasites were isolated from blood samples obtained throughout the infection period.
Horse IC3 failed to develop evidence of neurologic disease after i.v. infection. Prior to euthanasia, this horse was treated for 8 days with dexamethasone at a dose that is sufficient to suppress immune responses and induce a relapse of equine infectious anemia virus in carrier horses (15), but there was no evidence of exacerbation of parasite infection in this horse on the basis of clinical signs or evaluation of tissues obtained at necropsy. The severe hepatic lipidosis observed in this horse was attributed to the effects of corticosteroids.
In the absence of specific adaptive immune responses, SCID horses infected with S. neurona develop persistent parasitemia and widespread dissemination of parasites throughout peripheral (non-CNS) tissues. However, these horses do not develop fulminant CNS infection, neuropathology, or clinical neurological abnormalities. This may be the result of enhanced innate immune responses controlling parasite replication in neuronal tissues or because of a requirement for adaptive responses for neuroinvasion or neurovirulence. The pathogenesis of S. neurona infection in SCID horses appears to be very similar to that of infection in SCID mice. Further investigations with SCID mouse and horse models are important to help define the role of the immune response in the pathogenesis of CNS disease in horses.
Acknowledgments
This work was funded by a grant from the Grayson-Jockey Club Research Foundation.
REFERENCES
- 1.Cheadle, M. A., S. M. Tanhauser, J. B. Dame, D. C. Sellon, M. Hines, P. E. Ginn, R. J. MacKay, and E. C. Greiner. 2001. The nine-banded armadillo (Dasypus novemcinctus) is an intermediate host for Sarcocystis neurona. Int. J. Parasitol. 31:330-335. [DOI] [PubMed] [Google Scholar]
- 2.Cheadle, M. A., C. A. Yowell, D. C. Sellon, M. Hines, P. E. Ginn, A. E. Marsh, J. B. Dame, and E. C. Greiner. 2001. The striped skunk (Mephitis mephitis) is an intermediate host for Sarcocystis neurona. Int. J. Parasitol. 31:843-849. [DOI] [PubMed] [Google Scholar]
- 3.Cohen, N. D., and R. J. MacKay. 1997. Interpreting immunoblot testing of cerebrospinal fluid for equine protozol myeloencephalitis. Comp. Cont. Ed. Pract. Vet. 19:1176-1181. [Google Scholar]
- 4.Cutler, T. J., R. J. MacKay, P. E. Ginn, E. C. Greiner, R. Porter, C. A. Yowell, and J. B. Dame. 1999. Are Sarcocystis neurona and Sarcocystis falcatula synonymous? A horse infection challenge. J. Parasitol. 85:301-305. [PubMed] [Google Scholar]
- 5.Denkers, E. Y., R. T. Gazzinelli, D. Martin, and A. Sher. 1993. Emergence of NK1.1+ cells as effectors of IFN-gamma dependent immunity to Toxoplasma gondii in MHC class I-deficient mice. J. Exp. Med. 178:1465-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubey, J. P. 2001. Migration and development of Sarcocystis neurona in tissues of interferon gamma knockout mice fed sporocysts from a naturally infected opossum. Vet. Parasitol. 95:341-351. [DOI] [PubMed] [Google Scholar]
- 7.Dubey, J. P. 2001. Parasitemia and early tissue localization of Sarcocystis neurona in interferon gamma gene knockout mice fed sporocysts. J. Parasitol. 87:1476-1479. [DOI] [PubMed] [Google Scholar]
- 8.Dubey, J. P., and D. S. Lindsay. 1998. Isolation in immunodeficient mice of Sarcocystis neurona from opossum (Didelphis virginiana) faeces, and its differentiation from Sarcocystis falcatula. Int. J. Parasitol. 28:1823-1828. [DOI] [PubMed] [Google Scholar]
- 9.Dubey, J. P., D. S. Lindsay, W. J. Saville, S. M. Reed, D. E. Granstrom, and C. A. Speer. 2001. A review of Sarcocystis neurona and equine protozoal myeloencephalitis (EPM). Vet. Parasitol. 95:89-131. [DOI] [PubMed] [Google Scholar]
- 10.Dubey, J. P., W. J. Saville, D. S. Lindsay, R. W. Stich, J. F. Stanek, C. A. Speert, B. M. Rosenthal, C. J. Njoku, O. C. Kwok, S. K. Shen, and S. M. Reed. 2000. Completion of the life cycle of Sarcocystis neurona. J. Parasitol. 86:1276-1280. [DOI] [PubMed] [Google Scholar]
- 11.Fenger, C. K., D. E. Granstrom, A. A. Gajadhar, N. M. Williams, S. A. McCrillis, S. Stamper, J. L. Langemeier, and J. P. Dubey. 1997. Experimental induction of equine protozoal myeloencephalitis in horses using Sarcocystis sp. sporocysts from the opossum (Didelphis virginiana). Vet. Parasitol. 68:199-213. [DOI] [PubMed] [Google Scholar]
- 12.Hayward, A. R., K. Chmura, and M. Cosyns. 2000. Interferon-gamma is required for innate immunity to Cryptosporidium parvum in mice. J. Infect. Dis. 182:1001-1004. [DOI] [PubMed] [Google Scholar]
- 13.Kang, H., and Y. Suzuki. 2001. Requirement of non-T cells that produce gamma interferon for prevention of reactivation of Toxoplasma gondii infection in the brain. Infect. Immun. 69:2920-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knowles, D. P., Jr., L. S. Kappmeyer, and L. E. Perryman. 1994. Specific immune responses are required to control parasitemia in Babesia equi infection. Infect. Immun. 62:1909-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kono, Y., K. Hirasawa, Y. Fukunaga, and T. Taniguchi. 1976. Recrudescence of equine infectious anemia by treatment with immunosuppressive drugs. Nat. Inst. Anim. Health Q. 16:8-15. [PubMed] [Google Scholar]
- 16.Long, M. T., M. T. Mines, D. P. Knowles, S. M. Tanhauser, J. B. Dame, T. J. Cutler, R. J. MacKay, and D. C. Sellon. 2002. Sarcocystis neurona: parasitemia in a severe combined immunodeficient (SCID) horse fed sporocysts. Exp. Parasitol. 100:150-154. [DOI] [PubMed] [Google Scholar]
- 17.MacKay, R. J. 1997. Equine protozoal myeloencephalitis. Vet. Clin. N. Am. Equine Pract. 13:79-96. [DOI] [PubMed] [Google Scholar]
- 18.Marsh, A. E., B. C. Barr, J. Lakritz, R. Nordhausen, J. E. Madigan, and P. A. Conrad. 1997. Experimental infection of nude mice as a model for Sarcocystis neurona-associated encephalitis. Parasitol. Res. 83:706-711. [DOI] [PubMed] [Google Scholar]
- 19.Perryman, L. E., T. C. McGuire, and T. B. Crawford. 1978. Maintenance of foals with combined immunodeficiency: causes and control of secondary infections. Am. J. Vet. Res. 39:1043-1047. [PubMed] [Google Scholar]
- 20.Perryman, L. E., K. I. O'Rourke, and T. C. McGuire. 1988. Immune responses are required to terminate viremia in equine infectious anemia lentivirus infection. J. Virol. 62:3073-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saville, W. J., R. W. Stich, S. M. Reed, C. J. Njoku, M. J. Oglesbee, A. Wunschmann, D. L. Grover, A. L. Larew-Naugle, J. F. Stanek, D. E. Granstrom, and J. P. Dubey. 2001. Utilization of stress in the development of an equine model for equine protozoal myeloencephalitis. Vet. Parasitol. 95:211-222. [DOI] [PubMed] [Google Scholar]
- 22.Scharton-Kersten, T. M., T. A. Wynn, E. Y. Denkers, S. Bala, E. Grunvald, S. Hieny, R. T. Gazzinelli, and A. Sher. 1996. In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J. Immunol. 157:4045-4054. [PubMed] [Google Scholar]
- 22a.Sellon, D. C., D. P. Knowles, E. C. Greiner, M. T. Long, M. T. Hines, T. Hochstatter, M. Veti, K. Gillis, and J. B. Dame. 2004. Depletion of natural killer cells does not result in neurologic disease due to Sarcocystis neurona in mice with severe combined immunodeficiency. J. Parasitol. 90:782-788. [DOI] [PubMed] [Google Scholar]
- 23.Shin, E. K., L. E. Perryman, and K. Meek. 1997. A kinase-negative mutation of DNA-PK(CS) in equine SCID results in defective coding and signal joint formation. J. Immunol. 158:3565-3569. [PubMed] [Google Scholar]
- 24.Suzuki, Y. 2002. Host resistance in the brain against Toxoplasma gondii. J. Infect. Dis. 185(Suppl. 1):S58-S565. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki, Y. 2002. Immunopathogenesis of cerebral toxoplasmosis. J. Infect. Dis. 186(Suppl. 2):S234-S240. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki, Y., H. Kang, S. Parmley, S. Lim, and D. Park. 2000. Induction of tumor necrosis factor-alpha and inducible nitric oxide synthase fails to prevent toxoplasmic encephalitis in the absence of interferon-gamma in genetically resistant BALB/c mice. Microbes Infect. 2:455-462. [DOI] [PubMed] [Google Scholar]
- 27.Tanhauser, S. M., C. A. Yowell, T. J. Cutler, E. C. Griner, R. J. MacKay, and J. B. Dame. 1999. Multiple DNA markers differentiate Sarcocystis neurona and Sarcocystis falcatula. J. Parasitol. 85:221-228. [PubMed] [Google Scholar]
- 28.Wiler, R., R. Leber, B. B. Moore, L. F. VanDyk, L. E. Perryman, and K. Meek. 1995. Equine severe combined immunodeficiency: a defect in V(D)J recombination and DNA-dependent protein kinase activity. Proc. Natl. Acad. Sci. USA 92:11485-11489. [DOI] [PMC free article] [PubMed] [Google Scholar]